Abstract
Despite effective chemotherapy, schistosomiasis remains a major public health problem in the developing world, with at least 200 million active infections resulting in significant morbidity. Rapid reinfection after treatment, accompanied by extensive residual morbidity, mandates alternative control strategies, including vaccine development. Paramyosin, a myofibrillar protein found only in invertebrates, has been widely studied as a vaccine candidate for both Schistosoma mansoni and Schistosoma japonicum. Recently, we demonstrated that Th2-biased immune responses to paramyosin are associated with resistance to reinfection with S. japonicum in humans; however, challenges in the pilot-scale production of schistosome paramyosin have hampered further studies of this promising vaccine candidate. Here we report a method for the pilot-scale expression and purification of recombinant S. japonicum paramyosin (rSj97). rSj97 was extracted from Escherichia coli inclusion bodies and purified with sequential anion-exchange, hydroxyapatite, and size exclusion chromatography. The purified rSj97 was >95% pure as judged by sodium dodecyl sulfate-polyacrylamide gel electrophoretic analysis and was free of significant endotoxin contamination. We demonstrate that, like native paramyosin, rSj97 adopts an alpha-helical coiled-coil tertiary structure and binds immunoglobulin and collagen. Naïve mice infected with S. japonicum produce anti-rSj97 immunoglobulin G (IgG) antibodies as early as 4 weeks postinfection, while sera collected from S. japonicum-infected individuals contain anti-rSj97 IgE antibodies. Our method for pilot-scale production of recombinant full-length paramyosin will facilitate preclinical evaluation of paramyosin as a vaccine for schistosomiasis.
Schistosomiasis remains a major public health concern in the developing world, with 200 million individuals infected and 600 million at risk of infection (6). The disease is caused by parasitic helminths of the genus Schistosoma and is prevalent in sub-Saharan Africa, Asia, Latin America, and the Middle East. Severe morbidity and mortality are associated with end-organ liver or urinary tract pathology, while recent work has highlighted subtle morbidities associated with the chronic nature of the disease, such as anemia and malnutrition (11). Because of rapid reinfection, the prevalence of infection and morbidity has not been adequately reduced despite effective chemotherapy with praziquantel. For this reason, “vaccine-linked chemotherapy” has been advocated as an alternative control strategy (1). Even with a nonsterilizing, suboptimal vaccine, mathematical models predict significant long-term reductions in infection prevalence and intensity when this approach is targeted either to humans (3) or to livestock for the zoonotic species Schistosoma japonicum (25). These models justify the search for an antischistosomal vaccine to effect durable reductions in the prevalence and intensity of infection and to limit subtle morbidities that persist despite continued treatment.
Paramyosin, a 97-kDa muscular protein found exclusively in invertebrates, is a recognized priority vaccine candidate for both Schistosoma mansoni and S. japonicum (2). Paramyosin adopts a coiled-coil dimer structure composed of two parallel alpha-helices wrapped in a left-handed, supercoiled twist (10). Paramyosin was identified as the major immunogen in S. mansoni freeze-thawed larvae, a crude parasite preparation that confers significant protection in murine challenge studies (14). Independently, S. japonicum paramyosin (Sj97) was identified as the target of a monoclonal immunoglobulin E (IgE) antibody that conferred protection upon passive immunization of mice (12). In addition to its subtegumental location, paramyosin is also expressed on the surfaces of schistosomula (9), providing a target for immune attack via antibody-dependent cellular cytotoxicity, presumably mediated by eosinophils (12). Surface-expressed paramyosin has been implicated in immune evasion strategies due to its Ig (17), collagen (13), and complement protein (5) binding activities.
Murine immunization studies using both biochemically purified and recombinant paramyosins have consistently demonstrated significant protection from challenge infection. Studies with S. mansoni demonstrate a 24 to 56% reduction in worm burdens, while protection against S. japonicum ranged from 32 to 86% (reviewed in reference 8). Maximal protection (62 to 86%) against S. japonicum was observed by using biochemically purified paramyosin followed by challenge with the Philippine strain of S. japonicum (20).
Subsequently, several immunoepidemiologic studies conducted in areas where schistosomiasis is endemic have associated antigen-specific immune responses to paramyosin with resistance to infection. In a cohort study in Brazil, uninfected individuals had threefold-higher levels of IgG antibody to paramyosin than stool-positive individuals. Importantly, after antischistosomal treatment, individuals who remained stool negative showed elevated antiparamyosin antibodies over 32 months compared to those who continued to excrete eggs (4). Recently, we demonstrated in a longitudinal treatment-reinfection study in Leyte, Philippines, that Th2-biased cytokine responses to Sj97 predict a significantly longer time to reinfection and a 30 to 41% lower intensity of reinfection with S. japonicum following treatment with praziquantel (15).
Further development of paramyosin as a vaccine candidate for schistosomiasis has been halted due to the inability to express and purify this protein at a pilot scale. Specifically, poor yields and insolubility in bacterial and yeast expression systems (19, 27) have resulted in paramyosin being “shelved” from the vaccine priority list (1). Here we report a robust process for the pilot-scale production of recombinant full-length S. japonicum paramyosin (rSj97) with assessment of the protein's structure, binding properties, and antigenicity.
