Abstract
High levels of Lactobacillus, the dominant genus of the healthy human vaginal microbiota, have been epidemiologically linked to a reduced risk of infection following exposure to the sexually transmitted pathogen Neisseria gonorrhoeae. In this work, a cell culture model of gonococcal infection was adapted to examine the effects of lactobacilli on gonococcal interactions with endometrial epithelial cells in vitro. Precolonization of epithelial cells with Lactobacillus jensenii, Lactobacillus gasseri ATCC 33323, or L. gasseri ATCC 9857 reduced gonococcal adherence by nearly 50%. Lactobacilli also inhibited gonococcal invasion of epithelial cells by more than 60%, which was independent of the effect on adherence. Furthermore, lactobacilli were able to displace adherent gonococci from epithelial cells, suggesting that these organisms have potential as a postexposure prophylactic. Thus, vaginal lactobacilli have the ability to inhibit gonococci at two key steps of an infection, which might have a significant effect in determining whether the gonococcus will be able to successfully establish an infection following exposure in vivo.
Neisseria gonorrhoeae (gonococcus), the causative agent of gonorrhea, is an obligate human pathogen that infects an estimated 62 million people per year worldwide (www.who.org). Gonorrhea is treatable with antibiotics; however, the disease is still prevalent due in part to the high frequency of asymptomatic and subclinical infections in women, providing a significant reservoir for transmission. Untreated gonococcal infections ascend the reproductive tract in up to 45% of infected women and can lead to disseminated infection, pelvic inflammatory disease, ectopic pregnancy, or sterility. Thus, gonococcal infections place a significant health burden on our society.
In the initial phase of a gonococcal infection in a female host, N. gonorrhoeae adheres to receptors on epithelial cells of the genital tract. Type IV pili are the primary adhesins (28), although several additional surface structures can also mediate adherence, including lipooligosaccharide (8), porin (30), and opacity proteins (Opa and PII) (17, 31). The process of adherence is a crucial step in mounting a successful infection; if the pathogen cannot adhere, it can be swept away from the epithelial cell surface by the flow of vaginal fluid. While the flow of vaginal fluid (on average, 1.55 g/8h) is not as rapid as that of urine, if the pathogen is nonadherent, it will eventually be cleared or killed by the antimicrobial agents present in the vaginal mucus (11, 33).
The indigenous microbiota plays an important role in protecting the host from colonization by incoming pathogens. Lactobacillus is the predominant genus in the vaginal (37) and endocervical microbial communities (33) and is present at concentrations of 107 to 108 CFU/ml of vaginal fluid in healthy postmenarchal/premenopausal women (22). Lactobacillus jensenii and Lactobacillus gasseri are two of the most common species present, as determined by culture-independent techniques (19, 37). Communities dominated by lactobacilli seemed to be more resilient and less susceptible to altered microbial states such as bacterial vaginosis (BV) (37). BV, which is in part defined as a lack of lactobacilli, has been correlated with an increased risk of sexually transmitted infections (STIs) (32). This suggests that women who have large numbers of vaginal lactobacilli are less susceptible to STIs, such as gonorrhea and chlamydia, than women with BV.
While the epidemiological evidence suggests that the presence of lactobacilli is correlated with a reduced susceptibility to gonococcal infections, there has been no direct evidence linking precolonization by lactobacilli and effects on N. gonorrhoeae at any step in the infection process in cell culture or in vivo. Two studies have shown that lactobacilli and their products, such as hydrogen peroxide, can affect the growth of N. gonorrhoeae in vitro (24, 27); however, these experiments were conducted in the absence of host cells, so any inhibitory effects lactobacilli might have on gonococcal interactions with epithelial cells would not have been observed. In vivo, interactions between lactobacilli and gonococci are likely different due to the presence of the host. In this work, we hypothesized that lactobacilli affect gonococcal adherence to and invasion of epithelial cells, and we have adapted a tissue culture model of gonococcal infection to investigate the effect of lactobacilli on these interactions.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
N. gonorrhoeae MS11 (P+ Opa− [26]) was grown at 37°C in a humidified 5% CO2 environment on GC agar (Accumedia, Lansing, MI) with supplements (14) and VCNT inhibitor (vancomycin, colistin methane sulfonate, nystatin, and trimethoprim; Becton Dickinson and Co., Sparks, MD) (GCV). L. jensenii ATCC 25258 (hydrogen peroxide-producing strain[27] of human vaginal origin) and L. gasseri ATCC 33323 (hydrogen peroxide-producing strain [27] of human origin from unknown tissue) and L. gasseri ATCC 9857 (of human vaginal origin) were grown at 37°C in 5% CO2 on MRS agar (Difco, Sparks, MD).
Hydrogen peroxide production.
All Lactobacillus strains were tested for hydrogen peroxide production utilizing the plate assay developed by Eschenbach et al. (9) and modified by Felten et al. (10). Briefly, MRS agar plates were made containing 0.25 mg/ml 3,3′,5,5′-tetramethylbenzidine (TMB; Sigma-Aldrich, St. Louis, MO) and 0.01 mg/ml horseradish peroxidase (HRP; Sigma-Aldrich) (MRS-TMB-HRP). Each strain was streaked for isolated colonies on MRS-TMB-HRP plates and on control MRS plates lacking TMB that contained 0.01 mg/ml HRP and 225 μl of ethanol (diluent for TMB). The plates were incubated for 48 h at 37°C in 5% CO2. Hydrogen peroxide produced by the lactobacilli reacts with HRP to produce O2, which then reacts with TMB to form a dark blue precipitate. Colonies of hydrogen peroxide-producing strains are stained dark blue.
