Abstract
Mycobacterium tuberculosis possesses a diversity of potential virulence factors including complex branched lipids such as the phenolic glycolipid PGL-tb. PGL-tb expression by the clinical M. tuberculosis isolate HN878 has been associated with a less efficient Th1 response and increased virulence in mice and rabbits. It has been suggested that the W-Beijing family is the only group of M. tuberculosis strains with an intact pks1-15 gene, required for the synthesis of PGL-tb and capable of producing PGL-tb. We have found that some strains with an intact pks1-15 do not produce PGL-tb while others may produce a variant of PGL-tb. We examined the early host cytokine response to infection with these strains in vitro to better understand the effect of PGL-tb synthesis on immune responses. In addition, we generated a PGL-tb-producing H37Rv in order to determine the effect of PGL-tb production on the host immune response during infection by a strain normally devoid of PGL-tb synthesis. We observed that PGL-tb production by clinical M. tuberculosis isolates affected cytokine production differently depending on the background of the strain. Importantly, while ectopic PGL-tb production by H37Rv suppressed the induction of several pro- and anti-inflammatory cytokines in vitro in human monocytes, it did not lead to increased virulence in infected mice and rabbits. Collectively, our data indicate that, while PGL-tb may play a role in the immunogenicity and/or virulence of M. tuberculosis, it probably acts in concert with other bacterial factors which seem to be dependent on the background of the strain.
Mycobacterium tuberculosis, the causative agent of tuberculosis (TB), is an extremely well-adapted human pathogen. Infecting one-third of the world's population, M. tuberculosis remains the most prevalent of all potentially fatal bacterial infections worldwide (3, 11, 28). The reasons for the immense success of this pathogen in human populations are still not completely understood. The genome sequence of this bacterium includes an extensive repertoire of genes involved in lipid biosynthesis and degradation (5). Hence, it is not surprising that approximately 60% of the dry weight of M. tuberculosis is made up of lipids, mainly associated with the cell wall, including phenolic glycolipid (PGL-tb) (6, 9, 26, 27, 29). As many lipid moieties are surface exposed, it is likely that these large and complex molecules interact with components of the host immune response. Recently, considerable effort has been invested in identifying the lipid factors that contribute to immune activation and pathogenesis (8, 18, 29).
PGL-tb was originally identified in “Mycobacterium canetti” (9, 37). Its production by a subset of M. tuberculosis clinical isolates has been associated with increased virulence of the bacilli in animal infection models. Studies of aerosol infection in mice have shown that there is about a 90-day median difference in survival time between animals infected with a W-Beijing HN878 strain that produces PGL-tb and an isogenic one that cannot (29). This increased virulence has been associated with the induction of a suboptimal murine Th1 immune response (18, 20, 21, 29, 32).
The majority of clinical isolates, as well as the laboratory strains H37Rv and Erdman, are deficient in PGL-tb production (4, 6, 8, 17, 29, 30). A 7-base-pair deletion in a polyketide synthase gene (pks1-15), which gives rise to a frameshift mutation, has been associated with the inability of M. tuberculosis isolates to synthesize PGL-tb (6). This pks1-15 polymorphism is present in the laboratory strains H37Rv and Erdman and many clinical isolates, including CDC1551 and Mt103 (6). Members of the W-Beijing family of strains are unusual in having an intact pks1-15 gene, and many of these strains retain the ability to produce PGL-tb (18, 29, 30). However, recently, some W-Beijing isolates that possess an intact pks1-15 gene were shown to be deficient in PGL-tb synthesis, indicating that not all members of this family are producers of this lipid and suggesting other molecular mechanisms for their success (30).
Studies from this laboratory have shown that PGL-tb production by W-Beijing strains results in a reduced production of tumor necrosis factor alpha (TNF-α) and interleukin-12 (IL-12) by M. tuberculosis-infected human monocytes (18). This observation provided a link between the hypervirulence of these strains in experimental animals and their association with suboptimal Th1 immunity during infection. To further investigate the role of PGL-tb in the host immune response to M. tuberculosis infection, we have characterized a larger set of clinical isolates for the presence of an intact pks1-15 gene and for subsequent PGL-tb production. We then compared a representative set of PGL-tb-producing strains for their ability to induce cytokine production following infection of human peripheral blood monocytes and peripheral blood mononuclear cells (PBMCs). We also studied a recombinant H37Rv strain which was engineered to produce PGL-tb and investigated the role of ectopic PGL-tb production on (i) the growth of the bacilli in vitro and in vivo, (ii) the early host cytokine response in vitro, and (iii) the virulence of the strain in infected animals.
MATERIALS AND METHODS
Bacterial strains.
M. tuberculosis clinical isolates were selected from among the Public Health Research Institute TB Center's archived collection of over 20,000 isolates, all of which have been characterized by IS6110 restriction fragment length polymorphism analysis and assigned to strain families as previously described (36). A maximally diverse cohort of 318 isolates was selected based on genetic diversity, as defined by principal genetic groupings (PGGs) and known single nucleotide polymorphism (SNP) data (14, 31). Briefly, strains are separated into PGGs according to SNPs in codon 463 of katG (katG463) and gyrA95. PGG1 strains have katG463 CTG and gyrA95 ACC. PGG2 strains have katG463 CGC and gyrA95 ACC, and PGG3 strains have katG463 CGC and gyrA95 AGC. Based on our initial screen of the 318 clinical isolates, nine strains that represented several SNP clusters were selected for further analysis. These include CDC1551, CN1, GD46, HN878, HR59, N4, NHN5, W4, and W451 (14, 15). Also analyzed were the laboratory strains Erdman and H37Rv. Strains HN878, NHN5, W4, W451, and N4 are W-Beijing strains based on specific genetic markers (16). CN1 is related to the W-Beijing strains based on other markers (16). GD46 and HR59 are non-W-Beijing members of PGG1 (14). CDC1551 and Erdman belong to PGG2, and H37Rv belongs to PGG3 (14).
Molecular beacon probes and oligonucleotide primers.
The sequences for all molecular beacon probes and oligonucleotide primers are listed in Table 1. Molecular beacon probes were obtained from Biosearch Technologies (Novato, CA), and oligonucleotide primers were obtained from Integrated DNA Technologies (Coralville, IA). Molecular beacons (35) and oligonucleotide primers were designed using the Beacon Designer 2.0 software (Premier Biosoft International, Palo Alto, CA) and mfold, the Zuker DNA folding program (http://mfold.bioinfo.rpi.edu/cgi-bin/dna-form1.cgi). The probes and primers were validated using the guidelines set out in the molecular beacon website (http://www.molecular-beacons.org). Single-probe reactions were conducted first to confirm target specificity. The probes were then combined and used in a duplex format, with each probe labeled with a different fluorophore. Oligonucleotide primer and molecular beacon probe sequences for sigA were designed and validated as described previously (10).