MATERIALS AND METHODS
Cloning and fermentation.
Adult worms were prepared by perfusing strain ICR mice that had been infected with cercariae shed from snails collected in Mindoro, Philippines. The full-length S. japonicum paramyosin gene was amplified from reverse-transcribed adult worm mRNA by using gene-specific primers with ligation-independent cloning (LIC)-compatible overhangs (forward primer, GGTATTGAGGGTCGCATGATGAATCACGATACAGAATCTC; reverse primer, AGAGGAGAGTTAGAGCCTACATCATACTTGTTGCTCTCATTC). The PCR product was annealed with pET-32 Xa/LIC (Novagen, EMD Biosciences, San Diego, CA) downstream of a thioredoxin fusion tag according to the manufacturer's instructions. This plasmid preparation, designated pSj97, was amplified in Escherichia coli strain NovaBlue (Novagen), purified by anion exchange (Plasmid maxi kit; Qiagen, Hilden, Germany), and stored at −80°C. The plasmid was sequenced using standard dye terminator techniques and gene-specific primers.
pSj97 was transformed into the E. coli expression host BL21(DE3) (Novagen) and plated onto LB plates supplemented with 100 μg/ml carbenicillin (Novagen) for selection. Pilot-scale expression was performed using a 10-liter batch-fed fermentor (Microferm T-136; New Brunswick Scientific, New Brunswick, NJ) controlled at 37°C and pH 7.0, with a 900-rpm agitation speed, and supplemented with oxygen (10 liters/min). Eight liters of prewarmed culture medium (Terrific Broth supplemented with 100 μg/ml carbenicillin, 5 mM MgSO4, and Sigma Antifoam 204 as needed) was inoculated with 1 liter of an overnight pSj97/BL21 culture and allowed to reach an optical density (A600) of 30, corresponding to the late-log phase. The culture was then induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) and expressed for 5 h. Fifty milliliters of feed medium (30% glycerol, 90 g/liter yeast extract) was added every hour for the duration of fermentation. The culture was harvested and clarified by centrifugation at 7,900 × g for 30 min, and the cell pellet was stored at −80°C.
Inclusion body preparation.
rSj97 accumulated in the insoluble fraction and was extracted from inclusion bodies with urea. Briefly, 500 mg of wet cell paste was thawed, resuspended in 5 liters of buffer A (phosphate-buffered saline [PBS], 1% Triton X-100 [pH 7.4]), lysed by high-pressure cell disruption (Microfluidizer M110Y; Microfluidics, Newton, MA), and clarified by centrifugation at 7,900 × g for 30 min. The supernatant was discarded, and the pellet was resuspended in 3 liters of PBS and then disrupted and centrifuged as described above. The final pellet was resuspended in 1 liter of buffer B (8 M urea, 10 mM Tris-Cl, 10 mM dithiothreitol [DTT] [pH 8.0]), incubated for 1 h at room temperature with stirring, and filtered through 0.8-μm- and 0.45-μm-pore-size filters (Corning, NY) prior to chromatography.
Chromatographic purification.
All buffers utilized for chromatography were made with lipopolysaccharide (LPS)-free water and filtered through 0.45-μm- and/or 0.22-μm-pore-size membrane filters. Chromatography systems and columns were sanitized in 0.5 M NaOH and extensively rinsed in LPS-free water prior to equilibration in buffer, except for anion-exchange columns, which were sanitized in 2% acetic acid-24% ethanol.
(i) Capture.
One liter of the inclusion body preparation containing rSj97 was loaded onto a 2-liter-column-volume (CV) anion-exchange column (MacroPrep High Q; Bio-Rad, Hercules, CA) preequilibrated in buffer C (4 M urea, 10 mM Tris-Cl, 1 mM DTT [pH 8.0]), washed with the equilibration buffer, and eluted using a 10-liter-CV linear gradient with buffer D (1 M NaCl, 4 M urea, 10 mM Tris-Cl, 1 mM DTT [pH 8.0]). Fractions were characterized by electrophoresis using precast 4 to 15% gradient polyacrylamide gels (Bio-Rad). Fractions containing rSj97 were pooled (volume, 2 liters) and then divided into two equal portions (volume, 1 liter).
(ii) Intermediate purification.
In two successive chromatography runs, 1 liter of pooled eluate from the capture step was loaded onto a ceramic hydroxyapatite (CHT) column (CHT type I; Bio-Rad) containing 80 ml of packed resin preequilibrated in buffer E (10 mM potassium phosphate, 16 ppm CaCl2, 1 mM DTT [pH 7.0]). The column was washed in equilibration buffer and eluted with a 10-liter-CV linear gradient in buffer F (500 mM potassium phosphate, 16 ppm CaCl2,1 mM DTT [pH 7.0]). Fractions containing rSj97 from each CHT chromatography run were pooled (volume, 625 ml).
(iii) Concentration.
Post-CHT chromatography fractions were concentrated on a 120-ml-CV anion-exchange column (MacroPrep High Q; Bio-Rad) by step elution. The anion-exchange column was preequilibrated in buffer G (10 mM sodium phosphate [pH 7.4]), washed, and eluted with an upward flow of 100% buffer H (0.3 M NaCl, 10 mM sodium phosphate [pH 7.4]). Fractions containing rSj97 were pooled (volume, 150 ml).
