Abstract
Many uropathogenic Escherichia coli (UPEC) strains produce both hemolysin (Hly) and cytotoxic necrotizing factor type 1 (CNF1), and the loci for these toxins are often linked. The conclusion that Hly and CNF1 contribute to urovirulence is supported by the results of epidemiological studies associating the severity of urinary tract infections (UTIs) with toxin production by UPEC isolates. Additionally, we previously reported that mouse bladders and rat prostates infected with UPEC strain CP9 exhibit a more profound inflammatory response than the organs from animals challenged with CP9cnf1 and that CNF1 decreases the antimicrobial activities of polymorphonuclear leukocytes. More recently, we created an Hly mutant, CP9ΔhlyA1::cat, and showed that it was less hemolytic and destructive for cultured bladder cells than CP9 was. Here we evaluated the relative effects of mutations in hlyA1 or cnf1 alone or together on the pathogenicity of CP9 in a mouse model of ascending UTI. To do this, we constructed an hlyA1-complemented clone of CP9ΔhlyA1::cat and an hlyA1 cnf1 CP9 double mutant. We found that Hly had no influence on bacterial colonization of the bladder or kidneys in single or mixed infections with the wild type and CP9ΔhlyA1::cat but that it did provoke sloughing of the uroepithelium and bladder hemorrhage within the first 24 h after challenge. Finally, we confirmed that CNF1 expression induces bladder inflammation and, in particular, as shown in this study, submucosal edema. From these data, we speculate that Hly and CNF1 may be largely responsible for the signs and symptoms of cystitis in humans infected with toxigenic UPEC.
Urinary tract infections (UTIs) include infections of the bladder (cystitis) and/or kidney (pyelonephritis) and account for more than 7 million office visits each year by otherwise healthy women (23). Extraintestinal pathogenic Escherichia coli (ExPEC) is the causative agent of at least 80% of all uncomplicated UTIs. ExPEC strains that cause a UTI are called uropathogenic E. coli (UPEC). UPEC strains typically are members of phylogenetic group B2 or D and often exhibit specific O:K:H serotypes, as well as various combinations of virulence factors, including, among others, adhesins or fimbriae, siderophore systems, and toxins. The virulence-associated genes in UPEC are frequently clustered together in “pathogenicity islands” (PAIs) (25), and many UPEC isolates harbor more than one PAI. For instance, the prototypic UPEC clinical strain J96 (O4:K6) carries two PAIs, PAI IJ96 and PAI IIJ96 (58). PAI IJ96 is over 170 kb long and contains operons encoding alpha-hemolysin (hly) and Pap fimbriae (pap). PAI IIJ96 is 110 kb long and also contains an hly operon in addition to genes encoding Prs fimbriae (prs) and cytotoxic necrotizing factor type 1 (CNF1) (cnf1) (6). Here, we hypothesized that the link between PAI IIJ96 and the urovirulence of E. coli strains may largely reflect the fact that these strains produce both alpha-hemolysin (referred to as hemolysin [Hly] here) and CNF1. Indeed, epidemiological studies have consistently shown that UPEC strains that make CNF1 also produce Hly (5, 17, 19, 36, 66). Moreover, Real and coworkers reported that CNF1/Hly-expressing UPEC strains are more often isolated from humans with hemorrhagic UTIs who also have higher urinary levels of proinflammatory cytokines, such as interleukin-8 and monocyte chemoattractant protein 1, than UPEC strains that do not make these toxins (51). Landraud et al. provided a genetic explanation for the apparent linkage between CNF1 and Hly synthesis by UPEC; they demonstrated that a cnf1 gene and an hly operon in the prototypic UPEC strain J96 not only are cotranscribed but also are coregulated in vitro (37).
CNF1 is a ∼115-kDa cytoplasmic protein (8, 15, 16, 20) that is present in certain diarrheagenic as well as uropathogenic strains of E. coli (2-4, 9, 10, 45). This molecule is a member of a bacterial toxin family that modifies the function of key regulatory molecules within the Rho family of GTP-binding proteins in mammalian cells (1). Specifically, CNF1 catalyzes the deamidation of a glutamine residue at position 63 in RhoA (22, 57) and at position 61 in both Cdc42 and Rac1 (38, 39). These deamidation reactions cause constitutive activation of RhoA, Rac, and Cdc42 (i.e., they maintain these GTPases in the GTP-bound state), which in turn triggers a myriad of effects on the target cell. Specifically, activation of RhoA, Rac, and/or Cdc42 can evoke actin cytoskeleton rearrangements (27, 28, 50, 52, 53), cell cycle abnormalities (14, 21, 24), and alterations in host cell gene signaling pathways that involve certain nuclear transcription factors (39, 48). Of relevance to this study, we previously found that CNF1+ UPEC strains elicit a more intense inflammatory response than isogenic CNF1− mutants elicit in a mouse model of ascending UTI (55), as well as in a rat model of prostatitis (54). We also discovered that UPEC strain CP9 survives better than a CNF1− isogenic mutant of this strain in the presence of both human (55) and mouse polymorphonuclear leukocytes (13).
Hly is a heat-labile extracellular protein made by a large proportion of ExPEC isolates (31). This toxin forms pores in the membranes and lyses a number of mammalian cell types, including red blood cells (RBCs) (hence, the name) (for reviews, see references 35 and 65). The Hly operon is comprised of, in order from the promoter, hlyC (which encodes a novel homodimeric lysyl-acyltransferase responsible for the fatty acid acylation step needed to render the prohemolysin active), hlyA (which encodes the Hly protein), hlyB (which encodes an integral membrane ATPase), and hlyD (which encodes an accessory or membrane fusion protein) (for reviews, see references 29 and 34). The acylated active Hly is exported through the outer membrane protein conduit TolC (for a review, see reference 29). The circumstantial evidence that Hly is associated with the virulence of ExPEC in general and UPEC in particular is compelling, but defining the actual role that Hly may play in pathogenicity has been elusive (40, 46, 61, 64). In a previous study, we constructed an hlyA mutant with a mutation in the operon located proximal to cnf1 in strain CP9 (referred to below as hlyA1 to distinguish it from hlyA2 in the operon not linked to cnf1) (61). We found that this mutation dramatically reduced the hemolytic activity of CP9, as well as its capacity to damage uroepithelial cells in tissue culture and in three-dimensional uro-organoids (61). From these results, we concluded that the hly1 operon linked to cnf1 conferred almost all of the hemolytic properties to CP9.
