Abstract
The Streptococcus pneumoniae ComDE two-component signaling system controls the development of genetic competence in the bacterium and affects virulence in models of pneumonia and bacteremia. We have investigated the impact of the competence pathway during colonization of the nasopharynx, the principal ecological niche of the pneumococcus. Previous work showed that deletion of the pneumococcal CiaRH signaling system inhibited colonization and increased expression of genes required for competence. We anticipated that signaling by the competence pathway might similarly reduce carriage. Consistent with this expectation, a comE deletion that blocked transformation increased colonization fitness such that the mutant outcompeted the wild type in an infant rat model of asymptomatic carriage. Deletion of comD—immediately upstream of comE and likewise required for competence—similarly increased colonization fitness if the orientation of the antibiotic resistance cassette inserted into the comD locus was such that it reduced transcription of comE. However, an alternative comD deletion mutation that caused an increase in comE transcription impaired colonization instead. Activation of the competence system through a comE(D143Y) mutation did not affect colonization, but an inability to secrete the competence-stimulating peptide due to deletion of comAB produced a density-dependent reduction in colonization fitness. These results suggest a model in which signaling by the unactivated form of ComE reduces colonization fitness compared to that of bacteria in which it is either activated or absent entirely, with the most substantial fitness gain accompanying deletion of comE. This observation demonstrates that the pneumococcus incurs a substantial fitness cost in order to retain a functional competence regulatory system.
Natural competence for genetic transformation in the gram-positive pathogen Streptococcus pneumoniae is triggered by a peptide pheromone signaling system through a molecular pathway that has been well described (reviewed in reference 5). The role of this pathway in the overall biology of this pathogen, however, is less well characterized. Competence is triggered by activation of the ComDE two-component signaling system in response to accumulation of competence-stimulating peptide (CSP) outside the bacterium. CSP is produced as a prepeptide encoded by comC and is exported by the ComAB transporter and cleaved to generate the mature peptide (11, 12). The ComD histidine kinase is then activated by CSP and signals through its cognate response regulator, ComE, to induce the expression of an early group of genes (4, 26, 27, 35). These induced genes include comAB and comCDE themselves, resulting in a positive feedback loop that promotes synchronized development of competence throughout the population in response to increasing levels of CSP. ComE also induces expression of comX, which encodes an alternative sigma factor that then stimulates expression of a larger group of late-phase genes, including those required for DNA uptake and recombination (18, 20, 21).
Several pieces of evidence have recently suggested that the competence circuit regulates traits that may be important for aspects of pneumococcal physiology beyond genetic exchange. Microarray profiling revealed that activation of the competence pathway increased the expression of more than 120 genes, or approximately 6% of the bacterial genome (27). Fewer than 30 of these genes were required for transformation (27), suggesting that other phenotypic characteristics may be coregulated with the induction of competence. The competence pathway was recently linked to the virulence of this pathogen. Mutants lacking the ComD receptor were shown to be attenuated in models of both pneumonia and bacteremia (2, 16), while deletion of the ComAB peptide transporter decreased virulence in the bacteremia model but not in a pneumonia model (2, 16). Perturbation of the competence system in the opposite direction by administration of synthetic CSP has also been shown to produce a similar effect, improving survival of mice infected intravenously with S. pneumoniae (24). The competence pathway has been linked to several additional aspects of pneumococcal biology that are likely to be relevant to survival in vivo, including bacterial aggregation and biofilm formation (25, 27, 34).
The impact of the competence system on pneumococcal colonization of the upper respiratory tract, however, has not been well characterized. Because asymptomatic carriage in the upper respiratory tract represents the environment in which S. pneumoniae exists most commonly and from which the bacterium spreads to colonize new hosts, we investigated the effect of the competence pathway on colonization of this site. Our earlier work had demonstrated that deletion of the pneumococcal CiaRH two-component system strongly attenuates such colonization (29). Consistent with other studies of the CiaRH system (7, 8, 10), this ciaRH deletion also increased expression of genes in the competence regulon (29). Repression of competence genes by the CiaRH system involves the activity of the HtrA protease (30), which also contributes to the colonization potential of the organism (29). These observations motivated our further examination of the contribution of the competence system during colonization. Because the ciaRH and htrA mutations that impaired colonization derepressed competence, we sought to evaluate the effects of increased competence signaling as well as decreased competence during colonization.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Bacterial strains used in this study are described in Table 1. S. pneumoniae was grown in C+Y medium (14) at pH 7.4 or plated on tryptic soy agar spread with 5,000 U catalase (Worthington Biochemicals, Freehold, NJ) and incubated in candle extinction jars at 37°C. Antibiotics were used at the following concentrations: streptomycin, 500 μg/ml; kanamycin, 500 μg/ml; erythromycin, 1 μg/ml; and novobiocin, 5 μg/ml.
TABLE 1.
Pneumococcal strains used in this study
| Strain | Characteristicsa | Reference |
|---|---|---|
| D39 | Serotype 2 clinical isolate | 1 |
| P143 | R6x laboratory strain, but spontaneous Smr mutant | 30 |
| P1139 | Serotype 3 strain 0100993; ciaRH::ermAM | 33 |
| 706 | Novr | 15 |
| P1301 | D39, but Novr by transformation with 706 DNA | 30 |
| P1302 | D39, but Smr by transformation with P143 DNA | This work |
| P1304 | D39, but Smr by transformation with P1302 DNA | This work |
| P1310 | D39 recovered from murine intraperitoneal passage | Weiserb |
| P1474 | P143, but comD::Janus by transformation with ligated PCR products; Kmr | This work |
| P1534 | P1310, but Smr by transformation with P1304 rpsL PCR product | This work |
| P1535 | P1310, but comDfor::Janus by transformation with P1474 comD PCR product; Kmr | This work |
| P1537 | P1310, but comAB::Janus by transformation with ligated PCR products; Kmr | This work |
| P1538 | P1310, but comE::Janus by transformation with ligated PCR products; Kmr | This work |
| JKP102 | P1310, but Janus inserted downstream of comE with ligated PCR products; Kmr | This work |
| JKP105 | P1534, but Janus inserted downstream of comE with ligated PCR products; Kmr | This work |
| JKP109 | P1310, but ciaRH::ermAM by transformation with P1139 ciaRH PCR product; Emr | This work |
| JKP114 | JKP109, but comAB::Janus by transformation with P1537 comAB PCR product; Kmr Emr | This work |
| JKP116 | JKP109, but comE::Janus by transformation with P1538 comE PCR product; Kmr Emr | This work |
| JKP158 | P1310, but comDrev::Janus by transformation with ligated PCR products; Kmr | This work |
| MSP110 | JKP105, but Janus replaced with PCR product carrying comE(D143Y); Smr | This work |
| TMP133 | P1310, but comDE::Janus by transformation with ligated PCR products; Kmr | This work |
Emr, erythromycin resistant; Kmr, kanamycin resistant; Novr, novobiocin resistant; Smr, streptomycin resistant.