(iv) Final polishing.
The final chromatographic purification step utilized a 600-ml-CV size exclusion column (Superdex 200 prep grade; Amersham Pharmacia, Uppsala, Sweden) equilibrated in buffer I (0.05% sodium dodecyl sulfate [SDS], 0.3 M NaCl, 10 mM sodium phosphate). In preparation for size exclusion chromatography, SDS was added to the sample at a final concentration of 0.05%, and DTT was added at a final concentration of 20 mM immediately prior to loading. Using a sample load of 6 ml, sequential size exclusion chromatography runs were performed until the entire concentrated sample was processed. Fractions exclusively containing full-length rSj97 were pooled and quantified using a bicinchoninic acid (BCA)-based colorimetric assay (BCA protein assay kit; Pierce, Rockford, IL), together with aliquots from sequential chromatographic purification steps.
(v) Final processing, formulation, and lyophilization.
Fractions containing purified rSj97 were concentrated via tangential flow filtration (Minimate 5k; Pall, East Hills, NY) to 0.4 mg/ml, and excess SDS was removed by detergent exchange and cold precipitation. Briefly, sucrose and Tween 20 were added to final concentrations of 3% and 0.005%, respectively. The solution was then incubated overnight at 4°C, centrifuged at 10,397 × g for 30 min at 4°C, and filtered through 0.45-μm-pore-size filters at 4°C. The solution was aseptically filtered through sterilizing-grade (pore size, 0.22 μm) membrane systems, dispensed in 1-ml aliquots into LPS-free vials, and lyophilized (AdVantage; VirTis, Gardiner, NY). Vials were stored indefinitely at −80°C.
Assays for rSj97 purity, identity, stability, and residual SDS.
Lyophilized rSj97 in vials was resuspended in 1 ml of LPS-free water. The protein concentration was assessed by a BCA-based protein assay with bovine serum albumin as the standard (Pierce). Protein purity was assessed by 4 to 15% SDS-polyacrylamide gel electrophoresis (PAGE) and colloidal Coomassie staining (sensitivity, <50 ng of protein; Gelcode Blue; Pierce). To confirm the identities of expression products, bands from stained SDS-PAGE gels were excised and subjected to trypsin digestion and nano-liquid chromatography-tandem mass spectrometry-based peptide sequencing (ProtTech, Norristown, PA). Stability was evaluated by resuspending lyophilized rSj97 in 1 ml of LPS-free water and then incubating the suspension at different temperatures (4°C, −20°C) for 1 month prior to characterization by SDS-PAGE. Residual SDS was quantified using a quantitative colorimetric assay as described elsewhere (22).
Structural analysis.
Secondary and tertiary protein structure analyses were performed on 0.6 nM solutions of rSj97 in the presence and absence of 0.05% SDS using a circular dichroism (CD) spectropolarimeter (J-185; Jasco Inc., Easton, MD) with a 0.2-mm-path-length cuvette with temperature control at 25°C (Jasco Peltier Type). Raw data in millidegrees were converted to ellipticity and used to calculate helix fractions (21) and the presence of a coiled-coil tertiary structure (28).
LPS assay.
LPS (endotoxin) levels were determined using a colorimetric Limulus amebocyte lysate assay according to the manufacturer's instructions (Lonza Bioscience, Basel, Switzerland).
Ig and collagen binding assays.
rSj97 was characterized for IgG and collagen binding by enzyme-linked immunosorbent assay (ELISA)-based assays. For Ig binding, 96-well microtiter plates were coated overnight at 4°C with 2.5 μg of either rSj97 or the E. coli thioredoxin fusion tag/well in bicarbonate buffer (pH 9.6). Plates were then blocked for 1 h at 37°C with 5% nonfat milk in PBS, washed, and incubated with increasing amounts (0 to 3.2 mg/ml) of human nonspecific IgG (Sigma, St. Louis, MO) for 1 h at 37°C. The plates were then washed, incubated with anti-human IgG conjugated with alkaline phosphatase (Sigma) for 40 min at 37°C, washed, and developed with p-nitrophenyl phosphate (Sigma) for 15 min at room temperature. Quantification was performed using a UV spectrophotometer (Molecular Devices, Toronto, Canada) at a wavelength of 405 nm, and results were reported as optical densities.
Collagen binding was performed on commercially available microtiter wells coated with type II and type III equine collagen (Corgenix, Broomfield, CO) and incubated with increasing amounts (0 to 12.5 μg/ml) of either rSj97 or the E. coli thioredoxin fusion tag for 1 h at 37°C. The wells were washed and incubated with rabbit antithioredoxin (Sigma) for 1 h at 37°C. After a wash, the wells were first incubated with an alkaline phosphatase-conjugated anti-rabbit antibody (Sigma) for 50 min at 37°C and then developed and quantified as described above.