In this investigation, we sought to determine what effect the expression of Hly and CNF1, alone and in combination, has on the uropathogenicity of CP9 in mice infected intraurethrally. Therefore, we completed generation of a set of cnf1 and hlyA1 mutants by creating an hlyA1-complemented clone of CP9ΔhlyA1::cat and a CP9 cnf1 hlyA1 double mutant. We then used these clones and our CP9 wild-type strain and CP9cnf1 mutant to perform a comparative evaluation of the impact of these strains on colonization and the pathology of the bladders specifically and the kidneys in a subset of animals at 1, 3, and 5 days after intraurethral infection. The results of these studies revealed, for the first time, a clear role for Hly in the induction of damage to the superficial cell layers of the murine bladder, as well as hemorrhage in the bladder tissue, but only during the first day after infection. Furthermore, we not only validated our previous observations concerning CNF1-mediated exacerbation of inflammation in UPEC-infected bladders but also extended the findings to reveal the continued impact of the toxin 5 days after intraurethral inoculation of UPEC and the striking effect of CNF1 on elicitation of submucosal edema in the bladder.
MATERIALS AND METHODS
Strains and media.
The UPEC strains and plasmids used in this study are listed in Table 1. CP9 is an E. coli blood isolate obtained from a patient with sepsis hospitalized at the National Institutes of Health (56). This strain is of the 04/K54/H5 serotype and is characterized by its capacity to grow in 80% normal human serum, produce Hly and CNF1, and express P (class I PapG adhesin), Prs (class III PapG adhesin), and type I pili. CP9 also contains a 36.2-kb cryptic plasmid (pJEG) but does not make aerobactin. The media used in this study were Luria-Bertani (LB) broth and LB agar (Difco, Becton-Dickinson, Sparks, MD) that were supplemented, as needed, with ampicillin (100 μg/ml) or chloramphenicol (30 μg/ml).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype | Reference or source |
|---|---|---|
| E. coli isolates | ||
| CP9 (O4:H5:K54) (parent) | hlyA1+hlyA2+cnf1+ | 56 |
| CP9cnf1 | hlyA1+hlyA2+cnf1 | 55 |
| CP9ΔhlyA1::cat | hlyA1hlyA2+cnf1+ | 61 |
| CP9cnf1ΔhlyA1::cat | hlyA1hlyA2+cnf1 | This study |
| CP9ΔhlyA1::cat(pKG8) | hlyA1+hlyA2+cnf1+, pKG8 | This study |
| CP9ΔhlyA1::cat(pQE30) | hlyA1hlyA2+cnf1+, pQE30 | This study |
| Plasmids | ||
| pQE30 | Ampr, low-copy-number E. coli expression vector | QIAGEN |
| pKG8 | hlyA1 cloned into pQE30 | This study |
Generation of the CP9cnf1ΔhlyA1::cat mutant and an hlyA1-complementing clone of CP9ΔhlyA1::cat.
As mentioned above, CP9 carries two hly genes. The hlyA1 gene is located in an operon upstream of cnf1 (unpublished observation), as it is in other UPEC strains, such as J96 (6, 33, 37, 62). In this investigation, we introduced a nonpolar mutation in hlyA1 into the isogenic CP9cnf1 mutant strain by using the lambda red recombination technique (12), with the modifications described below. Oligonucleotides KG03F and KG04R (Table 2) were used to PCR amplify the chloramphenicol acetyltransferase gene (cat) sequence in pACYC184 (New England Biolabs, Ipswich, MA). Oligonucleotides KG01F and KG02R (Table 2) were designed using the previously published hly operon sequence of UPEC strain J96 (GenBank accession no. M10133) and were used to add sequences that flank hlyA1 to the cat gene. The resulting 720-bp PCR product, which was generated to create a precise in-frame deletion of hlyA1 and for insertion of cat in place of this gene (Fig. 1A), was gel purified with a Qiaex II gel extraction kit (QIAGEN, Valencia, CA) and then transformed into CP9cnf1 by electroporation at 2.5 V, 200 Ω, and 25 μF. Hly mutants were selected on medium with 30 μg/ml chloramphenicol and screened for acquisition of the cat gene by PCR (primers KG03F and KG04R [Table 2]), and then colonies of the mutant were evaluated on tryptic soy agar with 5% ovine blood (Remel Inc., Lenexa, KS) for reduced hemolysis. Insertion of cat into the hlyA1 locus was verified by amplification of PCR products with primers that extended from within cat downstream (KG03F and KG08R [Table 2]) or upstream (KG07F and KG04R [Table 2]) into the hlyA1-flanking regions. Primers specific for the regions flanking hlyA1 (KG07F and KG08R [Table 2]) were used to confirm replacement of the 3.1-kb hlyA1 gene with the 660-bp cat gene.
TABLE 2.
Oligonucleotide primers
| Primera | Sequence | Target | Reference |
|---|---|---|---|
| KG01F | 5′-CAGATTTCAATTTTTCATTAACAGGTTAAGAGGTAATTAAATGGAGAAAAAAATCACTGG-3′ | 40 bp upstream of hlyA1 and first 20 bp of cat | 61 |
| KG02R | 5′-CAGCCCAGTAAGATTGCTATTATTTAAATTAATAAATTACGCCCCGCCCTGCC-3′ | 5′ last 33 bp of cat and 20 bp downstream of hlyA1 | 61 |
| KG03F | 5′-ATGGAGAAAAAAATCACTGG-3′ | cat | 61 |
| KG04R | 5′-TTACGCCCCGCCCTGCC-3′ | cat | 61 |
| KG07F | 5′-CCATTAGAGGTTCTTGGGC-3′ | Upstream of hlyA1 | 61 |
| KG08R | 5′-GGAATAAACCAGGTAAAGTC-3′ | Downstream of hlyA1 | 61 |
| KG09F | 5′-ATACTGTATCGGGTATTTTATC-3′ | hlyA1 | 61 |
| KG09R | 5′-TATGGTCATCACCATCCGC-3′ | hlyA1 | This study |
| KG10R | 5′-GATACCAACTCATTGTACTCA-3′ | cnf1 | 61 |
| KG11F | 5′-GATTAATGGTGATACTTATGAAG-3′ | cnf1 | This study |
| KG11R | 5′-GCCATGGTTATCAAAGCGATC-3′ | cnf1 | This study |
| KG12F | 5′-ATACTGTATCGGGTATTTTATC-3′ | Upstream of NcoI site, hlyA1 | This study |
| KG13R | 5′-TATGGTCATCACCATCCGC-3′ | Upstream of NcoI site, hlyA1 | This study |
F, forward; R, reverse.