The production of hydrogen peroxide by L. jensenii, L. gasseri ATCC 33323, L. gasseri ATCC 9857, and N. gonorrhoeae individually and in coculture was also measured in cell culture medium using an assay described previously (7, 20, 21) with some modifications. Briefly, lactobacilli and gonococci were swabbed from fresh plate cultures into Dulbecco's modified Eagle medium (DMEM) without phenol red (Invitrogen, Carlsbad, CA) supplemented with 5% fetal calf serum (FCS; Invitrogen), GC supplement II [GC SII; 12.4 μM Fe(NO3)3], and 110 mM sodium pyruvate. Bacterial concentrations were determined spectrophotometerically and diluted to 107 CFU/ml for lactobacilli and 106 CFU/ml for gonococci. Serial dilutions were plated on the appropriate selective medium (lactobacilli, MRS medium; N. gonorrhoeae, GCV medium) to quantify inocula. Bacterial cultures were incubated at 37°C in 5% CO2 for 3 h and were then centrifuged for 10 min at 3,428.5 × g (3,000 rpm) and filtered through a 0.22-μm-pore-size membrane. A total of 0.75 ml of peroxide assay buffer (5.0 mM K2HPO4, 1.0 mM KH2PO4, 140 mM NaCl, 0.5 mM glucose, with phenol red [final concentration, 0.46 mM]and HRP [final concentration, 0.046 U/m] added immediately prior to the assay) was placed into a borosilicate glass tube to which 0.25 ml of the sample supernatants was added. The samples were incubated for 30 min at 37°C, and then NaOH (final concentration, 0.004 N) was added to stop the reaction. The absorbance at 610 nm was determined spectrophotometrically, and the amount of hydrogen peroxide produced under each condition was determined by comparison to an H2O2 standard curve generated in the same assay.
Cell culture.
The human endometrial epithelial cell line Hec-1-B (ATCC HTB-113) was grown in DMEM (Invitrogen) supplemented with 5% FCS at 37°C in 5% CO2. Cell culture assays were carried out in 24-well cell culture plates with Hec-1-B cells grown to 50 to 90% confluence. Adherence and invasion assays were as described previously (2, 6) with modifications as follows.
Adherence assay 1.
Lactobacilli were swabbed from fresh plate cultures into DMEM (without phenol red) supplemented with 5% FCS, GC SII, and 110 mM sodium pyruvate (DMEM-5% FCS-GC SII medium). Bacterial concentrations were determined spectrophotometerically, and diluted in DMEM-5% FCS-GC SII medium to a concentration of 4 × 107 CFU/ml. Serial dilutions of this inoculum were plated on MRS agar. Hec-1-B cells were precolonized with 250 μl of the lactobacilli inoculum for a multiplicity of infection (MOI) of 100, and the infected epithelial cells were incubated at 37°C in 5% CO2 for 3 h. The epithelial cells were then washed 5 times with phosphate buffered saline (PBS) to remove nonadherent lactobacilli. A mock infection was conducted as a control in which the epithelial cells were incubated with 250 μl of DMEM-5% FCS-GC SII medium for 3 h and then washed five times with phosphate-buffered saline (PBS) prior to the introduction of gonococci. Gonococci were swabbed from fresh plate cultures into DMEM-5% FCS-GC SII medium. Bacterial concentrations were determined spectrophotometerically and diluted in this medium to a concentration of 4 × 106 CFU/ml. Serial dilutions of this inoculum were plated on GCV agar. Hec-1-B cells precolonized with adherent lactobacilli, along with the control, were then infected with 250 μl of the N. gonorrhoeae inoculum (MOI of 10), and the epithelial cells were incubated at 37°C in 5% CO2 for 3 h. Following incubation, the medium was removed, and the infected epithelial cells were washed five times with sterile PBS to remove nonadherent bacteria. The epithelial cells and cell-associated bacteria were lifted with 1 ml of PBS containing 5 mM EDTA. Serial dilutions were plated on GCV agar to determine the number of cell-associated CFU. The number of CFU of gonococci adherent to epithelial cells precolonized with lactobacilli was then normalized to the number of cell-associated CFU from the control, which lacked lactobacilli.
Adherence assay 2.
L. jensenii was swabbed from fresh culture plates into DMEM-5% FCS-GC SII medium. Bacterial concentrations were determined and diluted in this medium to 4 × 106 CFU/ml and 4 × 107 CFU/ml. Serial dilutions of each inoculum were plated on MRS agar. One row of six wells containing 105 Hec-1-B cells was precolonized with 250 μl of a lactobacillus inoculum of 4 × 106 CFU/ml (MOI of 10), and a second row of wells containing 105 Hec-1-B cells was precolonized with 250 μl of a lactobacillus inoculum of 4 × 107 CFU/ml (MOI of 100). A third row was used as a mock infection in which the medium on the epithelial cells was replaced with 250 μl of fresh DMEM-5% FCS-GC SII medium. The infected epithelial cells were then incubated for 1 h at 37°C in 5% CO2. Gonococci were swabbed from fresh plate cultures into DMEM-5% FCS-GC supp II medium. Bacterial concentrations were determined spectrophotometerically and diluted to 4 × 106 CFU/ml. Serial dilutions of this inoculum were plated on GCV agar. Hec-1-B cells precolonized with adherent and nonadherent lactobacilli, along with the control containing no lactobacilli, were then infected with 250 μl of the N. gonorrhoeae inoculum (MOI of 10), and the infected epithelial cells were incubated at 37°C in 5% CO2 for 3 h. Following incubation, the infected Hec-1-B cells were split into two sets; one set was to calculate the total number of CFU of bacteria, and the other set was to calculate the cell-associated (adherent) number of CFU. For the first set, the supernatant (0.5 ml) from each well was placed in a sterile tube. The Hec-1-B cells and cell-associated bacteria were then lifted with 500 μl of PBS-EDTA and added to the sterile tube containing the unattached bacteria. Serial dilutions were then plated on the appropriate selective medium to determine the total number of CFU/well. For the second set, the supernatant was removed, and the epithelial cells were washed five times with sterile PBS. The epithelial cells and cell-associated bacteria were then lifted with 1 ml of PBS-EDTA and placed in a sterile tube. Serial dilutions were plated on selective medium to obtain the number of cell-associated (adherent) CFU/well. The adherence frequency was calculated by dividing the number of cell-associated CFU/well by the total number of CFU/well. The adherence frequencies were then normalized to the control, which contained no lactobacilli.
Invasion assays.
