Abstract
In heterothallic ascomycete fungi, idiomorphic alleles at the MAT locus control two sex pheromone-receptor pairs that function in the recognition and chemoattraction of strains with opposite mating types. In the ascomycete Gibberella zeae, the MAT locus is rearranged such that both alleles are adjacent on the same chromosome. Strains of G. zeae are self-fertile but can outcross facultatively. Our objective was to determine if pheromones retain a role in sexual reproduction in this homothallic fungus. Putative pheromone precursor genes (ppg1 and ppg2) and their corresponding pheromone receptor genes (pre2 and pre1) were identified in the genomic sequence of G. zeae by sequence similarity and microsynteny with other ascomycetes. ppg1, a homolog of the Saccharomyces α-factor pheromone precursor gene, was expressed in germinating conidia and mature ascospores. Expression of ppg2, a homolog of the a-factor pheromone precursor gene, was not detected in any cells. pre2 was expressed in all cells, but pre1 was expressed weakly and only in mature ascospores. ppg1 or pre2 deletion mutations reduced fertility in self-fertilization tests by approximately 50%. Δppg1 reduced male fertility and Δpre2 reduced female fertility in outcrossing tests. In contrast, Δppg2 and Δpre1 had no discernible effects on sexual function. Δppg1/Δppg2 and Δpre1/Δpre2 double mutants had the same phenotype as the Δppg1 and Δpre2 single mutants. Thus, one of the putative pheromone-receptor pairs (ppg1/pre2) enhances, but is not essential for, selfing and outcrossing in G. zeae whereas no functional role was found for the other pair (ppg2/pre1).
Gibberella zeae (Schwein.) Petch (anamorph: Fusarium graminearum Schwabe sensu lato) is the most important causal agent of Fusarium head blight (also termed scab) of wheat and barley (32) and also causes stalk rot and ear rot of maize and crown rot of carnation (30). In addition to direct yield loss, G. zeae can reduce grain quality and harvested grain often is contaminated with mycotoxins such as deoxynivalenol and zearalenone (13). G. zeae is globally distributed and comprises multiple phylogenetic lineages (34, 35).
G. zeae is a homothallic fungus, and strains originating from a single haploid nucleus can successfully complete the sexual cycle without a mating partner. This process may be advantageous for maximizing the production of ascospores, which are important as inoculum for initiating disease epidemics of wheat (17). Other species in the genus Gibberella are heterothallic, and strains of these species must cross with a strain of the opposite mating type to produce perithecia, complete meiosis, and produce ascospores. G. zeae is capable of outcrossing facultatively under laboratory conditions (7), and laboratory crosses have been used to generate genetic maps (18, 22, 27). Although the evidence is indirect, G. zeae apparently outcrosses at a significant rate in North American field populations (41, 51). Thus, both selfing and outcrossing are important in the life cycle of this fungus.
Sexual development in filamentous ascomycetes is controlled by the mating type (MAT) locus (10, 49). In heterothallic ascomycete fungi, there are two idiomorphic alleles. One allele, MAT1-1, encodes three proteins, MAT1-1-1, MAT1-1-2, and MAT1-1-3, while the MAT1-2 allele encodes only a single protein, MAT1-2-1 (10, 49). In G. zeae, the MAT locus is rearranged such that both alleles are adjacent on the same chromosome and opposite mating types do not exist (28, 49). However, both idiomorphs must function for homothallic sexual reproduction to occur. Deletion of either the MAT1-1 or the MAT1-2 coding region in G. zeae results in strains that are obligately heterothallic (28). Such mutants are useful for forcing outcrossing in genetic experiments.
Heterothallic ascomycetes possess two different diffusible pheromone peptides and corresponding G-protein-coupled receptors that function in the recognition and chemoattraction of strains of opposite mating types (2, 5, 14). The particular pheromone and receptor expressed depends on the allele at the MAT locus, although neither the pheromone nor the receptor is encoded at this locus. The structure and sequence of the pheromones and their receptors are broadly conserved in heterothallic ascomycetes such as Saccharomyces cerevisiae (26), Neurospora crassa (4, 25), Podospora anserina (11), Cryphonectria parasitica (48, 52), and Magnaporthe grisea (44). The pheromone peptides are encoded by two classes of pheromone precursor genes. One class, typified by the Saccharomyces α-factor precursor gene, contains a secretion signal and multiple tandem copies of a short peptide that are flanked by Kex2 protease-processing sites. The other class, typified by the Saccharomyces a-factor precursor gene, contains a CAAX carboxylation and farnesylation motif and produces a lipopeptide pheromone.
Pheromones and receptors are essential for sexual fertility in the heterothallic ascomycetes that have been studied. Deletion of the pre-1 pheromone receptor gene of N. crassa caused female sterility of the mat A mating type because trichogynes were incapable of directional growth and fusion with spermatia (23). Deletion of either pheromone precursor gene caused male sterility of the corresponding mating type because spermatia could no longer attract female trichogynes (24). Similar results were obtained with pheromone deletion mutants of P. anserina (11) and C. parasitica (48).
In the homothallic ascomycete Sordaria macrospora, both types of pheromones and their cognate receptors have been reported (31, 37) but no effect on fruiting body or ascospore development was produced by a single mutation of any pheromone precursor gene or receptor gene (31). However, the double pheromone mutant exhibited drastically reduced self-fertility and the double receptor mutant was completely sterile (31). In the homothallic ascomycete Emericella nidulans (Aspergillus nidulans), a single mutation of either receptor resulted in greatly reduced self-fertility and the double receptor mutant was self-sterile (42). However, the double mutants could outcross, suggesting that the pheromone receptors are required specifically for self-fertilization (42).
The mechanism of sexual fertilization and the role of pheromones are not known for G. zeae or any other Gibberella species. Information on the sex pheromones in G. zeae may provide insights into the mechanism of fertilization and could identify opportunities for reducing the ascospore inoculum of this economically important pathogen. Our objectives in this study were to identify and characterize the putative pheromone precursor genes and the corresponding pheromone receptor genes in G. zeae and to determine their roles in sexual reproduction in this homothallic fungus. Putative pheromone precursor genes and receptor genes were identified by sequence similarity and microsynteny with other ascomycetes, while the expression of the putative pheromone precursor genes and receptor genes was assayed with green fluorescent protein (GFP) reporter constructs. Deletion mutants were constructed, and their sexual function was tested in three assays: self-fertilization tests, obligate outcrossing tests using heterothallic MAT deletion strains, and facultative outcrossing using GFP-marked strains to monitor the outcrossing frequency.
MATERIALS AND METHODS
Fungal strains and methods.
G. zeae (anamorph: F. graminearum sensu lato lineage 7) wild-type strain Z3639, isolated in Kansas (6), and mutants derived from it were stored as frozen conidial suspensions in 15% glycerol at −70°C (Table 1). Standard laboratory methods and culture media for Fusarium spp. were used (30). Carboxymethyl cellulose (CMC) liquid culture medium was described previously (8). The sexual stage was induced in 3- to 5-day-old cultures on 6-cm carrot agar plates by applying 500 μl of an aqueous 2.5% Tween 60 solution containing 1 × 105 propagules (spermatia) of the male strain and then knocking down the aerial mycelium with a bent glass rod while rotating the plate several times (7). Mock fertilizations were performed similarly but with sterile Tween 60 solution lacking spermatia. Plates were incubated at 24°C with a 12-h photoperiod provided by cool white fluorescent lights.
TABLE 1.
