Abstract
Enterohemorrhagic Escherichia coli (EHEC) is a food-borne pathogen that causes hemorrhagic colitis and acute renal failure. We used a germ-free mouse model to investigate the role of host factors, Shiga toxin 2 (Stx2), and bacterial strain in disease due to EHEC. Germ-free male and female Swiss-Webster mice that were 3 days to 12 weeks old were orally inoculated with 1 of 10 EHEC strains or derivatives of two of these strains with Stx2 deleted. All inoculated mice became infected regardless of the inoculum dose. All bacterial strains colonized the intestines, reaching levels of 109 to 1012 CFU/g of feces by 4 days after inoculation. Seven of the 10 wild-type strains caused disease. However, the two Stx2 deletion mutants, unlike the Stx2+ parental strains, did not cause disease. The clinical signs of disease in mice included lethargy, dehydration, polyuria, polydypsia, and death. Postmortem examination of affected mice revealed dehydration and luminal cecal fluid accumulation. Histologic examination revealed close adherence of bacteria to the intestinal epithelium in the ileum and cecum but not in the colon. Other lesions included progressive renal tubular necrosis, glomerular fibrin thrombosis, and red blood cell sludging. The severity of disease varied according to the bacterial strain and age, but not sex, of the host. This study demonstrated that EHEC colonizes germ-free mice in large numbers, adheres to the intestinal epithelium, and causes luminal cecal fluid accumulation and progressive renal failure. The disease in mice was Stx2 and bacterial strain dependent. This animal model should be a useful tool for studying the pathogenesis of renal disease secondary to EHEC infection.
Enterohemorrhagic Escherichia coli (EHEC), a food-borne bacterial pathogen of humans, is a normal inhabitant of the ruminant intestine. This organism is most often transmitted to humans in undercooked meat, but it has also caused outbreaks associated with contaminated vegetables (5, 28), fruit juices (6), and drinking water (41). The disease manifestations include watery or bloody diarrhea and can be severe enough to cause hospitalization and even death (18). In addition, some affected children (and rarely adults) develop hemolytic-uremic syndrome (HUS), a rapidly progressive, often fatal form of renal failure that is attributed to expression of Shiga toxins (Stx) by pathogenic bacteria (2, 18). HUS is defined by a triad of symptoms that includes hemolysis, uremia, and thrombocytopenia. It is a rare complication of disease due to EHEC, but its importance is amplified by its severity, tendency to affect young children, and the absence of any specific treatment or preventative measure. Thus, the significance of disease due to EHEC is derived from its food-borne nature and its potentially severe consequences.
Both HUS and hemorrhagic colitis caused by EHEC have been attributed to the production of Stx by pathogenic bacteria (2, 50) referred to as Stx-producing E. coli (STEC). The Stxs are macromolecular cytotoxins that are phage encoded and consist of five B subunits and one A subunit (32). The B subunits bind to the host cell surface receptor, globotriaosylceramide (Gb3), allowing entry of the active A subunit. The A subunit, a glycosylase, cleaves rRNA at a specific adenine residue, inhibiting protein synthesis and causing cell death. Several Stx variants have been described, and all have similar effects on cultured cells; however, most studies suggest that Shiga toxin 2 (Stx2) is the most pathogenic form in vivo (10, 24, 51, 54).
Several different mouse models have been used to investigate the pathogenesis of EHEC. These models can be separated into infection models, in which mice are orally inoculated with live EHEC, and injection models, in which mice are parenterally inoculated with Stx, bacteria, or bacterial fractions, often in combination with lipopolysaccharide or other mediators. The infection models include simple models in which EHEC or STEC strains are orally inoculated into conventional or specific-pathogen-free (SPF) mice (7, 29, 40), streptomycin treatment models in which streptomycin-resistant strains of EHEC or STEC are orally inoculated into mice that are then treated with streptomycin to suppress some of their normal enteric microbiota (3, 12, 24, 27, 35, 44, 53, 54), and germ-free models (1, 17, 48, 49).
Over the past few years, there have been several reports demonstrating that germ-free mice are readily susceptible to colonization by EHEC and develop renal tubular necrosis, as do streptomycin-treated mice (1, 17, 48, 49). However, this model has not been extensively used. This is surprising because germ-free mice are relatively inexpensive, easy to work with, susceptible to freshly isolated EHEC strains without a need to induce antibiotic resistance, and free of the potentially confounding effects of the normal enteric microbiota. The enteric microorganisms, sometimes referred to as the microbiome (23), have been shown to influence host immunologic and physiologic responses, as well as alter host-bacterium interactions and the pathogenicity of cocolonizing bacteria, and thus they may unpredictably alter the effects of infectious agents in vivo (23). Germ-free mice permit study of pathogens in the absence of confounding microbiota and also offer the potential for reconstitution with specific, known microorganisms, thus limiting experimental variability. Here we describe studies that extended the characterization of germ-free mice as an animal model for studying EHEC-associated disease.
MATERIALS AND METHODS
Mice.
Germ-free Swiss-Webster mice of both sexes were used in this study. On the day of inoculation, the mice ranged from 3 days to 12 weeks old. Mice were raised in our germ-free colony, housed in soft-sided bubble isolators, and fed autoclaved water and laboratory chow ad libitum. All mice remained bacteriologically sterile, except for those that were inoculated with EHEC. EHEC-infected mice were colonized only by the inoculated EHEC strain and not by any other bacterial species. Periodic testing for fungi and viruses failed to reveal any microorganisms other than the infecting EHEC strain. The absence of bacterial contamination was determined by aerobic and anaerobic culturing on blood agar plates and by Gram staining of feces (from live mice) and/or cecal contents (at necropsy).
In some experiments mice were placed in sterile microisolator cages within the germ-free isolators. The microisolator cages were then aseptically removed from the isolators and kept in a laminar flow hood for the duration of the experiment. These mice received sterile food, water, and bedding like the mice in the germ-free isolators, and they remained sterile (except for the infecting EHEC strain) throughout the course of the experiment.
Bacterial strains.
The wild-type EHEC and STEC strains used were 86-24, EDL933, DEC8B, DEC10B, TW14359, TW04863, MI02-102, MI04-43, MI06-31, and Sakai. The phenotypic characteristics of the strains used are shown in Table 1. Bacteria were grown on LB agar plates or in LB broth with shaking at 37°C. In addition, isolates recovered from mice were streaked onto sorbitol-MacConkey agar plates. All wild-type strains were obtained from the culture collection of the STEC center at Michigan State University (http://www.shigatox.net/cgi-bin/stec/index). Strains with the stx2 gene deleted (Δstx2) were constructed by replacement of the stx2 gene in the 933W prophage with a kanamycin resistance cassette in strains 86-24 and EDL933 using the λ Red recombination system (8, 9). The method used has been described previously in detail (52). The Δstx2 strains did not express Stx2, but lysogeny functions were not altered. The primers used for synthesis of the replacement cassette were 5′CTGTTCGCGCCGTGAATGAAGAGAGTCAACCAGAATGTTATGGACAGCAAGCGAACCG and 3′TTCCTGTCAACTGAGCACTTTGCAGTAACGGTTGCAGATTCAGAAGAACTCGTCAAGAAG.
TABLE 1.