Precolonization of the Hec-1-B cells with L. jensenii was conducted as described above for adherence assay 2, with the lactobacillus inoculum at a concentration of 4 × 107 CFU/ml. After incubation for 1 h, 250 μl of N. gonorrhoeae (4 × 106 CFU/ml) was added to the Hec-1-B cells precolonized with lactobacilli and incubated for 7 h. Following incubation, wells were divided into three sets. For the first set (total CFU), the supernatant (0.5 ml) was removed and placed in a sterile tube. The Hec-1-B cells and cell-associated bacteria were lifted with 500 μl of PBS-EDTA, and this suspension was added to the tube of supernatant. Serial dilutions were plated on selective medium to determine the total number of CFU/well. For the second set (cell-associated CFU), the epithelial cells were washed five times with PBS to remove nonadherent bacteria, and the Hec-1-B cells with adherent bacteria were then lifted with 1 ml of PBS-EDTA. Serial dilutions were plated on selective medium to determine the number of cell-associated CFU/well. The third set of wells (intracellular CFU) was washed five times with PBS to remove nonadherent bacteria, treated with 75 mg/liter gentamicin (Gm) in DMEM, and then incubated at 37°C in 5% CO2 for 1 h to kill extracellular bacteria. The Hec-1-B cells (with intracellular bacteria) were then washed five times with PBS and lifted with 1 ml of PBS-EDTA. Serial dilutions were plated on selective medium to determine the number of Gmr CFU/well. The invasion frequency is defined as the ratio of the number of Gmr CFU/well to the number of cell-associated CFU/well.
Microscopy.
Hec-1-B cells were seeded into six-well cell culture plates containing sterilized glass coverslips (25 mm; Fisher Scientific). Once the epithelial cells reached confluence, an adherence assay as described above for adherence assay 2 was carried out with some modifications. Briefly, at 3 h postinfection (p.i.), nonadherent bacteria in the supernatant were removed and placed on glass microscope slides and Gram stained. The bacteria were then visualized by light microscopy (magnification, ×1,000) and photographed using a digital still camera (Cyber-shot DSC-P50; Sony). The epithelial cells with adherent bacteria were washed five times with PBS and then fixed with 100% methanol for 5 min. The coverslips were then removed from the well, placed on microscope slides, and Gram stained. The bacteria adherent to the Hec-1-B cells were visualized by light microscopy (magnification, ×1,000) and photographed using a digital still camera. The number of adherent lactobacilli was determined for 30 consecutive Hec-1-B cells in each replicate. Three replicate experiments, carried out in duplicate, were conducted, and the adherent bacterial counts were then averaged.
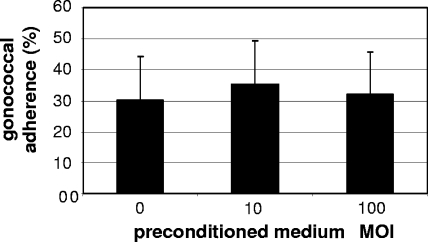
Preconditioned medium.
Hec-1-B cells were infected with L. jensenii at an MOI of 10 or 100 and incubated for 1 h at 37°C in 5% CO2. The supernatant from the infected epithelial cells was removed, centrifuged at 16,000 × g (14,000 rpm) for 30 s to pellet the bacteria, and then filtered through a 0.22-μm-pore-size membrane. These preconditioned media were then inoculated with 106 CFU of gonococci and used to infect fresh Hec-1-B cells (MOI of 10). The infected Hec-1-B cells were incubated at 37°C in 5% CO2, and at 3 h p.i. the epithelial cells with adherent bacteria were washed, lifted, and plated as described above for adherence assays.
Statistical analysis.
All data were analyzed by an unpaired Student t test. P values of less than 0.05 were considered statistically significant and are indicated by an asterisk in each of the graphs.
RESULTS
Conditions for N. gonorrhoeae and Lactobacillus coculture.
L. jensenii and L. gasseri are two of the most prevalent Lactobacillus species recovered from the vaginal tract of healthy women (1, 4, 5, 36) and were therefore chosen for this study. Previous research showed that L. jensenii ATCC 25258 and L. gasseri ATCC 33323 had the potential to kill or inhibit the growth of N. gonorrhoeae in vitro (27). Thus, it was necessary to identify conditions under which both species could grow in order to study the effects on gonococcal interactions with epithelial cells. Each bacterial strain was grown separately in DMEM-5% FCS-GC SII medium, and dilutions were plated at intervals to determine the number of viable CFU/ml. Next, N. gonorrhoeae (starting at 106 CFU/ml) was cocultured with L. jensenii, L. gasseri ATCC 33323, or L. gasseri ATCC 9857 at a ratio of 1:10 (gonococci to lactobacilli) and plated for viable CFU at intervals to determine the effect of coculture on growth of each bacterial strain. As shown in Fig. 1, the growth of N. gonorrhoeae in the presence of L. jensenii was similar to that observed when the strains were grown separately. Similar results were observed when N. gonorrhoeae was grown in the presence of L. gasseri (data not shown). Each Lactobacillus strain also survived similarly in the presence and absence of gonococci (data not shown). These results establish that gonococci and lactobacilli survive and grow in coculture under these conditions.
FIG. 1.
Growth of N. gonorrhoeae in the presence or absence of L. jensenii. N. gonorrhoeae (106 CFU/ml inoculum) was grown in the presence (□) or absence (♦) of L. jensenii (107 CFU/ml inoculum). Serial dilutions were plated on selective medium at 2-h intervals to quantify CFU. Data are the averages of three independent experiments. Error bars indicate standard deviations.
Hydrogen peroxide production in cell culture medium.
Two of the strains used in this work, L. jensenii ATCC 25258 and L. gasseri ATCC 33323, were previously reported to kill N. gonorrhoeae in an agar overlay assay due to the production of hydrogen peroxide (13, 27). Under the conditions of our assays, the lactobacilli did not inhibit the growth of gonococci (Fig. 1). Therefore, the Lactobacillus strains used in our study were tested for hydrogen peroxide production by an MRS-TMB plate assay (9, 10). Using this method, in which hydrogen peroxide production is visualized by the production of a dark blue precipitate, L. jensenii and L. gasseri ATCC 33323 were confirmed to be hydrogen peroxide producers (solid dark blue), while L. gasseri ATCC 9857 produced modest levels of hydrogen peroxide (blue after a prolonged incubation). Next, hydrogen peroxide production by lactobacilli grown under our cell culture conditions in both the presence and absence of gonococci was examined. When incubated for 3 h in DMEM-5% FCS-GC SII medium, L. jensenii, L. gasseri ATCC 33323, L. gasseri ATCC 9857, and N. gonorrhoeae all produced undetectable levels of hydrogen peroxide (less than 0.1 mM). When incubated in coculture with N. gonorrhoeae in DMEM-5% FCS-GC SII medium for 3 h, L. jensenii, L. gasseri ATCC 33323, and L. gasseri ATCC 9857 again produced undetectable levels of hydrogen peroxide. The lack of detectable levels of hydrogen peroxide is consistent with our observations that, when cocultured in DMEM-5% FCS-GC SII medium, lactobacilli do not kill gonococci and with the findings of other researchers that at neutral pH, hydrogen peroxide-producing lactobacilli do not inhibit the growth of gonococci (35).