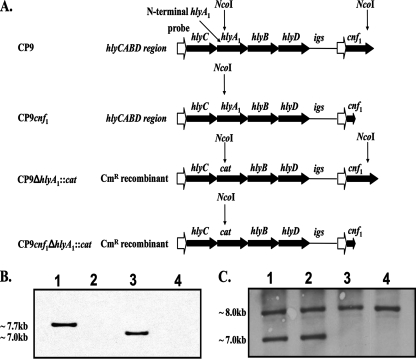
FIG. 1.
Diagram of the CP9 hly operon upstream of cnf1 and Southern blot analyses. (A) The mutated or wild-type Hly operon, the intergenic sequence (igs), and the cnf1 gene from the chromosome of strains CP9, CP9cnf1, CP9ΔhlyA1::cat, and CP9cnf1ΔhlyA1::cat are indicated by large filled arrows. Promoter regions (open arrows) are also shown. Sites of NcoI cleavage (indicated by vertical arrows) of chromosomal DNA were predicted from database sequences (J96 hly operon, GenBank accession no. M10133; cnf1, GenBank accession no. X70670; pACYC184 cat gene, GenBank accession no. X06403; J96 igs, GenBank accession no. X70670). (B) Southern blot to detect the NcoI-NcoI cnf1 fragment. Lane 1, CP9; lane 2, CP9cnf1; lane 3, CP9ΔhlyA1::cat; lane 4, CP9cnf1ΔhlyA1::cat. As expected, the fragment of the chromosome that contained the cnf1 gene was discernible in strains CP9 and CP9ΔhlyA1::cat (lanes 1 and 3, respectively). The cnf1-specific band size (∼7.7 kb) was reduced (∼7.0 kb) in the CP9ΔhlyA1::cat strain because hlyA1 was replaced by the smaller cat gene. A cnf1-specific band was absent in the cnf1 mutants (lanes 2 and 4) because the cnf1 probe (KG11F and KG11R) binds to a region of cnf1 that is absent in the mutant strains (due to internal deletion of a 1.2-kb BclI fragment). (C) Southern blot to detect the NcoI-NcoI hlyA fragment(s). Lane 1, CP9; lane 2, CP9cnf1; lane 3, CP9ΔhlyA1::cat; lane 4, CP9cnf1ΔhlyA1::cat. As anticipated, two copies of hlyA were evident in strains CP9 and CP9cnf1 (lanes 1 and 2, respectively), but only one band was apparent in the hlyA1 mutants (lanes 3 and 4) due to deletion of one hlyA gene locus.
A Southern blot analysis (ECL direct nucleic acid labeling and detection system; Amersham Biosciences, Piscataway, NJ) of NcoI-digested chromosomal DNA (predicted digests [Fig. 1A]) from the CP9cnf1 hlyA1 mutant compared to the chromosomal DNA of CP9cnf1 was performed (Fig. 1B). As anticipated, the cnf1 band was evident in the blot for wild-type strain CP9 and the CP9 hlyA1 mutant (Fig. 1B, lanes 1 and 3, respectively) but not in the blot for either CP9cnf1 (Fig. 1B, lane 2) or the cnf1 hlyA1 double mutant (Fig. 1B, lane 4). A probe specific for the hlyA1 fragment upstream of an internal NcoI site was PCR amplified (KG12F and KG13R [Table 2]). The two native hlyA genes were found on NcoI fragments that were approximately 7 and 8 kb long in the wild-type chromosomal digest and the cnf1 mutant (Fig. 1C, lanes 1 and 2, respectively). The cnf1-linked hlyA1 gene was not present in either the CP9ΔhlyA1::cat mutant (Fig. 1C, lane 3), as previously reported (61), or the newly constructed CP9cnf1ΔhlyA1::cat mutant (Fig. 1C, lane 4). The location of the hlyA1 mutation in the hly operon adjacent to the cnf1 locus was confirmed by performing long-range PCR with Pfu TURBO (Stratagene, La Jolla, CA) and the following primer sets: KG03F-KG10R and KG09F-KG10R (Fig. 2A).
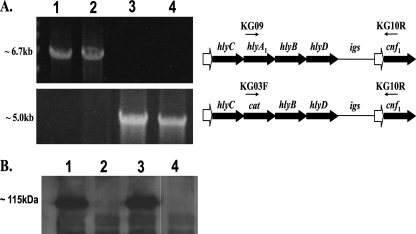
FIG. 2.
Long-range PCR. (A) Primers KG09 and KG10R (arrows above the hemolysin operon) were used to test for the presence of an hlyA1-containing PCR fragment amplified from hlyA1 to the cnf1 gene on the chromosome of the following strains: CP9 (lane 1), CP9cnf1 (lane 2), CP9ΔhlyA1::cat (lane 3), and CP9cnf1ΔhlyA1::cat (lane 4). PCR products (∼6.7 kb) were present for CP9 and CP9cnf1 but not for CP9ΔhlyA1::cat and CP9cnf1ΔhlyA1::cat, as shown in the top panel of the ethidium bromide-stained agarose gel on the left. Primers KG03F and KG10R were used to verify the replacement of hlyA1 with cat upstream of the cnf1 gene in mutant strains CP9ΔhlyA1::cat and CP9cnf1ΔhlyA1::cat (∼5.0-kb amplified PCR product from cat to cnf1 in the bottom panel of the ethidium bromide-stained agarose gel). (B) Western blot analysis with polyclonal anti-CNF1 serum as a probe was used to verify that wild-type levels of CNF1 (lane 1) were produced by CP9ΔhlyA1::cat (lane 3) and that, as expected, strains CP9cnf1 and CP9cnf1ΔhlyA1::cat did not produce CNF1 (lanes 2 and 4, respectively).
The CP9cnf1ΔhlyA1::cat mutant grew at wild-type levels and exhibited a reduced hemolytic phenotype on blood agar plates compared to CP9 (not shown). As expected, the cnf1 hlyA1 double mutant did not produce CNF1, as shown by Western blot analysis (Fig. 2B) and HEp-2 cell multinucleation assays of equal concentrations of sonicated cellular protein (data not shown) (Fig. 2B, lanes 1, 2, and 3, have been shown previously [61] and are included for comparison with lane 4 [cnf1 hlyA1 double mutant]). Protein concentrations were determined by using the methods described for a BCA protein assay kit from Pierce (Rockford, IL). Western blot analysis was done as described elsewhere with goat polyclonal anti-CNF1 serum as the probe (44).