EHEC strains used in this study
| Strain | Source (reference) | O antigen | H antigen | Stx expression |
|---|---|---|---|---|
| 86-24 | Outbreak (14) | O157 | H7 | Stx2 |
| EDL933 | Outbreak (38) | O157 | H7 | Stx1, Stx2 |
| DEC10B | Sporadic casea | O26 | H11 | Stx1 |
| DEC8B | Sporadic casea | O111 | H8 | Stx1, Stx2 |
| Sakai | Outbreak (28) | O157 | H7 | Stx1, Stx2 |
| TW14359 | Outbreak (26) | O157 | H7 | Stx2 |
| 93-111 | Outbreak (26) | O157 | H7 | Stx1, Stx2 |
| MI02-102 | Sporadic case (this study) | O157 | H7 | Stx2 |
| MI04-43 | Outbreak (this study) | O157 | H7 | Stx2 |
| MI06-31 | Outbreak (this study) | O157 | H7 | Stx2 |
| 86-24::Δstx2 | This study | O157 | H7 | None |
| EDL933::Δstx2 | This study | O157 | H7 | Stx1 |
Strain information is available at http://www.shigatox.net/cgi-bin/stec/index.
Experimental design.
The study included four separate experiments, as follows: (i) the course of disease due to EHEC strain 86-24 over 3 weeks was examined using adult mice of both sexes; (ii) disease in infant mice was examined; (iii) the role of Stx2 was examined; and (iv) the virulence of 10 EHEC strains was compared.
Adult and weanling mice were infected by oral inoculation of between 1 × 102 and 1 × 106 CFU of broth-cultured bacteria. For this, bacteria were grown in Luria broth to mid-logarithmic phase, quantified by determining the optical density at 600 nm, collected by centrifugation, and resuspended to a concentration of 1 × 103 to 1 × 107 CFU/ml in sterile phosphate-buffered saline. Mice were given 0.1 ml by using a gavage tube. Infant mice (3 days old) were infected by contact with infected dams that had been inoculated orally as described above. Uninfected mice and mice inoculated with 86-24 or 86-24::Δstx2 were euthanized 4 days or 1, 2, or 3 weeks after inoculation. All other mice were euthanized 1 week after inoculation. Some mice became moribund or died prior to the scheduled necropsy date, and these mice were necropsied early. All experimental groups contained at least five mice.
Just prior to necropsy, mice were deeply anesthetized, blood was collected for complete blood count and blood chemistry evaluation, and urine was collected for determination of the specific gravity. Mice were then euthanized and weighed, and the cecum was aseptically removed and weighed. Feces and cecal contents were collected for quantitative EHEC culturing, Gram staining, and aerobic and anaerobic culturing to demonstrate the absence of microorganisms other than EHEC. Quantitative counts were determined by plating serial 10-fold dilutions on LB agar plates, and the presence of EHEC was confirmed by the presence of white colonies on sorbitol-MacConkey agar. For Stx determination, cecal contents were stored at −20°C.
To enumerate bacteria in tissue, 15 mice were inoculated with strain 86-24 and euthanized 4 or 7 days postinoculation (PI). At necropsy, 10 to 30 mg of ileum and colon was weighed and homogenized, and the numbers of CFU per gram were calculated by plating serial 10-fold dilutions. Samples contained submucosa and muscularis, as well as mucosa. They included both adherent and nonadherent bacterial populations, except that feces were removed from the colon before homogenization. For samples of the cecal wall, adherent bacteria were quantified similarly except that the tissue was vigorously washed three times in sterile phosphate-buffered saline to remove nonadherent bacteria before it was weighed and homogenized.
For histologic examination, all levels of the gastrointestinal tract, as well as the heart, lungs, thymus, lymph nodes, liver, kidneys, adrenal, spleen, and brain, were immersion fixed in formalin. The kidneys and intestine were examined histologically for all mice, and other organs were examined for a subset of mice. Histologic lesions were present only in kidneys and intestines. Complete blood counts and blood chemistries were determined by the University of Michigan Animal Diagnostic Laboratory or by I-STAT (Heska Corp., Fort Collins, CO).
For Stx2 determination an enzyme-linked immunosorbent assay test kit was used according to the manufacturer's instructions (Premier EHEC; Meridian Biosciences, Cincinnati, OH). Commercially available Stx2 was used as a standard for quantification in this assay (Sigma-Aldrich, St. Louis, MO). Nonquantitative detection of Stx1 and Stx2 in cecal contents was performed by using STAT-test (Immunocard-STAT EHEC; Meridian Biosciences, Cincinnati, OH). Tissues used for histology were embedded in paraffin, cut in 5-μm sections, and stained with hematoxylin and eosin or Steiner stain. All histologic scoring was done by a single pathologist (K.A.E.) who was blinded to the origin of the sections. Animal experiments were approved by the University of Michigan Laboratory Animal Care and Use Committee.
Quantification of disease.
Clinical disease was defined as the presence of any of the following: dehydration, lethargy, weakness, or ruffled hair coat. In addition, polyuria and polydypsia were assessed by using increased water consumption and rapid turnover of bedding due to increased urination. The precise amount of water consumed by each mouse per day was not determined, but the water consumption per cage containing three affected mice increased from 25 ml/day to more than 100 ml/day by the end of the first week. Acute tubular necrosis was defined as the presence of necrotic renal tubules in histologic sections. Glomerular lesions were defined as fibrin thrombosis, sludged red blood cells, or both within glomerular capillary lumens. Each mouse was scored positive or negative for a characteristic, and the percentage of mice affected was calculated for each treatment group.
Statistics.
Quantitative data were analyzed with a Mann-Whitney U test. Multiple groups were compared by using analysis of variance and Fisher's least significant difference test. Percentages were compared by using Fisher's exact test (for comparisons of two groups) or the chi-square test (for comparisons of more than two groups). A P value of <0.05 was considered significant. Results are expressed as means ± standard deviations below.
RESULTS
Disease due to virulent EHEC in germ-free mice.
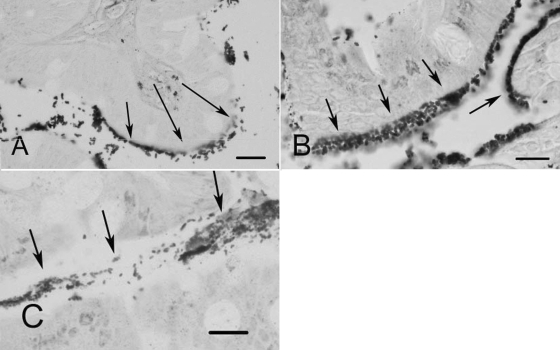
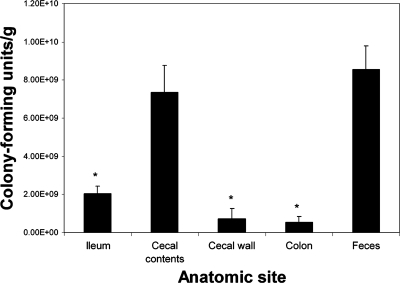
Adult mice of both sexes were inoculated orally with strain 86-24 and euthanized 4 days or 1, 2, or 3 weeks PI. All mice that were inoculated with EHEC became colonized, and uninoculated mice remained sterile throughout the experiment. By 4 days PI the bacterial density was 10.54 ± 0.20 log10 CFU/g feces, and this density or a greater density was maintained throughout the duration of the experiment. The bacterial density in feces was the same regardless of the inoculation dose. One week after inoculation the bacterial density in mice inoculated with 102 CFU (10.6 ± 0.5 log10 CFU/g of feces) did not differ significantly from the density in mice inoculated with 104 CFU (10.3 ± 0.30 log10 CFU/g of feces) or 106 CFU (11.0 ± 1.0 log10 CFU/g of feces). Histologic evaluation of bacterial colonization revealed that in the cecum and distal ileum, bacteria were present in the lumen and had adhered to the epithelium (Fig. 1A and B). In the colon, in contrast, many bacteria were present in the lumen, but few bacteria had adhered to the epithelial surface (Fig. 1C). To further evaluate colonization at different sites within the intestine, the adherent and nonadherent bacteria in the cecal contents, feces, ileum, and colon were quantified, and the adherent bacteria in the washed cecal mucosa were quantified (Fig. 2). The numbers of CFU per gram were significantly greater in the cecal contents and feces than in the ileum and colon, and the luminal bacterial populations in the cecum were significantly larger than populations of bacteria adhering to the cecal wall.
