Abstract
We recently characterized the histidine kinase receptor genes of Candida lusitaniae. For the present study, we have further investigated the role of SSK1 and SKN7, encoding response regulators. The results of functional analysis of mutants indicated that Ssk1p is involved in osmotolerance and pseudohyphal development, whereas Skn7p appears crucial for oxidative stress adaptation.
It is now accepted that two-component signal transduction pathways are essential for stress adaptation, morphogenesis, quorum sensing, and the regulation of virulence in fungi (28). In Saccharomyces cerevisiae, the two-component signaling system contains different proteins that work together in a phosphorelay pathway consisting of the sensor histidine kinase receptor (HKR) Sln1p, the histidine-containing phosphotransfer (HPt) protein Ypd1p, and the two response regulators (RR) Ssk1p and Skn7p. Differences in stimulus determine which RR is activated or inactivated. The Ssk1p branch of this pathway regulates a large palette of physiological processes, among which the Hog1-mediated osmoadaptation is the most documented. The Skn7p branch is involved in oxidative stress adaptation, cell wall biosynthesis, and the cell cycle (for a review, see references 9 and 15).
The budding yeast Candida lusitaniae (teleomorph, Clavispora lusitaniae) is an emerging pathogen which is characterized by its propensity to develop resistance to antifungal agents during treatment (14, 22, 26). Furthermore, this opportunistic yeast can efficiently switch to pseudohyphal growth (13). As a laboratory model, C. lusitaniae is an experimentally tractable haploid organism offering formal genetic tools based upon a complete sexual cycle reproducible in vitro (11, 23, 31) and an integrative transformation system for gene disruption (10, 30, 31).
We recently characterized three C. lusitaniae genes (SLN1, NIK1, and CHK1) potentially encoding HKRs (6) and showed that Sln1p is crucial (i) for oxidative stress adaptation when C. lusitaniae strains grow as budding yeast and (ii) in the early steps of pseudohyphal development, especially in hyperosmotic and oxidative conditions. For the current study, we characterized downstream elements, such as HPt and RR proteins. Blast analysis (2) of the C. lusitaniae database (Broad Institute Fungal Genome) revealed a single gene, YPD1, encoding a putative HPt protein, and two genes, SSK1 and SKN7, potentially encoding two-component RRs. The characterization of these genes and their corresponding proteins is reported in Table S1 and Fig. S1 in the supplemental material. Null mutants were constructed for both SSK1 and SKN7 genes by using an improved integrative transformation system based upon the “URA3-blaster” strategy (6, 25) (see Fig. S2 in the supplemental material). All disruptant and reintegrant strains are listed in Table S2 in the supplemental material. However, we failed to obtain homologous integration of the YPD1 disruption cassette at the corresponding target locus. The results of Southern blot analysis revealed that Ura+ transformants derived either from ectopic integrations of the disruption cassette (50%) or from gene replacement at the ura3 locus (50%) (see Fig. S3 in the supplemental material). This result suggested that the ypd1Δ mutant could be inviable in C. lusitaniae, as reported in several other fungal species (3, 4, 12, 19).
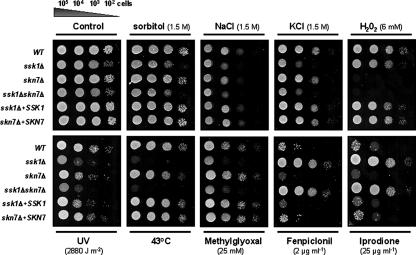
We first analyzed the growth, osmotolerance, drug sensitivities, and oxidative stress responses of ssk1Δ, skn7Δ, and ssk1Δ skn7Δ mutants. The single mutant ssk1Δ and the double mutant ssk1Δ skn7Δ exhibited increased generation times compared to that of the wild type when cultured in yeast extract-peptone-dextrose (YPD) or yeast nitrogen base medium (see Table S2 in the supplemental material). The growth of both the ssk1Δ and the ssk1Δ skn7Δ mutants is slightly affected on high-osmolarity medium (1.5 M sorbitol, NaCl or KCl) (Fig. 1), indicating that C. lusitaniae Ssk1p could be involved in the growth and in the capacity for adaptation to hyperosmotic conditions of yeast cells. Furthermore, the ssk1Δ and the ssk1Δ skn7Δ mutants exhibited hypersensitivity to UV irradiation (2,880 J·m−2) and high temperature (43°C), implying that Ssk1p is also essential for responses to these signals. Moreover, both the ssk1Δ and the ssk1Δ skn7Δ mutants were hypersensitive to methylglyoxal (25 mM), suggesting that Ssk1p is required to protect cells from this metabolic by-product (1, 20). Interestingly, both the skn7Δ and the ssk1Δ skn7Δ mutants exhibited strong sensitivities to H2O2, suggesting that Skn7p has a role in the regulation of oxidative stress in C. lusitaniae (Fig. 1).