Adherence of N. gonorrhoeae to epithelial cells precolonized with lactobacilli.
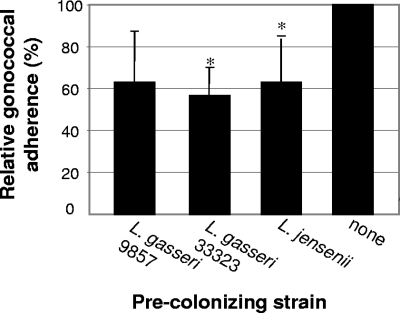
Epidemiological evidence suggests that there is a reduced risk of developing an infection following exposure to an STI, such as gonorrhea, when lactobacilli are present in large numbers (32). Therefore, the effect of precolonization of epithelial cells with lactobacilli on the adherence of N. gonorrhoeae was examined. Hec-1-B cells, a human endometrial epithelial cell line, were infected with lactobacilli at an MOI of 100 or cultured in DMEM-5% FCS-GC SII medium (control). At 3 h p.i., nonadherent lactobacilli were removed by washing. Epithelial cells precolonized with lactobacilli and the control epithelial cells were then infected with gonococci at an MOI of 10. At 3 h p.i., the Hec-1-B cells were again washed to remove nonadherent bacteria, lifted, and plated on selective medium. The adherence of each Lactobacillus strain to the epithelial cells after exposure to N. gonorrhoeae was as follows (as percentages of the inoculum): L. jensenii, 0.73%; L. gasseri ATCC 33323, to 0.61%; and L. gasseri ATCC 9857, to 0.93%. The average number of CFU/well of N. gonorrhoeae adherent to Hec-1-B cells precolonized with each Lactobacillus strain was compared to the average number of CFU/well of gonococci adherent in the control, and these results are shown in Fig. 2. When Hec-1-B cells were precolonized with L. jensenii, gonococcal adherence dropped to 63.0% ± 21.9% (P = 0.043) of that determined for the control. Precolonization with L. gasseri ATCC 33323 reduced gonococcal adherence to 56. 6% ± 13.8% (P = 0.003), and precolonization with L. gasseri ATCC 9857 reduced gonococcal adherence to 63.0% ± 24.6% (P = 0.059). Thus, in the presence of two of the adherent strains of Lactobacillus tested, the adherence of N. gonorrhoeae was significantly decreased compared to the control.
FIG. 2.
Adherence of N. gonorrhoeae to Hec-1-B cells precolonized with lactobacilli. Epithelial cells were incubated with lactobacilli for 3 h. Nonadherent lactobacilli were removed prior to the addition of gonococci, and the Hec-1-B cells were incubated for an additional 3 h. Relative adherence is the percentage of adherent gonococci in wells containing lactobacilli divided by the percentage of adherent gonococci in the control. The data are averages of three independent experiments performed in duplicate. Error bars indicate standard deviations. *, statistically significant.
Since the human vaginal tract contains lactobacilli in the fluid and mucosal layer that are nonadherent to the epithelia and because adherence is a dynamic process with bacteria attaching and then detaching from the host cells (29), we next developed an approach to examine the adherence of N. gonorrhoeae in a system more closely mimicking this natural state. In the experiments described above, nonadherent lactobacilli were washed off prior to the addition of gonococci, leaving fewer than 105 CFU of lactobacilli adherent to epithelial cells in the well. It has been reported that there are 107 to 108 Lactobacillus CFU/ml of vaginal fluid in healthy premenopausal women (22). Therefore, gonococcal adherence to Hec-1-B cells precolonized with lactobacilli was next examined without removing the nonadherent lactobacilli.
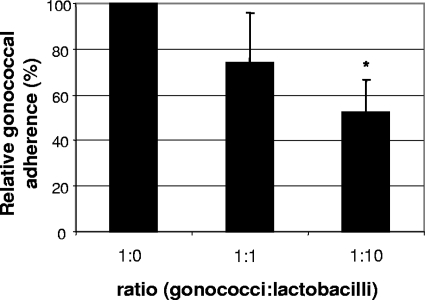
Hec-1-B cells were inoculated with L. jensenii at an MOI of 10 or 100 and incubated for 1 h. Hec-1-B cells were incubated with sterile DMEM-5% FCS-GC SII medium for 1 h as a control. Gonococci were then added at an MOI of 10, and the infected epithelial cells were incubated for an additional 3 h. The relative gonococcal adherence frequencies are shown graphically in Fig. 3. At a 1:1 ratio of N. gonorrhoeae to L. jensenii, gonococcal adherence was reduced to 73.9% ± 22.1% (P = 0.11) of the no-Lactobacillus control, whereas at the ratio 1:10 N. gonorrhoeae to L. jensenii the adherence of N. gonorrhoeae was reduced to 52.5% ± 14.2% (P = 0.004). Thus, inhibition of gonococcal adherence increased with the number of lactobacilli present.
FIG. 3.
Adherence of N. gonorrhoeae to Hec-1-B cells in the presence of adherent and nonadherent lactobacilli. Relative adherence is the percentage of adherent gonococci in wells containing lactobacilli divided by the percentage of adherent gonococci in the control. The data are the averages of three independent experiments performed in triplicate. Ratios of gonococci to lactobacilli were 1:0, 106 gonococci to no lactobacilli; 1:1, 106 gonococci to 106 lactobacilli; 1:10, 106 gonococci to 107 lactobacilli. At 3 h p.i. the number of CFU of N. gonorrhoeae doubled, and the total number of CFU of gonococci in the control was not significantly different from that of the Lactobacillus-treated samples (P value of 0.324 for 1:1 ratio and of 0.536 for 1:10 ratio). Error bars indicate standard deviations. Student's t test P values were 0.11 for 1:1 and 0.004 for 1:10. *, statistically significant.