From the collection of J. Weiser, University of Pennsylvania.
Construction of mutant strains.
In order to analyze the contribution of the competence regulatory system to fitness for colonization, mutants were constructed with both decreased and increased activity of this signaling system. An isolate of the type 2 pneumococcal strain D39 that had been passaged by murine intraperitoneal infection was chosen as the wild-type background in which to conduct these experiments. A streptomycin resistance marker was introduced into this strain by transformation with a PCR product carrying an rpsL(K56T) mutation in order to distinguish this strain during mixed colonization assays. Deletions in the competence genes comAB, comD, and comE were constructed by inserting the Janus cassette (32) into these loci, using PCR ligation mutagenesis (17) and resulting in kanamycin resistance. In order to analyze polar effects of its insertion, the Janus cassette was inserted into comD in each direction by interchanging the restriction enzyme sites engineered into the primers used to amplify DNA regions flanking comD. A control strain was also constructed with the Janus cassette similarly inserted in the intergenic region downstream of the 3′ end of comE. A ciaRH::ermAM insertion-deletion mutation was transferred into the type 2 background by transformation with a PCR product carrying this construct. To activate the competence system, overlap-extension PCR was used to generate a comE(D143Y) PCR product, which was used to replace the Janus cassette that had been inserted downstream of comE; isolates were sequenced to screen for the comE(D143Y) mutation in the flanking region of DNA. PCR primers used in the construction of these strains are listed in Table 2. The presence of each mutation was confirmed by sequencing. Transformation assays in vitro confirmed that both comD deletions and the comE deletion prevented development of competence even in the presence of exogenous CSP. The ΔcomDE double mutant was likewise unable to become competent in the presence of CSP, whereas the comAB deletion mutant was able to be transformed in response to exogenous CSP. Insertion of the Janus cassette downstream of comE did not affect the transformation profile of the bacterium.
TABLE 2.
PCR primers used in this study
| Usage | Primer name | Primer sequence (5′-3′) |
|---|---|---|
| PCR ligation mutagenesis | ||
| comDfor 5′-flanking region | COMCDE3380R26 | TGTAAAAAATAGAGCCAATCTTTCTG |
| COMCDE2781 (BamHI) | ACGAGGATCCAAAAATGAACAATAACCGTCCC | |
| comDfor 3′-flanking region | COMCDE1560 (ApaI) | AGCAGGGCCCTTAGAAACAGAGATGGAAGGCAG |
| COMCDE798F23 | CTTGAAATAGGACAACGATGGT | |
| comDrev 5′-flanking region | COMCDE3380R26 | TGTAAAAAATAGAGCCAATCTTTCTG |
| COMCDE2781 (ApaI) | AGCAGGGCCCAAAAATGAACAATAACCGTCCC | |
| comDrev 3′-flanking region | COMCDE1560 (BamHI) | ACGAGGATCCTTAGAAACAGAGATGGAAGGCAG |
| COMCDE798F23 | CTTGAAATAGGACAACGATGGT | |
| comAB 5′-flanking region | COMAB1786F23 | TTTTGTTTAGTGATTGGGGTAAG |
| COMAB2467 (BamHI) | ACGAGGATCCGAGAGCAGACCATTTTTTTGTTC | |
| comAB 3′-flanking region | COMAB4432 (ApaI) | AGCAGGGCCCAATACCAAGAAGGGGCAGAGGG |
| COMAB5281R20 | TAGCGAACAGAATCACCGAC | |
| comE 5′-flanking region | COMCDE2305R25 | TTTCGTTTCAGATATGGTAAGTACG |
| COMCDE1441 (BamHI) | ACGAGGATCCATATTCTCTCTAGTCTCACTTGATGTTC | |
| comE 3′-flanking region | COMCDE757 (ApaI) | AGCAGGGCCCTCTCAAAAGTGATTGACAATTAGC |
| COMCDE6F25 | AATGCTATGGTACAATTACTGATGG | |
| comDE 5′-flanking region | COMCDE3380R26 | TGTAAAAAATAGAGCCAATCTTTCTG |
| COMCDE2781 (BamHI) | ACGAGGATCCAAAAATGAACAATAACCGTCCC | |
| comDE 3′-flanking region | COMCDE757 (ApaI) | AGCAGGGCCCTCTCAAAAGTGATTGACAATTAGC |
| COMCDE6F25 | AATGCTATGGTACAATTACTGATGG | |
| 5′-Flanking region downstream of comE | COMCDE2305R25 | TTTCGTTTCAGATATGGTAAGTACG |
| COMCDE733 (BamHI) | ACGAGGATCCTGCTAATTGTCAATCACTTTTGAG | |
| 3′-Flanking region downstream of comE | COMCDE698 (ApaI) | AGCAGGGCCCAGAAGGTCCGTTGGTCAAGG |
| COMCDE6F25 | AATGCTATGGTACAATTACTGATGG | |
| Overlap-extension PCR | ||
| comE(D143Y) 5′ region | COMCDE2389R23 | GGAACTAGCCTATTTTGACGAGG |
| COMCDE1073F34 | GAAATAATCTACAACATCTTCATTTTCAAGTAAC | |
| comE(D143Y) 3′ region | COMCDE1106R74DY | GTTACTTGAAAATGAAGATGTTGTAGATTATTTCTACTACAATTACAAGGGAAATGATTTAAAAATTCCTTACC |
| COMCDE112F26 | AGGATAAGTATGATATGATTGAGCAC | |
| Amplification of other loci | ||
| Janus cassette | JANUS765F32 | GATCGGATCCGTTTGATTTTTAATGGATAATG |
| JANUS2109R30 | ACCTGGGCCCCTTTCCTTATGCTTTTGGAC | |
| rpsL locus | RPSL871F25 | CGGTACTTTTTACTTTTGGTCTCTC |
| RPSL1430R22 | TCTTTATCCCCTTTCCTTATGC | |
| ciaRH::Janus construct | CIARH288F22 | AACGTCGTTTGTTGGCTGAGGC |
| CIARH3977R21 | CGGTCCATCATATCTTGGTGC |
In vitro competitive growth assays.
S. pneumoniae strains were grown individually at 37°C in C+Y medium for 2 h after inoculation from colonies scraped off tryptic soy agar plates, after which time all cultures had entered log-phase growth. Strains were then diluted in fresh medium and mixed in a 1:1 ratio to achieve an overall optical density reading at 620 nm (OD620) of approximately 0.04. Colony counts were performed on serial dilutions of aliquots taken after 0, 1.5, 3, and 4.5 h of growth in mixed cultures at 37°C. Plating on selective medium containing either kanamycin or streptomycin was used to distinguish competing strains.
Nasal colonization model.