FIG. 1.
Sensitivity of the wild-type strain, representative single mutants ssk1Δ and skn7Δ, double mutant ssk1Δ skn7Δ, and reintegrant strains ssk1Δ+SSK1 and skn7Δ+SKN7 to different stresses. All strains were grown overnight in YPD liquid medium, the cells were counted, and then dilutions (105 to 102 cells) were spotted onto YPD plate supplemented or not (control) with 1.5 M sorbitol, NaCl or KCl, 6 mM of H2O2, 25 mM methyglyoxal, 2 μg ml−1 fludioxonil, or 25 μg ml−1 iprodione. To test UV sensitivity, cells on YPD plates were exposed to UV for 12 s (2,880 J m−2). Each plate was further incubated for 2 days at 35°C and photographed. For high-temperature sensitivity, spotted cells were incubated for 1 day at 43°C and photographed. This experiment was done in triplicate, and pictures show representative plates for each condition. WT, wild type.
Neither hypersensitivity nor resistance of mutants toward the clinical antifungals amphotericin B, 5-flucytosine, and fluconazole (FLC) was observed. The cell-mediated immunities (CMIs) of all null mutants were similar to those observed for the wild-type strain (amphotericin B CMI, ≤1 μg·ml−1; 5-flucytosine CMI, ≤4 μg·ml−1; and FLC CMI, ≤8 μg·ml−1) (data not shown). Because in the filamentous fungi Neurospora crassa and Cochliobolus heterostrophus, mutations of HKR or RR genes are responsible for severe dicarboximide and phenylpyrrole resistance (16, 17, 24), we tested the effects of these antifungal drugs on our null-mutant strains. Both the ssk1Δ and the ssk1Δ skn7Δ mutants were resistant to fenpiclonil and iprodione (Fig. 1). These data demonstrate that in C. lusitaniae cells, as with the NIK1 gene disruption (6), the ssk1 deletion confers resistance to these compounds.
We next assessed the effects of the deletion of SSK1 and SKN7 on the morphological changes occurring during the sexual reproduction of C. lusitaniae, notably during conjugation (11). All mutants were still able to reproduce sexually in vitro when mated unilaterally or bilaterally (crosses are listed in the mating test section of the supplemental material) with the appropriate opposite mating type. Our data imply that, contrary to the human pathogen Cryptococcus neoformans, in which the Tco1p HKR governs sexual reproduction via the Ssk1p branch (3), the RR proteins of C. lusitaniae do not play an obvious role in the mating process.
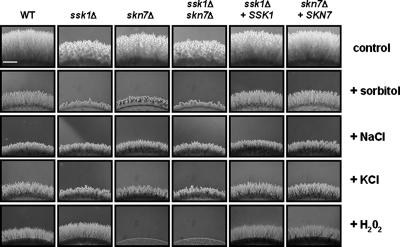
We finally investigated the capacity of the mutants to differentiate pseudohyphae as described in reference 6. Figure 2 shows representative pictures of pseudohyphal formation after 48 h of growth on yeast carbon base (YCB) medium supplemented or not with the most discriminatory concentration of sorbitol (1 M), NaCl (0.5 M), KCl (0.5 M), or H2O2 (1 mM). Those concentrations were used because higher and lower concentrations were either too toxic or had no effect on the pseudohyphal development of the wild-type strain. The lengths of pseudohyphae emerging from the edge of the colonies are reported in Table S4 in the supplemental material. Homogeneously distributed pseudohyphae were obtained for all strains on unsupplemented YCB medium, but both the ssk1Δ and the ssk1Δ skn7Δ mutants displayed a reproducible reduction of pseudohyphal length. This reduction in pseudohyphal development could result either from a delay in the morphogenetic transition induced by the mutation or from global growth defects. The addition of 1 M sorbitol produced a homogeneous reduction of the pseudohyphal growth of the wild-type strain and strongly inhibited the pseudohyphal development of the ssk1Δ, skn7Δ, and ssk1Δ skn7Δ mutants. Similar results were observed in the presence of 0.5 M KCl, whereas all strains presented equivalent pseudohyphal growth when NaCl (0.5 M) was added. These observations imply that both the SSK1 and the SKN7 gene play key roles in pseudohyphal development when C. lusitaniae is grown on high-osmolarity medium. The addition of 1 mM H2O2 produced a similar reduction of the pseudohyphal formation of the wild-type and complemented strains and completely inhibited the pseudohyphal development of the skn7Δ and the ssk1Δ skn7Δ mutants. Intriguingly, the pseudohyphal growth is 10 to 20% greater in the ssk1Δ mutant than in the wild type in these conditions.