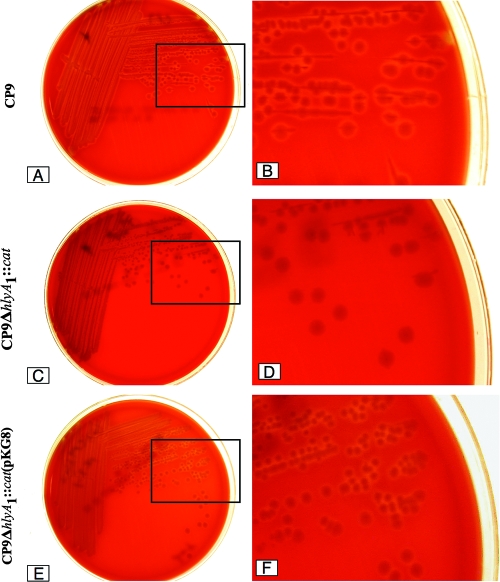
A complementing clone of the CP9ΔhlyA1::cat mutant was constructed as follows. The hlyA1 gene was amplified by PCR from the chromosome of UPEC strain CP9 and then ligated into pQE30 (QIAGEN, Valencia, CA) at the KpnI and BamHI enzyme sites. The clone was confirmed by restriction analysis and PCR with primers for the hlyA1 gene (KG09F and KG09R). The resulting plasmid, pKG8, was transformed into CP9ΔhlyA1::cat by electroporation and selected by plating preparations on LB agar that contained ampicillin. That complementation had occurred was evident from the zones of hemolysis surrounding colonies of CP9ΔhlyA1::cat(pKG8) on blood agar plates supplemented with ampicillin that were larger than the zones that were observed around colonies of the mutant CP9ΔhlyA1::cat (Fig. 3) or colonies of a strain transformed with the cloning vector pQE30 (not shown). On average, the hemolytic halos around CP9ΔhlyA1::cat(pKG8) colonies appeared to be smaller than those surrounding CP9 (Fig. 3), an observation that indicates that the mutant was complemented at less-than-wild-type levels.
FIG. 3.
Blood agar plate assay for hemolytic activity. (A) CP9 plate. (B) Enlargement of the section indicated by a box on the CP9 plate. (C) CP9ΔhlyA1::cat plate. (D) Enlargement of the section indicated by a box on the CP9ΔhlyA1::cat plate. (E) CP9ΔhlyA1::cat(pKG8) plate. (F) Enlargement of the section indicated by a box on the CP9ΔhlyA1::cat(pKG8) plate. Strains were streaked on plates, incubated overnight at 37°C, and photographed digitally with a Nikon Coolpix 995 camera.
Mouse UTI model.
The ascending UTI mouse model was used essentially as previously described (26, 55), with the modifications summarized below. The bacterial inocula used for infection of mice were prepared by culturing the strains in static LB broth for 48 h at 37°C, growth conditions that have been reported to enhance expression of type 1 pili (47). The extent of type 1 pilus expression was confirmed by mannose-sensitive agglutination of 1% baker's yeast suspended in Dulbecco's phosphate-buffered saline (PBS) (Cambrex BioSciences/Lonza, Walkersville, MD) (41, 42). The bacterial concentration was then adjusted to an A600 of ∼0.85, and 6 ml of each suspension was pelleted by centrifugation (10,000 × g for 5 min at 4°C) and resuspended in 0.5 ml of sterile saline (Baxter Healthcare Corporation, Deerfield, IL) to obtain a bacterial density of 109 CFU/ml. Each inoculum was pipetted into an adapter tube (6 in.) with a male/female line (Edwards Lifesciences, Irvine, CA) connected to a 30-gauge sterile needle (Becton Dickinson & Co, Franklin Lakes, NJ).
Four- to six-week-old C3H/HeOuJ female mice (Jackson Laboratory, Bar Harbor, ME) were used in these experiments because we and other workers (11) have previously found that this lipopolysaccharide-responsive strain of mice is readily infected by UPEC strains (55). Animals were quarantined for at least 1 week after receipt and were allowed access to water and food ad libitum. Animals were not given antibiotics to prevent loss of the hlyA1 complementing plasmid from strain CP9ΔhlyA1::cat(pKG8). The reason for this omission was that in preliminary experiments with antibiotic-free mice, the pKG8 plasmid was retained in the bacterial strain, as ascertained by comparison of colony counts of homogenates of CP9ΔhlyA1::cat(pKG8)-infected bladders on LB agar with and without ampicillin.
Mice were anesthetized with isoflurane (Hospira, Inc., Lake Forest, IL), and a 1-in. (2.5-cm) sterile polyethylene catheter (inside diameter, 0.28 mm; outside diameter, 0.61 mm; Intramedic, Becton Dickinson, Sparks, MD) was inserted intraurethrally into each animal. Prior to infection, a urine sample was collected from each mouse to test for preexisting bacteriuria, and mice in which the bacterial levels were >102 cells/ml were excluded from the study. The needle fitted to the inoculating apparatus was inserted into the catheter, and 25 μl of each inoculum (2.5 × 107 CFU) was delivered over a 20-s period into the bladder of an anesthetized animal. Immediately after inoculation, the catheter was withdrawn from the mouse, and no further manipulations were carried out until completion of the study. At 1, 3, or 5 days postchallenge, urine was collected by catheterization, serially diluted in PBS, and plated on LB agar to determine the bacterial load. Mice were then euthanized in groups of 10 by using an isoflurane overdose. Kidneys and bladders were aseptically removed, homogenized in PBS, and serially diluted for viable counting, and the number of bacteria was expressed as the number of CFU per bladder or per kidney. Alternatively, some of the samples were prepared for histological examination. Control animals were instilled with PBS. In each experiment, at least one mouse was sacrificed immediately after inoculation, and the kidneys were aseptically removed for assessment of quantitative vesicoureteral reflux (32). All animal experiments were performed in accordance with the principles outlined in the Guide for the Care and Use of Laboratory Animals (49).
Histopathology.