FIG. 1.
(A) Histologic section of the ileum from a mouse infected with 86-24. There are bacteria adhering to the epithelial surface (arrows) and in the lumen. Steiner stain was used. Bar = 10 μm. (B) Cecum. There are bacteria adhering to the epithelium (arrows). Steiner stain was used. Bar = 10 μm. (C) Colon. Bacteria are present in the lumen (arrows), but there are few bacteria adhering to the epithelium. Steiner stain was used. Bar = 10 μm.
FIG. 2.
Number of CFU per gram of tissue or intestinal contents. Sections of ileum and colon were weighed and homogenized, and 10-fold dilutions were plated as described in the text. The cecal wall was washed vigorously three times to remove nonadherent bacteria, weighed, homogenized, and plated as described above. Feces and cecal contents were weighed, homogenized, and plated as described above. An asterisk indicates that the results are significantly different from the results for the cecal contents and feces (P < 0.05). The error bars indicate the standard errors of the means. There is no significant difference between the ileum, colon, and cecal wall or between the cecal contents and feces.
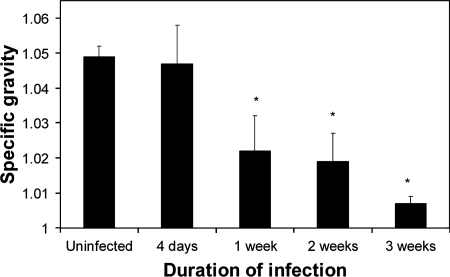
The clinical signs in infected mice included lethargy, ruffled fur (a nonspecific indicator of disease in mice), dehydration, polyuria, polydypsia, and death. The signs became evident in EHEC-infected mice between 4 and 7 days after inoculation, and the severity and prevalence of the signs increased over time. By 14 days PI, 7 of 12 mice inoculated with strain 86-24 were either dead or moribund. The hematology values determined for selected mice included hematocrit and blood urea nitrogen (BUN) values. In uninfected mice, the BUN values ranged from 10 to 35 mg/dl (mean ± standard deviation, 22 ± 8 mg/dl). In infected mice, the BUN level was elevated in 3/13 mice examined 1 week after inoculation (range of all 13 values, 19 to 369 mg/dl), but because of marked individual variation the mean (50 ± 96 mg/dl) did not differ from that of uninfected mice (P = 0.8843). BUN values were elevated in 5/7 mice examined 2 or 3 weeks after inoculation (range of the five values, 34 to 115 mg/dl; mean ± standard deviation, 69 ± 31 mg/dl; P < 0.0001 compared to uninfected mice). In contrast to the BUN values, urine specific gravity was a consistent marker of renal disease in EHEC-infected mice. The specific gravity was decreased in 2 of 15 infected mice by 4 days PI and in 24 of 26 infected mice by 7 days PI (Fig. 3).
FIG. 3.
Urine specific gravity for mice inoculated with 86-24 and examined 4 days to 3 weeks after inoculation. The error bars indicate the standard errors of the means. An asterisk indicates that the results are significantly different from the results for uninfected mice (P < 0.05).
The hematocrit values for mice euthanized 1 week after inoculation (55% ± 13%) were significantly elevated compared to those for uninfected mice (45% ± 6%; P = 0.0415), likely due to dehydration. However, by 2 to 3 weeks after inoculation, the hematocrit values did not differ from those of uninfected mice (48% ± 6%). Anemia in the chronically infected mice could have been masked by hemoconcentration due to dehydration, which was evident clinically in all mice euthanized 2 to 3 weeks PI.
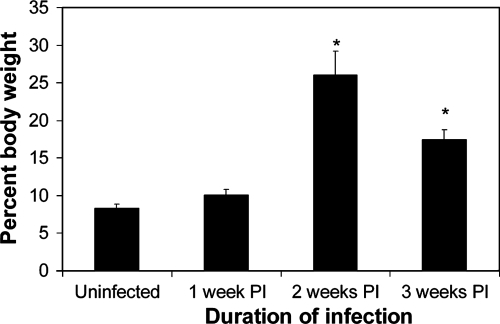
Other than dehydration, the most striking gross postmortem finding for EHEC-infected mice was markedly enlarged, fluid-filled ceca. The cecal wall was thin, and the lumen contained abundant watery fluid. The ceca of uninfected germ-free mice (5.1 to 12.5% of body weight; n = 18) are normally larger than those of mice with a normal microbiota (0.9 to 2.3% of body weight; n = 13). However, in EHEC-infected mice, the ceca accounted for as much as 32.3% of the body weight due to marked fluid accumulation. The cecal fluid accumulation was mild 1 week after inoculation, but it became marked by 2 to 3 weeks after inoculation (Fig. 4). Distended ceca had thin walls, some of these ceca had lymphoid follicular development, and there were adherent bacteria, as described above; otherwise, there were no histologic lesions.
FIG. 4.
Cecal weight as a percentage of body weight for mice inoculated with 86-24 and euthanized 1 to 3 weeks after inoculation. The error bars indicate the standard errors of the means. An asterisk indicates that the results are significantly different from the results for uninfected mice (P < 0.02).
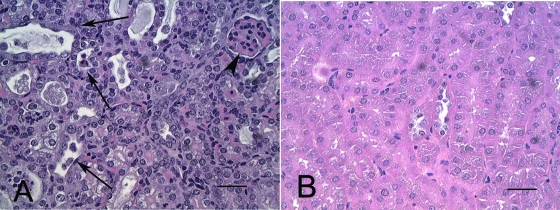
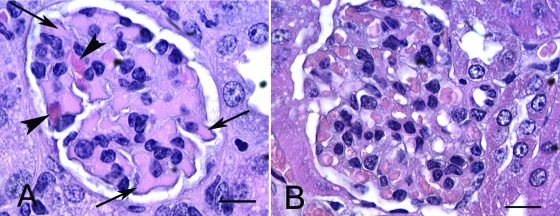
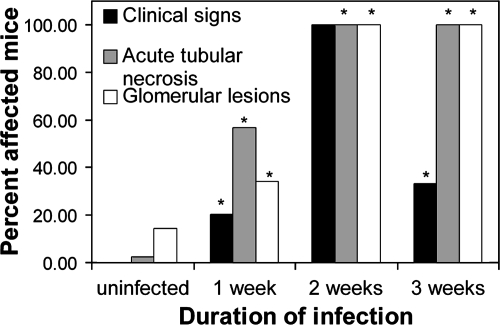
Histologically, major lesions were confined to the kidneys and consisted of acute tubular necrosis (Fig. 5) and glomerular capillary red blood cell sludging, sometimes accompanied by fibrin thrombi (Fig. 6). Tubular necrosis and glomerular thrombosis were multifocal and randomly distributed in mice euthanized 1 week PI, and they became more generalized as the infection progressed. The clinical signs of disease and histologic evidence of renal disease in EHEC-infected mice increased in severity as well as prevalence over the 3-week course of the experiment (Fig. 7). There were no differences in bacterial colonization, clinical signs, or disease lesions between male and female mice (data not shown).