Each bacterial inoculum was prepared by growth to an OD620 of approximately 0.3, washed, and resuspended in phosphate-buffered saline. Strains for use in competitive colonization experiments were mixed in a 1:1 ratio. Randomized groups of 10 to 14 newborn Sprague-Dawley rats (Taconic, Germantown, NY) were inoculated intranasally with 2 × 106 to 6 × 106 CFU of each strain. Colonization was monitored by serial washings of the nasopharynx of each animal on days 1, 2, 4, and 7 following inoculation, as previously described (29, 36). The density of bacteria in the recovered fluid was measured by colony counting; selective media were used to distinguish strains in competitive colonization assays. The relative strength of each competitive interaction was estimated by calculating a competitive index, defined as the ratio of CFU/ml of streptomycin-resistant to kanamycin-resistant bacteria recovered in nasal washings on day 7 for two competing strains divided by the ratio in the inoculum. All procedures were performed in accordance with institutional animal care guidelines.
Transformation assays.
Pneumococcal strains were inoculated into C+Y medium from colonies scraped off tryptic soy agar plates and grown for 2 h at 37°C before being diluted in fresh medium to achieve an OD620 of approximately 0.01. At target growth densities, aliquots were removed and mixed with P1301 DNA (conferring novobiocin resistance) at a final concentration of 2 μg/ml. Samples were incubated at 30°C for 40 min before the addition of 2 U/ml DNase I (Roche) and then at 37°C for 90 min. Transformation efficiency was calculated as the ratio of the number of colonies counted on serial dilutions plated in the presence of novobiocin to that in the absence of antibiotics. Where appropriate, CSP was added to samples at a final concentration of 1 μg/ml and samples were incubated for 10 min at 30°C before the addition of transforming DNA. Assays for the Trt (transformation resistant to trypsin) phenotype, which indicates competence activation, were performed as previously described (30). In these assays, transformation is measured in the presence of exogenous trypsin, which digests extracellular CSP and prevents induction of competence unless mutational activation of the pathway turns it on downstream of the detection of CSP (15).
RNA isolation and RT.
RNA was prepared, using a hot phenol lysis protocol as previously described (29), from in vitro cultures grown in C+Y medium at pH 7.4 to an OD620 of approximately 0.08. Purified RNA samples in 50 μl distilled H2O were digested with 1 U Turbo DNase (Ambion, Austin, TX) for 60 min at 37°C. An additional 1 U of DNase was added to each sample after the first 30 min of the incubation. DNase was inactivated using 6 μl DNase inactivation reagent (Ambion) according to the manufacturer's protocol. RNA aliquots were reverse transcribed by first mixing 2 μl of RNA with 1 μl random nonamers (50 μM; Sigma, Saint Louis, MO), 1 μl of 10 mM deoxynucleotide triphosphates (Invitrogen, Carlsbad, CA), and 9 μl distilled H2O. The mixtures were heated to 70°C for 10 min and chilled on ice. To each sample was then added 1 μl Superscript III reverse transcriptase (Invitrogen), 4 μl 5× first-strand synthesis buffer, 1 μl 0.1 M dithiothreitol, and 1 μl RNase Out (Invitrogen). Replicate samples were processed without reverse transcriptase. For reverse transcription (RT), samples were incubated at room temperature for 5 min before being moved to a heat block equilibrated at 42°C. Immediately after transfer, the block setting was adjusted to 50°C for 60 min of incubation with an initial temperature gradient. Samples were finally heated to 70°C for 15 min, followed by digestion with 0.5 μl RNase H for 20 min at 37°C.
Quantitative PCR.
cDNA samples were analyzed by quantitative PCR, using primers and probes designed for comE. Transcript levels for comE were normalized to those of rpoA as an internal control. The sequences of the primers and probes used for comE were 5′-AGCTCATTCGTCATTACAATCCTTACG-3′, 5′-CAAAATCTAGGGCTGATACCTGGT-3′, and 5′-6-carboxyfluorescein-TTTGCGACTCTAACATATAAAT-nonfluorescent quencher-3′. The sequences of the primers and probes used for rpoA were 5′-CAGAAGATGCTTTAGGGCTTTCAG-3′, 5′-CATCACTTCAGTTGACTTAGCAATCTCA-3′, and 5′-6-carboxyfluorescein-TCAAGATGTTCTGTCAAAATA-nonfluorescent quencher-3′. Dilutions of cDNA samples were mixed with 900 nM of each primer and 45 nM of probe in TaqMan universal PCR master mix (Applied Biosystems, Foster City, CA). Quantitative real-time PCR was performed using a model 7500 sequence detection system (Applied Biosystems). Serial dilutions of cDNA prepared from pneumococcal cultures stimulated with CSP to activate competence were used to generate standard curves for relative quantitation.
RESULTS
Construction of ComE activated mutant.
We sought to generate a pneumococcal strain containing a point mutation activating competence signaling for evaluation in a nasal colonization model. Such mutations in comD and comE have been described previously in the background of unencapsulated laboratory strains (7, 15, 22, 30) but not in an encapsulated background, as required for testing in vivo. During screening for a strain activated by a targeted comE(K38E) mutation, a clone was identified that displayed the Trt phenotype, indicating activation of the competence pathway despite degradation of CSP by trypsin. Although this isolate did not have the comE(K38E) change, sequencing revealed an alternative, comE(D143Y) mutation. This mutation is located just within the start of the predicted LytTR DNA binding domain of ComE (23) and does not involve the aspartate residue in the receiver domain on which phosphorylation of ComE is predicted to occur.
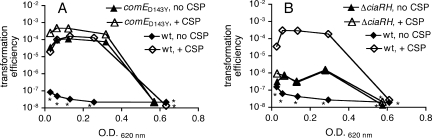
The comE(D143Y) mutation was generated again in the wild-type background, using overlap-extension PCR, in order to verify that the observed Trt phenotype arose from this mutation rather than from a change elsewhere in the genome. A comE(D143Y) PCR product was used to replace the counterselectable Janus cassette that had been placed downstream of comE because deletion of comE itself prevents subsequent transformation. Transformants were screened by sequencing to identify a clone in which the comE(D143Y) mutation had been incorporated through recombination within the flanking regions of the PCR product. This strain was designated MSP110 and displayed the predicted Trt phenotype (data not shown). Consistent with competence activation, the comE(D143Y) strain developed a high level of spontaneous competence during growth in vitro (Fig. 1A). In contrast, its wild-type parent strain became competent only in response to exogenous CSP. Both strains became refractory to stimulation with CSP at high densities. These data support the conclusion that the comE(D143Y) mutation causes a response similar to that triggered by exposure to CSP.
FIG. 1.
Transformation efficiencies of wild-type S. pneumoniae strain P1534 and comE(D143Y) strain MSP110 (A) or ΔciaRH strain JKP109 (B) in the presence and absence of exogenous CSP-1. No transformed bacteria were detected for the samples marked with asterisks; symbols for these data points represent the limit of detection of the assay, which decreases as the total number of bacteria in the sample rises.
Effects of competence deletion mutations on colonization.