The bladder and kidneys from selected euthanized infected and control mice were removed and immediately placed in 5% phosphate-buffered formalin (Fisher Scientific, Pittsburgh, PA) for at least 12 h. The resulting formalin-fixed tissues were then embedded in paraffin and cut into 5-μm sections (Histoserv, Rockville, MD), and the resulting slides were stained with hematoxylin and eosin or with Giemsa stain for better visualization of bacteria. Four stained sections per organ per animal were prescreened, and then single organ sections were graded in a blinded manner by one of us (R. Conran, a pathologist) for each of the following histopathologic parameters: interstitial edema, hemorrhage, leukocyte infiltration, and uroepithelial destruction. A semiquantitative severity scale (0 to 5 for each parameter) was used for these evaluations. For each of the four categories assessed, average scores (for five samples) of less than 1 were considered a mild effect; scores of 2 to 3 were classified as a moderate effect, and scores greater than 4 were categorized as a severe effect of the strain on the histopathological feature.
In vitro alpha-hemolytic activity determination.
Overnight cultures of CP9 or CP9ΔhlyA1::cat were diluted 1:100 with LB broth (optical density at 600 nm, ∼0.03) and incubated at 37°C (considered time zero). One-milliliter samples were taken hourly from time zero to 48 h, and the bacterial cells were pelleted by centrifugation (13,000 rpm, 10 min). The pellets were washed once with PBS and resuspended in 1 ml PBS. Three hundred microliters of each pellet or supernatant was mixed with 300 μl of 4% sheep RBCs (Hemostat Laboratories, Dixon, CA). Each sample was then incubated at 37°C for 1 h, and the cells were pelleted by centrifugation (13,000 rpm, 5 min). Two hundred microliters of each resultant supernatant was carefully transferred into an untreated flat-bottom 96-well microtiter plate (Corning Incorporated, Corning, NY) and read at 405 nm (A405) using a plate reader (Bio-Tek Instruments, Winooski, VT). RBCs mixed with water were used as a positive control, while RBCs with PBS or LB medium served as negative controls. Images of the microtiter assay plates were obtained, and the intensity of the red color in a well correlated with the A405 measurement. Samples were tested for alpha-hemolytic activity no more than 5 h after the time of collection, and each determination was done in triplicate.
Western blotting.
Bacterial pellets were obtained as described above for the hemolysis assay. A Western analysis was carried out essentially as previously described (43), with the following modifications. Pellets were concentrated twofold by resuspension in 0.5 ml PBS and then disrupted by sonication. Bacterial extracts were then clarified by centrifugation. The protein concentrations of clarified toxin extracts were determined by absorbance at 280 nm utilizing a NanoDrop 1000 spectrophotometer (Nanodrop Technologies LLC, Wilmington, DE), and the concentration of each extract was adjusted to 1 mg/ml. The CP9 or CP9ΔhlyA1::cat pellet lysates were then subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (4 to 20% Tris-glycine gel; Invitrogen, Carlsbad, CA). After electrophoretic separation, the proteins were transferred to Optitran nitrocellulose membranes with a Trans-Blot SD semidry electrophoretic transfer cell (Bio-Rad), and the membranes were then blocked overnight in 5% dry milk in PBS-0.1% Tween 20. Membranes were incubated with polyclonal goat anti-CNF sera (1:10,000) (44), washed in PBS-0.1% Tween 20, and then incubated with horseradish peroxidase-conjugated porcine anti-goat sera (1:10,000) (Roche). Reactive proteins were detected by enhanced chemiluminescence (GE HealthCare, Buckinghamshire, United Kingdom).
HEp-2 cell multinucleation assay.
HEp-2 multinucleation assays were performed as previously described (44), with the following modifications. Cells were seeded at a concentration of 5 × 104 cells well−1 into 96-well plates (Costar) and incubated for 4 h at 37°C with 5% CO2. Dilutions (1:5) of clarified sonic lysates from bacterial cells harvested at 2 to 48 h (see above) were applied to HEp-2 cells at a starting concentration of 20 μg per well. Cells were incubated for 72 h at 37°C in the presence of 5% CO2, fixed, and stained with Hema-3 (Fisher Scientific), and the degree of multinucleation was determined by light microscopy.
Immunofluorescence.
Five-micrometer sections of infected or uninfected murine bladders were immunostained to determine the presence of uroplakin or E. coli as previously described (61). Briefly, fixed tissue sections were deparaffinized and then heated in a microwave oven to optimize antigen retrieval. Next, nonspecific binding sites on the treated sections were blocked with 1% bovine serum albumin (Sigma, St. Louis, MO) and then incubated for 1 h at room temperature with the appropriate primary antibody, either goat anti-uroplakin III antibody (Santa Cruz Biotechnology, Santa Cruz, CA) or rabbit anti-E. coli antibody (Biodesign, Saco, ME). The dilution used for each antibody was selected based on the manufacturer's recommendation. The slides were subsequently incubated for 1 h at room temperature with AlexaFluor 488- or 555-conjugated secondary antibody (Molecular Probes, Eugene OR) and mounted with Fluoromount-G (Southern Biotechnologies Associates, Inc., Birmingham, AL). All samples were analyzed with an Olympus BX60 microscope with a BX-FLA reflected-light fluorescence attachment. Images were acquired with a SPOT RT charge-coupled device digital camera (Diagnostic Instrument, Inc., Miami, FL) and assembled for presentation using Adobe Photoshop.
Statistical analysis.
One-way analysis of variance (ANOVA) was used to identify statistically significant differences in the geometric mean numbers of CFU/ml of bacteria among the groups of mice. When experiments were done in triplicate, two-way ANOVA was used after adjustment for differences among experiments. Separate ANOVA analyses were done for urine, bladder, and kidney data for the different groups of mice. Statistical significance for histopathological analyses was also determined by one-way ANOVA, followed by Tukey's post hoc pairwise comparisons across the set of strains used in this investigation. P values less than 0.05 were considered statistically significant.
RESULTS
CP9 colonization versus CP9ΔhlyA1::cat colonization in single- and mixed-strain infection studies over time.