TABLE 1.
Molecular beacon and primer sequences
| Oligonucleotide | Sequence (5′-3′)a |
|---|---|
| pks1-15-F | GCATCTGATCTTGGAAGAGG |
| pks1-15-R | AACGCCTCAGCCGATCTC |
| pks1-15c-MB | FAM-CGCGAGCGGCCGTCGATGGTGCCGTGTCGCG-Dabcyl |
| pks1-15-MB | HEX-CGAGCAGCCGCGGGCCGCGGCCGTCTGCTCG-Dabcyl |
| sigA-F | TGCAGTCGGTGCTGGACAC |
| sigA-R | CGCGCAGGACCTGTGAGCGG |
| sigA-MB | HEX-CCTCGCGTCGAAGTTGCGCCATCCGAGCGAGG-Dabcyl |
Underlined sequences represent stem structure of the molecular beacons. FAM, fluorescein; HEX, hexachlorofluorescein; Dabcyl, 4-([4-{dimethylamino}phenyl]azo)-benzoyl.
RT-PCR for detection of an intact pks1-15.
Real-time PCR (RT-PCR) was performed using an Mx4000 thermofluorocycler (Stratagene, La Jolla, CA). The pks1-15 molecular beacon targets the region of a 7-base-pair deletion in H37Rv compared to the partially sequenced W-Beijing strain 210, and the pks1-15c molecular beacon targets a region common to all sequenced M. tuberculosis genomes as well as Mycobacterium bovis. Both molecular beacons target sequences in an amplification product generated with the same primer pair. Each 50-μl duplex RT-PCR mixture contained 25 μl Brilliant QPCR master mix (Stratagene, La Jolla, CA), 0.5 μM of each primer, 0.2 μM of pks1-15 molecular beacon, 0.2 μM of pks1-15c molecular beacon, and 1 μl of purified genomic DNA (used at approximately 15 to 150 ng/μl). The remainder of the 50-μl reaction mixture was made up of nuclease-free water. Genomic DNA was isolated as previously described (36). The thermocycling program used was 1 cycle of 95°C for 10 min followed by 45 cycles of 95°C for 30 seconds, 63°C for 30 seconds, and 72°C for 30 seconds, while monitoring the fluorescence signal output during the 63°C step.
Isolation of RNA.
Bacterial cultures were grown in triplicate in Middlebrook 7H9 medium (Becton Dickinson, Sparks, MD) supplemented with 10% oleic acid-albumin-dextrose-catalase (BD Biosciences, Sparks, MD), 0.2% glycerol, and 0.05% Tween 80 to late exponential phase. Cultures were centrifuged for 5 min at 5,000 rpm and 4°C. The bacterial pellet was immediately frozen on dry ice and then resuspended in 1 ml Tri reagent containing 1 μl polyacryl carrier (Molecular Research Center, Cincinnati, OH). This was transferred to a 2-ml bead beater tube containing 0.5 ml zirconia beads, and the cells were disrupted in a bead beater for 1 min three times with 2-minute cooling intervals on ice. After a 5-min incubation on ice, the supernatant was transferred to a 1.5-ml microcentrifuge tube and centrifuged at 14,000 rpm for 15 min at 4°C, followed by transfer of the supernatant to a new 1.5-ml tube. This step was repeated once more, and 100 μl 1-bromo-3-chloropropane (Molecular Research Center, Cincinnati, OH) was added to the supernatant. The tube was vortexed and left on ice for 10 min, followed by centrifugation at 14,000 rpm for 15 min at 4°C. The aqueous phase was transferred to a new tube containing 10 μl polyacryl carrier, and 600 μl isopropanol was added, followed by overnight incubation at −20°C to precipitate the RNA. The RNA was harvested by centrifugation (14,000 rpm, 15 min) and washed once with 75% ethanol, and the pellet was air dried before resuspension in diethyl pyrocarbonate-H2O. DNase treatment was carried out with the Turbo DNase I kit (Ambion, Austin, TX) by following the manufacturer's instructions, and the DNase I was removed using the RNeasy minikit (Qiagen, Valencia, CA) by following the manufacturer's instructions.
RT-PCR for detection of the pks1-15 mRNA transcript.
To generate cDNAs, each 20-μl reverse transcriptase PCR mixture contained 450 ng RNA, 2 μl PCR buffer, 1.25 mM MgCl2, 0.5 mM deoxynucleoside triphosphates, 0.5 μM reverse transcriptase primer, 20 units RNase inhibitor (Applied Biosystems, Foster City, CA), and 50 units murine leukemia virus reverse transcriptase (Applied Biosystems, Foster City, CA), and the remainder of the reaction mixture was made up with diethyl pyrocarbonate-H2O. Reaction mixtures without reverse transcriptase were used as a negative control. The samples were heated at 42°C for 30 min, followed by 99°C for 5 min. The resulting cDNA was used for quantitative RT-PCR as described above for the genomic DNA reactions. For pks1-15 the molecular beacon used was pks1-15c-MB (Table 1) and the thermal cycling program used is described above. For sigA, the thermal cycling program used was 1 cycle of 95°C for 10 min followed by 45 cycles of 95°C for 30 seconds, 55°C for 30 seconds, and 72°C for 30 seconds while monitoring the fluorescence signal output during the 55°C step. The oligonucleotide primer and molecular beacon sequences for sigA were described previously and are shown in Table 1 (10).
Standard curves were generated to calculate the relative copy numbers for each gene. This was accomplished by using the threshold cycle (CT) value for each sample and applying the equation relative copy number = 10(CT − y intercept)/slope, where the slope and y-intercept values were generated from the standard curve using Mx4000 software (Stratagene, La Jolla, CA). Each sample was assayed three times, and the average number of pks1-15 transcripts was divided by the average number of sigA transcripts to obtain the normalized number of pks1-15 transcripts for each strain.
Extraction of lipids and TLC.