FIG. 5.
Hematoxylin- and eosin-stained sections of kidneys from a mouse inoculated with 86-24 and euthanized 1 week PI (A) and from an uninfected mouse (B). (A) Renal tubules are necrotic, and many contain cellular debris (arrows). Glomeruli (arrowheads) are distended with fibrin and red blood cells. (B) Tubules are not affected. Bars = 25 μm.
FIG. 6.
(A) Section from a mouse inoculated with 86-24 and euthanized 1 week PI. A glomerulus with fibrin thrombi (arrows) and entrapped red blood cells (arrowheads) is shown. (B) Glomerulus from an uninfected mouse containing red blood cells but no thrombi. Bars = 10 μm.
FIG. 7.
Prevalence of disease over the 3-week course of infection of germ-free mice with 86-24. Clinical disease, acute tubular necrosis, and glomerular lesions were defined as described in Materials and Methods. Error bars are not included because the values are percentages rather than means. An asterisk indicates that the results are significantly different from the results for uninfected mice (P < 0.05, chi-square analysis).
Effect of age on disease in mice colonized by 86-24.
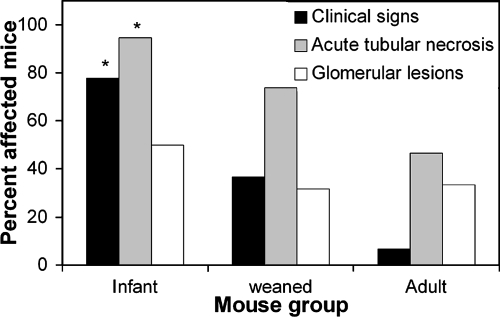
Mice were separated into three age groups: infant mice (colonized by contact with infected dams at 3 days of age), weaned mice (colonized by direct oral inoculation at 3 to 5 weeks of age), and adult mice (colonized by direct oral inoculation at 6 to 12 weeks of age). All mice were inoculated with strain 86-24 and euthanized 1 week after inoculation. The density of bacteria in feces of infant mice (10.96 ± 1.64 log10 CFU/g of feces) was not statistically different from the density of bacteria in feces of adult mice (11.09 ± 1.05 log10 CFU/g) or in feces of weaned mice (10.62 ± 0.32 log10 CFU/g of feces). Infant mice were significantly more susceptible to disease due to 86-24 than weaned and adult mice. In contrast to adult and weaned mice, all infant mice were dead or moribund by 1 week PI, and the incidence of clinical disease and the incidence of acute tubular necrosis were significantly greater in this group (Fig. 8).
FIG. 8.
Prevalence of disease in infant mice inoculated at 3 days of age, weaned mice (3 to 5 weeks of age), and adult mice (more than 5 weeks of age). Clinical disease, acute tubular necrosis, and glomerular lesions were defined as described in Materials and Methods. Error bars are not included because the values are percentages rather than means. An asterisk indicates that the results are significantly different from the results for adult mice (P < 0.05, chi-square analysis).
Role of Stx2 in disease due to EHEC.
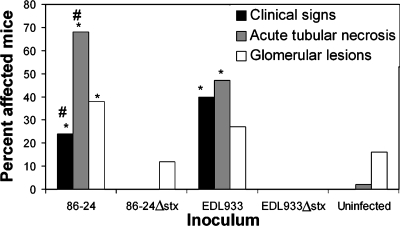
Infant, weanling, or adult mice were inoculated with 86-24 or EDL933 or with the 86-24 or EDL933 Δstx2 isogenic mutant. Mice were euthanized 1, 2, or 3 weeks after bacterial inoculation. In all cases, inoculated mice were colonized by the inoculating strain, and the fecal bacterial density for mice colonized by 86-24::Δstx2 (mean ± standard deviation, 11.30 ± 1.44 log10 CFU/g feces) or EDL933::Δstx2 (10.27 ± 0.15 log10 CFU/g) did not differ from the density for mice colonized by 86-24 (10.58 ± 1.03 log10 CFU/g) or EDL933 (11.48 ± 1.28 log10 CFU/g). Clinical signs and histologic lesions of renal disease developed in mice inoculated with 86-24 or EDL933 but not in mice inoculated with 86-24::Δstx2 or EDL933::Δstx2 regardless of the age of the host or the duration of infection (Fig. 9). Adult, weanling, and infant mice inoculated with either of the Δstx2 mutants remained clinically normal throughout the 3-week observation period, and there was no change in the BUN or hematocrit values or the urine specific gravity (data not shown). Only mice colonized by 86-24 or 86-24::Δstx2 were examined more than 1 week PI, and cecal size could be evaluated only for these mice. In contrast to the results for the ceca of mice infected with 86-24 (21.7% ± 6.8% of body weight; P < 0.0001 compared to uninfected mice), the cecal size for mice colonized by 86-24::Δstx2 (5.7% ± 1.1%) was not greater than the cecal size for uninfected mice (9.7% ± 3.5%).
FIG. 9.
Prevalence of disease in mice inoculated with 86-24, EDL933, or a Δstx2 mutant. All mice were examined 1 week after inoculation. Clinical disease, acute tubular necrosis, and glomerular lesions were defined as described in Materials and Methods. Error bars are not included because the values are percentages rather than means. An asterisk indicates that the results are significantly different from the results for uninfected mice. A number sign indicates that the results are significantly different from the results for mice infected with the Δstx2 mutant.
Virulence of EHEC strains in germ-free mice.
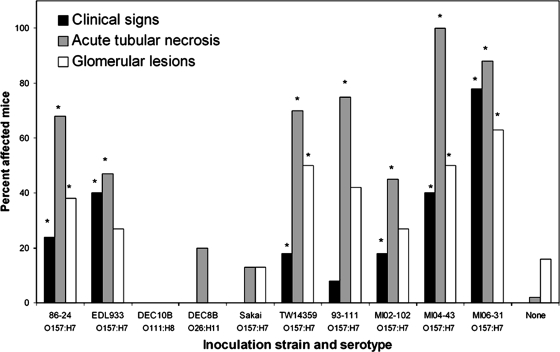
We compared the virulence for germ-free mice of eight different O157 and two non-O157 strains of EHEC (Table 1). Four of the strains expressed both Stx1 and Stx2, one expressed only Stx1, and five expressed only Stx2. Mice were inoculated as described above with 1 of the 10 strains and euthanized 1 week later. With one exception, these strains colonized mice at similar densities (mean ± standard deviation for the nine strains, 10.4 ± 1.9 log10 CFU/g feces). The one exception was DEC8B, a non-O157 strain that colonized at a somewhat lower fecal density (7.73 ± 5.52 log10 CFU/g feces; P < 0.001 compared to all other strains). In spite of the similar fecal bacterial densities, there were marked differences in disease induction by five strains. Seven of the eight O157 strains, but neither of the non-O157 strains, caused disease in mice (Fig. 10). Among the disease-causing strains, there was marked variation in the onset and severity of disease. Strains MI04-43 and MI06-31 caused the most rapidly progressive disease, causing death by 5 days PI in 3/10 and 9/15 mice, respectively. Strain 86-24 also caused severe disease and death. By 7 days PI, 5/37 mice inoculated with 86-24 were moribund or had died. Strains EDL933, TW04863, and MI02-102 did not cause mice to become moribund or die within the 1-week sacrifice interval, but they did cause clinical signs and histologic lesions of acute renal failure.