The competence-inactivating comAB, comD, and comE mutations as well as the competence-activating comE(D143Y) mutation were tested in an infant rat model of colonization. A control strain in which the Janus cassette had been inserted into the intergenic region immediately downstream of comE was tested in parallel to assess potential fitness effects or downstream polar effects associated with this resistance marker. Inoculated individually, each of these strains was able to colonize at levels that were not substantially different from those seen with the wild type (data not shown). In order to assess more sensitively the differences in fitness for colonization, competitive assays were then performed in which strains were inoculated together with a reference strain.
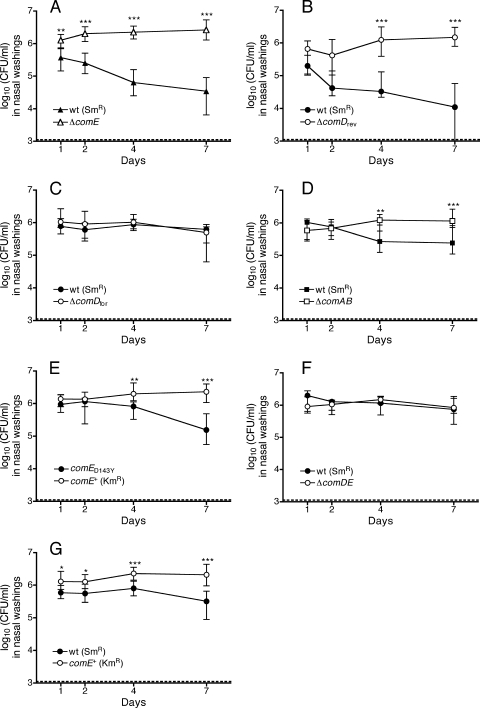
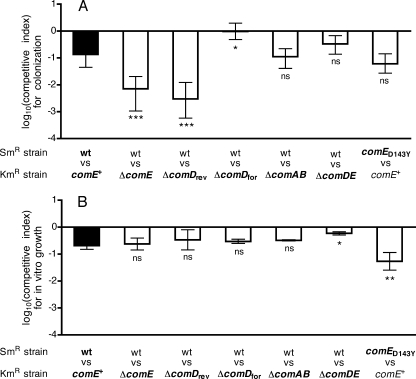
In the competitive model, the ΔcomE strain steadily outcompeted an isogenic streptomycin-resistant (Smr) wild-type strain (Fig. 2A). A kanamycin-resistant control strain with the Janus cassette downstream of comE (Kmr comE+) did not show a competitive effect of the same magnitude (Fig. 2G). The latter competition, between the Kmr comE+ strain and the Smr wild-type strain, is hereafter referred to as the reference competition and served as a baseline for evaluation of the specific fitness effects associated with mutations in the competence pathway. Comparisons between strains were made on the basis of the log competitive index (Fig. 3A), where a value of zero would indicate no difference between a pair of strains. The log competitive index of −0.87 for the reference competition was significantly less than the neutral value of 0 (P < 0.0001; Wilcoxon signed-rank test) and indicates a small advantage for the Kmr comE+ strain over the Smr wild type during colonization. This may reflect a fitness cost associated with the rpsL mutation conferring streptomycin resistance, as documented for other bacteria (3). The log competitive index of −2.15 seen with the ΔcomE strain was significantly lower than that of the reference competition (P < 0.001) and indicates a fitness advantage attributable to deletion of comE.
FIG. 2.
Competitive colonization assays of wild-type S. pneumoniae strain P1534 (Smr) versus the competence mutant strains P1538 (ΔcomE) (A), JKP158 (ΔcomDrev) (B), P1535 (ΔcomDfor) (C), P1537 (ΔcomAB) (D), and TMP133 (ΔcomDE) (F). (E) Competitive colonization of the Smr competence-activated comE(D143Y) strain MSP110 versus the Kmr comE+ control. (G) Reference competition of the Smr wild-type strain P1534 versus the Kmr comE+ control strain JKP102, which has the Janus cassette in the intergenic region downstream of comE. Values are displayed as medians and interquartile ranges for colonization of at least 11 pups with each pair of strains. Differences in colonization levels between pairs of competing strains at each time point were assessed using the Kruskal-Wallis test with Dunn's correction (*, P < 0.05; **, P < 0.01; ***, P < 0.001). The dotted lines represent the limit of detection of the assay.
FIG. 3.
(A) Competitive indices based on colonization levels at day 7 for the experiments shown in Fig. 2. Values are shown as medians and interquartile ranges. The competitive index for each pair of competing strains was compared with that for the reference competition (filled bar [wild type versus comE+ strain]), using the Kruskal-Wallis test with Dunn's correction (*, P < 0.05; ***, P < 0.001; ns, not significant). (B) Competitive indices for growth in vitro. Values represent means ± standard deviations based on at least three independent determinations. The competitive index for each pair of strains was compared with that for the reference competition (filled bar [wild type versus comE+ strain]), using analysis of variance with Dunnett's multiple comparison correction (*, P < 0.05; **, P < 0.01; ns, not significant).
To determine whether the apparent advantage of the ΔcomE strain might be explained by differential recovery of the mutant and wild-type strains during the washing process, we dissected nasal tissues 8 days after inoculation to directly assess colonization levels. A log competitive index favoring the ΔcomE strain (median, −2.38; range, −1.56 to −3.37) was also observed with tissue samples, with a value similar to that obtained from nasal washings. To assess the potential impact of in vivo recombination between strains during mixed colonization, we examined 96 colonies that were recovered from nasal washings on plates without either streptomycin or kanamycin throughout the course of the experiment. These colonies were tested to determine their resistance patterns. No colonies were found that were either sensitive to both antibiotics or resistant to both antibiotics. It is therefore unlikely that competition from an in vivo recombinant that may have gained fitness through the restoration of a streptomycin-sensitive rpsL allele explains the displacement of the streptomycin-resistant wild type during mixed colonization.
We expected that blockade of the competence pathway by deletion of comD—located immediately upstream of comE and also required for competence—would result in an enhancement of fitness similar to that with comE deletion. This effect was not observed with a comD deletion mutant constructed with the Janus cassette inserted in the same direction as transcription of the comCDE locus (ΔcomDfor) (Fig. 2C and 3A). Instead, the ΔcomDfor mutation caused a significant increase in competitive index relative to the reference competition value. Because the competitive index is calculated from the ratio of streptomycin-resistant to kanamycin-resistant bacteria, this elevated value indicates a loss of colonization fitness in the kanamycin-resistant ΔcomDfor strain. When the comD deletion was constructed with the Janus cassette in the reverse orientation (ΔcomDrev) (Fig. 2B and 3A), however, the resulting strain showed an enhanced colonization fitness similar to that of the ΔcomE strain.
A strain with a deletion spanning both comD and comE was also tested in the colonization model. This ΔcomDE mutation did not cause a change in the competitive index relative to the reference competition value (Fig. 2F and 3A). The interpretation of this result, however, is complicated by the fact that this mutation, unlike the comD and comE single mutations, also significantly slowed the growth of the bacteria in vitro, as described below (Fig. 3B).