Strains were cultured in Middlebrook 7H9 liquid medium containing 10% ADC (0.2% dextrose, 0.5% bovine serum albumin fraction V, 0.0003% beef catalase). Cultures of each strain (16 ml) were grown to exponential phase and labeled by incubation with 0.625 μCi/ml [1-14C]propionate (specific activity of 54 Ci/mol) for 24 h with continuous shaking. Lipids were extracted by adding 2 volumes of CH3OH and 1 volume of CHCl3 to 0.8 volume of culture to yield a homogeneous one-phase mixture. After 2 days, the mixture was partitioned into two phases by adding 1 volume of H2O-CHCl3 (1:1, vol/vol). The organic phase was recovered. The extraction was repeated once by adding 1 volume of CHCl3 to the aqueous phase. The organic phases were pooled, washed twice with water, and dried before analysis. Lipid extracts from each culture were resuspended in 100 μl of CHCl3, and approximately 30 μl was spotted onto a silica gel 60 thin-layer chromatography (TLC) plate (20 by 20 cm; Merck, Darmstadt, Germany). The TLC plate was developed in CHCl3-CH3OH (95:5 or 90:10) and visualized with a Typhoon PhosphorImager (Amersham Biosciences, Piscataway, NJ) or by spraying the plate with a 0.2% anthrone solution in concentrated H2SO4, followed by heating. PGL-tb production in each strain was quantified by comparing the number of counts per minute obtained in the PGL-tb region to the total number of counts per minute. For H37Rv and H37Rv::pPET1, cultures were not labeled with [1-14C]propionate and PGL-tb was visualized by spraying with 1% α-naphthol spray solution, followed by heating.
Construction of H37Rv producing PGL-tb (H37Rv::pPET1).
The pPET1 plasmid containing the functional pks1-15 gene as well as a hygromycin resistance cassette for selection was electroporated into M. tuberculosis strain H37Rv (6). For electroporations, H37Rv was grown to an optical density at 540 nm of 0.2 in Middlebrook 7H9 liquid medium supplemented with 10% oleic acid-albumin-dextrose-catalase, 0.2% glycerol, and 0.05% Tween 80. The culture was then centrifuged at 4,000 rpm, washed twice with sterile 10% glycerol, and then resuspended in 1 ml 10% glycerol. Fifty-microliter aliquots were stored at −80°C. Bacteria were thawed, added to an electroporation cuvette (Bio-Rad, Hercules, CA) with approximately 1.5 μg pPET1, and electroporated using an model 2510 electroporator (Eppendorf, Westbury, NY) at 600 Ω, 10 μF, and 2,500 V. Transformants were selected on media containing hygromycin (50 μg/ml), and PGL-tb was produced by H37Rv::pPET1, as detected by TLC analysis.
Human monocyte and PBMC infections.
PBMCs were isolated from fresh human blood (buffy coat) (New Jersey Blood Center, East Orange, NJ) by Ficoll-Paque separation (24) and plated at a density of 3 × 106 PBMCs per well in a 24-well plate. For the preparation of the monocytes, cells were resuspended in RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 1% human serum AB (Gemini Bioproducts, Calabasas, CA) and allowed to adhere for 2 h and then nonadherent cells were removed by washing as described previously (24). Both adherent monocytes and PBMCs were cultured in RPMI 1640 supplemented with 20% human serum prior to infection. Adherent monocytes were infected with M. tuberculosis at a multiplicity of infection of 1:1 (bacilli/monocytes). Monocytes were lysed by sonic disruption as described previously (19), and dilutions were spotted on Middlebrook 7H11 agar (Becton Dickinson, Sparks, MD) to assay for intracellular growth of the bacilli. Culture supernatants were removed at 5, 24, or 48 h postinfection, filtered using 0.22-μm microcentrifuge filter tubes to remove virulent organisms (Millipore, Billerica, MA), removed from the biosafety level 3 facility, and stored at −80°C for secreted-cytokine assays.
Detection of secreted cytokines by Luminex assay.
Culture supernatants were probed using a multiplex human cytokine Luminex panel (IL-1β, IL-2, IL-5, IL-6, IL-10, IL-12 p70, IL-13, IL-17, gamma interferon [IFN-γ], TNF-α) (Bio-Rad, Hercules, CA) according to the manufacturer's instructions. For the H37Rv and H37Rv::pPET1 strains, the Luminex cytokine panel used was IL-1β, IL-6, IL-10, IL-12 p70, IL-13, IL-17, IFN-γ, monocyte chemoattractant protein 1, TNF-α, and macrophage inflammatory protein 1β. The assay was read using a Bio-Plex 200 system (Bio-Rad, Hercules, CA), and data and statistical analyses were carried out using the Prism 4 program (GraphPad, San Diego, CA).
Mouse aerosol infections.
Mice were infected as described previously (19). Female 8-week-old (C57BL/6 × DBA/2)F1 (B6D2F1) mice free of common viral pathogens were acquired from Charles River Laboratories (Wilmington, MA). For each experiment, a stock vial of M. tuberculosis was sonicated using a water bath sonicator (Laboratory Supplies; model G112SPIT) for 30 seconds to disperse clumps and diluted in 1× phosphate-buffered saline containing 0.05% Tween 80 to the final concentration of approximately 3 × 106 cells/ml in 10 ml. Mice were exposed to the aerosol for 20 min using a Lovelace nebulizer (In-Tox Products, Albuquerque, NM). This implanted between 20 and 50 bacilli in the lungs of the mice, as confirmed by plating lung homogenates 3 h after infection (34). Quantification of CFU in lung, spleen, and liver tissue was carried out as follows. Mouse lungs were removed without the large bronchi prior to homogenization, and right-lung and whole-spleen tissues were homogenized in 1× phosphate-buffered saline plus 0.05% Tween 80, followed by plating 10-fold serial dilutions on Middlebrook 7H11 agar. Viable colonies were counted at 21 days.
Rabbit aerosol infections.
New Zealand White rabbits were acquired from Millbrook Farm (Amherst, MA) and infected as previously described (33). Briefly, for each experiment, a suspension of M. tuberculosis was prepared at 5 × 106 to 10 × 106 CFU/ml. Rabbits were exposed to the aerosol for 20 min using a custom-built aerosol infection module (CH Technologies). The aerosol delivery resulted in implantation of approximately 3.7 log10 bacilli in the lungs of the rabbits, as confirmed by plating lung homogenates 3 h after infection. Homogenates were prepared from lung tissue from each segment of the left and right lungs (excluding the large airways) in saline containing 0.05% Tween 80. The amount of tissue used for homogenization was approximately 30% of total lung weight. At time zero and at 4 and 8 weeks, two rabbits per group were used, and at 12 weeks, four rabbits per group were used. Growth of M. tuberculosis in infected lungs was monitored by homogenizing the lungs in 0.9% NaCl solution containing 0.05% Tween 80 and plating 10-fold dilutions on Middlebrook 7H11 agar. Colonies were counted after incubation at 37°C for 15 days.
RESULTS
Presence of an intact pks1-15 gene in M. tuberculosis clinical isolates.