To evaluate the efficiency of the purification process, the collagen binding assay was performed using aliquots containing rSj97 obtained from each purification step. Serial dilutions of fully purified rSj97 were utilized as a standard curve. We defined 1.0 U of collagen binding activity as equal to a purified rSj97 concentration of 12.5 μg/ml. Optical density values obtained for the intermediate purification steps were transformed to activity units based on the standard curve.
Mouse infection and IgG recognition.
Ten healthy female outbred Kunming strain mice, 6 to 8 weeks old, were infected with 40 Chinese-strain S. japonicum cercariae using the coverslip method (16). Whole blood was collected by retro-orbital bleeding and was used for the preparation of serum at several time points: prior to infection and 2, 4, and 6 weeks thereafter.
Sera collected from each time point were pooled and analyzed for antibody recognition to rSj97 as follows. Microtiter plates were coated overnight at 4°C with 0.5 μg per well of either rSj97 or E. coli thioredoxin in carbonate buffer, pH 9.6. Plates were then blocked with 5% skim milk in PBS (blocking buffer) for 1 h at 37°C, washed once in blocking buffer, and incubated with 100 μl of a 1:100 dilution (in PBS) of the various serum pools for 1 h at 37°C. All serum pools were analyzed in triplicate. Plates were then washed four times in blocking buffer and incubated with 100 μl of a 1:5,000 dilution of a goat anti-mouse IgG antibody conjugated with horseradish peroxidase (AbD SeroTec, United Kingdom) for 1 h at 37°C. Plates were washed four times in blocking buffer and developed with 100 μl of the substrate (3,3′,5,5′-tetramethylbenzidine; Dazhi-Bio, Nanjing, China) for 15 min at room temperature. The reaction was stopped by addition of 50 μl of 2 M sulfuric acid, and the product was quantified using a UV spectrophotometer at a 450-nm wavelength.
Recognition of rSj97 by human antibodies.
Sera from S. japonicum-infected patients were collected as part of a longitudinal study conducted in villages where schistosomiasis was endemic in Leyte, Philippines, as previously described (15). Briefly, S. japonicum-infected patients were treated with praziquantel at baseline, and phlebotomies were performed 4 weeks thereafter. Whole blood was collected, and serum was separated and stored at −80°C until it was thawed for analysis.
Recognition of rSj97 by human IgE was measured using a high-throughput bead-based multiplex platform (Bioplex; Bio-Rad) similar to that used in our previous study (23). Briefly, 100 μg of rSj97 was coupled to 1.25 ×107 microspheres according to the manufacturer's instructions (Luminex, Austin, TX), aliquoted for single use, and lyophilized. Because rSj97 can bind the Fc region of immunoglobulins, we blocked these antibody binding sites by pretreating rSj97-coupled beads with 2.8 mg/ml of nonspecific human IgG (Sigma) for 30 min at room temperature prior to sample incubation. Seventy-four haphazardly selected patient samples and samples from 16 uninfected North American controls were tested in this assay. Individual sera were diluted 1:20 in assay buffer (PBS, 1 mg/ml bovine serum albumin, 0.05% Tween 20, 0.05% sodium azide) and incubated with blocked rSj97 beads for 30 min at room temperature. After several washes, a biotinylated anti-human IgE detection antibody (1:100; Pharmingen, San Diego, CA) was added and incubated for 30 min at room temperature. Plates were developed in streptavidin-phycoerythrin (1:500; Pharmingen), and the fluorescence was quantified in a BioPlex 100 analyzer (Bio-Rad). All liquid handling procedures were carried out by a high-speed pipetting robot (Tecan, Research Triangle Park, NC).
RESULTS
Cloning and fermentation.
The nucleotide sequence of pSj97 (GenBank accession no. EU488866) differed from the published S. japonicum paramyosin sequence (GenBank accession no. U11825), which resulted in two amino acid substitutions, one at position 371 (D to E) and one at position 790 (M to T).
Full-length S. japonicum paramyosin was cloned from reverse-transcribed worm mRNA and annealed into a pET32 Xa/LIC vector downstream of a thioredoxin fusion tag. The plasmid, pSj97, was transformed into the E. coli expression host BL21(DE3) and cultured in Terrific Broth supplemented with carbenicillin. Pilot-scale expression was performed in an oxygen-sparged 10-liter fermentor batch fed with enrichment medium every hour. Typical yields were 600 g of wet cell paste per fermentation. SDS-PAGE separation of cell lysates demonstrated a prominent, induced protein of 118 kDa. Liquid chromatography-tandem mass spectrometry-based peptide sequencing of this protein identified 36 peptides from S. japonicum paramyosin spanning 92% of the entire expected sequence and 4 peptides from E. coli thioredoxin.
Purification.
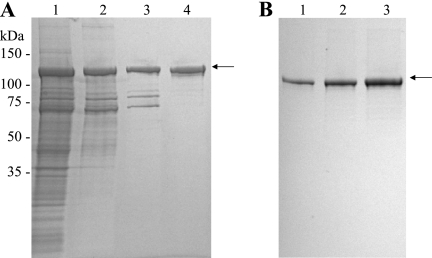
Following high-pressure cell disruption, rSj97 partitioned with the insoluble protein fraction but was solubilized by resuspension in the presence of urea. This inclusion body preparation was subjected to a two-step chromatographic process involving anion-exchange chromatography and chromatography with CHT, a mixed anion-cation exchanger (Fig. 1A). Importantly, rSj97 remained soluble despite the removal of urea during CHT purification. Pooled fractions obtained from CHT chromatography contained full-length rSj97 and several lower-molecular-weight (lower-MW) species (Fig. 1A, lane 3).