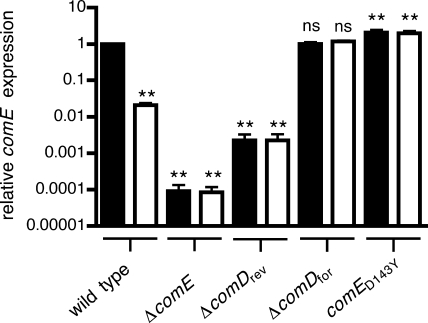
Impact of competence mutations on comE expression.
Because the difference observed between the ΔcomDfor and ΔcomDrev strains suggested the likelihood of a polar effect on the downstream comE gene, comE transcript levels were measured by quantitative RT-PCR (Fig. 4). The ΔcomDfor mutation resulted in constitutive expression of comE at a level similar to that achieved by the wild type activated by CSP. The ΔcomDrev mutation, in contrast, reduced the baseline level of comE transcripts to below that of the unstimulated wild type and prevented induction of comE expression in response to CSP. As anticipated, the competence-activating comE(D143Y) mutation resulted in increased expression of comE, even in the absence of stimulation by exogenous CSP.
FIG. 4.
Relative levels of comE transcripts in strains P1534 (wild type), P1538 (ΔcomE), JKP158 (ΔcomDrev), P1535 (ΔcomDfor), and MSP110 [comE(D143Y)], measured by quantitative RT-PCR. Values are normalized to transcript levels for the wild type after stimulation with exogenous CSP (assigned a value of 1) and represent means ± standard deviations for RNAs isolated from three independent cultures of each strain. No transcript was detected for the ΔcomE strain; values shown for this strain represent the limit of detection of the assays. Expression levels were compared to that of the wild type after treatment with CSP, using analysis of variance with Dunnett's multiple comparison correction (**, P < 0.01; ns, not significant). CSP treatment (filled bars) caused a significant increase in comE expression for the wild type (P < 0.01) but did not significantly change comE transcript levels for any of the other strains.
Density-dependent interaction of ΔcomAB and wild-type strains during colonization.
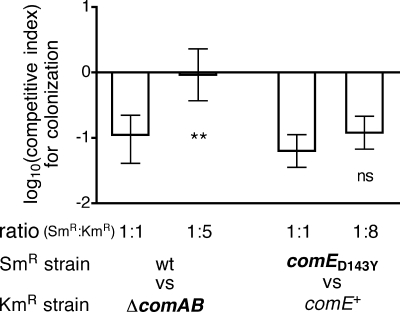
Deletion of comAB did not significantly affect colonization compared to that of the reference competition (Fig. 2D and 3A) when the ΔcomAB and wild-type strains were inoculated at equivalent levels. Because comAB encodes the transporter required for export and processing of CSP, the ΔcomAB strain does not develop spontaneous competence yet retains the ability to respond to CSP released by other bacteria. During cocolonization with bacteria that have the potential to develop competence, such stimulation might mask differences between the ΔcomAB strain and the wild type. However, because the ΔcomAB strain cannot participate in the autocatalytic loop by which activation of the competence system promotes further release of CSP, the potential for cross-stimulation should be reduced if the ratio of wild-type to ΔcomAB bacteria is decreased. We therefore determined the competitive index for cocolonization with the wild-type and ΔcomAB strains inoculated at a 1:5 ratio. Under these conditions, the competitive index was significantly higher than that observed with inoculation of the same strains at a 1:1 ratio (Fig. 5), corresponding to increased recovery of the streptomycin-resistant wild-type strain relative to that of the ΔcomAB strain. This finding suggests that the ΔcomAB mutation reduces colonization fitness unless stimulation from CSP released by the wild type is available to activate competence in the ΔcomAB strain.
FIG. 5.
Effect of inoculum ratio on competitive colonization with S. pneumoniae. Competitive indices are based on colonization levels at day 7 for the strain pairs P1534 (Smr wild type)-P1537 (Kmr ΔcomAB) and MSP110 [Smr comE(D143Y)]-JKP102 (Kmr comE+ control). Inoculum ratios were varied as indicated; data for 1:1 inoculation experiments are from experiments shown in Fig. 3. Values are shown as medians and interquartile ranges. Differences in competitive indices at the two inoculum ratios were assessed using the Kruskal-Wallis test with Dunn's correction (**, P < 0.01; ns, not significant).
Effects of competence-activating comE(D143Y) mutation on colonization.
Considering the competitive advantage associated with comE deletion, the possibility that the comE(D143Y) mutation might impair colonization was also investigated. Because the comE(D143Y) strain was resistant to streptomycin, the Kmr comE+ strain was used as a reference competitor for this assay (Fig. 2E). Comparison was again made to the reference competition between the Smr wild-type strain and the Kmr comE+ control strain. The competitive index obtained with the comE(D143Y) strain was not significantly different from that of the reference competition (Fig. 3A).
Dual colonization with the comE(D143Y) strain and the Kmr comE+ control strain presents another situation in which cross-stimulation may reduce the differences between strains. In this case, activation of the competence circuit in the comE(D143Y) strain may cause enhanced release of CSP and thereby promote increased development of competence in the control strain. We therefore tested whether reducing the ratio of comE(D143Y) to control strain bacteria would impact the outcome of competitive colonization. Although a small increase in the competitive index was observed, this difference was not significant (Fig. 5). It is possible, however, that even at a reduced density the comE(D143Y) strain is able to stimulate competence in the control strain, because unlike the ΔcomAB strain, for which a density-dependent effect was observed, the control strain has a normal competence circuit and should be able to participate in autocatalytic activation of competence once it begins to be triggered.
Competence mutations and growth in vitro.
Competence activation through exposure to CSP is known to delay pneumococcal growth (24). We therefore tested the impact of our set of competence mutations during competitive growth in vitro to determine whether the effects seen during colonization might be attributed to general effects on growth rate. A competitive index for growth in vitro was defined as the ratio of CFU/ml of streptomycin-resistant versus kanamycin-resistant bacteria for the two strains after 4.5 h of growth divided by the ratio immediately after mixing. The competitive indices consistently showed that kanamycin-resistant strains containing the Janus cassette grew better in vitro than the isogenic streptomycin-resistant control strain (Fig. 3B). Deletion of comAB, comD, or comE did not significantly change the competitive index relative to that of the reference competition in vitro. The comDE deletion, however, was associated with a significant increase in the competitive index in vitro, reflecting reduced recovery of the kanamycin-resistant ΔcomDE strain relative to the streptomycin-resistant control. The comE(D143Y) competence-activating mutation also significantly decreased recovery of the mutant strain (streptomycin resistant in this case) relative to the Kmr comE+ control strain following in vitro growth. Because the pattern of competitive indices measured in vitro did not mirror that seen in vivo, we concluded that the fitness effects of the comE and comD mutations are specific for colonization. The slow growth of the ΔcomDE and comE(D143Y) strains in vitro, however, may have affected the ability of these two strains to compete during nasal colonization.