FIG. 10.
Prevalence of disease in mice inoculated with 1 of 10 EHEC strains. Clinical disease, acute tubular necrosis, and glomerular lesions were defined as described in Materials and Methods. Error bars are not included because the values are percentages rather than means. An asterisk indicates that the values are greater than the values for uninfected mice (P < 0.05, Fisher's exact test).
Severe renal disease (>50% incidence) was found only in mice colonized by TW14359, TW04863, MI04-43, or MI06-31. Strains 86-24, MI02-102, and EDL933 caused moderate renal disease (>25% but <50% incidence) (Fig. 10). Stx1, Stx2, or both were detected in cecal contents, depending on the inoculum strain (Table 2). Measurable levels of Stx2 were not found in cecal contents of uninfected mice or of mice infected with the Δstx2 mutants. There was no correlation between the Stx2 concentration in vivo and the severity of disease in mice (Table 2).
TABLE 2.
Disease induction and Stx production in vivo by the EHEC strains used in this study
| Strain | Disease in micea | Stx in cecal contentsb | Cecal Stx2 concn (ng/ml of cecal contents) |
|---|---|---|---|
| 86-24 | Moderate | Stx2 | 921 ± 111 |
| EDL933 | Moderate | Stx1, Stx2 | 6,529 ± 4,432c |
| DEC8B | Minimal | Stx1, Stx2 | 1,909 ± 714 |
| DEC10B | Minimal | ND | ND |
| TW14359 | Severe | Stx2 | 5,843 ± 1,518c |
| 93-111 | Severe | ND | ND |
| MI02-102 | Moderate | Stx2 | 8,694 ± 2,611c |
| MI04-43 | Severe | ND | ND |
| MI06-31 | Severe | Stx2 | 5,321 ± 3,807 |
| Sakai | Minimal | Stx1, Stx2 | 1,872 ± 828 |
The severity estimates are based on the incidence of disease in mice. Severe, more than 50% affected; moderate, 25 to 50% affected; minimal, less than 20% affected (see Fig. 10).
Based on a qualitative determination of the presence of Stx1 and Stx2. ND, not determined.
P < 0.05 compared to 86-24.
DISCUSSION
EHEC (and enterobacteria in general) do not normally colonize the mouse intestine, which is largely colonized by gram-positive anaerobes (4, 21, 31). When conventional or SPF mice are inoculated with live EHEC, bacteria are detectable only briefly in the feces, and bacterial counts fall to undetectable levels within a few days (7, 24, 29, 40). Generally, signs of disease and lesions are minimal or not detectable (29, 40). Conlan et al. (7) reported death in mice inoculated with one of nine EHEC strains tested, but neither clinical signs nor lesions were reported in their study. A second study reported rapid onset of renal and colonic lesions, neurologic signs, and death in SPF mice that were orally inoculated with several strains of EHEC O157, but the mice in that study became bacteremic and intestinal colonization was not documented (19).
The most widely used infection model is the streptomycin-treated mouse model, in which a subset of the normal enteric microbiota is suppressed by streptomycin treatment and mice are orally inoculated with streptomycin-resistant EHEC strains (3, 12, 24, 27, 35, 44, 53, 54). These mice are highly susceptible to colonization by less than 100 CFU of EHEC, and colonization persists for as long as the mice are treated with streptomycin. Signs of disease and lesions vary according to the bacterial strain, laboratory, and other (unknown) factors and range from no disease or lesions (12, 44) to acute renal tubular necrosis and death (53, 54). A few reports have also included descriptions of intestinal epithelial necrosis (44), cerebral hemorrhage (12), or brain edema (22). The residual microbiota in streptomycin-treated mice have not been described and likely differ between laboratories (and even between individual mice), possibly accounting for some of the differences in the outcomes of the experiments.
Several reports have described disease due to EHEC in germ-free mice, as described in this study (1, 17, 48, 49). Like streptomycin-treated mice, germ-free mice are exquisitely susceptible to colonization by EHEC, and the colonization is indefinite. Descriptions of signs and lesions are inconsistent. Lesions that have been reported include renal tubular necrosis (48), necrosis of colonic epithelial cells (16), and neurologic signs or lesions (16, 48). Most previous studies with germ-free mice, however, did not investigate or report clinical signs or lesions (1, 17, 49). The role of age and sex in the susceptibility of mice to EHEC has not been investigated previously with any model.
The results of this study are consistent with the results of previous reports for streptomycin-treated and germ-free mice in several respects. First, we demonstrated that germ-free mice are exquisitely susceptible to colonization by EHEC and that inoculation of as few as 100 bacteria results in a bacterial density of 109 CFU/g of feces or more by 1 day PI and persistence until death of the mouse or (in the case of nonpathogenic strains) for the duration of the experiment. We also demonstrated that the principal outcome (and probably the cause of death) of pathogenic EHEC infection in mice is acute renal tubular necrosis and that vascular lesions are subtle in mice and are confined to multifocal glomerular thrombosis and red blood cell sludging. Previous studies in which kidneys were examined generally demonstrated that there was renal tubular necrosis in infected mice, although the reported severity of disease varied between studies (3, 12, 24, 44, 53, 54). A few studies showed that there were consistent vascular or glomerular lesions (12, 16, 48), and the descriptions of these lesions varied between studies, confirming our findings that glomerular disease is present but mild in EHEC-infected mice. Several studies that used an injection model, in which Stx or Stx in combination with lipopolysaccharide was injected parenterally into mice, described glomerular lesions (11, 13, 15, 20, 45), supporting our finding that virulent EHEC is capable of inducing glomerular disease in mice.
In addition to renal lesions, we report several new findings concerning intestinal colonization of infected mice here. First, we showed that bacteria are present throughout the lower intestine but adhere to the cecal and ileal mucosa and not to the colonic mucosa. Culture of the ileum, cecal contents, cecal wall, and colon detected bacteria at all sites and demonstrated that bacterial populations adhered to the cecal wall. Adherence was morphologically detectable only in histologic sections of the ileum and cecum of the mouse and was largely absent in the colon. This distribution of colonization in mice has not been described previously and could explain the failure of some previous studies to detect adherence, because only the colon was examined (29), while other studies reported adherence in the ceca of infected mice (30). We also showed that although mice do not develop diarrhea due to EHEC, chronic infection is accompanied by marked luminal fluid accumulation in the cecum. The restriction of fluid accumulation to the ceca without diarrhea in these mice could be due to the fact that bacterial adherence occurs largely in the cecum, inducing fluid loss there but not in the colon. Mice are desert animals with very efficient fluid reabsorption mechanisms (46). In EHEC-infected mice, since bacterial adherence is confined to the cecum, the fluid lost into the cecum is likely reabsorbed in the colon, resulting in the absence of diarrhea. Thus, germ-free mice do develop enteric disease due to EHEC, although the lesions develop more slowly and the distribution is different than that in humans, likely due to differences in the pattern of adherence of EHEC and in the physiology of mice and humans.