FIG. 1.
(A) Chromatographic purification of rSj97. Pooled fractions containing rSj97 were analyzed by SDS-PAGE after inclusion body preparation (lane 1), a capture step (anion-exchange chromatography) (lane 2), an intermediate step (hydroxyapatite chromatography) (lane 3), and a polishing step (size exclusion chromatography) (lane 4). (B) Purity assessment of rSj97. Lane 1, 0.5 μg; lane 2, 1.0 μg; lane 3, 2.0 μg. Electrophoresis was performed on 4 to 15% gradient polyacrylamide gels under reducing conditions, and the gel was stained with colloidal Coomassie G-250. The arrow indicates full-length rSj97 with the thioredoxin fusion protein.
Removal of these lower-MW species constituted the major challenge in the purification of rSj97; standard anion, cation, size exclusion, and metal chelate chromatography all failed to resolve rSj97 from these contaminants.
To inform our process development, we sequenced these lower-MW species and determined that they encode carboxy-terminal fragments of S. japonicum paramyosin. Because paramyosin adopts a coiled-coil tertiary structure, we hypothesized that the truncated paramyosin species were interacting with full-length rSj97 as coiled-coil dimers, thus accounting for the failure of our earlier attempts at resolving the full-length protein. Paramyosin dimers can be dissociated only under highly denaturing conditions, including 7 M guanidine hydrochloride or 9.5 M urea at 65°C (26). While size exclusion chromatography using these denaturing conditions did achieve resolution of rSj97 (data not shown), these chaotropes pose substantial difficulties for scale-up. We successfully separated full-length paramyosin by employing size exclusion chromatography in the presence of 0.05% SDS (Fig. 1A).
Postchromatography processing of rSj97 involved concentration by tangential flow filtration and removal of excess SDS by cold precipitation. rSj97 was sterile filtered and lyophilized as 1-ml aliquots in polypropylene vials. Resuspension yielded 280 mg of rSj97 per ml. Endotoxin, residual SDS, and stability assessments showed that rSj97 contained 0.07 endotoxin units/ml of LPS and 0.01% SDS and showed no evidence of degradation for at least 1 month at 4°C (data not shown). rSj97 constituted >95% of the final product, as assessed by SDS-PAGE (Fig. 1B). A summary of the protein yields and functional activities of the various purification steps is shown in Table 1. Five hundred grams of E. coli wet cell paste yielded 22.4 mg of purified rSj97.
TABLE 1.
Summary of rSj97 purification
| Purification step | Total protein (mg)a | Recovery (%)b | Activity (U)c |
|---|---|---|---|
| Inclusion body preparation | 6,570 | 100 | 0.0184 |
| Anion exchange | 1,234 | 18.8 | 0.209 |
| Hydroxyapatite | 506.3 | 7.71 | 0.367 |
| Size exclusion | 39 | 0.594 | 0.816 |
| Final processing | 22.4 | 0.341 | 1.00 |
Protein content was assessed by a BCA-based colorimetric assay.
Calculated as the percentage of the protein mass of intermediate purification steps relative to the yield from the inclusion body preparation.
Calculated from an ELISA-based collagen binding assay. Serial dilutions of rSj97 were utilized as a standard curve, with 1.0 U defined as the optical density corresponding to 12.5 μg/ml of purified rSj97.
Characterizations of rSj97.
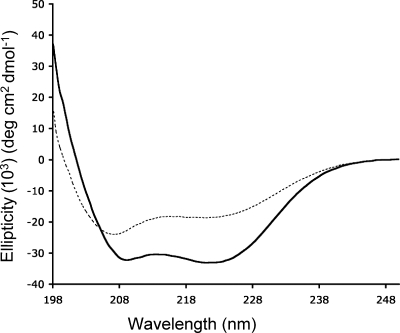
In consideration of the denaturing conditions employed during purification, we evaluated the secondary and tertiary structure of rSj97 using CD spectroscopy. Resuspended rSj97 from lyophilized vials exhibited a CD spectrum consistent with a predominantly alpha-helical protein (Fig. 2) with an alpha-helix content of 88% (21). Addition of SDS to 0.05% perturbed the CD spectrum, and the helix content dropped to 50%.
FIG. 2.
rSj97 adopts an alpha-helical coiled-coil conformation as assessed by CD. A 0.6 nM concentration of rSj97 resuspended in buffer in the absence (solid line) and in the presence (dashed line) of 0.05% SDS was analyzed on a Jasco J-185 CD spectropolarimeter using a 0.2-mm-path-length cuvette. The ellipticity ratio (220 nm/207 nm) suggests that rSj97 adopts a coiled-coil tertiary protein structure as described in Results.