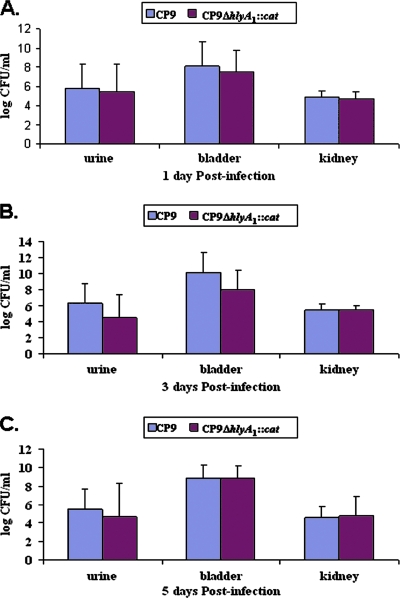
The overall objective of these experiments was to assess the relative importance of Hly and CNF1 individually and together in the urovirulence of UPEC strain CP9. We began our investigation by evaluating the importance of Hly in colonization because we previously tested the impact of a mutation in cnf1 on bacterial colonization and histopathology in a mouse UTI model (55) and in a rat acute prostatitis system (54). As noted above, these previous studies revealed a significant role for CNF1 in the acute inflammation of the mouse bladder (55) and rat prostate (54), but the investigations did not reveal a statistically significant effect of CNF1 on bacterial persistence in the mouse UTI model or the rat prostrate after single-strain challenge. However, in mixed infections with wild-type strain CP9 and CP9cnf1, CP9 outcompeted the mutant through day 9 of infection in the urine and in the bladder and kidney tissues of C3H/HeOuJ mice (55). In contrast, the growth advantage of CP9 over CP9cnf1 was not evident in rat prostates (54). Here, we inoculated groups of 30 mice with CP9 or CP9ΔhlyA1::cat intraurethrally using 2.5 × 107 CFU and then euthanized groups of 10 animals/strain at days 1, 3, and 5 postinfection. Bacterial counts in the urine and in bladder and kidney homogenates were obtained for five mice; kidneys and bladders from the other five animals were prepared for histological evaluation. The experiment was done three times. As shown in Fig. 4, no statistically significant differences in colony counts were observed in the urine, bladder, or kidneys of CP9-infected mice and CP9ΔhlyA1::cat-infected mice.
FIG. 4.
Kinetics of UTI of C3H/HeOuJ mice with CP9 and the CP9ΔhlyA1::cat isogenic mutant. Fifteen female C3H/HeOuJ mice were inoculated via the urethra with 2.5 × 107 CFU of either CP9 or CP9ΔhlyA1::cat. After 1, 3, and 5 days, mice were euthanized and urine samples, as well as bladders and kidneys, were collected, homogenized, and plated on LB agar for bacterial enumeration. (A) Log CFU/ml for 1 day postinfection. (B) Log CFU/ml for 3 days postinfection. (C) Log CFU/ml for 5 days postinfection. Each bar indicates the geometric mean for a sample, and the error bars indicate one standard deviation above the geometric mean.
Next, we asked whether coinoculation of CP9 and CP9ΔhlyA1::cat would permit us to discern differences in fitness between these strains that were not apparent in the single-strain animal challenge studies described above. For this purpose, we introduced equal numbers of CFU/ml of the parent CP9 strain and the hlyA1 mutant intraurethrally into C3H/HeOuJ female mice as described in Material and Methods. Urine and bladders from the challenged animals were collected at 1 and 3 days postinfection, and colonies of the strains in the mixture were distinguished by using the chloramphenicol resistance of the hlyA1 mutant on antibiotic-supplemented LB agar. We found that the CP9ΔhlyA1::cat counts were comparable to the strain CP9 counts when the organisms were coinoculated and were at the levels observed in the single-strain UTI model (Table 3 and data not shown). One caveat to interpretation of the results of these mixed-infection studies is that it is conceivable that the Hly made by the wild-type strain could have complemented, and hence obscured, a defect in the fitness of the mutant. Nevertheless, these results, together with the results of the single-strain challenge experiments, strongly indicate that expression of Hly does not influence the extent of UPEC strain CP9 colonization.
TABLE 3.
Quantitative culture of CP9 UPEC strains in C3H/HeOuJ mouse samples collected 1 day postchallenge
| Strain | n | Mean log10 CFU/ml (SD)a
|
|
|---|---|---|---|
| Urine | Bladder | ||
| CP9 (O4:H5:K54) (parent) | 15 | 5.81 (2.51) | 8.12 (2.54) |
| CP9ΔhlyA1::cat | 15 | 5.42 (2.91) | 7.42 (2.37) |
| CP9cnf1 | 10 | 5.77 (2.09) | 7.13 (1.32) |
| CP9cnf1ΔhlyA1::cat | 10 | 5.75 (2.22) | 7.33 (0.34) |
| CP9ΔhlyA1::cat(pKG8) | 10 | 6.43 (2.57) | 8.98 (1.29) |
| CP9ΔhlyA1::cat(pQE30) | 10 | 5.51 (2.41) | 7.59 (0.73) |
There were no statistically significant differences among urine counts or among bladder counts as assessed by ANOVA.
Infection with hlyA1-complemented CP9ΔhlyA1::cat mutant.
To further define any possible role that Hly may play in the pathogenesis of UPEC-mediated UTI, a CP9ΔhlyA1::cat complemented mutant, CP9ΔhlyA1::cat(pKG8), was created by subcloning the hlyA1 gene into the pQE30 vector. To ensure that pQE30, the plasmid (Table 1) used for this complementation, was not itself the cause of the increase in the hemolytic effect or any other unwanted effects, we transformed the cloning vector alone into CP9ΔhlyA1::cat. The growth curves for CP9ΔhlyA1::cat, CP9ΔhlyA1::cat(pKG8), and CP9ΔhlyA1::cat(pQE30) in LB media were similar (data not shown). When we tested CP9ΔhlyA1::cat(pKG8) and CP9ΔhlyA1::cat(pQE30) in the mouse model of UTI, we observed no statistically significant differences in bacterial counts in the urine samples or the infected bladder homogenates at 1 day postinfection for the mice given the different strains (Table 3). Moreover, the numbers of CFU/ml in the urine or bladder tissues were similar to those seen 1 day after challenge with strain CP9 or CP9ΔhlyA1::cat (Fig. 4 and Table 3).
Bacterial colonization of the bladder by CP9cnf1ΔhlyA1::cat and CP9cnf1.
Since our primary goal was to investigate the relative contributions of CNF1 and Hly in the urovirulence of UPEC strain CP9, we examined the impact of a cnf1 hlyA1 double mutant on colony counts in the urine and bladders of C3H/HeOuJ mice 1 day after challenge. As a control, we included a set of mice inoculated with the previously examined CP9cnf1 isogenic mutant (55). We observed no statistically significant differences in colony counts in the urine or bladder homogenates of animals infected with CP9cnf1ΔhlyA1::cat and animals infected with the CP9cnf1 mutant; moreover, the numbers were similar to those for the wild type at 1 day after challenge (Table 3). In aggregate, these findings suggest that virulence factors of UPEC strain CP9 other than Hly and CNF1 are integral for bacterial colonization of the bladder, at least as evaluated at 1 day after infection (we did not test all strains after 3 and 5 days).