In this study we showed that while sex does not affect susceptibility to EHEC-associated disease, age is an important factor. Mice of all ages were susceptible to infection and disease, but infant mice were the most susceptible to both clinical disease and renal lesions. This finding correlates with the increased incidence of EHEC-associated HUS in children compared to adults (2, 18). We also showed that while (as previously described for mice [37]) BUN is an insensitive marker of renal disease, urine specific gravity is a sensitive indicator of the onset of renal failure and correlates well with histologic evidence of renal disease. This has not been shown previously in a mouse model of EHEC.
Our investigations demonstrated that Stx2 is necessary but not sufficient to induce both renal disease and cecal fluid accumulation in mice. Deletion of Stx2 abrogated pathogenicity both in 86-24, which produces only Stx2, and in EDL933, which produces both Stx1 and Stx2. In the latter case, Stx1 alone was insufficient to induce disease in mice colonized by EDL933::Δstx2. The results of previous studies performed by other investigators support our findings, although to our knowledge, no one has used Stx2 deletion mutants to test directly the role of Stx2 in renal disease. Several studies have demonstrated that plasmid-mediated expression of Stx2 confers pathogenicity on laboratory strains of E. coli (24, 25, 36, 54), and a number of studies have demonstrated that antibodies directed against Stx2 provide protection (24, 42, 43, 54); both of these findings provide indirect evidence of the role of Stx2 in renal disease. Donohue-Rolf et al. (10) showed that isogenic Stx2-negative mutants failed to cause neurologic lesions or signs in gnotobiotic piglets, but renal lesions were not described. Finally, several studies have demonstrated the nephrotoxic effect of parenterally injected Stx1 or Stx2 (11, 13, 15, 20, 25, 45, 51), again providing indirect evidence that Stx is at least partially responsible for renal disease.
Because the only strain in this study that expressed Stx1 alone, DEC10B, did not cause disease in mice, we could not evaluate Stx1 directly. EDL933::Δstx2 did not cause disease in mice in spite of the presence of Stx1 in cecal contents, suggesting that Stx1 does not contribute to disease in this model, but the evidence remains indirect. Our results are compatible with previous studies cited above showing that Stx2 is necessary for disease in orally infected mice, but studies performed with other models suggest a role for Stx1 in disease. For example, Sjogren et al. (47) observed enhanced severity of enteric disease in rabbits infected with RDEC that was engineered to express Stx1. Also, direct injection studies with mice have shown that parenteral Stx1 alone, as well as Stx2, causes renal disease (20, 51). Definitive evidence of the in vivo pathogenicity of Stx1 awaits identification of a pathogenic strain that produces Stx1 alone.
Unlike a previous report (40), we found no evidence of a role for Stx2 in colonization. In our hands, both Δstx2 mutants colonized as well as the wild-type parental strains. This finding is compatible with one report which demonstrated that Stx2 did not influence colonization in a rabbit model (39), but it differs from the results of the study of Robinson et al. (40), in which challenge of conventional SPF mice with mutants deficient in Stx2 resulted in less colonization than challenge with the wild-type parent strain. However, because Robinson et al. used SPF mice with a full complement of intestinal microbiota, our study may not be directly comparable. EHEC did not cause disease in SPF mice, and the colonization density in SPF mice was low and decreased rapidly over the course of the experiment, indicating that neither the wild type nor the Stx mutant colonized well. It is possible that in the presence of normal murine microbiota, Stx2 provides a competitive advantage to EHEC strains. If this is the case, we would not expect differences in colonization between mice monocolonized with an Stx deletion mutant and mice colonized by the wild-type parent. However, in the presence of other intestinal microbiota, the Stx deletion mutant might be cleared faster than the wild type. Confirmation of this hypothesis awaits direct competition studies with Δstx and wild-type bacteria and/or studies with mice having a defined flora.
In spite of the dependence of disease on the presence of Stx2 in the two strains examined in this study, several other EHEC strains that did produce Stx2 failed to cause disease in mice, demonstrating that while Stx2 is necessary to cause disease, it is not sufficient. In addition, although the severity of disease in mice varied for the bacterial strains, the concentration of Stx2 in the cecum of infected mice did not correlate with the severity of disease. These results suggest that strain-specific factors in addition to toxin production contribute to disease due to EHEC in mice. Disease did correlate with O157 serotype in this study, consistent with the prominence of O157 serotypes in clinical disease (18), but only two non-O157 strains were examined in this study, precluding definitive interpretation.
Stx-independent differences in pathogenicity of EHEC strains have been described previously. The manifestations of clinical outbreaks associated with Stx2-producing strains vary (26), suggesting that, as we have shown in mice, production of Stx2 alone does not completely account for the variation in pathogenicity among strains. A few studies have compared the pathogenicities of different EHEC strains in streptomycin-treated mice (24, 44, 53), and the results have been similar to our results, showing differences in disease outcome between EHEC strains, all of which produce Stx2. One possible explanation for these differences is genetic diversity among pathogenic strains. Marked genomic diversity among Stx2-producing O157 strains has been described by several authors (33, 34), and a recent study suggested that differences in disease outcome may be due to genotypic diversity among strains, regardless of Stx2 (26). Genetic comparison of strains that differ in virulence is likely to reveal specific factors that suppress or enhance Stx-associated disease.
Acknowledgments
This project was supported in part by funds from NIAID, NIH, Department of Health and Human Services, under contract N01-AI-30058 and by Public Health Service grant AI11459-10.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 28 April 2008.