An ellipticity ratio (θ220/θ207) greater than 1 is a marker for coiled-coil structures (28). Using this measure, rSj97 had an ellipticity ratio of 1.13, suggesting that the recombinant protein adopts a coiled-coil configuration. Addition of 0.05% SDS reduces the ellipticity ratio to 0.775, indicating a loss of coiled-coil conformation. These results are consistent with our purification experience, in which full-length rSj97 was resolved from the lower-MW paramyosin fragments by size exclusion chromatography only in the presence of 0.05% SDS.
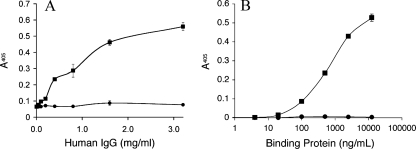
Further characterization of rSj97 involved functional binding assays for Ig (17) and collagen (13), properties that are characteristic of native paramyosin. rSj97, but not the thioredoxin fusion tag, bound human Ig (isotype IgG) nonspecifically in a dose-dependent manner (Fig. 3A). Similarly, rSj97, but not thioredoxin, bound to type II/III equine collagen in a dose-dependent fashion (Fig. 3B). Preincubation of rSj97 with 0.05% SDS completely abolished binding to collagen (data not shown). These results suggest that rSj97 has functional binding capacities similar to those of native paramyosin.
FIG. 3.
rSj97 binds IgG and collagen. (A) Microtiter plates were coated with 2.5 μg of either rSj97 (squares) or the thioredoxin fusion tag (circles)/well, incubated with increasing amounts of nonspecific human IgG, and probed with anti-human IgG. (B) Commercially available collagen-coated microtiter strips were incubated with increasing amounts of either rSj97 (squares) or thioredoxin (circles) and probed with an antithioredoxin antibody. Both assays were developed in an alkaline phosphatase-p-nitrophenyl phosphate substrate system, quantified at a wavelength of 405 nm, and presented as optical densities. Error bars, standard errors.
Antibody recognition.
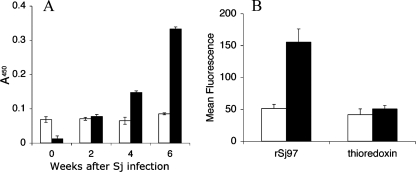
Sera obtained from infected mice and from patients living in an area of S. japonicum endemicity in Leyte, Philippines, were assayed for the presence of antibodies targeting rSj97. Infected mice recognized rSj97 and generated specific IgG as early as 4 weeks postinfection, with an enhanced response at 6 weeks (Fig. 4A). Similarly, anti-rSj97 IgE antibody levels were threefold higher in individuals treated for schistosomiasis than in uninfected, unexposed controls (Fig. 4B).
FIG. 4.
rSj97 is recognized by antibodies in sera from infected mice and humans. (A) Outbred Kunming mice (n = 10) were infected with a Chinese strain of S. japonicum and bled several weeks postinfection. An ELISA was performed on plates coated with either rSj97 (solid bars) or the thioredoxin fusion tag (open bars). (B) Human sera were collected from individuals with schistosomiasis in Leyte, Philippines, 4 weeks posttreatment (solid bars) (n = 74). Uninfected sera (open bars) (n = 16) were obtained from North American controls. Immunoassays for anti-rSj97 isotype IgE were performed on a bead-based multiplex platform as described in Materials and Methods. Error bars, standard errors.
DISCUSSION
Vaccine development remains a promising long-term approach to the control of schistosomiasis, particularly for developing countries that face challenges in implementing and maintaining praziquantel-based control programs. Numerous vaccine candidates have been identified and subsequently prioritized by the WHO; however, the development of several candidates, including paramyosin, has been hampered due to unexpected difficulties in recombinant expression and scale-up. Despite substantial production difficulty, work with both animal models and humans has strongly supported paramyosin as a lead vaccine candidate for S. mansoni and S. japonicum.
Motivated by our recent study implicating Th2-biased immune responses and resistance to reinfection with S. japonicum in humans (15), we developed a pilot-scale process to produce recombinant paramyosin. We obtained sufficient yields of rSj97 in a bacterial expression system using a pET-32 plasmid construct containing an N-terminal thioredoxin fusion tag. rSj97 accumulated in the insoluble fraction and was solubilized in 8 M urea. Previous reports on paramyosin purification utilized the interaction between polyhistidine expression tags and metal ion affinity resins to capture the recombinant protein (19); unfortunately, this process was inefficient with our inclusion body preparation. Instead, capture and intermediate purifications were achieved using ion-exchange-based chromatography processes consistent with the numerous ionic amino acids within the heptad repeats characteristic of coiled-coil proteins.
An unexpected challenge during purification was the presence of truncated paramyosin expression products that persisted despite multiple chromatographic separation attempts. We hypothesized that these lower-MW species formed heterodimeric coiled-coils with the full-length protein. To overcome this interaction, SDS was added during size exclusion chromatography. As supported by CD data, the addition of SDS abolishes the coiled-coil dimer conformation of paramyosin, allowing for separation by size and subsequently the isolation of the full-length rSj97. Importantly, rSj97 refolds into a coiled-coil structure upon removal of excess SDS by cold precipitation. This final step is necessary to produce full-length rSj97 that is substantially free from lower-MW species and will facilitate regulatory acceptance in preparation for preclinical studies.