FIG. 2.
Pseudohyphal-growth ability of mutants. Amounts of 106 cells (contained in drops of 5 μl) of the wild-type strain, representative single mutants ssk1Δ and skn7Δ, double mutant ssk1Δ skn7Δ, and reintegrant strains ssk1Δ+SSK1 and skn7Δ+SKN7 were spotted onto YCB solid medium supplemented or not with discriminatory concentrations of sorbitol (1 M), NaCl (0.5 M), KCl (0.5 M), and H2O2 (1 mM). Observations were made 48 h after spotting. This experiment was done in triplicate, and pictures show representative structures observed for each sample. The lengths of pseudohyphae emerging from the edge of the colonies are reported in Table S4 in the supplemental material. WT, wild type. Bar, 300 μm.
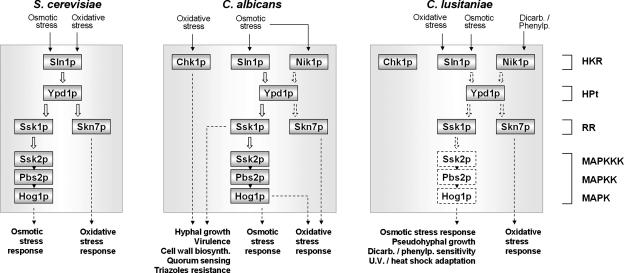
In summary, C. lusitaniae, like Candida albicans, possesses two RR proteins which could act as downstream or cross-talk elements of the Sln1p-, Nik1p-, and Chk1p-mediated transduction pathways. However, despite these similarities, the results of functional analysis of the C. lusitaniae mutants described above also reveal significant differences (Fig. 3). First, only the skn7Δ mutant is hypersensitive to oxidant conditions in C. lusitaniae, whereas both the skn7Δ and the ssk1Δ mutants are affected by oxidative stress in C. albicans (5, 21, 29). Second, while C. albicans chk1Δ and ssk1Δ mutants are hypersensitive toward FLC (7), the corresponding C. lusitaniae mutants are not (6; this study). Finally, contrary to findings for C. albicans, where the absence of SSK1 and SKN7 results in dramatic defects in true-hypha formation (5, 29), only SSK1 appears to be involved in pseudohyphal differentiation in C. lusitaniae. This difference clearly reveals that a set of specific genes controls the switch between true- and pseudohyphae. However, we cannot exclude the possibility that the HKR-mediated signaling of C. albicans behaves similarly to that of C. lusitaniae when C. albicans is undergoing pseudohyphal formation. Finally, the results of the current study (in addition to those of our previous study [6]) show that in spite of a close conservation of the His-to-Asp phosphorelay components (Sln1p, Nik1p, Chk1p, Ypd1p, Ssk1p, and Skn7p), the transduction pathways are differently regulated in C. albicans and C. lusitaniae (Fig. 3).
FIG. 3.
Models for the action of Ssk1p and Skn7p in C. lusitaniae and comparison with simplified models for S. cerevisiae (9) and C. albicans (8, 18, 27). A possible mechanism that incorporates our experimental results from this study and our previous study (6) is proposed. The RR protein Ssk1p could operate downstream of the Sln1p HKR and the HPt protein Ypd1p to regulate the osmotic stress response, pseudohyphal growth, UV adaptation, and the heat shock response. Genes encoding Ssk2p, Pbs2p, and Hog1p mitogen-activated protein kinase cascade components (dotted boxes) are located in the C. lusitaniae genome (unpublished data) and probably act downstream of Ssk1p. In addition, the Nik1p-Ypd1p-Ssk1p-mediated transduction pathway probably regulates dicarboximide (Dicarb.) and phenylpyrrole (Phenylp.) sensitivity. The RR protein Skn7p acts in oxidative stress adaptation, possibly through Sln1p and Ypd1p elements. Dotted arrows indicate mechanisms or interactions not yet fully elucidated. MAPK, mitogen-activated protein kinase; MAPKK, MAPK kinase; MAPKKK, MAPK kinase kinase.