G. zeae strains used in this study
| Genotype | No. of strains | Description |
|---|---|---|
| Z3639 | 1 | Wild-type Z3639 |
| Δmat1-1 | 1 | Deletion of mat1-1-1 |
| Δmat1-2 | 1 | Deletion of mat1-2 |
| Δppg1 | 11 | Deletion of ppg1 |
| Δppg2 | 19 | Deletion of ppg2 |
| Δpre1 | 11 | Deletion of pre1 |
| Δpre2 | 13 | Deletion of pre2 |
| GFPZ3639 | 1 | Z3639 constitutively expressing GFP |
| GFPΔppg1 | 1 | Δppg1 constitutively expressing GFP |
| GFPΔpre2 | 1 | Δpre2 constitutively expressing GFP |
| Δppg1GFPR | 4 | ppg1 replaced with GFP reporter cassette for ppg1 expression |
| Δppg2GFPR | 9 | ppg2 replaced with GFP reporter cassette for ppg2 expression |
| Δpre1GFPR | 4 | pre1 replaced with GFP reporter cassette for pre1 expression |
| Δpre2GFPR | 7 | pre2 replaced with GFP reporter cassette for pre2 expression |
| Δppg1/Δmat1-1 | 1 | Δppg1 Δmat1-1-1 double mutant |
| Δppg1/Δmat1-2 | 1 | Δppg1 Δmat1-2 double mutant |
| Δppg2/Δmat1-1 | 1 | Δppg2 Δmat1-1-1 double mutant |
| Δppg2/Δmat1-2 | 1 | Δppg2 Δmat1-2 double mutant |
| Δppg1/Δppg2 | 3 | Δppg1 Δppg2 double mutant |
| Δppg1/Δpre2 | 8 | Δppg1 Δpre2 double mutant |
| Δpre1/Δpre2 | 3 | Δpre1 Δpre2 double mutant |
| ppg1-ect | 1 | ppg1 ectopic insertion mutant with intact ppg1 |
| CNPΔppg1 | 7 | Complementation of ppg1 with native promoter |
| CSPΔppg1 | 5 | Complementation of ppg1 under ICL promoter |
| CSP2SΔppg1 | 8 | Complementation of ppg1 without signal peptide under ICL promoter |
Identification of pheromone precursor and pheromone receptor genes of G. zeae.
The pheromone precursor genes and receptors are named differently in different fungi. For G. zeae, we use ppg1 for the putative homolog of the Saccharomyces α-factor pheromone precursor gene and ppg2 for the homolog of the a-factor pheromone precursor gene. The putative cognate pheromone receptors are designated pre2 and pre1, respectively. To identify G. zeae ppg1, the sequences of ppg1 of S. macrospora (37), ccg4 of N. crassa (4), and mf2-1 of M. grisea (44) were used in a BlastP search of the F. graminearum genome database (http://www.broad.mit.edu/annotation/fungi/Fusarium/). The other pheromone precursor gene (ppg2) is less conserved across species and is too short (less than 25 amino acids in S. macrospora, N. crassa, and M. grisea) to be found in a BlastP search. Instead, microsynteny near mf1-1 of M. grisea (44) and mfa-1 of N. crassa (25) was used to identify ppg2 of G. zeae. Two putative pheromone receptor genes, pre1 and pre2, were identified from the database following BlastP searches with the pheromone receptor genes of N. crassa, pre1 and pre2 (38), and E. nidulans, preA and preB (15).
DNA manipulations.
DNA was extracted by a cetyltrimethylammonium bromide (CTAB) procedure (30). Standard procedures were used for restriction endonuclease digestions, agarose gel electrophoresis, Southern hybridizations, and Northern hybridizations (40).
Targeted gene deletion.
ppg1, ppg2, pre1, pre2, mat1-1-1, and mat1-2 were deleted by split marker recombination (9) with slight modifications. Both the 5′ and 3′ flanking regions of a target gene were amplified by PCR with the F1/R2 and F3/R4 primer sets (Table 2) and an amplification protocol of 2 min at 94°C, followed by 30 cycles of 30 s at 94°C, 1 min at 55°C, and 1 min at 72°C and 10 min at 72°C for a final extension. The PCR products were purified with the DNA Purification System (Promega, Madison, WI) by following the manufacturer's instructions. The hygromycin phosphotransferase cassette (HYG; 1.4 kb) was amplified with the HYG-1F/2R primers from pIGPAPA (20) and purified with the same system. The HYG sequence for ppg1 deletion was amplified from pCSN43 (46) by PCR with the GNT-F1 and GNT-R4 primers. In this construct, the hygromycin resistance gene was flanked by a trpC promoter and trpC terminator and the size of amplicon is ∼2 kb. The three amplicons were fused by PCR in a 25-μl reaction mixture containing 1 μl of purified 5′ flanking amplicon (100 ng/μl), 1 μl of 3′ flanking amplicon (100 ng/μl), 3 μl of HYG amplicon (100 ng/μl), 2 μl of deoxynucleoside triphosphates (each at 2.5 mM), 2.5 μl of 10× PCR buffer including MgCl2, 1 U of ExTaq polymerase (Takara Bio Inc., Japan), and 15.25 μl of water. The PCR amplification conditions were 2 min at 94°C, followed by 10 cycles of 30 s at 94°C, 20 min at 58°C, and 5 min at 72°C and 10 min at 72°C for a final extension. One microliter of this amplification mixture was reamplified as a template in a PCR with F1-NT/YG-R4 and HY-F3/R4-NT primer sets and a 50-μl reaction volume. The PCR conditions were 2 min at 94°C, followed by 30 cycles of 30 s at 94°C, 1 min at 60°C, and 90 s at 72°C and 10 min at 72°C for a final extension. The amplification products were combined and used to directly transform G. zeae protoplasts by a polyethylene glycol-mediated method (29). The percent deletion of the open reading frame was 100, 100, 100, 100, 86, and 100% for mat1-1-1, mat1-2, ppg1, ppg2, pre1, and pre2, respectively. The presence of single copies of each insertion was confirmed by Southern blotting. The numbers of independent transformants obtained for the deletion mutant classes are listed in Table 1. Δppg1/Δmat1-1, Δppg1/Δmat1-2, Δppg2/Δmat1-1, Δppg2/Δmat1-2, Δppg1/Δppg2, Δppg1/Δpre2, and Δpre1/Δpre2 double mutants were generated by sexual crosses between single mutants. Multiple strains in a double-mutant class were derived from the same single-mutant parents.
TABLE 2.
Oligonucleotides used in this study
| Name | Sequence (5′→3′) |
|---|---|
| PPG1-F1 | CGCGTCTGACAAGTAAAAGGAGAAAC |
| PPG1-F1-NT | GCGTCTGACAAGTAAAAGGCGAAACCAAATGGCAA |
| PPG1-R2 | AAAAAGTGCTCCTTCAATATCATCTTCTGAGGCGGCTAGCGTCAAAATGGA |
| PPG1-F3 | CTTGTTTAGAGGTAATCCTTCTTTCTAGAGTAAGTTTGGTTATCGACGCAGAG |
| PPG1-R4 | CTAGCGCACAAGGCATCAACT |
| PPG1-R4-NT | CTGCCCCACCATCTCAGACGC |
| PPG1-R2-GFP | CCTCGCCCTTGCTCACCATGTTGGGCGCCGTACTTGTCG |
| PPG1-R-TRPCP | GCTCCTTCAATATCATCTTCTGGGATATGCTGCTTGGCGTCAAC |
| PPG1-F1-ICL | TTCATACCACACCTGCCCACCGCGCCCAACATGAAGTACTCC |
| PPG1M2-F1-ICL | TTCATACCACACCTGCCCACCATGCCCTGGTGCACCTGGAAA |
| PPG2-F1 | GGCCGCCAAAGACCTAAGC |
| PPG2-F1-NT | GCGGTGGCAGCATTGTTCTACG |
| PPG2-R2 | TTGACCTCCACTAGCTCCAGCCAAGCCGGCCAGAACGATACGTC |
| PPG2-F3 | GAATAGAGTAGATGCCGACCGCGGGTTCCGCCACGAGGACGCCA |
| PPG2-R4 | GCGAGGAGGGCGGTTGTGTTGTTA |
| PPG2-R4-NT | GGGGCTCTTCATCGTCCTCATCAT |
| PPG2-R2-GFP | CGCCCTTGCTCACCATTTTGAAAGTTGGGGTTGAAAGACTTAGA |
| PRE1-F1 | GCTGAGACGCGATAGGGTAGGAA |
| PRE1-F1-NT | TCAACCTCACCACGTCCCTCAACA |
| PRE1-R2 | TTGACCTCCACTAGCTCCAGCCAAGCCGCGGGAAGCGCAAGGAC |
| PRE1-F3 | GAATAGAGTAGATGCCGACCGCGGGTTCGGCATCATCTGCGAGC |
| PRE1-R4 | GACGAACGTATGCGAAATGGAGAC |
| PRE1-R4-NT | AAGGGCTTGGTTATGGCGGTTGG |
| PRE1-F5 | CCTGGACCGGGAACAACTATCACT |
| PRE1-R6 | AGGCCATGCGACCCAACTG |
| PRE1-R2-GFP | CGCCCTTGCTCACCATGTTGGGACGTCGACCGTGATGTGGAAG |
| PRE2-F1 | GCGCAGCAGGCAGCAGAA |
| PRE2-F1-NT | GAATGGGCCTGGCTGCGTGAT |
| PRE2-R2 | TTGACCTCCACTAGCTCCAGCCAAGCCGGCAAGAAGACACGGGA |