In order to identify strains that have the potential to produce PGL-tb, we first sought to determine the distribution of an intact pks1-15 gene among a cohort of diverse clinical isolates. A total of 318 strains belonging to different PGGs based on katG and gyrA mutations were analyzed using a duplex RT-PCR (data not shown) (14, 15). Among the strains, 134 belonged to PGG1, which is composed of W-Beijing and other strain families, 144 belonged to PGG2, and 40 belonged to PGG3. An intact pks1-15 gene was detected only in PGG1 (Table 2). A disrupted pks1-15 among PGG1 strains was restricted to M. bovis, M. bovis BCG, and M. africanum isolates, confirming previous observations (22).
TABLE 2.
Distribution of intact pks1-15 gene clusters among isolates in this study
| PGG | No. of:
|
% pks1-15
|
||
|---|---|---|---|---|
| Strains | RFLPb families | Intact | Disrupted | |
| 1 | 134 | 53 | 89.6 | 10.4a |
| 2 | 144 | 79 | 0 | 100 |
| 3 | 40 | 26 | 0 | 100 |
Strains belonging to PGG1 with a disruption in pks1-15 were restricted to M. bovis, M. bovis BCG, and M. africanum isolates. All M. tuberculosis isolates belonging to PGG1 have an intact pks1-15 gene cluster.
RFLP, restriction fragment length polymorphism.
Expression of pks1-15 mRNA in M. tuberculosis clinical isolates.
Eleven strains out of the initial 318 were selected on the basis of genetic diversity and clinical importance for analysis of the expression of the pks1-15 gene by real-time quantitative PCR. This selection included three strains that had a disruption in pks1-15 and eight that possessed an intact pks1-15. We found that, in the strains that had a disruption in pks1-15, the transcript for this gene was still produced (Fig. 1). Also, all clinical isolates that had an intact pks1-15 produced the mRNA. The amount of transcript produced varied between strains, and this variation did not have an apparent genetic relationship. For example, strains HN878, W4, and W451, all closely related W-Beijing strains based on genetic markers (16), produced pks1-15 mRNA in very different quantities (Fig. 1). Thus, the levels of production of pks1-15 mRNA seemed to be dependent on other strain-specific factors even within the same PGG.
FIG. 1.
Expression of pks1-15 mRNA in selected clinical isolates. pks1-15 mRNA levels produced relative to sigA mRNA were assessed for nine genetically diverse clinical isolates as well as two laboratory strains. Black bars represent strains possessing an intact pks1-15 gene cluster. White bars represent strains having a disruption in pks1-15. A two-tailed paired t test was used for statistical analysis (*, P < 0.05 in comparison to HN878; ≠, P < 0.01 in comparison to HN878).
Detection of PGL-tb production by TLC.
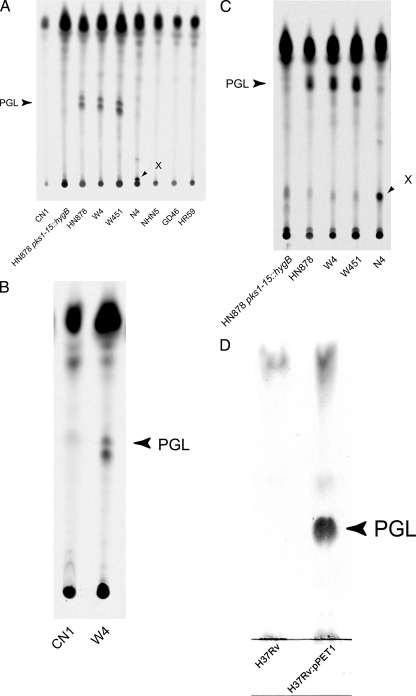
Because a functional pks1-15 is a requirement for PGL-tb production (6), strains possessing a functional pks1-15 were assayed for production of PGL-tb by TLC. As controls, the W-Beijing strain HN878 and a recombinant mutant harboring a mutation within pks1-15 (HN878 pks1-15::hygB) (29) were included. This was accomplished by two methodologies. First, the lipid extracts of the various strains were run on a TLC plate and stained for the presence of glycolipids (data not shown). Second, the cultures were incubated with [1-14C]propionic acid and lipid extracts were analyzed by TLC and detected radiometrically after fractionation on a TLC plate. This assay is specific for methyl-branched fatty acids such as PGL-tb, which are lipids derived from propionate (30). The two methods had a high concordance of PGL-tb production results for all the strains assessed. We found that PGL-tb was produced by the W-Beijing strains W4, HN878, and W451 (Fig. 2A). Quantification of radioactivity incorporated in PGL-tb revealed that these three strains synthesized similar amounts of the lipid. In contrast, other closely related and genetically distant strains did not synthesize PGL-tb despite possessing an intact pks1-15 (Fig. 2A). Strain CN1 grew poorly in media without Tween, and, to confirm the absence of PGL-tb production, lipids were also extracted from CN1 and W4 grown in the presence of Tween and fractionated by TLC (Fig. 2B). We also noted that the W-Beijing strain N4 produced a new compound with mobility suggestive of polarity greater than that of PGL-tb (Fig. 2A and C). This new glycolipid exhibited the same blue color as PGL-tb following anthrone staining (data not shown). The lipid was labeled with [1-14C]propionic acid, and preliminary structural characterization suggests that it may correspond to a structural variant of PGL-tb (data not shown). This compound was not detected in any of the other M. tuberculosis strains analyzed and to our knowledge has not been previously identified. As shown previously, when H37Rv was transformed with a functional pks1-15 (carried on pPET1), PGL-tb production was restored (Fig. 2D) (6, 26, 27).
FIG. 2.
TLC analysis of lipids extracted from selected M. tuberculosis clinical isolates possessing an intact pks1-15 gene. (A) Analysis of PGL-tb (PGL) production by the various W-Beijing strains. In this experiment, culturing was performed without adding 0.05% Tween 80. Under these growth conditions, CN1 grew very poorly. (B) Absence of PGL-tb production in CN1. To confirm the absence of PGL-tb production in CN1, the experiment was repeated with cultures grown with 0.05% Tween 80. Again no PGL-tb was detected in the CN1 culture. (C) Identification of a new glycolipid produced by strain N4. Glycolipid X was labeled with [1-14C]propionate and produced a blue color with anthrone. In contrast, the faint bands with similar mobility observed with the other strains were not stained with anthrone. PGL-tb is indicated, and an additional glycolipid produced only by strain N4 is shown as X. (D) Production of PGL-tb by H37Rv::pPET1. Glycolipids were stained with α-naphthol and visualized by heating. Lipids were extracted from [1-14C]propionate-labeled cultures of M. tuberculosis isolates (A to C), dissolved in CHCl3, and run in CHCl3-CH3OH (95:5) (A, B, and D) or CHCl3-CH3OH (90:10) (C).