Supplementary Material
Acknowledgments
We acknowledge the Broad Institute Fungal Genome Initiative for making the complete genome sequence of C. lusitaniae available. We thank Denise Zickler and Sandrine Castella for critical reading of the manuscript.
Footnotes
Published ahead of print on 11 April 2008.
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Aguilera, J., S. Rodriguez-Vargas, and J. A. Prieto. 2005. The HOG MAP kinase pathway is required for the induction of methylglyoxal-responsive genes and determines methylglyoxal resistance in Saccharomyces cerevisiae. Mol. Microbiol. 56228-239. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 253389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bahn, Y. S., K. Kojima, G. M. Cox, and J. Heitman. 2006. A unique fungal two-component system regulates stress responses, drug sensitivity, sexual development, and virulence of Cryptococcus neoformans. Mol. Biol. Cell 173122-3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banno, S., R. Noguchi, K. Yamashita, F. Fukumori, M. Kimura, I. Yamaguchi, and M. Fujimura. 2007. Roles of putative His-to-Asp signaling modules HPT-1 and RRG-2, on viability and sensitivity to osmotic and oxidative stresses in Neurospora crassa. Curr. Genet. 51197-208. [DOI] [PubMed] [Google Scholar]
- 5.Calera, J. A., X. J. Zhao, and R. Calderone. 2000. Defective hyphal development and avirulence caused by a deletion of the SSK1 response regulator gene in Candida albicans. Infect. Immun. 68518-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapeland-Leclerc, F., P. Paccallet, G. Ruprich-Robert, D. Reboutier, C. Chastin, and N. Papon. 2007. Differential involvement of histidine kinase receptors in pseudohyphal development, stress adaptation, and drug sensitivity of the opportunistic yeast Candida lusitaniae. Eukaryot. Cell 61782-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chauhan, N., M. Kruppa, and R. Calderone. 2007. The Ssk1p response regulator and Chk1p histidine kinase mutants of Candida albicans are hypersensitive to fluconazole and voriconazole. Antimicrob. Agents Chemother. 513747-3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chauhan, N., J. P. Latge, and R. Calderone. 2006. Signalling and oxidant adaptation in Candida albicans and Aspergillus fumigatus. Nat. Rev. Microbiol. 4435-444. [DOI] [PubMed] [Google Scholar]
- 9.Chen, R. E., and J. Thorner. 2007. Function and regulation in MAPK signaling pathways: lessons learned from the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta 17731311-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Francois, F., F. Chapeland-Leclerc, J. Villard, and T. Noel. 2004. Development of an integrative transformation system for the opportunistic pathogenic yeast Candida lusitaniae using URA3 as a selection marker. Yeast 2195-106. [DOI] [PubMed] [Google Scholar]
- 11.Francois, F., T. Noel, R. Pepin, A. Brulfert, C. Chastin, A. Favel, and J. Villard. 2001. Alternative identification test relying upon sexual reproductive abilities of Candida lusitaniae strains isolated from hospitalized patients. J. Clin. Microbiol. 393906-3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furukawa, K., Y. Hoshi, T. Maeda, T. Nakajima, and K. Abe. 2005. Aspergillus nidulans HOG pathway is activated only by two-component signalling pathway in response to osmotic stress. Mol. Microbiol. 561246-1261. [DOI] [PubMed] [Google Scholar]
- 13.Goumar, A., A. Brulfert, C. Martin, J. Villard, and T. Noël. 2004. Selection and genetic analysis of pseudohyphae defective mutants with attenuated virulence in Candida lusitaniae. J. Med. Mycol. 143-11. [Google Scholar]
- 14.Hawkins, J. L., and L. M. Baddour. 2003. Candida lusitaniae infections in the era of fluconazole availability. Clin. Infect. Dis. 36e14-e18. [DOI] [PubMed] [Google Scholar]
- 15.Hohmann, S., M. Krantz, and B. Nordlander. 2007. Yeast osmoregulation. Methods Enzymol. 42829-45. [DOI] [PubMed] [Google Scholar]