| PRE2-F3 | GAATAGAGTAGATGCCGACCGCGGGTTCCGCCTCGATACCCCAA |
| PRE2-R4 | TCGCCACAATTCGGTTCCTGAT |
| PRE2-R4-NT | GGAACCCCGGTCGCCTCACA |
| PRE2-F5 | TGTCTTATCATGCTGGTCGTGCTC |
| PRE2-R6 | GGGAGAATCACAGCGACAGAGGTA |
| PRE2-R2-GFP | CGCCCTTGCTCACCATGTTGGGGTGGTATCTGCTTTTCGACTGG |
| MAT11-F1 | CTCCACTTGCGGCATCGTCTAC |
| MAT11-F1-NT | GCCCTGATGATGCTGTAAGTGTTA |
| MAT11-R2 | TTGACCTCCACTAGCTCCAGCCAAGCCGGAGGGAAAGGGGTGTG |
| MAT11-F3 | GAATAGAGTAGATGCCGACCGCGGGTTGCACATGTCGGGCACGG |
| MAT11-R4 | CTCCCAACGCTTACATCCTCTACC |
| MAT11-R4-NT | CCCGCCGCCCAGCCTACTC |
| ICL-F1 | GGGCCCCACACGGACTCAAAC |
| ICL-F1-NT | CCCCACACGGACTCAAACTGATGTTCGAGTC |
| ICL-R2-PPG1 | GGAGTACTTCATGTTGGGCGCGGTGGGCAGGTGTGGTATGAAA |
| ICL-R2-PPG1M2 | CCAGGTGCACCAGGGCATGGTGGGCAGGTGTGGTATGAAA |
| GFP-F1 | GGGGCCCCACACGGACTC |
| GFP-F1-NT | CCAGAGGTCCGATCGCCAATGA |
| GFP-R2 | TTGACCTCCACTAGCTCCAGCCAAGCCAGATGACACCGCGCGCG |
| GFP-F3 | CCATGGTGAGCAAGGGCGAGGAG |
| GFP-F3-PPG1 | ACGACAAGTACGGCGCCCAACATGGTGAGCAAGGGCGAGG |
| GFP-F3-PPG2 | CTTTCAACCCCAACTTTCAAAATGGTGAGCAAGGGCGAGG |
| GFP-F3-PRE1 | TCCACATCACGGTCGACGTCATGGTGAGCAAGGGCGAGG |
| GFP-F3-PRE2 | CGAAAAGCAGATACCACCATGGTGAGCAAGGGCGAGG |
| GFP-R4 | AGATGACACCGCGCGCGATAATTTA |
| HYG-F1 | GGCTTGGCTGGAGCTAGTGGAGG |
| HYG-R2 | AACCCGCGGTCGGCATCTACTCTA |
| HY-F3 | GATGTAGGAGGGCGTGGATATGT |
| YG-R4 | GAACCCGCTCGTCTGGCTAAG |
| HYG-F1-GFP | TATCGCGCGCGGTGTCATCTGGCTTGGCTGGATCTAGTGGAGG |
| GNT-F1 | CAGAAGATGATATTGAAGGAGC |
| GNT-R4 | CTAGAAAGAAGGATTACCTCT |
| GNT-F1-PPG1 | CAACGTTGACGCCAAGCAGCATATCCCAGAAGATGATATTGAAAG |
| GNT-R4-NT | CCTGTGCATTCTGGGTAAACGAC |
| GNT-R4A-NT | GTACCTGTGCATTCTGGGTAAACGACTCATAGGAG |
| rRNA-F | CATCCGGCACGCAAACCAC |
| rRNA-R | CGATGTCGCCGCTGTCAATG |
GFP-tagged strains for outcrossing.
The DNA fragment (3.4 kb) carrying the GFP and HYG cassettes was amplified from pIGPAPA with primers ICL-F1 and HYG-F1 and transformed into Z3639. Transformant GFPZ3639 constitutively expresses GFP and was used as the spermatial parent in crosses. GFP cassettes were combined with Δppg1 and Δpre2 mutants by crossing Δppg1 and Δpre2 mutants with GFPZ3639 to yield GFPΔppg1 and GFPΔpre2. GFP fluorescence was detected with an Axioplan2 microscope (Carl Zeiss, Thornwood, NY) with 480 ± 10 nm for excitation and 510 ± 10 nm for emission.
GFP reporter constructs.
We amplified a GFP sequence (0.97 kb) that has a terminator, but no promoter, from pIGPAPA. The GFP gene fragment was fused to the HYG cassette (1.4 kb), which was amplified from pIGPAPA with primers HYG-F1-GFP and HYG-R2 (GFP::HYG). The promoter region of the target gene was amplified with primers F1 and R2-GFP, and the 3′ flanking region was amplified with primers F3 and R4. After PCR purification, the promoter and the 3′ flanking region were fused with the GFP::HYG construct in a 25-μl reaction mixture, 1 μl of which was used to produce split markers. Following transformation with the two split markers, we recovered deletion mutants in which GFP expression was controlled by the native promoter of the target gene. Target gene expression was monitored by screening for GFP expression. The experiment was performed twice with four to nine independent transformants per class.
RNA isolation and reverse transcription (RT)-PCR.
Total RNA was isolated from vegetative mycelia (uninduced mycelia or mycelia 3 days after induction) or ascospores of wild-type and mutant strains by using TRIZOL reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. RNA concentration and purity were checked with an ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE). For RT-PCR, 1 μg of total RNA was used to synthesize first-strand cDNA with the 3′-full RACE Core Set (Takara Bio Inc., Japan) according to the manufacturer's instructions and 1 μl of the cDNA solution was used for RT-PCR. To normalize cDNA, the 60S ribosomal gene (FGSG_13664.3) was amplified with the rRNA-F/R primer set (Table 2). Twenty micrograms of total RNA was subjected to Northern hybridization with the PCR product of the target gene as a probe. The experiment was performed twice with one mutant strain per class.
Fertility tests.
For qualitative self-fertilization tests, cultures were mock fertilized to induce homothallic sexual development. The number and size of perithecia were noted after incubation for 10 to 14 days following induction.
For quantitative self-fertilization tests, there were three independent transformants for each mutant class and eight replicate plates for each transformant, and the experiment was performed twice. Photographs of each plate were taken to facilitate the counting of perithecia. The percentage of mature perithecia was calculated for each plate. Percentage data were given an arcsine square root transformation to improve the homogeneity of variance, and means were back-transformed for presentation. Data from both runs of the experiment were combined and analyzed as a completely randomized design (n = 16) with Minitab Version 14 (Minitab, Inc., State College, PA) to compare means for each mutant to the value for the wild-type control with Dunnett's two-tailed test (family error rate = 0.05).
For obligate outcrossing tests, heterothallic strains carrying a mat1-1 or mat1-2 deletion were used as females so that all ascospore progeny resulted from heterozygous crosses. Plates were fertilized with a suspension of conidia from cultures grown on complete medium. Crosses were scored qualitatively as either fertile or nonfertile. The experiment was performed twice with one mutant strain per class and three replicates.
For facultative outcrossing tests, conidia from GFP-expressing males were used as spermatia to fertilize non-GFP-expressing females. Ascospores resulting from self-fertilized perithecia did not express GFP, and ascospores resulting from heterozygous perithecia segregated 1:1 for GFP expression. Ten days after fertilization, crossing plates were inverted and the ascospores ejected were collected on the underside of the lid of a petri dish overnight. Ascospores were suspended in 1 ml of water and counted with a hemocytometer to determine the total number of progeny produced and the proportion of the progeny expressing GFP. Under the conditions of this study, the spermatial parent never produced mature homothallic perithecia or ascospores. Therefore, the outcrossing percentage was estimated as two times the percentage of GFP-expressing ascospores. The experiment was performed three times with three replicate plates per cross. There was one mutant strain per class. Data from the three runs were combined and analyzed as a randomized complete block design. Data were given an arcsine square root transformation and back-transformed for presentation. Means were compared to the control (Z3639 female/GFPZ3639 male) with Dunnett's test. The experiment was also performed once with ascospores as spermatia, and the results were analyzed as a completely randomized design with three replicates.