REFERENCES
- 1.Aiba, Y., H. Ishikawa, K. Shimizu, S. Noda, Y. Kitada, M. Sasaki, and Y. Koga. 2002. Role of internalization in the pathogenicity of Shiga toxin-producing Escherichia coli infection in a gnotobiotic murine model. Microbiol. Immunol. 46723-731. [DOI] [PubMed] [Google Scholar]
- 2.Amirlak, I., and B. Amirlak. 2006. Haemolytic uraemic syndrome: an overview. Nephrology 11213-218. [DOI] [PubMed] [Google Scholar]
- 3.Asahara, T., K. Shimizu, K. Nomoto, T. Hamabata, A. Ozawa, and Y. Takeda. 2004. Probiotic bifidobacteria protect mice from lethal infection with Shiga toxin-producing Escherichia coli O157:H7. Infect. Immun. 722240-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bibiloni, R., M. A. Simon, C. Albright, B. Sartor, and G. W. Tannock. 2005. Analysis of the large bowel microbiota of colitic mice using PCR/DGGE. Lett. Appl. Microbiol. 4145-51. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2006. Ongoing multistate outbreak of Escherichia coli serotype O157:H7 infections associated with consumption of fresh spinach—United States, September 2006. MMWR Morb. Mortal. Wkly. Rep. 551045-1046. [PubMed] [Google Scholar]
- 6.Cody, S. H., M. K. Glynn, J. A. Farrar, K. L. Cairns, P. M. Griffin, J. Kobayashi, M. Fyfe, R. Hoffman, A. S. King, J. H. Lewis, B. Swaminathan, R. G. Bryant, and D. J. Vugia. 1999. An outbreak of Escherichia coli O157:H7 infection from unpasteurized commercial apple juice. Ann. Intern. Med. 130202-209. [DOI] [PubMed] [Google Scholar]
- 7.Conlan, J. W., and M. B. Perry. 1998. Susceptibility of three strains of conventional adult mice to intestinal colonization by an isolate of Escherichia coli O157:H7. Can. J. Microbiol. 44800-805. [DOI] [PubMed] [Google Scholar]
- 8.Court, D. L., J. A. Sawitzke, and L. C. Thomason. 2002. Genetic engineering using homologous recombination. Annu. Rev. Genet. 36361-388. [DOI] [PubMed] [Google Scholar]
- 9.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 976640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donohue-Rolfe, A., I. Kondova, S. Oswald, D. Hutto, and S. Tzipori. 2000. Escherichia coli O157:H7 strains that express Shiga toxin (Stx) 2 alone are more neurotropic for gnotobiotic piglets than are isotypes producing only Stx1 or both Stx1 and Stx2. J. Infect. Dis. 1811825-1829. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez, G. C., M. F. Lopez, S. A. Gomez, M. V. Ramos, L. V. Bentancor, R. J. Fernandez-Brando, V. I. Landoni, G. I. Dran, R. Meiss, M. A. Isturiz, and M. S. Palermo. 2006. Relevance of neutrophils in the murine model of haemolytic uraemic syndrome: mechanisms involved in Shiga toxin type 2-induced neutrophilia. Clin. Exp. Immunol. 14676-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujii, J., T. Kita, S. Yoshida, T. Takeda, H. Kobayashi, N. Tanaka, K. Ohsato, and Y. Mizuguchi. 1994. Direct evidence of neuron impairment by oral infection with verotoxin-producing Escherichia coli O157:H- in mitomycin-treated mice. Infect. Immun. 623447-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomez, S. A., G. C. Fernandez, S. Vanzulli, G. Dran, C. Rubel, T. Berki, M. A. Isturiz, and M. S. Palermo. 2003. Endogenous glucocorticoids attenuate Shiga toxin-2-induced toxicity in a mouse model of haemolytic uraemic syndrome. Clin. Exp. Immunol. 131217-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffin, P. M., S. M. Ostroff, R. V. Tauxe, K. D. Greene, J. G. Wells, J. H. Lewis, and P. A. Blake. 1988. Illnesses associated with Escherichia coli O157:H7 infections. A broad clinical spectrum. Ann. Intern. Med. 109705-712. [DOI] [PubMed] [Google Scholar]
- 15.Ikeda, M., S. Ito, and M. Honda. 2004. Hemolytic uremic syndrome induced by lipopolysaccharide and Shiga-like toxin. Pediatr. Nephrol. 19485-489. [DOI] [PubMed] [Google Scholar]
- 16.Isogai, E., H. Isogai, K. Kimura, S. Hayashi, T. Kubota, N. Fujii, and K. Takeshi. 1998. Role of tumor necrosis factor alpha in gnotobiotic mice infected with an Escherichia coli O157:H7 strain. Infect. Immun. 66197-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeon, B., and K. Itoh. 2007. Production of shiga toxin by a luxS mutant of Escherichia coli O157:H7 in vivo and in vitro. Microbiol. Immunol. 51391-396. [DOI] [PubMed] [Google Scholar]
- 18.Karch, H., P. I. Tarr, and M. Bielaszewska. 2005. Enterohaemorrhagic Escherichia coli in human medicine. Int J. Med. Microbiol. 295405-418. [DOI] [PubMed] [Google Scholar]
- 19.Karpman, D., H. Connell, M. Svensson, F. Scheutz, P. Alm, and C. Svanborg. 1997. The role of lipopolysaccharide and Shiga-like toxin in a mouse model of Escherichia coli O157:H7 infection. J. Infect. Dis. 175611-620. [DOI] [PubMed] [Google Scholar]
- 20.Keepers, T. R., M. A. Psotka, L. K. Gross, and T. G. Obrig. 2006. A murine model of HUS: Shiga toxin with lipopolysaccharide mimics the renal damage and physiologic response of human disease. J. Am. Soc Nephrol. 173404-3414. [DOI] [PubMed] [Google Scholar]
- 21.Kuehl, C. J., H. D. Wood, T. L. Marsh, T. M. Schmidt, and V. B. Young. 2005. Colonization of the cecal mucosa by Helicobacter hepaticus impacts the diversity of the indigenous microbiota. Infect. Immun. 736952-6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurioka, T., Y. Yunou, and E. Kita. 1998. Enhancement of susceptibility to Shiga toxin-producing Escherichia coli O157:H7 by protein calorie malnutrition in mice. Infect. Immun. 661726-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ley, R. E., D. A. Peterson, and J. I. Gordon. 2006. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 124837-848. [DOI] [PubMed] [Google Scholar]
- 24.Lindgren, S. W., A. R. Melton, and A. D. O'Brien. 1993. Virulence of enterohemorrhagic Escherichia coli O91:H21 clinical isolates in an orally infected mouse model. Infect. Immun. 613832-3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindgren, S. W., J. E. Samuel, C. K. Schmitt, and A. D. O'Brien. 1994. The specific activities of Shiga-like toxin type II (SLT-II) and SLT-II-related toxins of enterohemorrhagic Escherichia coli differ when measured by Vero cell cytotoxicity but not by mouse lethality. Infect. Immun. 62623-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manning, S. D., A. S. Motiwala, A. C. Springman, W. Qi, D. W. Lacher, L. M. Ouellette, J. M. Mladonicky, P. Somsel, J. T. Rudrik, S. E. Dietrich, W. Zhang, B. Swaminathan, D. Alland, and T. S. Whittam. 10 March 2008. Variation in virulence among clades of Escherichia coli O157:H7 associated with disease outbreaks. Proc. Natl. Acad. Sci. USA. [Epub ahead of print] doi: 10.1073/pnas.0710834105. [DOI] [PMC free article] [PubMed]
- 27.Melton-Celsa, A. R., J. E. Rogers, C. K. Schmitt, S. C. Darnell, and A. D. O'Brien. 1998. Virulence of Shiga toxin-producing Escherichia coli (STEC) in orally-infected mice correlates with the type of toxin produced by the infecting strain. Jpn. J. Med. Sci. Biol 51(Suppl)S108-S114. [DOI] [PubMed] [Google Scholar]
- 28.Michino, H., K. Araki, S. Minami, S. Takaya, N. Sakai, M. Miyazaki, A. Ono, and H. Yanagawa. 1999. Massive outbreak of Escherichia coli O157:H7 infection in schoolchildren in Sakai City, Japan, associated with consumption of white radish sprouts. Am. J. Epidemiol. 150787-796. [DOI] [PubMed] [Google Scholar]