The number of adherent CFU per well of L. jensenii increased proportionally with the number of lactobacilli in the inoculum. At an MOI of 10, 4 × 104 CFU/well L. jensenii were adherent to the epithelial cells (an adherence frequency of ∼1% cell-associated lactobacilli/total lactobacilli), while at an MOI of 100, 3 × 105 CFU/well L. jensenii were adherent (an adherence frequency of ∼1%). When the first adherence assay (Fig. 2) and the second adherence assay (Fig. 3) are compared, the CFU of adherent lactobacilli per well inversely correlates with the gonococcal adherence frequency, suggesting that as the number of adherent lactobacilli increases, the number of adherent gonococci decreases correspondingly.
Gonococcal invasion of epithelial cells precolonized with lactobacilli.
After adherence has been attained in a gonococcal infection, a subpopulation of these bacteria invades the epithelial cells to transcytose to the subepithelial space. To determine whether lactobacilli could affect gonococcal invasion, a Gm protection assay was used in which Hec-1-B cells were first precolonized for 1 h with L. jensenii at an MOI of 100 prior to the addition of gonococci (MOI of 10). As a control, Hec-1-B cells were incubated with fresh DMEM-5% FCS-GC SII medium for 1 h prior to inoculation with gonococci. The frequency of invasion was determined as number of Gmr CFU/well per cell-associated CFU/well at 7 h p.i., thereby segregating the frequency of adherence from the frequency of invasion. To check for Gmr gonococci that are not intracellular, 4 × 106 CFU/ml gonococci were incubated for 1 h with Gm at the same concentration used in the invasion assay and then plated in dilutions from 101 to 105 for viable cell counts. There was less than 1 Gmr gonococcus in 4 × 106 CFU/ml, verifying that the gonococcal strain used in these experiments was not Gmr. The results of the invasion experiments show that gonococcal invasion was reduced to 39.46% ± 10.47% (P = 0.001) in the presence of L. jensenii compared to invasion in its absence. Interestingly, at 7 h after (gonococcal) infection when invasion was measured, the adherence frequency for the gonococci in coculture with L. jensenii was not significantly different from that of the control (data not shown). Thus, although gonococci eventually recover from the initial inhibition of adherence, these results indicate that lactobacilli affect both steps of gonococcal interactions with Hec-1-B cells.
Displacement of adherent gonococci by lactobacilli.
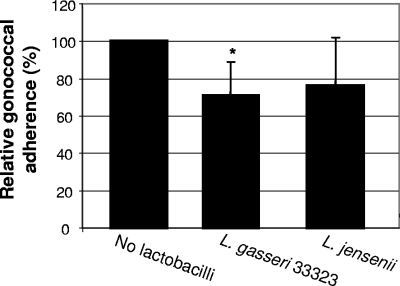
To determine whether lactobacilli had the potential to affect gonococcal interactions with host epithelial cells postexposure, we assessed the ability of lactobacilli to displace gonococci already adherent to epithelial cells. Hec-1-B cells were infected with N. gonorrhoeae at an MOI of 10, as for an adherence assay. At 3 h p.i., nonadherent gonococci were removed, and DMEM containing L. jensenii, L. gasseri ATCC 33323 (MOI of 100), or no bacteria (control) was added. Infected epithelial cells were incubated for an additional hour. Nonadherent bacteria were then removed, and Hec-1-B cells with adherent bacteria were lifted and plated on selective medium. After addition of L. gasseri ATCC 33323, the number of adherent gonococci was reduced by 28.7% (P = 0.019) compared to the control (Fig. 4). Displacement by L. jensenii was less consistent (P = 0.174), although treatment with either Lactobacillus species tended to reduce the number of adherent gonococci.
FIG. 4.
Displacement of N. gonorrhoeae adherent to Hec-1-B cells by lactobacilli. N. gonorrhoeae adherent to Hec-1-B cells after 3 h were then infected with L. gasseri ATCC 33323 or L. jensenii at an MOI of 100 for 1 h. Adherence data are presented as the percentages of adhered gonococci in the well divided by that of the control and are the averages of four or more experiments performed in triplicate. Error bars indicate standard deviations. Student's t test P values were 0.019 (L. gasseri ATCC 33323) and 0.174 (L. jensenii). *, statistically significant.
Spatial arrangement of lactobacilli and gonococci adherent to epithelial cells.
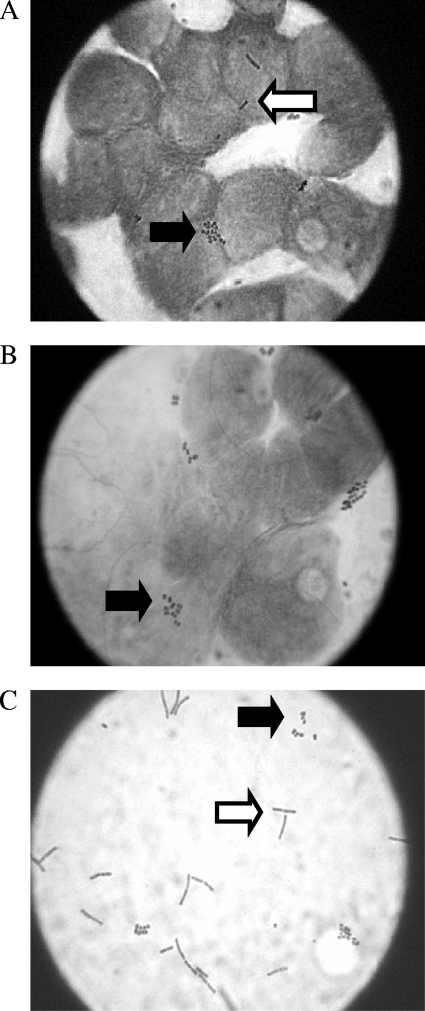
To visualize the spatial arrangement of gonococci and lactobacilli on the epithelial cell monolayer during a coinfection, adherence assays were conducted in which the epithelial cells were adherent to a glass coverslip, allowing for microscopic analysis. Adherent lactobacilli were observed either individually or in short chains (one to three bacilli) at an adherence frequency of 0.72 lactobacilli per epithelial cell, consistent with the adherence frequencies found using the plate count method. Gonococci were predominantly in microcolonies when adherent to epithelial cells in the presence or absence of lactobacilli; thus, counting of individual gonococci adherent to Hec-1-B cells was not possible. In all fields observed, lactobacilli and gonococci were found randomly dispersed on the epithelial cells and generally not in contact with each other (Fig. 5A and B).