Complementation of Δppg1.
Three constructs were made to complement ppg1. In the first, the entire ppg1 gene, including the native promoter and terminator, was amplified from G. zeae strain Z3639 with primers PPG1-F1 and PPG1-R-TRPCP and the Geneticin resistance gene cassette (GNT) was amplified from pII99 (33) with primers GNT-F1-PPG1 and GNT-R4. These two amplicons were fused (whole ppg1 cassette::GNT) after purification as described above. In the second, the ppg1 gene was amplified by PCR from Z3639 with PPG1-F1-ICL and PPG1-R-TRPCP as the primers. The resulting DNA fragment contained the entire ppg1 sequence, and the 3′ flanking region including the terminator. A strong constitutive promoter, ICL from isocitrate lyase of N. crassa, was amplified from pIGPAPA (20) by PCR with ICL-F1 and ICL-R2-PPG1. The ICL promoter fragment, the ppg1 genomic fragment, and the GNT cassette were fused (ICL promoter::ppg1::GNT). The third construct was similar to the second construct, except that the ppg1 gene fragment, amplified with primers PPG1M2-F1-ICL and PPG1-R-TRPCP, began at the second methionine codon of ppg1 and did not include the signal peptide. All three constructs were transformed into one Δppg1 mutant, and there were five to eight transformants per class. The self-fertility phenotype was determined in two experiments with three replications.
RESULTS
Identification of ppg1, ppg2, pre1, and pre2.
One ppg1 candidate sequence (Broad Institute; FGSG_05061.3) was identified by BlastP. This protein sequence had significant identity (28, 30, and 21%, respectively) with ppg1 of S. macrospora, ccg4 of N. crassa, and mf2-1 of M. grisea. Upstream of FGSG_05061.3, there are MAT binding motifs (CTTTG) at positions −434 and −483. The putative ppg1 gene contains four repeats of one decapeptide (WCTWKGQPCW) and five repeats of a second decapeptide (WCWWKGQPCW) that differs from the first decapeptide by a single amino acid (Fig. 1). All decapeptides, except for the first two, are bordered by a putative Kex2 protease site (KR).
FIG. 1.
Deduced sequence of the protein encoded by ppg1 (FGSG_05061.3) of G. zeae. This polypeptide contains two types of decapeptides. Four repeats of one type (WCTWKGQPCW) are underlined, and five repeats of a second type (WCWWKGQPCW) that differs from the first decapeptide by a single amino acid are in bold underlined type. The putative secretion signal sequence cleavage site is marked by an arrow. KR dipeptides, which are potential Kex2 protease-processing sites, are in bold type.
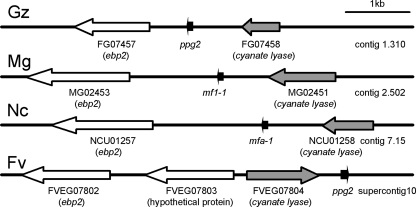
ppg2 could not be unambiguously identified in a BlastP search with ppg2 of S. macrospora, mfa-1 of N. crassa, or mf1-1 of M. grisea, because the sequences are short and have relatively low levels of sequence similarity. We compared the synteny of the N. crassa and M. grisea sequences flanking the mfa-1 and mf1-1 genes. Both genes are between cyanate lyase and ebp2 homologs, and both ppg2 homologues are transcribed in the same direction and in the same open reading frame (Fig. 2). In the Fusarium verticilloides genomic sequence, a putative ppg2 homolog was found in the same orientation with cyanate lyase but these two genes were inverted relative to the ebp2 homolog. These results suggest that microsynteny in this region generally is well conserved in these related ascomycete fungi.
FIG. 2.
Microsynteny near the ppg2 gene among different ascomycete species. The putative ppg2 gene of G. zeae (Gz) was between FG07457 (ebp2 homolog) and FG07458 (cyanate lyase homolog) in contig 1.310 of the Fusarium genome database developed by the Broad Institute. In the other fungal species (Mg, M. grisea; Nc, N. crassa), the ppg2 gene was between homologs of ebp2 and cyanate lyase. Note that the names for the ppg2 gene are different in these three fungal species. In the F. verticilloides (Fv) genomic sequence, a putative ppg2 homolog was found downstream of cyanate lyase but these two genes were inverted relative to the ebp2 homolog in the other fungi.
The 10-kb sequence flanking mfa-1 of N. crassa and mf1-1 of M. grisea was blasted against the genomic sequence to find the syntenous region of G. zeae. Contig 1.310 contains a cyanate lyase (FGSG_07458.3) and a homolog of ebp2 (FGSG_07457.3). A previously undescribed open reading frame was identified in the region between the two genes that contains a candidate ppg2 gene in the same orientation as in M. grisea and N. crassa (Fig. 3). The putative ppg2 gene encodes a peptide of 21 amino acids with a prenylation signal sequence (CAAX) at its C terminus. This signal sequence also is found in the precursors of several other fungal pheromones (1, 12, 36, 37, 44, 45). Upstream of the putative ppg2 gene, there are a putative TATA box, two CAAT boxes, and two putative MAT transcription factor binding sites.
FIG. 3.
Putative nucleotide sequence of the ppg2 gene of G. zeae. The sequence shown is from Fusarium genome database contig 1.310. The putative MAT transcription factor binding motif (GTTTC or CAAAG), CAAT box, and TATA box are underlined, and the prenylation signal sequence (CAAX) is boxed. The amino acid sequence is shown below the nucleotide sequence.
One pre1 candidate sequence (FGSG_07270.3) was identified in the genomic sequence. It had sequence identity (30 and 23%, respectively) to the preA gene in E. nidulans (GenBank DAA01795) and the pre1 gene of N. crassa (GenBank CAC86413.1). The putative G. zeae pre1 gene has a CAAAG motif at −440 and encodes a protein with seven transmembrane domains typical of G protein-coupled receptors.
One pre2 candidate sequence (FGSG_02655.3) was identified in the genomic sequence. It has sequence identity (34 and 22%, respectively) with the receptor gene for preB from E. nidulans (GenBank DAA01796) and pre2 from N. crassa (GenBank CAC86431.1). The G. zeae pre2 sequence has a CAAAG motif at positions −506 and −526 and seven transmembrane domains.
Gene expression.
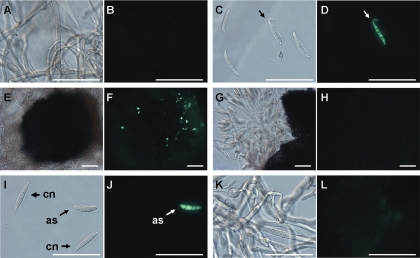
In four independent Δppg1GFPR mutants in which GFP expression was controlled by the native ppg1 promoter, GFP was expressed strongly in induced germinating conidia and mature discharged ascospores (Table 3, Fig. 4). Mycelia and conidia from colonies >10 days old on carrot agar media had a weak GFP signal. Young mycelia, perithecia, ungerminated conidia, and ascospores inside asci had no detectable GFP signal. We never observed GFP expression in the Δppg2GFPR reporter strains (nine mutants). GFP was weakly expressed in mature ascospores of Δpre1GFPR mutants (four mutants) and was more strongly expressed in Δpre2GFPR strains (seven mutants) by all mycelial ages or tissues from carrot agar except young mycelia prior to induction (Table 3).
TABLE 3.
Expression assays of putative pheromone precursors (ppg1 and ppg2) and receptors (pre1 and pre2) in different cell types of different ages on carrot agar
| Sample | ppg1a | ppg2 | pre1 | pre2 |
|---|---|---|---|---|
| 3 DAIb (without inductionc) | ||||
| Mycelia | −d | − | − | − |
| Ungerminated conidia | − | − | − | + |
| 6 DAI (3 days after induction) | ||||
| Mycelia | − | − | − | ++ |
| Ungerminated conidia | − | − | − | ++ |
| Germinating conidia | ++++ | − | − | ++ |
| Young perithecia | − | − | − | +++ |
| 13 DAI (10 days after induction) | ||||
| Mycelia | − | − | − | ++ |
| Ungerminated conidia | − | − | − | ++ |
| Germinating conidia | ++++ | − | − | ++ |
| Mature perithecia | − | − | − | ++ |
| Ascospores in perithecia | − | − | − | + |
| Ascospores discharged from perithecia | +++ | − | + | + |
| Old culture without induction (15 DAI) | ||||
| Mycelia | + | − | − | ++ |
| Ungerminated conidia | + | − | − | ++ |
| Germinating conidia | ++++ | − | − | ++ |
| Mature perithecia | − | − | − | ++ |
| Ascospores in perithecia | − | − | − | + |
| Ascospores discharged from perithecia | +++ | − | + | + |
ppg1, ppg2, pre1, and pre2 expression was assayed with GFP reporter constructs Δppg1GFPR, Δppg2GFPR, Δpre1GFPR, and Δpre2GFPR, respectively.