Growth of selected W-Beijing strains in adherent human monocytes.
Four W-Beijing strains (W4, W451, N4, and CN1) were selected for further analysis out of the initial 11 strains on the basis of their capacity to produce PGL-tb. During a 72-hour infection of adherent human monocytes, the PGL-tb-producing strains W4 and W451, as well as CN1 (which does not produce PGL-tb), grew similarly. In contrast, the strain producing the novel glycolipid N4 grew significantly faster (Fig. 3A). Thus, the presence or absence of PGL-tb did not appear to determine the growth rate of the M. tuberculosis strains in vitro in human monocytes.
FIG. 3.
Growth of selected W-Beijing strains in monocytes in vitro and cytokine production by infected PBMCs. (A) Growth in human monocytes in vitro of W-Beijing isolates W4, W451, N4, and CN1. Mtb, M. tuberculosis. (B) Cytokine levels in supernatants from PBMCs infected with clinical W-Beijing isolates. Values are expressed as percentages of activity relative to that for W4-infected PBMCs. One hundred percent activity corresponds to 70,089.9 pg/ml for IL-1β, 17.98 pg/ml for IL-5, 34,150.25 pg/ml for IL-6, and 282.94 pg/ml for IL-10. Each experiment was performed with monocytes or PBMCs obtained from a single donor. Results are means ± standard errors from three independent experiments (donors) performed in duplicate. A two-tailed paired t test was used for statistical analysis (*, P < 0.05; ≠, P < 0.01, ≈, P < 0.005 [in comparison to W4]).
Effect of W-Beijing strains on cytokine production by human PBMCs.
Previously, we showed that selected W-Beijing strains elicit less proinflammatory and Th1 type cytokines than the non-W-Beijing strains H37Rv and CDC1551 (18, 29). In order to determine if PGL-tb production by W-Beijing strains would have a reproducible effect on host cytokine production, human PBMCs were infected with the W-Beijing strains W4, W451, N4, and CN1. Supernatants from infected PBMCs were collected at optimal time points for each cytokine (24 and 48 h) postinfection. Despite the fact that both strains produced similar amounts of PGL-tb (Fig. 2A), the cytokine responses to W4 and W451 were similar, but not equivalent. Both strains elicited similar amounts of IL-2, IL-4, IL-5, IL-10, IL-12 p70, IL-13, IL-17, IFN-γ, and TNF-α but significantly different amounts of IL-1β and IL-6 (Fig. 3B and data not shown). N4, which produces a novel glycolipid, elicited amounts of IL-1β and other cytokines similar to amounts elicited by W4 and significantly more IL-10 (Fig. 3B and data not shown). CN1 elicited significantly lesser amounts of Th2 cytokines IL-5 and IL-10 than W4 and similar amounts of Th1 cytokines (Fig. 3B and data not shown).
Effect of PGL-tb production by recombinant H37Rv on growth in human monocytes and cytokine production by human PBMCs.
Previously, we reported that production of PGL-tb by the W-Beijing strain HN878 results in less efficient proinflammatory and Th1 cytokine responses in infected human and mouse macrophages than infection with HN878 pks1-15::hygB (18, 29). We sought to establish whether PGL-tb production in a non-W-Beijing strain would give rise to similar results. H37Rv and H37Rv::pPET1 (which produces PGL-tb) were used to infect human peripheral blood monocytes over 72 h. Both H37Rv and H37Rv::pPET1 grew similarly in infected human monocytes (Fig. 4A). This indicated that the presence of PGL-tb did not affect the intracellular growth of the bacilli, thus confirming our observations with W-Beijing strains. Next, H37Rv::pPET1 and wild-type H37Rv were used to infect human PBMCs and culture supernatants were collected at optimal time points postinfection for each cytokine (5 and 48 h) for analysis. Compared to the parental H37Rv, H37Rv::pPET1 elicited significantly less IL-1β, TNF-α, IL-10, and IL-17 and similar amounts of IL-6 and IL-12 (Fig. 4B), two cytokines whose response, we reported previously, was significantly reduced by PGL-tb-producing HN878 infecting human monocytes and in murine macrophages (18, 29). H37Rv and H37Rv::pPET1 also elicited similar amounts of IL-13, MCP-1, IFN-γ, and MIP-1β (data not shown). These results suggested that PGL-tb production by H37Rv had only a modest effect on the production of specific cytokines and did not produce a generalized suppression of Th1 immunity.
FIG. 4.
H37Rv and H37Rv::pPET1: in vitro growth in monocytes and cytokine production by infected PBMCs. (A) Growth in human monocytes in vitro of H37Rv and PGL-tb-producing H37Rv::pPET1. Mtb, M. tuberculosis. (B) Cytokine levels in supernatants from PBMCs infected with H37Rv or H37Rv::pPET1. Values are expressed as percentages of activity relative to that for H37Rv-infected PBMCs. One hundred percent activity corresponds to 44,828.7 pg/ml for IL-1β, 5,372.16 pg/ml for IL-6, 198.78 pg/ml for IL-10, 18.09 pg/ml for IL-12 p70, 159.38 pg/ml for IL-17, and 2,081.24 pg/ml for TNF-α. Each experiment was performed with monocytes or PBMCs obtained from a single donor. Results are means ± standard errors from six independent experiments (donors) performed in duplicate. A two-tailed paired t test was used for statistical analysis (*, P < 0.05; ≈, P < 0.005; ∝, P < 0.001 [in comparison to H37Rv]).
Effect of PGL-tb production by recombinant H37Rv on virulence in infected mice and rabbits.
Mice and rabbits were infected with H37Rv and H37Rv::pPET1 to determine if PGL-tb production by the recombinant H37Rv would result in increased virulence in these models. At 3 h postexposure, the numbers of bacilli in the lungs were similar (Fig. 5A and B). In general, H37Rv::pPET1 grew more slowly in the lungs and disseminated slower to the spleen, but the differences were not statistically significant. The highest bacillary load with both strains was observed at 28 days postinfection, followed by a reduction by 84 days (Fig. 5A and B). Ongoing survival experiments with infected mice showed little difference in mice infected with H37Rv or H37Rv::pPET1 at 300 days postinfection (data not shown). Gross pathology and histopathology were performed for the infected mouse lungs. No significant difference between the lungs of the two groups was observed (data not shown). Histopathological examination of the infected lungs revealed more and larger disorganized granulomas in the lungs of H37Rv::pPET1-infected mice than in lungs of H37Rv-infected mice at 4 weeks postinfection. By 8 and 12 weeks postinfection, lesions became heavily infiltrated with lymphocytes and there was no significant difference in the amounts, sizes, or organizations of the lesions in the lungs of H37Rv- and H37Rv::pPET1-infected mice (data not shown). Gross pathological examination of the lungs of rabbits infected with H37Rv at 4 weeks postinfection revealed multiple 2- to 3-mm lesions, whereas lesions in rabbits infected with H37Rv:pPET1 were fewer and smaller (Fig. 5C). This result contradicted the observation on the increased virulence of HN878 in rabbits compared to that of the PGL-tb-deficient isogenic strain HN878 pks1-15::hygB, indicating that the effect of PGL-tb on virulence in the rabbit may be strain dependent (32).