FIG. 5.
Gram stains from an adherence assay performed as for the experiment shown in Fig. 2 with Hec-1-B cells precolonized with 107 CFU of L. jensenii. (A) Gonococci and lactobacilli adherent to epithelial cells. (B) Gonococci adherent to epithelial cells in the absence of lactobacilli. (C) Arrangement of gonococci and lactobacilli in the supernatant. In all panels the white arrow points to lactobacilli, and the black arrow points to gonococci (Olympus model CHBS microscope; ×1,000 magnification).
Lactobacilli and gonococci do not coaggregate in coculture.
Previous reports have suggested that lactobacilli produce aggregation factors that could allow lactobacilli to aggregate with other bacterial species, thus sequestering pathogens and decreasing adherence (3, 15). To determine if coaggregation of lactobacilli with gonococci could be the mechanism of inhibition observed in the adherence assays, the supernatants of three separate adherence assays (performed in duplicate) were Gram stained and visualized by light microscopy. In all fields observed, lactobacilli and gonococci never formed coaggregates; a representative field of such an experiment is shown in Fig. 5C.
Lactobacillus-preconditioned medium does not inhibit gonococcal adherence.
Recently, Lactobacillus acidophilus was found to secrete a soluble compound that can reduce expression of virulence factors in Escherichia coli O157:H7 necessary for colonization of intestinal epithelial cells (18). To examine the possibility that lactobacilli secrete a soluble compound that inhibits gonococcal adherence to epithelial cells, gonococcal adherence assays were performed using Lactobacillus-preconditioned medium. L. jensenii at an MOI of 10 and an MOI of 100 was incubated in cell culture medium with Hec-1-B cells for 1 h and then removed by centrifugation and filtration. These media were then inoculated with gonococci at an MOI of 10 for an adherence assay using fresh Hec-1-B cells and compared to a control of gonococci inoculated in fresh medium. The results of these experiments show that the gonococcal adherence frequency was not statistically different from that of the control in either preconditioned medium at an MOI of 10 (P = 0.631) or preconditioned medium at an MOI of 100 (P = 0.884) (Fig. 6).
FIG. 6.
Adherence of gonococci in medium preconditioned with L. jensenii and Hec-1-B cells. Percent adherence is the ratio of adherent gonococci to the total number of gonococci for each condition. Number of lactobacilli used to condition the medium: fresh medium; no lactobacilli and fresh DMEM-5% FCS-GC SII; preconditioned medium at an MOI of 10, 106 CFU lactobacilli to 105 Hec-1-B cells; preconditioned medium at an MOI of 100, 107 CFU lactobacilli to 105 Hec-1-B cells. Data are the averages of four independent experiments performed in triplicate. Error bars indicate the standard deviations. P values are 0.631 (MOI of 10) and 0.884 (MOI of 100).
DISCUSSION
To successfully infect the female genital tract, gonococci must first adhere to the epithelia to avoid clearance by the continual flow of vaginal fluid. A reduction in the ability to adhere would significantly affect the outcome of an infection following exposure. Although lactobacilli have been implicated in protecting the female vaginal tract from exogenous pathogens, research on the direct effect of lactobacilli on STIs has been lacking. Therefore, we developed a coculture model of gonococcal infection that includes lactobacilli to examine their effect on gonococcal interactions with epithelial cells.
In our in vitro model of gonococcal infection, precolonization with lactobacilli reduced gonococcal adherence to epithelial cells by 40 to 50% (Fig. 2 and 3). This inhibition of adherence was dependent on the ratio of gonococci to adherent lactobacilli, with the inhibition becoming more pronounced in the presence of increased numbers of lactobacilli (Fig. 3). In a gonococcal infection, following adherence to epithelial cells, gonococci will invade these epithelial cells and transcytose to the subepithelial space. Our results show that gonococcal invasion of epithelial cells precolonized with L. jensenii was decreased by 60%. This inhibition of invasion was distinct from the inhibition of adherence, demonstrating that even the gonococci that overcome the inhibition of adherence are negatively affected in invasiveness by the presence of lactobacilli. The inhibition of adherence, along with the inhibition of invasion, suggests that colonization with lactobacilli could reduce the risk for infection by N. gonorrhoeae by potentially decreasing the amount of gonococci that adhere and invade the mucosal epithelia, thus providing a greater opportunity for the innate host defenses to prevent the establishment of an infection.
Due to the nature of N. gonorrhoeae as an obligate human pathogen, testing the biological relevance of the 50% reduction in gonococcal adherence caused by lactobacilli cannot be done in vivo. However, semen samples from males infected with gonorrhea on average contain 7 × 106 CFU per ejaculate (12), similar to the gonococcal inoculum used in these studies (6 × 106 CFU/well). Cervical aspirates of females with gonorrhea have been reported to contain 104 to 106 CFU/ml (16, 34). In our studies the average number of CFU of gonococci adherent to epithelial cells in the controls falls within this range (1 × 106 CFU/well), again lending support to the relevance of our experimental conditions. Thus, the inhibition of gonococcal adherence to and invasion of epithelial cells precolonized with lactobacilli may explain the epidemiological observations in which women with bacterial vaginosis (reduced numbers of lactobacilli) have an increased susceptibility for gonorrhea following exposure (32).
Lactobacilli are now being promoted as probiotic bacteria that have broad health benefits. Lactobacilli have the ability to displace adherent pathogens associated with urinary tract infections (23), as well as Gardnerella vaginalis biofilms associated with BV (25), suggesting potential as a treatment for these diseases. While it is unlikely that lactobacilli alone could be used as a treatment for gonorrhea, it is conceivable that lactobacilli could reduce the likelihood that gonococci would establish an infection when lactobacilli are directly administered to the vaginal tract shortly after exposure. Reid et al. (23) suggests a displacement of 20 to 50% would be clinically relevant for pathogens infecting the vaginal tract. Our results indicate that gonococcal displacement by L. gasseri ATCC 33323 is within this range at 28.7% (Fig. 5). Thus, the ability of lactobacilli to displace adherent gonococci in a cell culture model of infection suggests that lactobacilli have potential as a postexposure prophylactic for gonococcal infection.