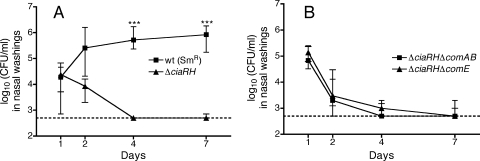
Competence activation is not responsible for ΔciaRH colonization defect.
Deletion of the CiaRH two-component system impairs pneumococcal colonization in the background of a serotype 3 strain and increases expression of the competence regulon (29). In the serotype 2 background used in this study, deletion of ciaRH also triggered spontaneous transformation that was not observed in the wild-type strain (Fig. 1B). The level of transformation measured in the ΔciaRH strain, however, was substantially lower than the level achieved by the wild type in the presence of CSP. We then asked whether this intermediate level of competence activation contributes to the colonization defect seen with ciaRH deletion. Second mutations inactivating competence were constructed in the context of a ciaRH deletion. As seen with the serotype 3 strain studied previously, the ΔciaRH mutation severely attenuated colonization in the serotype 2 background even in the absence of competition from another pneumococcal strain (Fig. 6A). Insertion of the Janus cassette to delete either comAB or comE did not correct the colonization defect caused by loss of ciaRH (Fig. 6B).
FIG. 6.
Nasal colonization with single strains of S. pneumoniae. (A) Wild type (P1534) and ΔciaRH (JKP109) strains; (B) ΔciaRH ΔcomAB (JKP114) and ΔciaRH ΔcomE (JKP116) strains. Values are shown as medians and interquartile ranges for colonization of groups of 10 to 13 pups with each strain. Differences in colonization levels between the wild-type and ΔciaRH strains at each time point were assessed using the Kruskal-Wallis test with Dunn's correction (***, P < 0.001); neither of the double mutant strains showed significant differences from the ΔciaRH strain at any of the time points. The dotted line represents the limit of detection of the assay.
DISCUSSION
We have investigated the influence of the pneumococcal competence signaling pathway on the ability of the bacterium to colonize the nasopharynx. Because asymptomatic carriage in the respiratory tract is the setting in which S. pneumoniae exists most frequently and from which it spreads to colonize new hosts, the impact of competence in this environment may be particularly important in defining the evolutionary pressures responsible for competence. We have found that deletion of the ComE response regulator, which is required for induction of competence, increases fitness for colonization above that of the wild type. This finding appears to contrast with earlier observations that competence inactivation through deletion of comD or comAB attenuated pneumococcal virulence in models of disease (2, 16) and may reflect differing requirements for asymptomatic carriage versus disease.
The magnitude of the competitive effect associated with the ΔcomE mutation was substantial, corresponding to an increase in relative abundance of the ΔcomE strain of 2.14 log10 units (nearly 140-fold) over 7 days. Of this increase, 1.28 log10 units (18.9-fold change) can be attributed directly to deletion of comE, as the excess effect beyond that seen with a control insertion of the resistance cassette directly downstream of comE. This change would correspond to a 50% daily increase in relative abundance of a comE null mutant in competition with wild-type S. pneumoniae. Pneumococcus therefore appears to incur a substantial fitness cost in order to maintain a functional comE gene compared to the potential advantage that might be gained by its elimination. The nature of the selective advantage for which ComE is nonetheless retained remains to be elucidated. These experiments, however, provide evidence that this function is unlikely to be utilization of the competence pathway for acquisition of extracellular DNA as a nutrient on the mucosal surface, as suggested for transformation in other bacteria (9, 28), because loss of this resource would be predicted to impair rather than improve colonization fitness.
The observation that comD deletion—also blocking competence—does not similarly enhance colonization unless the mutation is constructed in a manner that causes a polar decrease in comE transcription indicates that the signaling events that control fitness for colonization differ from those that regulate the development of competence. Several signaling models could be considered to explain such discordant effects of comD and comE deletions during colonization. (i) The unactivated form of ComE could regulate the transcription of an additional set of genes beyond those induced by activation of ComE in response to CSP. If such a set included genes affecting colonization, a deletion of comE causing loss of the ComE protein entirely would be predicted to impact colonization differently from a loss of comD, causing only inactivation of ComE. Although activation of the ComE response regulator is often presumed to correspond to its phosphorylation, this correspondence has not been demonstrated experimentally. Studies of two-component signaling in other organisms have revealed diversity in signaling architectures in which stimuli may regulate either kinase or phosphatase activities of sensor kinases and in which dephosphorylation occasionally may correspond to the “active” state of a response regulator (31). We therefore consider changes in ComE signaling in terms of its activation (i.e., the signaling state required for induction of genetic competence in response to detection of CSP by the ComD sensor kinase) rather than its phosphorylation. (ii) ComE could be activated by factors in vivo, independent of the ComD sensor kinase. In vitro, however, none of our mutants lacking comD displayed competence. Alternative phosphate donors that might activate ComE could include noncognate histidine kinases as well as the small-molecule phosphate donor acetyl phosphate (37). The efficiency of ComE activation through such an alternative pathway—if activation corresponds to phosphorylation—might be enhanced in the ΔcomD strain if ComD possesses, in addition to its kinase activity, a phosphatase activity that is able to dephosphorylate its cognate response regulator, such as that described for other sensor kinases (13, 19). The lack of effect on colonization seen with the competence-activating comE(D143Y) mutation, however, provides evidence against this model. (iii) ComD could interact with an alternative target, such as a noncognate response regulator. Such cross talk with another two-component signaling system could be facilitated in the absence of the cognate response regulator ComE. This model may be excluded because colonization enhancement is seen in the absence of ComD in the ΔcomDrev mutant.
Of these models, the first—involving effects of the unactivated form of ComE—is supported by the observation that the ΔcomDrev mutant displays enhanced colonization similar to that of the ΔcomE mutant. This ΔcomDrev mutation causes a polar decrease in comE transcription. In contrast, the ΔcomDfor mutation increases comE transcription and results in reduced colonization fitness. Because the ΔcomDfor strain lacks the ComD histidine kinase, increased comE transcription in this background is expected to result in overexpression of the unactivated form of the ComE response regulator. Consistent with this prediction, the ΔcomDfor strain does not display competence in either the presence or absence of CSP. Our results therefore suggest that increases in unactivated ComE impair colonization, while decreases in unactivated ComE enhance colonization.
Although this model would also predict enhanced colonization by the ΔcomDE double mutant strain, this strain unexpectedly displayed an in vitro growth deficit not seen with any of the individual mutations in comD or comE. The basis for this growth deficit is uncertain, but as a consequence, comparisons of the impact of competence mutations on colonization are most appropriately focused on those strains that do not affect growth in vitro. We cannot currently determine whether the growth deficit of the ΔcomDE strain counterbalances a colonization advantage associated with the loss of comE to produce the neutral result observed in vivo.