FIG. 5.
Effect of infection in mice and rabbits with H37Rv and H37Rv::pPET1. (A) Growth of H37Rv and H37Rv::pPET1 in infected mouse lungs and spleen. Tissue homogenates from H37Rv::pPET1-infected mice were plated also on hygromycin-containing medium to determine retention of the plasmid [H37Rv::pPET1(hygro)]. Error bars are present at each time point, but some are too small to be visible. (B) Growth of H37Rv and H37Rv::pPET1 in infected rabbit lungs. Error bars are present at each time point, but some are too small to be visible. (C) Gross pathology of rabbit lungs at 4 weeks postinfection.
DISCUSSION
In this study, we have demonstrated that PGL-tb production is highly restricted within the M. tuberculosis complex and possibly produced only by W-Beijing strains. We have identified a W-Beijing strain, N4, which synthesizes a novel glycolipid that may be a variant of PGL-tb. This strain elicited a cytokine response similar to that elicited by the PGL-tb-producing W4 strain but grew more rapidly in human monocytes. We showed that PGL-tb can exert a cytokine-modulatory effect in diverse genetic backgrounds of M. tuberculosis. However, this modulation of the host cytokine response is not the same for each strain producing PGL-tb, and PGL-tb-induced cytokine modulation does not necessarily confer hypervirulence.
Previously, we showed that PGL-tb-producing W-Beijing strains elicited less TNF-α and IL-12 from infected monocytes than non-W-Beijing strains (18). We sought to expand on those observations by asking if all W-Beijing strains producing PGL-tb would exert similar effects on host cytokine production. We have found that several W-Beijing clinical isolates, differing in their abilities to produce PGL-tb or other glycolipids, elicit largely similar cytokine responses but that specific proinflammatory cytokines are differentially induced. For example, the W-Beijing strains W4 and W451 both synthesize similar amounts of PGL-tb and elicit similar amounts of most Th1 and Th2 cytokines, but they differ significantly in their abilities to induce the proinflammatory cytokines IL-1β and IL-6. Also, CN1, a W-Beijing-related strain devoid of PGL-tb, elicits Th1 and proinflammatory cytokine responses largely similar to those elicited by W4 but significantly lesser amounts of Th2 cytokines IL-5 and IL-10. All of these W-Beijing strains elicited similar low levels of IL-12. These results indicate that PGL-tb production by W-Beijing strains likely contributes to regulation of the host cytokine response but does not exert a global suppressive effect. Regardless of their abilities to produce PGL-tb or the novel glycolipid, all of these clinical isolates are virulent and have likely adapted mechanisms to survive in their respective niches (2, 13; G. Kaplan, unpublished observations).
Our results also indicate that the ability of PGL-tb to modulate the host cytokine response is likely dependent on the background of the strain in which it is produced. In these studies, we found that H37Rv::pPET1 elicited significantly lesser amounts of particular cytokines than its parental strain, H37Rv. However, the cytokines differentially induced were not the same as those found previously to be differentially elicited by HN878 and its PGL-tb knockout HN878 pks1-15::hygB (29). H37Rv::pPET1 elicited significantly lesser amounts of pro- and anti-inflammatory cytokines (TNF-α, IL-1β, IL-10, IL-17) than H37Rv, but levels of IL-6 and IL-12, two cytokines shown previously to be affected by PGL-tb production in HN878, remained the same (18, 29). H37Rv has been shown to be relatively more immunogenic than HN878 (25). It is possible that PGL-tb, acting in concert with various other stimulatory and/or inhibitory protein and lipid factors, contributes to the specific responses elicited from the host but that, rather than being a dominant factor in suppressing cytokine production, PGL-tb merely adjusts or redirects a host response which is largely determined by the sum of all the factors produced by each strain.
In previous studies, PGL-tb was implicated in the hypervirulence of the clinical isolate HN878 (19, 29, 32). HN878-infected mice succumbed to infection much more rapidly than mice infected with the PGL-tb knockout, and, in a rabbit M. tuberculosis meningitis model, rabbits infected with HN878 exhibited more-severe neurological symptoms than those infected with HN878 pks1-15::hygB (29, 32). Here, we sought to determine if PGL-tb synthesis by H37Rv would result in hypervirulence of the strain. In contrast to HN878, H37Rv::pPET1, which produces PGL-tb, although able to modulate the host proinflammatory cytokine response to infection, did so in a manner different from HN878 (29). H37Rv::pPET1 was not more virulent in the mouse or rabbit model of infection, as judged by bacillary growth in lungs, dissemination of bacilli to other tissues, gross pathology, and survival. Rather, H37Rv::pPET1 may have even been less virulent. It is possible that the specific profile of cytokines differentially inhibited by H37Rv::pPET1 could determine this difference. Although we cannot exclude the possibility that the plasmid slightly impacted the virulence of H37Rv::pPET1, the large quantities of PGL-tb synthesized by this strain would be expected, if sufficient for hypervirulence, to override this effect and confer some increased virulence. The hypervirulence of HN878 was attributed to its failure to induce a strong Th1 cytokine response, including inducing lower levels of IL-12 (29). However, levels of IL-12 remained unchanged by PGL-tb production in H37Rv::pPET1. IL-12 is an important regulatory cytokine in M. tuberculosis infection. The cytokine has been shown to be required for T-cell IFN-γ production, thereby contributing to controlling bacillary growth and inhibition of lung pathology (7, 12, 23). In humans, polymorphisms in the IL-12 receptor are directly linked to an increased susceptibility to TB (1). Perhaps, by affecting the ability of HN878 to induce IL-12, PGL-tb induced a hypervirulence phenotype in infected animals.