In this study, L. jensenii and L. gasseri strains were chosen because these species are two of the most prevalent species recovered from healthy women and because they had been previously studied for their effects on N. gonorrhoeae in the absence of epithelial cells (1, 4, 5, 13, 27, 36). Each strain inhibited the adherence of N. gonorrhoeae to epithelial cells but adhered poorly to epithelial cells (average adherence frequency of ∼1%). When epithelial cells are precolonized with lactobacilli at an MOI of 100, there is on average 1 adherent Lactobacillus per epithelial cell. Thus, it is unlikely that the mechanism for exclusion of gonococci is direct competition for receptors on the epithelial cells. However, the number of adherent lactobacilli is inversely correlated with the gonococcal adherence frequency: as the number of adherent lactobacilli increases, the gonococcal adherence frequency decreases. This suggests that it is the adherent lactobacilli that play an important role in the observed inhibition of gonococcal adherence. Our results also show that the mechanism of inhibition of adherence is not due to coaggregation of lactobacilli with gonococci (Fig. 5C), growth inhibition (Fig. 1), or the secretion of a stable soluble compound into the medium by either the epithelial cells or lactobacilli before inoculation with gonococci (Fig. 6). Further study will be necessary to identify the mechanisms of inhibition, which will be fundamental in the development of Lactobacillus-based treatments or preventatives for gonococcal infection.
In summary, we have adapted a tissue culture model of gonococcal infection to include a major constituent of the human vaginal tract, lactobacilli. This approach will be essential in elucidating the role of the indigenous microbiota in the early, and therefore preventable, steps of a gonococcal infection. The observation that lactobacilli inhibit gonococcal adherence and invasion and could even displace adherent gonococci is intriguing; however, the mechanisms by which these events occur are not yet clear. While coaggregation, the secretion of soluble inhibitory molecules, and growth inhibition can be ruled out as possible mechanisms, there are still several hypotheses to test. Possible mechanisms might include a contact-inducible change in the epithelial cells due to the adherence of lactobacilli that would reduce susceptibility to gonococci or the secretion of a short-lived inhibitory factor produced by lactobacilli directly in response to incoming gonococci. This model will be indispensable in future studies to examine the molecular basis for the observed inhibition of gonococcal interactions with epithelial cells.
Acknowledgments
We thank Robert Britton and his lab for assistance, advice in working with lactobacilli, and critical reading of the manuscript. We also thank Martha Mulks, Todd Ciche, and Hilary Phelps for critical reading of the manuscript and Kathleen Musick for technical assistance with cell culture assays.
This work was supported financially by National Institute of Health grant AI064292 (to C.G.A.), the Mary F. Dye Fellowship (to R.R.S.), and the Michigan State University Center for Microbial Pathogenesis.
Editor: A. Camilli
Footnotes
Published ahead of print on 14 April 2008.
REFERENCES
- 1.Antonio, M. A., S. E. Hawes, and S. L. Hillier. 1999. The identification of vaginal Lactobacillus species and the demographic and microbiologic characteristics of women colonized by these species. J. Infect. Dis. 1801950-1956. [DOI] [PubMed] [Google Scholar]
- 2.Arvidson, C. G., R. Kirkpatrick, M. T. Witkamp, J. A. Larson, C. A. Schipper, L. S. Waldbeser, P. O'Gaora, M. Cooper, and M. So. 1999. Neisseria gonorrhoeae mutants altered in toxicity to human fallopian tubes and molecular characterization of the genetic locus involved. Infect. Immun. 67643-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boris, S., J. E. Suarez, and C. Barbes. 1997. Characterization of the aggregation promoting factor from Lactobacillus gasseri, a vaginal isolate. J. Appl. Microbiol. 83413-420. [DOI] [PubMed] [Google Scholar]
- 4.Boyd, M. A., M. A. Antonio, and S. L. Hillier. 2005. Comparison of API 50 CH strips to whole-chromosomal DNA probes for identification of Lactobacillus species. J. Clin. Microbiol. 435309-5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burton, J. P., P. A. Cadieux, and G. Reid. 2003. Improved understanding of the bacterial vaginal microbiota of women before and after probiotic instillation. Appl. Environ. Microbiol. 6997-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du, Y., J. Lenz, and C. G. Arvidson. 2005. Global gene expression and the role of sigma factors in Neisseria gonorrhoeae in interactions with epithelial cells. Infect. Immun. 734834-4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duane, P. G., J. B. Rubins, H. R. Weisel, and E. N. Janoff. 1993. Identification of hydrogen peroxide as a Streptococcus pneumoniae toxin for rat alveolar epithelial cells. Infect. Immun. 614392-4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards, J. L., and M. A. Apicella. 2002. The role of lipooligosaccharide in Neisseria gonorrhoeae pathogenesis of cervical epithelia: lipid A serves as a C3 acceptor molecule. Cell. Microbiol. 4585-598. [DOI] [PubMed] [Google Scholar]
- 9.Eschenbach, D. A., P. R. Davick, B. L. Williams, S. J. Klebanoff, K. Young-Smith, C. M. Critchlow, and K. K. Holmes. 1989. Influence of the normal menstrual cycle on vaginal tissue, discharge, and microflora. J. Clin. Microbiol. 27251-256.2915019 [Google Scholar]
- 10.Felten, A., C. Barreau, C. Bizet, P. H. Lagrange, and A. Phillipon. 1999. Lactobacillus species identification, H2O2 production, and antibiotic resistance and correlation with clinical status. J. Clin. Microbiol. 37729-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Godley, M. J. 1985. Quantitation of vaginal discharge in healthy volunteers. Br. J. Obstet. Gynaecol. 92739-742. [DOI] [PubMed] [Google Scholar]