DAI, days after inoculation.
Induction by knockdown of mycelia with 500 μl of 2.5% Tween 60 solution to induce sexual development.
−, GFP fluorescence signal not detected; +, weak fluorescence; ++, moderate fluorescence; +++, strong fluorescence; ++++, very strong fluorescence; NT, not tested.
FIG. 4.
GFP expression assay for ppg1 in the Δppg1GFPR mutant strain on carrot agar. (A and B) Four-day-old mycelia without induction. (C and D) Conidia 3 days after induction. Only germinating conidia (arrow) expressed GFP. (E and F) Young perithecium 3 days after induction. GFP expression from the perithecium was not detected, but there were a few associated germinating conidia expressing GFP. (G and H) Squashed perithecium 10 days after induction. Ascospores in asci did not express GFP. (I and J) Conidia (cn) and freshly discharged ascospore (as) from a perithecium 10 days after induction. Only the ascospore expressed GFP. (K and L) Mycelia 15 days old without induction. (A, C, E, G, I, and K) Bright-field microscopy. (B, D, F, H, J, and L) Same specimen, fluorescence microscopy. Scale bars, 50 μm.
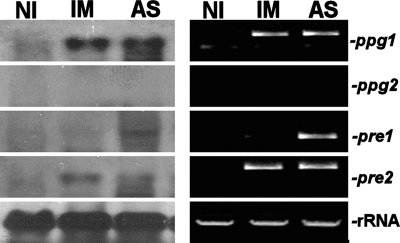
The expression of ppg1, pre1, and pre2 was confirmed by Northern blotting and RT-PCR of RNA from induced mycelial cultures or ascospores, but ppg2 expression was not detected (Fig. 5).
FIG. 5.
Analysis of transcript levels of pheromone precursor genes ppg1 and ppg2 and pheromone receptor genes pre1 and pre2 in G. zeae. Total RNA was extracted from 3-day-old fresh mycelia without induction (NI), 3-day-old mycelia after induction (IM), or ascospores (AS). Twenty micrograms was subjected to Northern analysis with the PCR product of the target gene serving as a probe (left panel), and 1 μg was used to synthesize first-strand RNA cDNA for RT-PCR (right panel).
Self-fertilization tests.
Δppg1 mutants (11 strains) had normal colony morphology on carrot agar and complete medium. They were self-fertile but produced fewer large perithecia than did the wild-type strain (Fig. 6). The Δpre2 (13 strains), Δppg1/Δppg2 (3 strains), Δpre1/Δpre2 (3 strains), and Δppg1/Δpre2 (8 strains) mutants all had the same phenotype as the Δppg1 mutant. However, the Δppg2 (19 strains) and Δpre1 (11 strains) mutants showed no obvious changes in perithecial production compared to the wild type.
FIG. 6.
Effect of ppg1 deletion on self-fertility of G. zeae growing on carrot agar 10 days after induction. (A) The wild-type Z3639 control and the Δppg1 mutant, with a reduced number of large mature perithecia. Scale bar, 1 cm. (B) Magnification showing fewer large mature perithecia (black arrowhead) and more small immature perithecia (white arrowhead) in the Δppg1 mutant strain than in Z3639. Scale bars, 1 mm.
Large and small perithecia of Δppg1 mutant strains were measured and then crushed to examine their contents microscopically. Immature perithecia contained no asci or ascospores and ranged from 24 to 98 μm (average, 58 μm) in diameter. Mature perithecia contained normal-appearing asci and ascospores, regardless of the mutant genotype, and ranged from 146 to 293 μm (average, 235 μm) in diameter. Immature perithecia never developed further, even if the cultures were incubated for 3 additional weeks. For counting purposes, 120 μm was considered the minimum diameter of a mature perithecium. The percentage of mature perithecia was reduced by approximately 50% in the Δppg1 and Δpre2 mutants but was not reduced significantly in the Δppg2 or Δpre1 mutant or the ppg1 ectopic-insertion control (Fig. 7).
FIG. 7.
Box plots of percent mature perithecia formed by self-fertilization of wild-type G. zeae and pheromone precursor gene ppg1 and ppg2 and pheromone receptor gene pre1 and pre2 deletion mutants of G. zeae. There are three independent transformants for each class. ppg1-ect is an ectopic-insertion mutant shown as a control. Each box plot shows the 75th and 25th percentiles (upper and lower borders of the box), the median (a solid line within a box), the mean (a dotted line within a box), the 90th and 10th percentiles (upper and lower horizontal lines outside of the box), and outliers (black circles). Box plots with asterisks indicate significant difference from the wild type by Dunnett's test.
Obligate outcrossing tests.
All single-pheromone mutants (Δppg1 and Δppg2), the double-pheromone mutant (Δppg1/Δppg2), and double mutants with mating type deletions (Δppg1/Δmat1-1, Δppg1/Δmat1-2, Δppg2/Δmat1-1, and Δppg2/Δmat1-2) could serve as either the male or the female parent in a cross (Table 4). Crosses were infertile only in pairings that lacked a functional copy of one or the other MAT idiomorph.
TABLE 4.
Male and female fertility of mutant strains in obligate outcrosses
| Male | Female
|
|||||
|---|---|---|---|---|---|---|
| Δmat1-1 | Δmat1-2 | Δppg1/Δmat1-1 | Δppg1/Δmat1-2 | Δppg2/Δmat1-1 | Δppg2/Δmat1-2 | |
| Z3639 (wild type) | +a | + | + | + | + | + |
| Δppg1 | + | + | + | + | + | + |
| Δppg2 | + | + | + | + | + | + |
| Δppg1/Δmat1-1 | −b | + | − | + | − | + |
| Δppg1/Δmat1-2 | + | − | + | − | + | − |
| Δppg2/Δmat1-1 | − | + | − | + | − | + |
| Δppg2/Δmat1-2 | + | − | + | − | + | − |
| Δppg1/Δppg2 | + | + | + | + | + | + |
| Mock fertilizationc | − | − | − | − | − | − |
+, ascospores produced.
−, no ascospores produced.
Female was self-fertilized but with no exogenous male conidia.
Facultative outcrossing tests.
Both conidia and ascospores functioned similarly as spermatia (Table 5). Outcrossing percentages generally were low but never zero. However, Δppg1 females had a >20-fold increase in facultative outcrossing, ranging from 35 to 87%. This effect was abolished by Δppg1 in the male or the additional deletion of pre2 (i.e., Δppg1/Δpre2) in the female.
TABLE 5.
Estimated facultative outcrossing percentages of ppg1 and pre2 deletion mutants
| Female | Malea | Estimated outcrossing percentageb
|
|
|---|---|---|---|
| Conidiac | Ascospores | ||
| Z3639 | GFPZ3639 | 0.3 | 1.7 |
| Z3639 | GFPΔppg1 | 0.2 | 0.3 |
| Z3639 | GFPΔpre2 | 1.0 | 3.9 |
| Δppg1 | GFPZ3639 | 35.1e | 61.7e |
| Δppg1 | GFPΔppg1 | 1.2 | 0.5 |
| Δppg1 | GFPΔpre2 | 53.1e | 87.8e |
| Δpre2 | GFPZ3639 | 0.4 | 0.3 |
| Δpre2 | GFPΔppg1 | 2.1 | 4.7 |
| Δpre2 | GFPΔpre2 | 0.9 | 1.5 |
| Δppg1/Δpre2 | GFPZ3639 | 0.3 | NTd |
| Δppg1/Δpre2 | GFPΔppg1 | 0.8 | NT |
| Δppg1/Δpre2 | GFPΔpre2 | 0.4 | NT |
Male strains were GFP labeled so GFP segregated 1:1 in heterozygous crosses.
The outcrossing frequency was estimated as twice the percentage of GFP-labeled ascospores produced.