Collectively, our data indicate that, while PGL-tb is an important virulence factor for specific strains of M. tuberculosis, including some W-Beijing strains, it probably acts synergistically with other protein and/or lipid virulence factors in other strains. While its cytokine-modulatory function is active in a non-W-Beijing strain, this does not necessarily confer hypervirulence. Thus, it is possible that PGL-tb is an important virulence factor only for W-Beijing strains. It is not known whether the immunosuppressive effect of PGL-tb is dominant over other mycobacterial stimulatory factors in vivo, and the impact of PGL-tb may be masked in a more immunogenic background. Elucidating the mechanism by which PGL-tb affects production of host leukocyte cytokines, including the activation or suppression of different signaling pathways, will contribute to the understanding of the diversity of immune stimulation and evasion properties employed by M. tuberculosis during infection.
Acknowledgments
We thank Dorothy Fallows for a critical reading of the manuscript and Heran Darwin for technical assistance with the H37Rv/H37Rv::pPET1 TLC.
This work was supported by NIH R01 grant AI66046 awarded to G.K.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 28 April 2008.
REFERENCES
- 1.Akahoshi, M., H. Nakashima, K. Miyake, Y. Inoue, S. Shimizu, Y. Tanaka, K. Okada, T. Otsuka, and M. Harada. 2003. Influence of interleukin-12 receptor B1 polymorphisms on tuberculosis. Hum. Genet. 112237-243. [DOI] [PubMed] [Google Scholar]
- 2.Bifani, P. J., B. Mathema, Z. Liu, S. L. Moghazeh, B. Shopsin, B. Tempalski, J. Driscol, R. Frothingham, J. M. Musser, P. Alcabes, and B. N. Kreiswirth. 1999. Identification of a W variant outbreak of Mycobacterium tuberculosis via population-based molecular epidemiology. JAMA 2822321-2327. [DOI] [PubMed] [Google Scholar]
- 3.Bloom, B. R., and C. J. L. Murray. 1992. Tuberculosis: commentary on a reemergent killer. Science 2571055-1064. [DOI] [PubMed] [Google Scholar]
- 4.Cho, S., J. Shin, M. Daffe, Y. Chong, S. Kim, and J. Kim. 1992. Production of monoclonal antibody to a phenolic glycolipid of Mycobacterium tuberculosis and its use in detection of the antigen in clinical isolates. J. Clin. Microbiol. 303065-3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, A. Krogh, J. McLean, S. Moule, L. Murphy, K. Oliver, J. Osborne, M. A. Quail, M.-A. Rajandream, J. Rogers, S. Rutter, K. Seeger, J. Skelton, R. Squares, S. Squares, J. E. Sulston, K. Taylor, S. Whitehead, and B. G. Barrell. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393537-544. (Erratum, 396: 190.) [DOI] [PubMed] [Google Scholar]
- 6.Constant, P., E. Perez, W. Malaga, M. A. Laneelle, O. Saurel, M. Daffe, and C. Guilhot. 2002. Role of the pks15/1 gene in the biosynthesis of phenolglycolipids in the Mycobacterium tuberculosis complex. Evidence that all strains synthesize glycosylated p-hydroxybenzoic methly esters and that strains devoid of phenolglycolipids harbor a frameshift mutation in the pks15/1 gene. J. Biol. Chem. 27738148-38158. [DOI] [PubMed] [Google Scholar]
- 7.Cooper, A. M., A. D. Roberts, E. R. Rhoades, J. E. Callahan, D. M. Getzy, and I. M. Orme. 1995. The role of interleukin 12 in acquired immunity to Mycobacterium tuberculosis infection. Immunology 84423-432. [PMC free article] [PubMed] [Google Scholar]
- 8.Cox, J. S., B. Chen, M. McNeil, and W. R. Jacobs, Jr. 1999. Complex lipid determines tissue-specific replication of Mycobacterium tuberculosis in mice. Nature 40279-83. [DOI] [PubMed] [Google Scholar]
- 9.Daffe, M., C. Lacave, M. Laneelle, and G. Laneelle. 1987. Structure of the major triglycosyl phenol-phthiocerol of Mycobacterium tuberculosis (strain Canetti). Eur. J. Biochem. 167155-160. [DOI] [PubMed] [Google Scholar]
- 10.Dawes, S. S., D. F. Warner, L. Tsenova, J. Timm, J. D. McKinney, G. Kaplan, H. Rubin, and V. Mizrahi. 2003. Ribonucleotide reduction in Mycobacterium tuberculosis: function and expression of genes encoding class Ib and class II ribonucleotide reductases. Infect. Immun. 716124-6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dye, C., S. Scheele, P. Dolin, V. Pathania, and M. C. Raviglione. 1999. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA 282677-686. [DOI] [PubMed] [Google Scholar]
- 12.Flynn, J., M. Goldstein, K. Triebold, J. Sypek, S. Wolf, and B. Bloom. 1995. IL-12 increases resistance of BALB/c mice to Mycobacterium tuberculosis infection. J. Immunol. 1552515-2524. [PubMed] [Google Scholar]
- 13.Freeman, R., M. Kato-Maeda, K. A. Hauge, K. L. Horan, E. Oren, M. Narita, C. K. Wallis, D. Cave, C. M. Nolan, P. M. Small, and G. A. Cangelosi. 2005. Use of rapid genomic deletion typing to monitor a tuberculosis outbreak within an urban homeless population. J. Clin. Microbiol. 435550-5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gutacker, M. M., B. Mathema, H. Soini, E. Shashkina, B. N. Kreiswirth, E. A. Graviss, and J. M. Musser. 2006. Single-nucleotide polymorphism-based population genetic analysis of Mycobacterium tuberculosis strains from 4 geographic sites. J. Infect. Dis. 193121-128. [DOI] [PubMed] [Google Scholar]
- 15.Gutacker, M. M., J. C. Smoot, C. A. Migliaccio, S. M. Ricklefs, S. Hua, D. V. Cousins, E. A. Graviss, E. Shashkina, B. N. Kreiswirth, and J. M. Musser. 2002. Genome-wide analysis of synonymous single nucleotide polymorphisms in Mycobacterium tuberculosis complex organisms: resolution of genetic relationships among closely related microbial strains. Genetics 1621533-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurepina, N., E. Likhoshvay, E. Shashkina, B. Mathema, K. Kremer, D. van Soolingen, P. Bifani, and B. N. Kreiswirth. 2005. Targeted hybridization of IS6110 fingerprints identifies the W-Beijing Mycobacterium tuberculosis strains among clinical isolates. J. Clin. Microbiol. 432148-2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee, T., S. Cho, K. Yoon, J. Shin, and J. Kim. 1991. Comparison of DNA fragment patterns between the phenolic glycolipid-tb producers and non-producers of Mycobacterium tuberculosis. Yonsei Med. J. 32243-249. [DOI] [PubMed] [Google Scholar]