The density-dependent colonization defect associated with the ΔcomAB strain provides further support for the hypothesis that unactivated ComE impairs colonization. In this strain, ComE activation would depend on cross-stimulation by CSP released by the cocolonizing wild-type strain. When the wild type is abundant, the potential for such stimulation would be increased, and under these conditions, colonization by the ΔcomAB strain was strong. With the wild type being less abundant, however, the potential for ComE activation in the ΔcomAB mutant would be reduced. Indeed, when the ratio of wild-type to ΔcomAB bacteria in the inoculum was reduced, the mutant strain displayed decreased fitness for colonization. The density dependence of the ΔcomAB colonization phenotype furthermore argues against the second signaling model presented above (i.e., in vivo activation of ComE through an alternative pathway). If activation of ComE during colonization were independent of CSP, a pattern consistent with cross-stimulation of the ΔcomAB strain by CSP released by the wild type would not be expected. The observation of this density-dependent effect also provides indirect evidence that the competence system is activated at some level in the wild type during colonization, because release of CSP would be necessary for cross-stimulation of the ΔcomAB strain. Although we have attempted to demonstrate this activation directly through transcriptome amplification and quantitative RT-PCR performed directly on nasal washing samples, the sensitivity of these assays has not been consistently high enough to measure transcript levels from these limited samples. It should be noted that similar cross-stimulation by extracellular CSP is unlikely to occur with the ΔcomD and ΔcomE mutants because these strains can neither respond to CSP nor participate in the autocatalytic loop that amplifies its production during competence.
The observation that the competence-activating comE(D143Y) mutation does not significantly impact colonization further supports the model that levels of the unactivated form of ComE, rather than the activated form, affect colonization potential. A density-dependent effect on fitness might also have been anticipated during cocolonization with the wild-type and comE(D143Y) strains if the latter increased the level of ComE activation in the former. Although the competitive index shifted slightly toward less strong colonization by the wild type when the abundance of the activated comE(D143Y) strain was reduced, this change was not significant. The potential to observe a density-dependent fitness effect may be reduced in this situation (compared to the competition between ΔcomAB and wild-type strains) because CSP from the comE(D143Y) strain may trigger a positive feedback loop, amplifying its production by the cocolonizing wild type even with a reduced population of comE(D143Y) bacteria. Alternatively, the level of spontaneous competence activation in the wild type during colonization may be high enough that additional stimulation by the comE(D143Y) strain does not produce a substantial effect. Finally, the finding that secondary mutations blocking competence do not restore colonization fitness to the ΔciaRH strain indicates that the colonization deficit of the ΔciaRH strain is not due to its enhanced competence activation and is likewise consistent with colonization being affected by the unactivated form of ComE.
Although the cause of the colonization advantage conferred by loss of unactivated ComE is not currently known, it appears likely to stem from transcriptional effects on an uncharacterized portion of the ComE regulon governed by unactivated ComE. The colonization advantage appears to be specific to the in vivo environment, as competitive indices measured during growth in vitro did not mirror the results of competition during colonization. We have examined the impact of mutations in the competence pathway on adherence to cultured epithelial cell lines and have not observed substantial changes associated with deletion of comE. Pneumococcal biofilm formation was recently linked to the induction of competence as well (25). Our observation of robust colonization by the ΔcomE strain, however, suggests either that such biofilms are not required for efficient colonization of the infant rat nasopharynx or that alternative pathways are sufficient to stimulate biofilm formation in vivo. S. pneumoniae has also been demonstrated to undergo allolysis, or fratricide, in which competent pneumococci lyse a subpopulation of noncompetent pneumococci present in the same culture (reviewed in reference 6). While this process is likely to be important in generating the pool of extracellular DNA available for transformation, our study suggests that such allolysis does not determine the outcome of competition between strains during colonization. Further work will be required to define the regulatory events controlled by the unactivated form of ComE and the impact of these events on colonization.
Acknowledgments
We thank J. Weiser for valuable discussions during the course of these experiments, S. King and J. Weiser for providing pneumococcal strain P1310, and T. Miller for technical assistance during the construction of strain TMP133.
This work was supported by Public Health Service grant K08 AI052129 from the National Institute of Allergy and Infectious Diseases to M.E.S. as well as by institutional funding from the Children's Hospital of Philadelphia.
Editor: A. Camilli
Footnotes
Published ahead of print on 28 April 2008.
REFERENCES
- 1.Avery, O. T., C. M. MacLeod, and M. McCarty. 1944. Studies on the nature of the chemical nature of the substance inducing transformation of pneumococcal types. J. Exp. Med. 79137-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartilson, M., A. Marra, J. Christine, J. S. Asundi, W. P. Schneider, and A. E. Hromockyj. 2001. Differential fluorescence induction reveals Streptococcus pneumoniae loci regulated by competence stimulatory peptide. Mol. Microbiol. 39126-135. [DOI] [PubMed] [Google Scholar]
- 3.Björkman, J., D. Hughes, and D. I. Andersson. 1998. Virulence of antibiotic-resistant Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 953949-3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng, Q., E. A. Campbell, A. M. Naughton, S. Johnson, and H. R. Masure. 1997. The com locus controls genetic transformation in Streptococcus pneumoniae. Mol. Microbiol. 23683-692. [DOI] [PubMed] [Google Scholar]
- 5.Claverys, J.-P., and L. S. Håvarstein. 2002. Extracellular-peptide control of competence for genetic transformation in Streptococcus pneumoniae. Front. Biosci. 71798-1814. [DOI] [PubMed] [Google Scholar]
- 6.Claverys, J.-P., B. Martin, and L. S. Håvarstein. 2007. Competence-induced fratricide in streptococci. Mol. Microbiol. 641423-1433. [DOI] [PubMed] [Google Scholar]
- 7.Echenique, J. R., C.-R. Sabine, and M.-C. Trombe. 2000. Competence regulation by oxygen in Streptococcus pneumoniae: involvement of ciaRH and comCDE. Mol. Microbiol. 36688-696. [DOI] [PubMed] [Google Scholar]