Histological examination of bladders.
Next, we evaluated the relative impact of Hly and CNF1 on acute inflammation and damage using bladder sections from mice infected with each of the members of the CP9 isogenic strain set. We used a scoring system for coded sections that ranged from 0 to 5 for each of the following histopathological parameters: interstitial edema, hemorrhage, leukocyte infiltration, and uroepithelial destruction. The pathologist evaluator of the slides was blinded to the bacterial strain used to infect the animal from which the bladder section slides were prepared. Five tissue blocks (one block per animal) for 1 day after infection with CP9 or each CP9-derived strain were analyzed. Additional sections from bladders and kidneys harvested 3 and 5 days after infection with CP9 or CP9ΔhlyA1::cat were also examined.
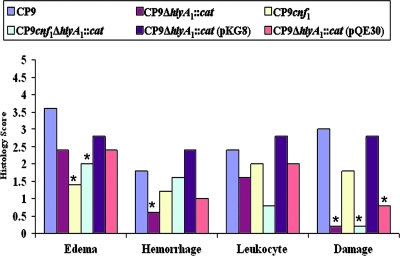
We found that bladders from CP9-challenged animals exhibited the highest levels of interstitial edema among the mice tested in this study (Fig. 5). The higher level of edema was evident on a representative slide taken from the tissue set scored (Fig. 6A). The fact that the interstitial edema was greatest for the parental CP9 strain and was statistically significantly different from the levels of the edema observed with the isogenic mutants CP9cnf1 and CP9cnf1ΔhlyA1::cat (Fig. 5) (P < 0.001 and P < 0.028, respectively) indicates that the submucosal swelling was largely due to CNF1 (Fig. 6, compare panels E and I with panel A). Additionally, CP9-provoked submucosal edema remained clearly apparent at day 3 after infection (Fig. 7, compare panel A with control panel E) and was still evident at day 5 (Fig. 7C).
FIG. 5.
Effect of CP9 strains on the histology of murine bladders. Groups of five female C3H/HeOuJ mice were inoculated via the urethra with 2.5 × 107 CFU of CP9, CP9ΔhlyA1::cat, CP9cnf1, CP9cnf1ΔhlyA1::cat, CP9ΔhlyA1::cat(pKG8), or CP9ΔhlyA1::cat(pQE30) and euthanized 1 day later. Bladders were removed, fixed in formalin, and processed for histological examination. Each sample was examined by light microscopy. A score of 0 to 5 was assigned to each bladder for edema, hemorrhage, leukocyte presence, and urothelial damage. Scores for each group of mice were combined, and the averages are indicated by the bars. An asterisk indicates that the tissue response to a strain was statistically significantly different from the response to the strain for which the response was greatest for the specific effect, as determined by ANOVA assessment followed by Tukey's post hoc pairwise comparisons.
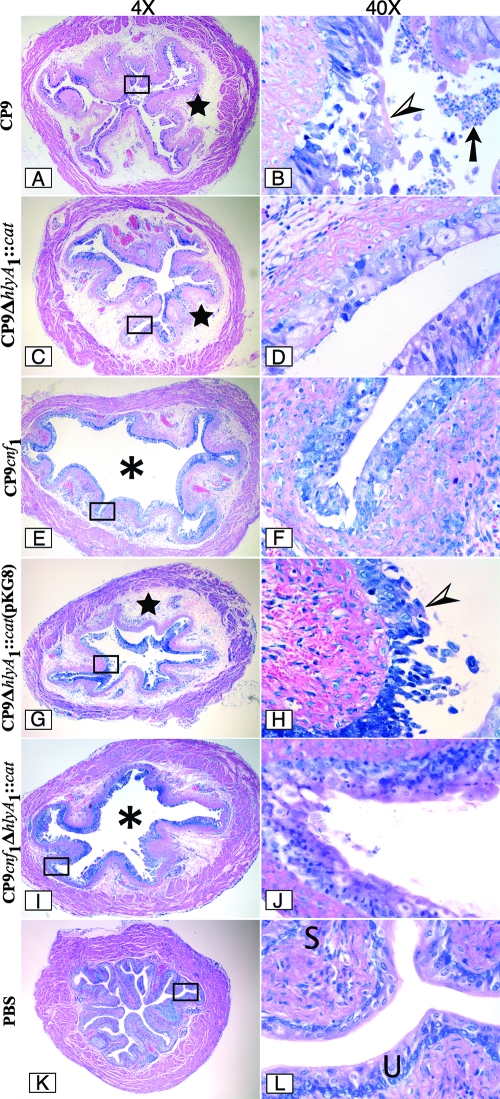
FIG. 6.
Infection of female C3H/HeOuJ mice with CP9 UPEC strains. Mice were inoculated with CP9 (A and B), CP9ΔhlyA1::cat (C and D), CP9cnf1 (E and F), CP9ΔhlyA1::cat(pKG8) (G and H), and CP9cnf1ΔhlyA1::cat (I and J) for 1 day, and then sections were cut, fixed with formalin, embedded in paraffin, stained with Giemsa stain, and analyzed by light microscopy. Images of a representative sample for each infection are shown at a magnification of ×4 on the left. The rectangles in the images indicate the sections that are shown at a magnification of ×40 on the right. The stars in panels A, C, and G indicate a high degree of edema. The asterisks in panels E and I indicate the luminal space. Note the presence of many inflammatory cells (arrow) in the luminal space in panel B. The arrowheads in panels B and H indicate the intense damage to the urothelium caused by CP9 and CP9ΔhlyA1::cat(pKG8), respectively. In contrast, the minimal damage caused by CP9ΔhlyA1::cat, CP9cnf1, and CP9cnf1ΔhlyA1::cat is shown in panels D, F, and J, respectively. Panels K and L show a murine bladder instilled with only PBS. U and S in panel L indicate the murine urothelium and submucosa, respectively.
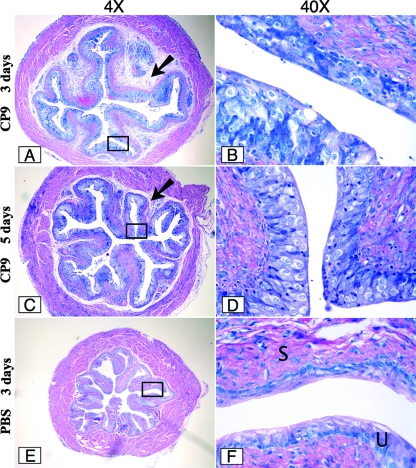
FIG. 7.