- 18.Manca, C., M. B. Reed, S. Freeman, B. Mathema, B. Kreiswirth, C. E. Barry III, and G. Kaplan. 2004. Differential monocyte activation underlies strain-specific Mycobacterium tuberculosis pathogenesis. Infect. Immun. 725511-5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manca, C., L. Tsenova, C. E. Barry III, A. Bergtold, S. Freeman, P. A. J. Haslett, J. M. Musser, V. H. Freedman, and G. Kaplan. 1999. Mycobacterium tuberculosis CDC1551 induces a more vigorous host response in vivo and in vitro, but is not more virulent than other clinical isolates. J. Immunol. 1626740-6746. [PubMed] [Google Scholar]
- 20.Manca, C., L. Tsenova, A. Bergtold, S. Freeman, M. Tovey, J. M. Musser, C. E. Barry III, V. H. Freedman, and G. Kaplan. 2001. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-alpha/beta. Proc. Natl. Acad. Sci. USA 985752-5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manca, C., L. Tsenova, S. Freeman, A. K. Barczak, M. Tovey, P. J. Murray, C. Barry, and G. Kaplan. 2005. Hypervirulent M. tuberculosis W/Beijing strains upregulate type I IFNs and increase expression of negative regulators of the Jak-Stat pathway. J. Interferon Cytokine Res. 25694-701. [DOI] [PubMed] [Google Scholar]
- 22.Marmiesse, M., P. Brodin, C. Buchrieser, C. Gutierrez, N. Simoes, V. Vincent, P. Glaser, S. T. Cole, and R. Brosch. 2004. Macro-array and bioinformatic analyses reveal mycobacterial ‘core’ genes, variation in the ESAT-6 gene family and new phylogenetic markers for the Mycobacterium tuberculosis complex. Microbiology 150(Pt. 2)483-496. [DOI] [PubMed] [Google Scholar]
- 23.Mendez-Samperio, P., H. E. Ayala-Verdin, and A. Trejo-Echeverria. 1999. Interleukin-12 regulates the production of bacille Calmette-Guerin-induced interferon-gamma from human cells in a CD40-dependent manner. Scand. J. Immunol. 5061-67. [PubMed] [Google Scholar]
- 24.Molloy, A., P. Meyn, K. Smith, and G. Kaplan. 1993. Recognition and destruction of bacillus Calmette-Guerin-infected human monocytes. J. Exp. Med. 1771691-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ordway, D., M. Henao-Tamayo, M. Harton, G. Palanisamy, J. Troudt, C. Shanley, R. J. Basaraba, and I. M. Orme. 2007. The hypervirulent Mycobacterium tuberculosis strain HN878 induces a potent TH1 response followed by rapid down-regulation. J. Immunol. 179522-531. [DOI] [PubMed] [Google Scholar]
- 26.Perez, E., P. Constant, F. Laval, A. Lemassu, M. A. Laneelle, M. Daffe, and C. Guilhot. 2004. Molecular dissection of the role of two methyltransferases in the biosynthesis of phenolglycolipids and phthiocerol dimycoserosate in the Mycobacterium tuberculosis complex. J. Biol. Chem. 27942584-42592. [DOI] [PubMed] [Google Scholar]
- 27.Perez, E., P. Constant, A. Lemassu, F. Laval, M. Daffe, and C. Guilhot. 2004. Characterization of three glycosyltransferases involved in the biosynthesis of the phenolic glycolipid antigens from the Mycobacterium tuberculosis complex. J. Biol. Chem. 27942574-42583. [DOI] [PubMed] [Google Scholar]
- 28.Raviglione, M. C., D. E. J. Snider, and A. Kochi. 1995. Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA 273220-226. [PubMed] [Google Scholar]
- 29.Reed, M. B., P. Domenech, C. Manca, H. Su, A. K. Barczak, B. N. Kreiswirth, G. Kaplan, and C. E. Barry III. 2004. A glycolipid of hypervirulent tuberculosis strains that inhibits the innate immune response. Nature 43184-87. [DOI] [PubMed] [Google Scholar]
- 30.Reed, M. B., S. Gagneux, K. DeRiemer, P. M. Small, and C. E. Barry III. 2007. The W-Beijing lineage of Mycobacterium tuberculosis overproduces triglycerides and has the DosR dormancy regulon constitutively upregulated. J. Bacteriol. 1892583-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sreevatsan, S., X. Pan, K. E. Stockbauer, N. D. Connell, B. N. Kreiswirth, T. S. Whittam, and J. M. Musser. 1997. Restricted structural gene polymorphism in the Mycobacterium tuberculosis complex indicates evolutionarily recent global dissemination. Proc. Natl. Acad. Sci. 949869-9874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsenova, L., E. Ellison, R. Harbacheuski, A. L. Moreira, N. Kurepina, M. B. Reed, B. Mathema, C. E. Barry III, and G. Kaplan. 2005. Virulence of selected Mycobacterium tuberculosis clinical isolates in the rabbit model of meningitis is dependent on phenolic glycolipid produced by the bacilli. J. Infect. Dis. 19298-106. [DOI] [PubMed] [Google Scholar]
- 33.Tsenova, L., R. Harbacheuski, E. Ellison, C. Manca, and G. Kaplan. 2006. Aerosol exposure system for rabbits: application to M. tuberculosis infection. J. Am. Biol. Safety Assoc. 117-14. [Google Scholar]
- 34.Tsenova, L., A. Moreira, E. Party, V. H. Freedman, and G. Kaplan. 1997. Aerosol infection of mice with mycobacteria using a nose-only exposure device. J. Am. Biol. Safety Assoc. 220-31. [Google Scholar]
- 35.Tyagi, S., and F. R. Kramer. 1996. Molecular beacons: probes that fluoresce upon hybridization. Nat. Biotechnol. 14303-308. [DOI] [PubMed] [Google Scholar]
- 36.van Embden, J. D., M. D. Cave, J. T. Crawford, J. W. Dale, K. D. Eisenach, B. Gicquel, P. W. Hermans, C. Martin, R. McAdam, T. M. Shinnick, and P. M. Small. 1993. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J. Clin. Microbiol. 31406-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Soolingen, D., T. Hoogenboezem, P. E. de Haas, P. W. Hermans, M. A. Koedam, K. S. Teppema, P. J. Brennan, G. S. Besra, F. Portaels, J. Top, L. M. Schouls, and J. D. van Embden. 1997. A novel pathogenic taxon of the Mycobacterium tuberculosis complex, Canetti: characterization of an exceptional isolate from Africa. Int. J. Syst. Bacteriol. 471236-1245. [DOI] [PubMed] [Google Scholar]