- 29.Mundy, R., F. Girard, A. J. FitzGerald, and G. Frankel. 2006. Comparison of colonization dynamics and pathology of mice infected with enteropathogenic Escherichia coli, enterohaemorrhagic E. coli and Citrobacter rodentium. FEMS Microbiol. Lett. 265126-132. [DOI] [PubMed] [Google Scholar]
- 30.Nagano, K., K. Taguchi, T. Hara, S. Yokoyama, K. Kawada, and H. Mori. 2003. Adhesion and colonization of enterohemorrhagic Escherichia coli O157:H7 in cecum of mice. Microbiol. Immunol. 47125-132. [DOI] [PubMed] [Google Scholar]
- 31.Nagura, T., S. Hachimura, S. Kaminogawa, T. Aritsuka, and K. Itoh. 2005. Characteristic intestinal microflora of specific pathogen-free mice bred in two different colonies and their influence on postnatal murine immunocyte profiles. Exp. Anim. 54143-148. [DOI] [PubMed] [Google Scholar]
- 32.O'Brien, A. D., V. L. Tesh, A. Donohue-Rolfe, M. P. Jackson, S. Olsnes, K. Sandvig, A. A. Lindberg, and G. T. Keusch. 1992. Shiga toxin: biochemistry, genetics, mode of action, and role in pathogenesis. Curr. Top. Microbiol. Immunol. 18065-94. [DOI] [PubMed] [Google Scholar]
- 33.Ogura, Y., K. Kurokawa, T. Ooka, K. Tashiro, T. Tobe, M. Ohnishi, K. Nakayama, T. Morimoto, J. Terajima, H. Watanabe, S. Kuhara, and T. Hayashi. 2006. Complexity of the genomic diversity in enterohemorrhagic Escherichia coli O157 revealed by the combinational use of the O157 Sakai OligoDNA microarray and the whole genome PCR scanning. DNA Res. 133-14. [DOI] [PubMed] [Google Scholar]
- 34.Ohnishi, M., J. Terajima, K. Kurokawa, K. Nakayama, T. Murata, K. Tamura, Y. Ogura, H. Watanabe, and T. Hayashi. 2002. Genomic diversity of enterohemorrhagic Escherichia coli O157 revealed by whole genome PCR scanning. Proc. Natl. Acad. Sci. USA 9917043-17048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paton, A. W., A. J. Bourne, P. A. Manning, and J. C. Paton. 1995. Comparative toxicity and virulence of Escherichia coli clones expressing variant and chimeric Shiga-like toxin type II operons. Infect. Immun. 632450-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paton, A. W., P. A. Manning, and J. C. Paton. 1995. Increased oral virulence of Escherichia coli expressing a variant Shiga-like toxin type II operon is associated with both A subunit residues Met4 and Gly102. Microb. Pathog. 19185-191. [DOI] [PubMed] [Google Scholar]
- 37.Quimby, F. W., and R. Luong. 2007. Clinical chemistry of the laboratory mouse, p. 171-216. In J. G. Fox, M. T. Davisson, F. W. Quimby, S. W. Barthold, C. E. Newcomer, and A. L. Smith (ed.), The mouse in biomedical research, 2nd ed., vol. 3. Academic Press, New York, NY. [Google Scholar]
- 38.Riley, L. W., R. S. Remis, S. D. Helgerson, H. B. McGee, J. G. Wells, B. R. Davis, R. J. Hebert, E. S. Olcott, L. M. Johnson, N. T. Hargrett, P. A. Blake, and M. L. Cohen. 1983. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N. Engl. J. Med. 308681-685. [DOI] [PubMed] [Google Scholar]
- 39.Ritchie, J. M., C. M. Thorpe, A. B. Rogers, and M. K. Waldor. 2003. Critical roles for stx2, eae, and tir in enterohemorrhagic Escherichia coli-induced diarrhea and intestinal inflammation in infant rabbits. Infect. Immun. 717129-7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robinson, C. M., J. F. Sinclair, M. J. Smith, and A. D. O'Brien. 2006. Shiga toxin of enterohemorrhagic Escherichia coli type O157:H7 promotes intestinal colonization. Proc. Natl. Acad. Sci. USA 1039667-9672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharma, S., P. Sachdeva, and J. S. Virdi. 2003. Emerging water-borne pathogens. Appl. Microbiol. Biotechnol. 61424-428. [DOI] [PubMed] [Google Scholar]
- 42.Sheoran, A. S., S. Chapman-Bonofiglio, B. R. Harvey, J. Mukherjee, G. Georgiou, A. Donohue-Rolfe, and S. Tzipori. 2005. Human antibody against Shiga toxin 2 administered to piglets after the onset of diarrhea due to Escherichia coli O157:H7 prevents fatal systemic complications. Infect. Immun. 734607-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sheoran, A. S., S. Chapman, P. Singh, A. Donohue-Rolfe, and S. Tzipori. 2003. Stx2-specific human monoclonal antibodies protect mice against lethal infection with Escherichia coli expressing Stx2 variants. Infect. Immun. 713125-3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shimizu, K., T. Asahara, K. Nomoto, R. Tanaka, T. Hamabata, A. Ozawa, and Y. Takeda. 2003. Development of a lethal Shiga toxin-producing Escherichia coli-infection mouse model using multiple mitomycin C treatment. Microb. Pathog. 351-9. [DOI] [PubMed] [Google Scholar]
- 45.Shimizu, K., K. Tanaka, A. Akatsuka, M. Endoh, and Y. Koga. 1999. Induction of glomerular lesions in the kidneys of mice infected with vero toxin-producing Escherichia coli by lipopolysaccharide injection. J. Infect. Dis. 1801374-1377. [DOI] [PubMed] [Google Scholar]
- 46.Singleton, G. R., and C. J. Krebs. 2007. The secret world of wild mice, p. 25-51. In J. G. Fox, M. T. Davisson, F. W. Quimby, S. W. Barthold, C. E. Newcomer, and A. L. Smith (ed.), The mouse in biomedical research, 2nd ed., vol. 1. Academic Press, New York, NY. [Google Scholar]
- 47.Sjogren, R., R. Neill, D. Rachmilewitz, D. Fritz, J. Newland, D. Sharpnack, C. Colleton, J. Fondacaro, P. Gemski, and E. Boedeker. 1994. Role of Shiga-like toxin I in bacterial enteritis: comparison between isogenic Escherichia coli strains induced in rabbits. Gastroenterology 106306-317. [DOI] [PubMed] [Google Scholar]
- 48.Taguchi, H., M. Takahashi, H. Yamaguchi, T. Osaki, A. Komatsu, Y. Fujioka, and S. Kamiya. 2002. Experimental infection of germ-free mice with hyper-toxigenic enterohaemorrhagic Escherichia coli O157:H7, strain 6. J. Med. Microbiol. 51336-343. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi, M., H. Taguchi, H. Yamaguchi, T. Osaki, A. Komatsu, and S. Kamiya. 2004. The effect of probiotic treatment with Clostridium butyricum on enterohemorrhagic Escherichia coli O157:H7 infection in mice. FEMS Immunol. Med. Microbiol. 41219-226. [DOI] [PubMed] [Google Scholar]
- 50.Tarr, P. I., C. A. Gordon, and W. L. Chandler. 2005. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 3651073-1086. [DOI] [PubMed] [Google Scholar]
- 51.Tesh, V. L., J. A. Burris, J. W. Owens, V. M. Gordon, E. A. Wadolkowski, A. D. O'Brien, and J. E. Samuel. 1993. Comparison of the relative toxicities of Shiga-like toxins type I and type II for mice. Infect. Immun. 613392-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tyler, J. S., M. J. Mills, and D. I. Friedman. 2004. The operator and early promoter region of the Shiga toxin type 2-encoding bacteriophage 933W and control of toxin expression. J. Bacteriol. 1867670-7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wadolkowski, E. A., J. A. Burris, and A. D. O'Brien. 1990. Mouse model for colonization and disease caused by enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 582438-2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wadolkowski, E. A., L. M. Sung, J. A. Burris, J. E. Samuel, and A. D. O'Brien. 1990. Acute renal tubular necrosis and death of mice orally infected with Escherichia coli strains that produce Shiga-like toxin type II. Infect. Immun. 583959-3965. [DOI] [PMC free article] [PubMed] [Google Scholar]