- 16.Izumitsu, K., A. Yoshimi, and C. Tanaka. 2007. Two-component response regulators Ssk1p and Skn7p additively regulate high-osmolarity adaptation and fungicide sensitivity in Cochliobolus heterostrophus. Eukaryot. Cell 6171-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones, C. A., S. E. Greer-Phillips, and K. A. Borkovich. 2007. The response regulator RRG-1 functions upstream of a mitogen-activated protein kinase pathway impacting asexual development, female fertility, osmotic stress, and fungicide resistance in Neurospora crassa. Mol. Biol. Cell 182123-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kruppa, M., and R. Calderone. 2006. Two-component signal transduction in human fungal pathogens. FEMS Yeast Res. 6149-159. [DOI] [PubMed] [Google Scholar]
- 19.Maeda, T., S. M. Wurgler-Murphy, and H. Saito. 1994. A two-component system that regulates an osmosensing MAP kinase cascade in yeast. Nature 369242-245. [DOI] [PubMed] [Google Scholar]
- 20.Maeta, K., S. Izawa, and Y. Inoue. 2005. Methylglyoxal, a metabolite derived from glycolysis, functions as a signal initiator of the high osmolarity glycerol-mitogen-activated protein kinase cascade and calcineurin/Crz1-mediated pathway in Saccharomyces cerevisiae. J. Biol. Chem. 280253-260. [DOI] [PubMed] [Google Scholar]
- 21.Menon, V., D. Li, N. Chauhan, R. Rajnarayanan, A. Dubrovska, A. H. West, and R. Calderone. 2006. Functional studies of the Ssk1p response regulator protein of Candida albicans as determined by phenotypic analysis of receiver domain point mutants. Mol. Microbiol. 62997-1013. [DOI] [PubMed] [Google Scholar]
- 22.Minari, A., R. Hachem, and I. Raad. 2001. Candida lusitaniae: a cause of breakthrough fungemia in cancer patients. Clin. Infect. Dis. 32186-190. [DOI] [PubMed] [Google Scholar]
- 23.Noel, T., A. Favel, A. Michel-Nguyen, A. Goumar, K. Fallague, C. Chastin, F. Leclerc, and J. Villard. 2005. Differentiation between atypical isolates of Candida lusitaniae and Candida pulcherrima by determination of mating type. J. Clin. Microbiol. 431430-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ochiai, N., M. Fujimura, T. Motoyama, A. Ichiishi, R. Usami, K. Horikoshi, and I. Yamaguchi. 2001. Characterization of mutations in the two-component histidine kinase gene that confer fludioxonil resistance and osmotic sensitivity in the os-1 mutants of Neurospora crassa. Pest Manag. Sci. 57437-442. [DOI] [PubMed] [Google Scholar]
- 25.Papon, N., T. Noel, M. Florent, S. Gibot-Leclerc, D. Jean, C. Chastin, J. Villard, and F. Chapeland-Leclerc. 2007. Molecular mechanism of flucytosine resistance in Candida lusitaniae: contribution of the FCY2, FCY1, and FUR1 genes to 5-fluorouracil and fluconazole cross-resistance. Antimicrob. Agents Chemother. 51369-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfaller, M. A., S. A. Messer, and R. J. Hollis. 1994. Strain delineation and antifungal susceptibilities of epidemiologically related and unrelated isolates of Candida lusitaniae. Diagn. Microbiol. Infect. Dis. 20127-133. [DOI] [PubMed] [Google Scholar]
- 27.Roman, E., D. M. Arana, C. Nombela, R. Alonso-Monge, and J. Pla. 2007. MAP kinase pathways as regulators of fungal virulence. Trends Microbiol. 15181-190. [DOI] [PubMed] [Google Scholar]
- 28.Santos, J. L., and K. Shiozaki. 2001. Fungal histidine kinases. Sci. STKE 2001RE1. [DOI] [PubMed] [Google Scholar]
- 29.Singh, P., N. Chauhan, A. Ghosh, F. Dixon, and R. Calderone. 2004. SKN7 of Candida albicans: mutant construction and phenotype analysis. Infect. Immun. 722390-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young, L. Y., C. M. Hull, and J. Heitman. 2003. Disruption of ergosterol biosynthesis confers resistance to amphotericin B in Candida lusitaniae. Antimicrob. Agents Chemother. 472717-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young, L. Y., M. C. Lorenz, and J. Heitman. 2000. A STE12 homolog is required for mating but dispensable for filamentation in Candida lusitaniae. Genetics 15517-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.