- 12.Isbey, S. F., T. M. Alcorn, R. H. Davis, J. Haizlip, P. A. Leone, and M. S. Cohen. 1997. Characterisation of Neisseria gonorrhoeae in semen during urethral infection in men. Genitourin. Med. 73378-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jerse, A. E., E. T. Crow, A. N. Bordner, I. Rahman, C. N. Cornelissen, T. R. Moench, and K. Mehrazar. 2002. Growth of Neisseria gonorrhoeae in the female mouse genital tract does not require the gonococcal transferrin or hemoglobin receptors and may be enhanced by commensal lactobacilli. Infect. Immun. 702549-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kellogg, D. S., Jr., W. L. Peacock, Jr., W. E. Deacon, L. Brown, and D. I. Pirkle. 1963. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J. Bacteriol. 851274-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kmet, V., and F. Lucchini. 1997. Aggregation-promoting factor in human vaginal Lactobacillus strains. FEMS Immun. Med. Microbiol. 19111-114. [DOI] [PubMed] [Google Scholar]
- 16.Lowe, T. L., and S. J. Kraus. 1976. Quantitation of Neisseria gonorrhoeae from women with gonorrhea. J. Infect. Dis. 133621-626. [DOI] [PubMed] [Google Scholar]
- 17.Makino, S., J. P. van Putten, and T. F. Meyer. 1991. Phase variation of the opacity outer membrane protein controls invasion by Neisseria gonorrhoeae into human epithelial cells. EMBO J. 101307-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medellin-Pena, M. J., H. Wang, R. Johnson, S. Anand, and M. W. Griffiths. 2007. Probiotics affect virulence-related gene expression in Escherichia coli O157:H7. Appl. Environ. Microbiol. 734259-4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pavlova, S. I., A. O. Kilic, S. S. Kilic, J. S. So, M. E. Nader-Macias, J. A. Simoes, and L. Tao. 2002. Genetic diversity of vaginal lactobacilli from women in different countries based on 16S rRNA gene sequences. J. Appl. Microbiol. 92451-459. [DOI] [PubMed] [Google Scholar]
- 20.Pericone, C. D., K. Overweg, P. W. M. Hermans, and J. N. Weiser. 2000. Inhibitory and bactericidal effects of hydrogen peroxide production by Streptococcus pneumoniae on other inhabitants of the upper respiratory tract. Infect. Immun. 683990-3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pick, E., and Y. Keisari. 1980. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J. Immunol. Methods 38161-170. [DOI] [PubMed] [Google Scholar]
- 22.Redondo-Lopez, V., R. L. Cook, and J. D. Sobel. 1990. Emerging role of lactobacilli in the control and maintenance of the vaginal bacterial microflora. Rev. Infect. Dis. 12856-872. [DOI] [PubMed] [Google Scholar]
- 23.Reid, G., C. Heinemann, M. Velraeds, H. C. van der Mei, and H. J. Busscher. 1999. Biosurfactants produced by Lactobacillus. Methods Enzymol. 310426-433. [DOI] [PubMed] [Google Scholar]
- 24.Saigh, J. H., C. C. Sanders, and W. E. Sanders, Jr. 1978. Inhibition of Neisseria gonorrhoeae by aerobic and facultatively anaerobic components of the endocervical flora: evidence for a protective effect against infection. Infect. Immun. 19704-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saunders, S., A. Bocking, J. Challis, and G. Reid. 2007. Effect of Lactobacillus challenge on Gardnerella vaginalis biofilms. Colloids Surf. B 55138-142. [DOI] [PubMed] [Google Scholar]
- 26.Segal, E., E. Billyard, M. So, S. Storzbach, and T. F. Meyer. 1985. Role of chromosomal rearrangement in N. gonorrhoeae pilus phase variation. Cell 40293-300. [DOI] [PubMed] [Google Scholar]
- 27.St. Amant, D. C., I. E. Valentin-Bon, and A. E. Jerse. 2002. Inhibition of Neisseria gonorrhoeae by Lactobacillus species that are commonly isolated from the female genital tract. Infect. Immun. 707169-7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swanson, J. 1973. Studies on gonococcus infection. IV. Pili: their role in attachment of gonococci to tissue culture cells. J. Exp. Med. 137571-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swidsinski, A., W. Mendling, V. Loening-Baucke, A. Ladhoff, S. Swidsinski, L. P. Hale, and H. Lochs. 2005. Adherent biofilms in bacterial vaginosis. Obstet. Gynecol. 1061013-1023. [DOI] [PubMed] [Google Scholar]
- 30.van Putten, J. P., T. D. Duensing, and J. Carlson. 1998. Gonococcal invasion of epithelial cells driven by P.IA, a bacterial ion channel with GTP binding properties. J. Exp. Med. 188941-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waldbeser, L. S., R. S. Ajioka, A. J. Merz, D. Puaoi, L. Lin, M. Thomas, and M. So. 1994. The opaH locus of Neisseria gonorrhoeae MS11A is involved in epithelial cell invasion. Mol. Microbiol. 13919-928. [DOI] [PubMed] [Google Scholar]
- 32.Wiesenfeld, H. C., S. L. Hillier, M. A. Krohn, D. V. Landers, and R. L. Sweet. 2003. Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin. Infect. Dis. 36663-668. [DOI] [PubMed] [Google Scholar]
- 33.Wilson, M. 2005. Microbial inhabitants of humans: their ecology and role in health and disease. Cambridge University Press, Cambridge, United Kingdom.
- 34.Young, H., S. K. Sarafian, A. B. Harris, and A. McMillan. 1983. Non-cultural detection of Neisseria gonorrhoeae in cervical and vaginal washings. J. Med. Microbiol. 16183-191. [DOI] [PubMed] [Google Scholar]
- 35.Zheng, H. Y., T. M. Alcorn, and M. S. Cohen. 1994. Effects of H2O2-producing lactobacilli on Neisseria gonorrhoeae growth and catalase activity. J. Infect. Dis. 1701209-1215. [DOI] [PubMed] [Google Scholar]
- 36.Zhou, X., S. J. Bent, M. G. Schneider, C. C. Davis, M. R. Islam, and L. J. Forney. 2004. Characterization of vaginal microbial communities in adult healthy women using cultivation-independent methods. Microbiology 1502565-2573. [DOI] [PubMed] [Google Scholar]
- 37.Zhou, X., C. J. Brown, Z. Abdo, C. C. Davis, M. A. Hansmann, P. Joyce, J. A. Foster, and L. Forney. 2007. Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women. ISME J. 1121-133. [DOI] [PubMed] [Google Scholar]