Pilot-scale production using 500 g of wet cell paste generated 22.4 mg of purified rSj97, enough to support several immunization experiments. All processes have been carefully chosen to support linear scale-up under good manufacturing practice standards according to facility capabilities. Our initial quality control assessments demonstrate high purity, good stability, low LPS contamination, and residual SDS levels below the levels found in an FDA-approved intravenous pharmaceutical, aldesleukin (7; Proleukin package insert; Novartis, East Hanover, NJ [http://www.pharma.us.novartis.com/product/pi/pdf/proleukin.pdf]). Characterization of rSj97 demonstrated levels of binding to collagen and immunoglobulins that were similar to those of native paramyosin, suggesting that the respective binding domains are maintained in the bacterially expressed protein.
Serological analysis of infected mouse and human sera confirm that rSj97 contains epitopes expressed during S. japonicum infection. The early recognition of paramyosin in mice suggests that the immune system encounters paramyosin early during infection. This is consistent with immunolocalization studies showing that paramyosin is found on the surfaces of larval-stage schistosomula, among other sites (9). Infected individuals also show enhanced antibody recognition of rSj97 compared to uninfected, unexposed controls. The threefold-higher mean IgE reactivity of infected individuals is striking, considering that helminth infections are often characterized by a “modified Th2 response,” with most patients exhibiting low IgE titers (18). We suspect that a boosting effect of praziquantel treatment, with consequent adult worm death 4 weeks prior to serum collection, contributed to these high anti-rSj97 IgE levels (24).
In conclusion, we have developed a pilot scale expression and purification scheme for recombinant full-length S. japonicum paramyosin. We have recently extended this work by achieving pilot-scale expression and purification of Sj97 without a thioredoxin fusion tag (GenBank accession no. EU488865) by using a kanamycin-resistant plasmid (see Fig. S1 in the supplemental material). Future work includes the utilization of plant-based culture media and enhancement of expression by synthesizing codon optimized/harmonized expression constructs. Together, these enhancements will enable future studies evaluating paramyosin as a potential vaccine for schistosomiasis and will facilitate large-scale, good manufacturing practice production of this promising vaccine candidate for livestock and/or human use.
Supplementary Material
Acknowledgments
This work was funded by National Institutes of Health grants R01 AI48123 and K23 AI52125.
We thank our field staff for their diligence and energy. We thank the study participants from Macanip, Buri, and Pitogo in Leyte, Philippines. We also thank Guanling Wu and Suhua Zhang at Nanjing Medical University for providing the S. japonicum-infected mouse sera and Francisco Tabicas for technical assistance with collagen binding assays.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 21 April 2008.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Bergquist, N. R., L. R. Leonardo, and G. F. Mitchell. 2005. Vaccine-linked chemotherapy: can schistosomiasis control benefit from an integrated approach? Trends Parasitol. 21112-117. [DOI] [PubMed] [Google Scholar]
- 2.Bergquist, R., M. Al-Sherbiny, R. Barakat, and R. Olds. 2002. Blueprint for schistosomiasis vaccine development. Acta Trop. 82183-192. [DOI] [PubMed] [Google Scholar]
- 3.Chan, M. S., M. E. Woolhouse, and D. A. Bundy. 1997. Human schistosomiasis: potential long-term consequences of vaccination programmes. Vaccine 151545-1550. [DOI] [PubMed] [Google Scholar]
- 4.Correa-Oliveira, R., E. J. Pearce, G. C. Oliveira, D. B. Golgher, N. Katz, L. G. Bahia, O. S. Carvalho, G. Gazzinelli, and A. Sher. 1989. The human immune response to defined immunogens of Schistosoma mansoni: elevated antibody levels to paramyosin in stool-negative individuals from two endemic areas in Brazil. Trans. R. Soc. Trop. Med. Hyg. 83798-804. [DOI] [PubMed] [Google Scholar]
- 5.Deng, J., D. Gold, P. T. LoVerde, and Z. Fishelson. 2003. Inhibition of the complement membrane attack complex by Schistosoma mansoni paramyosin. Infect. Immun. 716402-6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engels, D., L. Chitsulo, A. Montresor, and L. Savioli. 2002. The global epidemiological situation of schistosomiasis and new approaches to control and research. Acta Trop. 82139-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.FDA. 1991. List of orphan products designations and approvals. http://www.fda.gov/ohrms/dockets/dailys/00/mar00/030100/lst0094.pdf.