Conidia and ascospores were used to fertilize female cultures at a concentration of 1 × 105 spermatia/ml.
NT, not tested.
Significantly different from the control (Z3639 female/GFPZ3639 male) by Dunnett's test.
Complementation of Δppg1.
Δppg1 deletions were made with a construct that contained hyg as the selectable marker. To test for complementation, the entire ppg1 gene, including the 5′ and 3′ flanking regions, was fused with a Geneticin resistance cassette (GNT), and Geneticin-resistant transformants were selected following transformation into a Δppg1 mutant. All seven of the Geneticin-resistant transformants had a wild-type phenotype in self-fertilization tests. If the reintroduced ppg1 sequence was controlled by the constitutive ICL promoter from N. crassa (ICL promoter::ppg1::GNT), then partial complementation (70 to 80% mature perithecia produced) occurred in each of the five transformants. Thus, the timing and/or the level of ppg1 expression is important for full function.
Eight transformants carried the ppg1 gene construct lacking the initial signal peptide. Strains carrying this construct did not complement the ppg1 deletion and had the same phenotype as the Δppg1 mutants. Thus, the secretion signal peptide of ppg1 is essential for the ppg1 gene product to function properly.
DISCUSSION
Two putative pheromone precursor genes (ppg1 and ppg2) and two putative pheromone receptor genes (pre1 and pre2) were identified in the genomic sequence of G. zeae. ppg1, a homolog of the Saccharomyces α-factor pheromone precursor gene, was expressed strongly in germinating conidia and discharged ascospores but weakly, if at all, in other cells. The cognate receptor, pre2, was expressed in all cells except uninduced mycelia. In fertility tests with Δppg1 and Δpre2 mutants, the cognate pair enhanced both self-fertility and facultative outcrossing ability. Δppg1 mutants were successfully complemented by transformation with an intact ppg1 gene, thus confirming that ppg1 retains a functional role in this homothallic ascomycete fungus.
Expression of ppg2, a homolog of the a-factor pheromone precursor gene, was not detected in any cells. The cognate receptor, pre1, was expressed weakly and only in mature ascospores. In fertility tests with deletion mutants, the ppg2/pre1 pheromone-receptor pair had no detectable function in selfing or outcrossing. The lack of detectable function of the ppg2/pre1 cognate pair probably was not due to incorrect identification of the genes. There were many candidates for ppg2, and we relied on microsynteny to identify the correct sequence (Fig. 2). The selected candidate had the expected regulatory and prenylation sequences (Fig. 3), which strongly supports its identification as ppg2. There was only one candidate for pre1 in the genomic sequence, and it had the typical seven transmembrane domains of a G-protein-coupled receptor. Since ppg2 and pre1 homologs are functional in heterothallic ascomycetes (11, 23, 24), we hypothesize that the ppg2 and pre1 genes in G. zeae are nonfunctional vestiges of the evolutionarily recent change from a heterothallic to a homothallic life style in this fungus (49, 50).
Although the ppg1/pre2 pheromone-receptor pair is functional in G. zeae, neither the pheromones nor the receptors are essential for sexual development. In obligate outcrossing tests, Δppg1, Δppg2, and Δppg1/Δppp2 mutants were all fertile as males, thus demonstrating that pheromones are not absolutely required for male fertility in G. zeae (Table 4). Similarly, the full self-fertility of Δpre1 mutants and the partial self-fertility (∼50%) of Δpre2 mutants and Δpre1/Δpre2 double mutants demonstrate that neither of the pheromone receptors is essential for female fertility. This pattern differs from the heterothallic ascomycetes N. crassa, P. anserina, and C. parasitica, in which pheromones and receptors are essential for fertilization (11, 23, 24, 48). G. zeae also differs from the homothallic ascomycetes S. macrospora and E. nidulans, in which at least one functional pheromone receptor is required for self-fertilization (31, 42). Thus, G. zeae is the first ascomycete in which the dispensability of both pheromones and pheromone receptors for sexual development has been demonstrated.
The role of the ppg1/pre2 pheromone-receptor pair in G. zeae appears to be restricted to fertilization. Aside from a reduction in the percentage of mature perithecia, Δppg1 and Δpre2 mutants showed no consistent differences in colony morphology compared to the wild type. We tested combinations of Δppg1 and Δppg2 with Δmat1-1 or Δmat1-2 that might reveal postfertilization pheromone effects but found no evidence of interactions (Table 4). Most importantly, Δppg1, Δpre2, Δppg1/Δppg2, Δpre1/Δpre2, and Δppg1/Δpre2 mutants all had decreased percentages of mature perithecia but the mature perithecia were apparently normal in size and fecundity (Fig. 6). These results are similar to those obtained with P. anserina, in which the role of pheromones also is restricted to the fertilization step (11).
In contrast, pheromones and/or receptors appear to have additional functions in S. macrospora and E. nidulans. Single pheromone or pheromone receptor of mutants S. macrospora have no detectable defects in self-fertilization, but double mutants in which there was no functional cognate pair, Δppg1/Δppg2, Δpre1/Δppg1, and Δpre2/Δppg2, showed drastically reduced numbers of mature self-fertilized perithecia (31). These results imply that both cognate pairs function interchangeably in fertilization. However, deletion of both the pre1 and pre2 receptor genes in S. macrospora leads to a complete loss of self-fertility, showing that at least one functional receptor is required for sexual development and suggesting that the receptor can be activated even when both pheromones are absent in S. macrospora. In E. nidulans, single pheromone receptor mutants produce smaller cleistothecia with a reduced number of ascospores, which suggests a postfertilization role for the receptors (42). Double-receptor mutants completely lost the ability to self-fertilize, as in S. macrospora. This diversity of pheromone-receptor functions in homothallic ascomycetes is not surprising since the homothallic life cycle apparently has evolved independently and uniquely multiple times from a conserved ancestral heterothallic state (50).
In G. zeae, ppg1 and pre2 appear to play conventional roles in the chemoattraction of female cells by male cells. Deletion of ppg1 in the male strain dramatically decreased the effectiveness of spermatia for fertilizing Δppg1 females, which are efficient facultative outcrossers (Table 5). Deletion of the pre2 pheromone receptor gene in Δppg1 females (i.e., Δppg1/Δpre2) eliminated the ability of females to distinguish males with an intact ppg1 gene (Table 5). These results are consistent with the classic Neurospora model in which spermatia secrete pheromones to attract female trichogynes (2, 3, 4, 23, 24, 25).
The high expression level of ppg1 in induced germinating conidia and discharged ascospores, but not other cells, accords well with the chemoattraction model. The high expression in freshly discharged ascospores was unexpected and prompted us to test their ability to serve as spermatia. Interestingly, the ability of ascospores to function as spermatia was equal to or better than that of conidia (Table 5). Mature discharged ascospores expressed ppg1 constitutively, unlike conidia, which expressed ppg1 only when induced and germinating (Table 3). The role of ascospores as spermatia may be an underrecognized function.
We were not able to identify the female receptive structures or visually confirm chemoattraction of the female by the male. We intended to use the pre2 reporter constructs to identify potential receptive female structures, but this approach was not possible since pre2 is expressed in all cells except uninduced mycelia. We used methods similar to those described by Bistis (2, 3) for N. crassa but were unable to visually identify trichogynes associated with immature perithecia. If these structures occur at all, they are difficult to distinguish due to the relatively dense mycelial growth on carrot agar. Without a second pheromone-receptor pair for communication from the female structures to the male spermatium, it also is unknown how cell cycle synchronization is achieved prior to conjugation (2, 3, 4, 14). Further work is needed to identify the receptive female structures and how the spermatia transfer their nuclei to the female in this fungus.
The high facultative outcrossing rate in Δppg1 mutant females probably is due to a decrease in the competitiveness of conidia from the female strain as spermatia. On a facultative outcrossing plate, conidia produced in situ by the female parent usually outnumber the spermatia added from the fertilizing male parent. The deletion of ppg1 in the female parent may decrease the ability of conidia produced by the female strain to be recognized as spermatia. An alternative hypothesis is that absence of interfering pheromone peptides produced from female mycelia or perithecia increases the recognition of spermatia from the male strain. However, this hypothesis seems unlikely because these female structures do not appear to express ppg1 (Table 3; Fig. 4). It also does not explain why male spermatia would be favored over conidia from the female strain. The enhancing effect of the deletion of ppg1 in females could be useful for studies of pheromone function.