- 8.Echenique, J. R., and M. C. Trombe. 2001. Competence modulation by the NADH oxidase of Streptococcus pneumoniae involves signal transduction. J. Bacteriol. 183768-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finkel, S. E., and R. Kolter. 2001. DNA as a nutrient: novel role for bacterial competence gene homologs. J. Bacteriol. 1836288-6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guenzi, E., A. M. Gasc, M. A. Sicard, and R. Hakenbeck. 1994. A two-component signal-transducing system is involved in competence and penicillin susceptibility in laboratory mutants of Streptococcus pneumoniae. Mol. Microbiol. 12505-515. [DOI] [PubMed] [Google Scholar]
- 11.Håvarstein, L. S., D. B. Diep, and I. F. Nes. 1995. A family of bacteriocin ABC transporters carry out proteolytic processing of their substrates concomitant with export. Mol. Microbiol. 16229-240. [DOI] [PubMed] [Google Scholar]
- 12.Hui, F. M., L. Zhou, and D. A. Morrison. 1995. Competence for genetic transformation in Streptococcus pneumoniae: organization of a regulatory locus with homology to two lactococcin A secretion genes. Gene 15325-31. [DOI] [PubMed] [Google Scholar]
- 13.Keener, J., and S. Kustu. 1988. Protein kinase and phosphoprotein phosphatase activities of nitrogen regulatory proteins NTRB and NTRC of enteric bacteria: roles of the conserved amino-terminal domain of NTRC. Proc. Natl. Acad. Sci. USA 854976-4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lacks, S., and R. D. Hotchkiss. 1960. A study of the genetic material determining an enzyme activity in pneumococcus. Biochim. Biophys. Acta 39508-517. [DOI] [PubMed] [Google Scholar]
- 15.Lacks, S. A., and B. Greenberg. 2001. Constitutive competence for genetic transformation in Streptococcus pneumoniae caused by mutation of a transmembrane histidine kinase. Mol. Microbiol. 421035-1045. [DOI] [PubMed] [Google Scholar]
- 16.Lau, G. W., S. Haataja, M. Lonetto, S. E. Kensit, A. Marra, A. P. Bryant, D. McDevitt, D. A. Morrison, and D. W. Holden. 2001. A functional genomic analysis of type 3 Streptococcus pneumoniae virulence. Mol. Microbiol. 40555-571. [DOI] [PubMed] [Google Scholar]
- 17.Lau, P. C. Y., C. K. Sung, J. H. Lee, D. A. Morrison, and D. G. Cvitkovitch. 2002. PCR ligation mutagenesis in transformable streptococci: application and efficiency. J. Microbiol. Methods 49193-205. [DOI] [PubMed] [Google Scholar]
- 18.Lee, M. S., and D. A. Morrison. 1999. Identification of a new regulator in Streptococcus pneumoniae linking quorum sensing to competence for genetic transformation. J. Bacteriol. 1815004-5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lois, A. F., M. Weinstein, G. S. Ditta, and D. R. Helinski. 1993. Autophosphorylation and phosphatase activities of the oxygen-sensing protein FixL of Rhizobium meliloti are coordinately regulated by oxygen. J. Biol. Chem. 2684370-4375. [PubMed] [Google Scholar]
- 20.Luo, P., H. Li, and D. A. Morrison. 2003. ComX is a unique link between multiple quorum sensing outputs and competence in Streptococcus pneumoniae. Mol. Microbiol. 50623-633. [DOI] [PubMed] [Google Scholar]
- 21.Luo, P., and D. A. Morrison. 2003. Transient association of an alternative sigma factor, ComX, with RNA polymerase during the period of competence for genetic transformation of Streptococcus pneumoniae. J. Bacteriol. 185349-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin, B., M. Prudhomme, G. Alloing, C. Granadel, and J.-P. Claverys. 2000. Cross-regulation of competence pheromone production and export in the early control of transformation in Streptococcus pneumoniae. Mol. Microbiol. 38867-878. [DOI] [PubMed] [Google Scholar]
- 23.Nikolskaya, A. N., and M. Y. Galperin. 2002. A novel type of conserved DNA-binding domain in the transcriptional regulators of the AlgR/AgrA/LytR family. Nucleic Acids Res. 302453-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oggioni, M. R., F. Iannelli, S. Ricci, D. Chiavolini, R. Parigi, C. Trappetti, J.-P. Claverys, and G. Pozzi. 2004. Antibacterial activity of a competence-stimulating peptide in experimental sepsis caused by Streptococcus pneumoniae. Antimicrob. Agents Chemother. 484725-4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oggioni, M. R., C. Trappetti, A. Kadioglu, M. Cassone, F. Iannelli, S. Ricci, P. W. Andrew, and G. Pozzi. 2006. Switch from planktonic to sessile life: a major event in pneumococcal pathogenesis. Mol. Microbiol. 611196-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pestova, E. V., L. S. Håvarstein, and D. A. Morrison. 1996. Regulation of competence for genetic transformation in Streptococcus pneumoniae by an auto-induced peptide pheromone and a two-component regulatory system. Mol. Microbiol. 21853-862. [DOI] [PubMed] [Google Scholar]
- 27.Peterson, S. N., C. K. Sung, R. Cline, B. V. Desai, E. C. Snesrud, P. Luo, J. Walling, H. Li, M. Mintz, T. Getahun, P. C. Burr, Y. Do, S. Ahn, J. Gilbert, R. D. Fleischmann, and D. A. Morrison. 2004. Identification of competence pheromone responsive genes in Streptococcus pneumoniae by use of DNA microarrays. Mol. Microbiol. 511051-1070. [DOI] [PubMed] [Google Scholar]
- 28.Redfield, R. J. 1993. Genes for breakfast: the have-your-cake-and-eat-it-too of bacterial transformation. J. Hered. 84400-404. [DOI] [PubMed] [Google Scholar]
- 29.Sebert, M. E., L. M. Palmer, M. Rosenberg, and J. N. Weiser. 2002. Microarray-based identification of htrA, a Streptococcus pneumoniae gene that is regulated by the CiaRH two-component system and contributes to nasopharyngeal colonization. Infect. Immun. 704059-4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sebert, M. E., K. P. Patel, M. Plotnick, and J. N. Weiser. 2005. Pneumococcal HtrA protease mediates inhibition of competence by the CiaRH two-component signaling system. J. Bacteriol. 1873969-3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stock, A. M., V. L. Robinson, and P. N. Goudreau. 2000. Two-component signal transduction. Annu. Rev. Biochem. 69183-215. [DOI] [PubMed] [Google Scholar]
- 32.Sung, C. K., H. Li, J. P. Claverys, and D. A. Morrison. 2001. An rpsL cassette, Janus, for gene replacement through negative selection in Streptococcus pneumoniae. Appl. Environ. Microbiol. 675190-5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Throup, J. P., K. K. Koretke, A. P. Bryant, K. A. Ingraham, A. F. Chalker, Y. Ge, A. Marra, N. G. Wallis, J. R. Brown, D. J. Holmes, M. Rosenberg, and M. K. Burnham. 2000. A genomic analysis of two-component signal transduction in Streptococcus pneumoniae. Mol. Microbiol. 35566-576. [DOI] [PubMed] [Google Scholar]
- 34.Tomasz, A., and E. Zanati. 1971. Appearance of a protein “agglutinin” on the spheroplast membrane of pneumococci during induction of competence. J. Bacteriol. 1051213-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ween, O., P. Gaustad, and L. S. Håvarstein. 1999. Identification of DNA binding sites for ComE, a key regulator of natural competence in Streptococcus pneumoniae. Mol. Microbiol. 33817-827. [DOI] [PubMed] [Google Scholar]
- 36.Weiser, J. N., R. Austrian, P. K. Sreenivasan, and H. R. Masure. 1994. Phase variation in pneumococcal opacity: relationship between colonial morphology and nasopharyngeal colonization. Infect. Immun. 622582-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolfe, A. J. 2005. The acetate switch. Microbiol. Mol. Biol. Rev. 6912-50. [DOI] [PMC free article] [PubMed] [Google Scholar]