- 8.Gobert, G. N., and D. P. McManus. 2005. Update on paramyosin in parasitic worms. Parasitol. Int. 54101-107. [DOI] [PubMed] [Google Scholar]
- 9.Gobert, G. N., D. J. Stenzel, M. K. Jones, D. E. Allen, and D. P. McManus. 1997. Schistosoma japonicum: immunolocalization of paramyosin during development. Parasitology 11445-52. [DOI] [PubMed] [Google Scholar]
- 10.Kagawa, H., K. Gengyo, A. D. McLachlan, S. Brenner, and J. Karn. 1989. Paramyosin gene (unc-15) of Caenorhabditis elegans. Molecular cloning, nucleotide sequence and models for thick filament structure. J. Mol. Biol. 207311-333. [DOI] [PubMed] [Google Scholar]
- 11.King, C. H., K. Dickman, and D. J. Tisch. 2005. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet 3651561-1569. [DOI] [PubMed] [Google Scholar]
- 12.Kojima, S., M. Niimura, and T. Kanazawa. 1987. Production and properties of a mouse monoclonal IgE antibody to Schistosoma japonicum. J. Immunol. 1392044-2049. [PubMed] [Google Scholar]
- 13.Laclette, J. P., C. B. Shoemaker, D. Richter, L. Arcos, N. Pante, C. Cohen, D. Bing, and A. Nicholson-Weller. 1992. Paramyosin inhibits complement C1. J. Immunol. 148124-128. [PubMed] [Google Scholar]
- 14.Lanar, D. E., E. J. Pearce, S. L. James, and A. Sher. 1986. Identification of paramyosin as schistosome antigen recognized by intradermally vaccinated mice. Science 234593-596. [DOI] [PubMed] [Google Scholar]
- 15.Leenstra, T., L. P. Acosta, H. W. Wu, G. C. Langdon, J. S. Solomon, D. L. Manalo, L. Su, M. Jiz, B. Jarilla, A. O. Pablo, S. T. McGarvey, R. M. Olveda, J. F. Friedman, and J. D. Kurtis. 2006. T-helper-2 cytokine responses to Sj97 predict resistance to reinfection with Schistosoma japonicum. Infect. Immun. 74370-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis, F. 1998. Schistosomiasis, p. 19.1.5-19.1.6. In J. E. Coligan et al. (ed.), Current protocols in immunology. John Wiley & Sons, Inc., New York, NY.
- 17.Loukas, A., M. K. Jones, L. T. King, P. J. Brindley, and D. P. McManus. 2001. Receptor for Fc on the surfaces of schistosomes. Infect. Immun. 693646-3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maizels, R. M., and M. Yazdanbakhsh. 2003. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat. Rev. Immunol. 3733-744. [DOI] [PubMed] [Google Scholar]
- 19.McManus, D. P., J. Y. Wong, J. Zhou, C. Cai, Q. Zeng, D. Smyth, Y. Li, B. H. Kalinna, M. J. Duke, and X. Yi. 2001. Recombinant paramyosin (rec-Sj-97) tested for immunogenicity and vaccine efficacy against Schistosoma japonicum in mice and water buffaloes. Vaccine 20870-878. [DOI] [PubMed] [Google Scholar]
- 20.Ramirez, B. L., J. D. Kurtis, P. M. Wiest, P. Arias, F. Aligui, L. Acosta, P. Peters, and G. R. Olds. 1996. Paramyosin: a candidate vaccine antigen against Schistosoma japonicum. Parasite Immunol. 1849-52. [DOI] [PubMed] [Google Scholar]
- 21.Rohl, C. A., and R. L. Baldwin. 1997. Comparison of NH exchange and circular dichroism as techniques for measuring the parameters of the helix-coil transition in peptides. Biochemistry 368435-8442. [DOI] [PubMed] [Google Scholar]
- 22.Rusconi, F., E. Valton, R. Nguyen, and E. Dufourc. 2001. Quantification of sodium dodecyl sulfate in microliter-volume biochemical samples by visible light spectroscopy. Anal. Biochem. 29531-37. [DOI] [PubMed] [Google Scholar]
- 23.Solomon, J. S., C. P. Nixon, S. T. McGarvey, L. P. Acosta, D. Manalo, and J. D. Kurtis. 2004. Expression, purification, and human antibody response to a 67 kDa vaccine candidate for schistosomiasis japonica. Protein Expr. Purif. 36226-231. [DOI] [PubMed] [Google Scholar]
- 24.Webster, M., P. G. Fallon, A. J. Fulford, A. E. Butterworth, J. H. Ouma, G. Kimani, and D. W. Dunne. 1997. Effect of praziquantel and oxamniquine treatment on human isotype responses to Schistosoma mansoni: elevated IgE to adult worm. Parasite Immunol. 19333-335. [DOI] [PubMed] [Google Scholar]
- 25.Williams, G. M., A. C. Sleigh, Y. Li, Z. Feng, G. M. Davis, H. Chen, A. G. Ross, R. Bergquist, and D. P. McManus. 2002. Mathematical modelling of schistosomiasis japonica: comparison of control strategies in the People's Republic of China. Acta Trop. 82253-262. [DOI] [PubMed] [Google Scholar]
- 26.Woods, E. F. 1969. Subunit structure of oyster paramyosin. Biochem. J. 11339-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang, D. M., W. Q. Pan, L. Qian, M. Duke, L. H. Shen, and D. P. McManus. 2006. Investigation of recombinant Schistosoma japonicum paramyosin fragments for immunogenicity and vaccine efficacy in mice. Parasite Immunol. 2877-84. [DOI] [PubMed] [Google Scholar]
- 28.Zhou, N. E., C. M. Kay, and R. S. Hodges. 1992. Synthetic model proteins. Positional effects of interchain hydrophobic interactions on stability of two-stranded alpha-helical coiled-coils. J. Biol. Chem. 2672664-2670. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.