G. zeae must have a pheromone-independent alternate mechanism to activate the pheromone signal transduction pathway. Pheromone receptors are an integral upstream part of the receptor-G-protein-coupled mitogen-activated protein kinase cascade that mediates pheromone responses in ascomycetes (16). Sexual reproduction in G. zeae has an absolute requirement for pheromone pathway components such as both MAT idiomorphs (28) and for the MGV1 MAP kinase gene (21), but not for sex pheromones or receptors. In S. macrospora and E. nidulans, pheromone receptors are required but can apparently trigger the signal transduction cascade in the absence of pheromone peptides (31, 42). In G. zeae, the receptors are not required, so nonspecific triggering must occur downstream of the receptors. This phenomenon could be similar to STE5 gain-of-function mutations in S. cerevisiae in which pheromone pathway signaling is constitutively activated in the absence of pheromone or Gβγ (39, 43).
The pheromone-independent alternate activation mechanism clearly functions for external fertilization by spermatia, but it also might function as part of an internal self-fertilization mechanism. Trail and Common (47) reported thick, lipid-rich dikaryotic hyphae associated with perithecium production on carrot agar plates. The dikaryotic hyphae were produced in homothallic cultures, so the paired nuclei were presumed to be genetically identical. In detailed microscopic studies of homothallic production of perithecia in wheat stems, Guenther and Trail (19) again associated the production of perithecial initials with dikaryotic hyphae. Dikaryotic hyphae were produced from uninucleate hyphae within xylem vessels, pith cavities, and chlorenchyma tissues. Although the details are still unclear, these observations suggest that sexual developmental can occur without fertilization by external spermatia.
Induction of the sexual stage in G. zeae occurs in response to specific compounds or conditions in carrot agar cultures. Carrot agar is an excellent medium for induction of the sexual stage of G. zeae, and abundant perithecia usually are formed within 10 days (7). In contrast, few or no perithecia are produced on potato dextrose agar, complete medium, minimal medium, or CMC medium (data not shown). Germinating conidia and ascospores from induced cultures, i.e., cultures growing on carrot agar that have been fertilized with a spore suspension or mock fertilized with a 2.5% aqueous Tween 60 solution, expressed ppg1 at a high level. Germinating conidia from cultures grown on water agar, complete medium (solid or liquid), minimal medium (solid or liquid), minimal medium containing 10% of the normal nitrogen amount, and CMC liquid medium did not express ppg1 (data not shown). In N. crassa, the expression of these pheromone pathway genes is influenced by nitrogen starvation (4, 25). However, in G. zeae, low-nitrogen media such as water agar, CMC medium, and reduced-nitrogen minimal medium apparently are insufficient for induction. The GFP reporter strains for ppg1 and pre2 could be useful for identifying the inducing factors for the sexual stage of this fungus.
In conclusion, this study demonstrates that one of the pheromone-receptor pairs (ppg1/pre2) found in heterothallic ascomycetes enhances, but is not essential for, selfing and outcrossing in homothallic G. zeae, whereas the other pheromone-receptor pair (ppg2/pre1) no longer has any detectable function in sexual reproduction. Thus, a pheromone- and pheromone receptor-independent sexual triggering mechanism exists in this fungus, which makes it unique among ascomycetes. This alternate activation mechanism appears to be an evolutionarily recent adaptation since most other Gibberella species are heterothallic (30). Therefore, different portions of the ancestral pheromone signaling pathway in G. zeae may be under purifying selection pressure (ppg1 and pre2), under directional selection pressure (components of the signal transduction mechanism), or under no selection pressure at all (ppg2 and pre1). Characterization of recent molecular evolution in these genes may provide insights into the fundamental mechanisms underlying cell recognition and differentiation in fungi.
Acknowledgments
We thank Mizuho Nita for assistance with statistical analyses.
This is contribution 07-272-J from the Kansas Agricultural Experiment Station.
This material is based on work supported by the U.S. Department of Agriculture. This is a cooperative project with the U.S. Wheat & Barley Scab Initiative.
Mention of a trademark or a proprietary product does not constitute a guarantee or warranty of the product by the United States Department of Agriculture and does not imply its approval to the exclusion of other products that may also be suitable.
Footnotes
Published ahead of print on 23 May 2008.
REFERENCES
- 1.Anderegg, R. J., R. Betz, S. A. Carr, J. W. Crabb, and W. Duntze. 1988. Structure of the Saccharomyces cerevisiae mating hormone a-factor: identification of S-farnesyl cysteine as a structural component. J. Biol. Chem. 26318236-18240. [PubMed] [Google Scholar]
- 2.Bistis, G. N. 1981. Chemotropic interactions between trichogynes and conidia of opposite mating type in Neurospora crassa. Mycologia 73959-975. [Google Scholar]
- 3.Bistis, G. N. 1983. Evidence for diffusible, mating-type-specific trichogyne attractions in Neurospora crassa. Exp. Mycol. 7292-295. [Google Scholar]
- 4.Bobrowicz, P., R. Pawlak, A. Correa, D. Bell-Pedersen, and D. J. Ebbole. 2002. The Neurospora crassa pheromone precursor genes are regulated by the mating type locus and the circadian clock. Mol. Microbiol. 45795-804. [DOI] [PubMed] [Google Scholar]
- 5.Bölker, M., and R. Kahmann. 1993. Sexual pheromones and mating responses in fungi. Plant Cell 51461-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowden, R. L., and J. F. Leslie. 1992. Nitrate-nonutilizing mutants of Gibberella zeae (Fusarium graminearum) and their use in determining vegetative compatibility. Exp. Mycol. 16308-315. [Google Scholar]
- 7.Bowden, R. L., and J. F. Leslie. 1999. Sexual recombination in Gibberella zeae. Phytopathology 89182-188. [DOI] [PubMed] [Google Scholar]
- 8.Capellini, R. A., and J. L. Peterson. 1965. Macroconidium formation in submerged cultures by a nonsporulating strain of Gibberella zeae. Mycologia 57962-966. [Google Scholar]
- 9.Catlett, N. L., B.-N. Lee, O. C. Yoder, and B. G. Turgeon. 2003. Split-marker recombination for efficient targeted deletion of fungal genes. Fungal Genet. Newsl. 5011. [Google Scholar]
- 10.Coppin, E., R. Debuchy, S. Arnaise, and M. Picard. 1997. Mating types and sexual development in filamentous ascomycetes. Microbiol. Mol. Biol. Rev. 61411-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coppin, E., C. D. Renty, and R. Debuchy. 2005. The function of the coding sequences for the putative pheromone precursors in Podospora anserina is restricted to fertilization. Eukaryot. Cell 4407-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davey, J. 1992. Mating pheromones of the fission yeast Schizosaccharomyces pombe: purification and structural characterization of M-factor and isolation and analysis of two genes encoding the pheromone. EMBO J. 11951-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desjardins, A. E. 2006. Fusarium mycotoxins: chemistry, genetics, and biology. APS Press, St. Paul, MN.
- 14.Dohlman, H., and J. Thorner. 2001. Regulation of G protein-initiated signal transduction in yeast: paradigms and principles. Annu. Rev. Biochem. 70703-754. [DOI] [PubMed] [Google Scholar]
- 15.Dyer, P. S., M. Paoletti, and D. B. Archer. 2003. Genomics reveals sexual secrets of Aspergillus. Microbiology 1492301-2303. [DOI] [PubMed] [Google Scholar]
- 16.Elion, E. A. 2000. Pheromone response, mating and cell biology. Curr. Opin. Microbiol. 3573-581. [DOI] [PubMed] [Google Scholar]
- 17.Fernando, W. G. D., T. C. Paulitz, W. L. Seaman, P. Dutilleul, and J. D. Miller. 1997. Head blight gradients caused by Gibberella zeae from area sources of inoculum in wheat field plots. Phytopathology 87414-421. [DOI] [PubMed] [Google Scholar]
- 18.Gale, L. R., J. D. Bryant, S. Calvo, H. Giese, T. Katan, K. O'Donnell, H. Suga, M. Taga, T. R. Usgaard, T. J. Ward, and H. C. Kistler. 2005. Chromosome complement of the fungal plant pathogen Fusarium graminearum based on genetic and physical mapping and cytological observations. Genetics 171985-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guenther, J. C., and F. Trail. 2005. The development and differentiation of Gibberella zeae (anamorph: Fusarium graminearum) during colonization of wheat. Mycologia 97229-237. [DOI] [PubMed] [Google Scholar]
- 20.Horwitz, B. A., A. Sharon, S.-W. Lu, V. Ritter, T. M. Sandrock, O. C. Yoder, and B. G. Turgeon. 1999. A G protein alpha subunit from Cochliobolus heterostrophus involved in mating and appressorium formation. Fungal Genet. Biol. 2619-32. [DOI] [PubMed] [Google Scholar]
- 21.Hou, Z., C. Xue, Y. Peng, T. Katan, H. C. Kistler, and J.-R. Xu. 2002. A mitogen-activated protein kinase gene (MGV1) in Fusarium graminearum is required for female fertility, heterokaryon formation, and plant infection. Mol. Plant-Microbe Interact. 151119-1127. [DOI] [PubMed] [Google Scholar]
- 22.Jurgenson, J. E., R. L. Bowden, K. A. Zeller, J. F. Leslie, N. J. Alexander, and R. D. Plattner. 2002. A genetic map of Gibberella zeae (Fusarium graminearum). Genetics 1601451-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim, H., and K. A. Borkovich. 2004. A pheromone receptor gene, pre-1, is essential for mating type-specific directional growth and fusion of trichogynes and female fertility in Neurospora crassa. Mol. Microbiol. 521781-1798. [DOI] [PubMed] [Google Scholar]
- 24.Kim, H., and K. A. Borkovich. 2006. Pheromones are essential for male fertility and sufficient to direct chemotropic polarized growth of trichogynes during mating in Neurospora crassa. Eukaryot. Cell 5544-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim, H., R. L. Metzenberg, and M. A. Nelson. 2002. Multiple functions of mfa-1, a putative pheromone precursor gene of Neurospora crassa. Eukaryot. Cell 1987-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurjan, J. 1993. The pheromone response pathway in Saccharomyces cerevisiae. Annu. Rev. Genet. 27147-179. [DOI] [PubMed] [Google Scholar]
- 27.Lee, J., J. E. Jurgenson, J. F. Leslie, and R. L. Bowden. 2008. Alignment of genetic and physical maps of Gibberella zeae. Appl. Environ. Microbiol. 742349-2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee, J., T. Lee, Y.-W. Lee, S.-H. Yun, and B. G. Turgeon. 2003. Shifting fungal reproductive mode by manipulation of mating type genes: obligatory heterothallism of Gibberella zeae. Mol. Microbiol. 50145-152. [DOI] [PubMed] [Google Scholar]
- 29.Lee, T., Y.-K. Han, K.-H. Kim, S.-H. Yun, and Y.-W. Lee. 2002. TRI13 and TRI7 determine deoxynivalenol- and nivalenol-producing chemotypes of Gibberella zeae. Appl. Environ. Microbiol. 682148-2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leslie, J. F., and B. A. Summerell. 2006. The Fusarium laboratory manual. Blackwell Publishing, Ames, IA.
- 31.Mayrhofer, S., J. M. Weber, and S. Poggeler. 2006. Pheromones and pheromone receptors are required for proper sexual development in the homothallic ascomycete Sordaria macrospora. Genetics 1721521-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McMullen, M., R. Jones, and D. Gallenberg. 1997. Scab of wheat and barley: a re-emerging disease of devastating impact. Plant Dis. 811340-1348. [DOI] [PubMed] [Google Scholar]
- 33.Namiki, F., M. Matsunaga, M. Okuda, I. Inoue, K. Nishi, Y. Fujita, and T. Tsuge. 2001. Mutation of an arginine biosynthesis gene causes reduced pathogenicity in Fusarium oxysporum f. sp. melonis. Mol. Plant-Microbe Interact. 14580-584. [DOI] [PubMed] [Google Scholar]
- 34.O'Donnell, K., H. C. Kistler, B. K. Tacke, and H. H. Casper. 2000. Gene genealogies reveal global phylogeographic structure and reproductive isolation among lineages of Fusarium graminearum, the fungus causing wheat scab. Proc. Natl. Acad. Sci. USA 977905-7910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Donnell, K., T. J. Ward, D. M. Geiser, H. C. Kistler, and T. Aoki. 2004. Genealogical concordance between the mating type locus and seven other nuclear genes supports formal recognition of nine phylogenetically distinct species within the Fusarium graminearum clade. Fungal Genet. Biol. 41600-623. [DOI] [PubMed] [Google Scholar]
- 36.Olesnicky, N. S., A. J. Brown, S. J. Dowell, and L. A. Casselton. 1999. A constitutively active G-protein-coupled receptor causes self-compatibility in the mushroom Coprinus. EMBO J. 182756-2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pöggeler, S. 2000. Two pheromone precursor genes are transcriptionally expressed in the homothallic ascomycete Sordaria macrospora. Curr. Genet. 37403-411. [DOI] [PubMed] [Google Scholar]
- 38.Pöggeler, S., and U. Kuck. 2001. Identification of transcriptionally expressed pheromone receptor genes in filamentous ascomycetes. Gene 2809-17. [DOI] [PubMed] [Google Scholar]
- 39.Pryciak, P. M., and F. A. Huntress. 1998. Membrane recruitment of the kinase cascade scaffold protein Ste5 by the Gβγ complex underlies activation of the yeast pheromone response pathway. Genes Dev. 122684-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 41.Schmale, D. G., J. F. Leslie, K. A. Zeller, A. A. Saleh, E. J. Shields, and G. C. Bergstrom. 2006. Genetic structure of atmospheric populations of Gibberella zeae. Phytopathology 961021-1026. [DOI] [PubMed] [Google Scholar]
- 42.Seo, J.-A., K.-H. Han, and J.-H. Yu. 2004. The gprA and gprB genes encode putative G protein-coupled receptors required for self-fertilization in Aspergillus nidulans. Mol. Microbiol. 531611-1623. [DOI] [PubMed] [Google Scholar]
- 43.Sette, C., C. J. Inouye, S. L. Stroschein, P. J. Iaquinta, and J. Thorner. 2000. Mutational analysis suggests that activation of the yeast pheromone response mitogen-activated protein kinase pathway involves conformational changes in the Ste5 scaffold protein. Mol. Biol. Cell 114033-4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen, W.-C., P. Bobrowicz, and D. J. Ebbole. 1999. Isolation of pheromone precursor genes of Magnaporthe grisea. Fungal Genet. Biol. 27253-263. [DOI] [PubMed] [Google Scholar]
- 45.Spellig, T., M. Bolker, F. Lottspeich, R. W. Frank, and R. Kahmann. 1994. Pheromones trigger filamentous growth in Ustilago maydis. EMBO J. 131620-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Staben, C., B. Jensen, M. Singer, J. Pollock, M. Schechtman, J. Kinsey, and E. Selker. 1989. Use of a bacterial hygromycin B resistance gene as a dominant selectable marker in Neurospora crassa transformation. Fungal Genet. Newsl. 3679-81. [Google Scholar]
- 47.Trail, F., and R. Common. 2000. Perithecial development by Gibberella zeae: a light microscopy study. Mycologia 92130-138. [Google Scholar]
- 48.Turina, M., A. Prodi, and N. K. Van Alfen. 2003. Role of the Mf1-1 pheromone precursor gene of the filamentous ascomycete Cryphonectria parasitica. Fungal Genet. Biol. 40242-251. [DOI] [PubMed] [Google Scholar]
- 49.Yun, S.-H., T. Arie, I. Kaneko, O. C. Yoder, and B. G. Turgeon. 2000. Molecular organization of mating type loci in heterothallic, homothallic, and asexual Gibberella/Fusarium species. Fungal Genet. Biol. 317-20. [DOI] [PubMed] [Google Scholar]
- 50.Yun, S.-H., M. L. Berbee, O. C. Yoder, and B. G. Turgeon. 1999. Evolution of the fungal self-fertile reproductive life style from self-sterile ancestors. Proc. Natl. Acad. Sci. USA 965592-5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeller, K. A., R. L. Bowden, and J. F. Leslie. 2004. Population differentiation and recombination in wheat scab populations of Gibberella zeae in the United States. Mol. Ecol. 13563-571. [DOI] [PubMed] [Google Scholar]
- 52.Zhang, L., R. A. Baasiri, and N. K. Van Alfen. 1998. Viral expression of fungal pheromone precursor gene expression. Mol. Biol. Cell 18353-359. [DOI] [PMC free article] [PubMed] [Google Scholar]