Infection of female C3H/HeOuJ mice with the CP9 UPEC strain after 3 and 5 days. Groups of five mice were inoculated with CP9, and bladders were harvested after 3 days (A and B) and 5 days (C and D) and then processed as described in the legend to Fig. 6. Images of a representative sample for each infection are shown at a magnification of ×4 on the left. The rectangles in the images indicate the sections that are shown at a magnification of ×40 on the right. Panels E and F show a murine bladder instilled with only PBS and harvested after 3 days. U and S in panel F indicate the murine urothelium and submucosa, respectively. The arrows in panels A and C indicate the degree of interstitial edema at 3 and 5 days, respectively.
We also examined the extent of hemorrhage in the bladders caused by the strains used in these experiments. We found that loss of Hly, as exemplified by bladders from mice inoculated with CP9ΔhlyA1::cat, resulted in the largest decrease in the degree of hemorrhage in tissues (P < 0.041) (Fig. 5). When we assessed the slides obtained for sections of bladders infected with the various strains for the extent of leukocyte infiltration (Fig. 5), we noted that the tissue sections from mice inoculated with CP9 and the equivalent strain CP9ΔhlyA1::cat(pKG8) showed a higher influx of white cells than the tissue sections from mice inoculated with any of the other isogenic mutants. In particular, the differences were most apparent when we compared the CP9 and CP9ΔhlyA1::cat(pKG8) strains to the CP9cnf1ΔhlyA1::cat mutant, although the differences were not statistically significant (P < 0.278 and P < 0.104, respectively) (Fig. 5). Furthermore, histopathological examination also revealed significantly greater damage to the urothelium in the bladders of mice infected with CP9 than in the bladders of mice infected with the CP9ΔhlyA1::cat and CP9cnf1ΔhlyA1::cat mutants (P < 0.0001 for each strain) (Fig. 5 and Fig. 6, compare panel B with panels D and J). Additionally, we evaluated bladder sections from mice infected with the hlyA1-complemented CP9ΔhlyA1::cat mutant and the isogenic CP9ΔhlyA1::cat(pQE30) mutant for uroepithelial damage at 1 day postinfection. We observed that after reinstatement of the capacity of the hlyA1 mutant to produce Hly (Fig. 3), the complemented mutant could cause extensive uroepithelial damage (Fig. 6G and H). In contrast, markedly less destruction of the luminal surface of the bladders of mice infected with the isogenic CP9ΔhlyA1::cat(pQE30) mutant was detectable (data not shown) (the appearance of sections was similar to the appearance of sections in Fig. 6C and D). Moreover, all three strains that did not make Hly [CP9ΔhlyA1::cat, CP9cnf1ΔhlyA1::cat, and CP9ΔhlyA1::cat(pQE30)] caused statistically significantly lower levels of damage to the murine urothelium than the strains that produced Hly [CP9, CP9cnf1, and CP9ΔhlyA1::cat(pKG8)] (Fig. 5). Overall, these in vivo results are in agreement with our previous in vitro observations which showed that a mutation in the hlyA1 gene of strain CP9 resulted in attenuation of the uroepithelial damage that was evident soon after CP9 infection of 5637 bladder cells grown as monolayers or as three-dimensional organoids (61).
Surprisingly, at 3 days postinfection, our histopathologic observations of the murine bladders revealed no apparent urothelium injury in CP9-infected bladders (Fig. 7A and B). In fact, when we evaluated the slides containing bladder sections from mice infected with CP9 and mice infected with CP9ΔhlyA1::cat at 3 and 5 days postinfection, the uroepithelia in all sections appeared to be smooth and generally intact and much like that of the PBS-inoculated control (Fig. 7; data not shown for CP9ΔhlyA1::cat-infected sections). We concluded from analyses of the day 1, 3, and 5 sections that Hly-mediated effects on the bladder appeared to be limited to the first day of infection, whereas edema, largely attributable to CNF1, at least after 1 day of infection (Fig. 5), remained readily discernible at day 3 after challenge and was still apparent at day 5 postinoculation (Fig. 7).
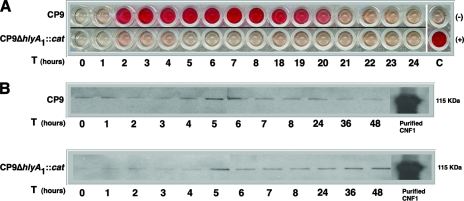
Alpha-hemolytic activity and CNF1 expression.
We next asked whether the differences in timing of the impact of Hly and CNF1 on infected murine bladders reflected variations in the kinetics of activity of these toxins in vitro. We observed that the CP9 alpha-hemolytic activity in culture supernatants, as determined by lysis of sheep RBCs, began at 2 h, remained constant until approximately 19 to 20 h, when it started to decrease, and then decreased to background levels by 22 h postinoculation (Fig. 8A). As expected, the CP9ΔhlyA1::cat mutant displayed little alpha-hemolytic activity throughout the assay (Fig. 8A). Furthermore, no alpha-hemolytic activity was seen in the PBS-resuspended pellets of the CP9 strain or its hly isogenic mutant at any time (data not shown). In contrast, CNF1 was detectable even at time zero and remained evident until 48 h, as ascertained by Western blot analysis (Fig. 8B). Moreover, the presence of the CNF1 protein coincided with the presence of CNF1 activity, as measured by a HEp-2 multinucleation assay, up to 48 h after culture inoculation (data not shown). Taken together, these data are consistent with our conclusion based on the results obtained with the murine UTI model that Hly has an impact only early in infection, while CNF1 effects are evident throughout the infection.
FIG. 8.
Alpha-hemolytic activity and CNF1 expression in CP9 strains. Samples were prepared and assays were carried out as described in Material and Methods. (A) Alpha-hemolytic activity of CP9 or CP9ΔhlyA1::cat supernatants collected hourly from 0 to 48 h was measured by mixing the samples with sheep RBCs. Results for 9 to 17 and 25 to 48 h are not shown. C(−) and C(+) were the negative and positive controls, respectively. (B) Western blot analyses of CP9 and CP9ΔhlyA1::cat bacterial cell lysates probed with polyclonal anti-CNF1 serum.
Immunofluorescence.
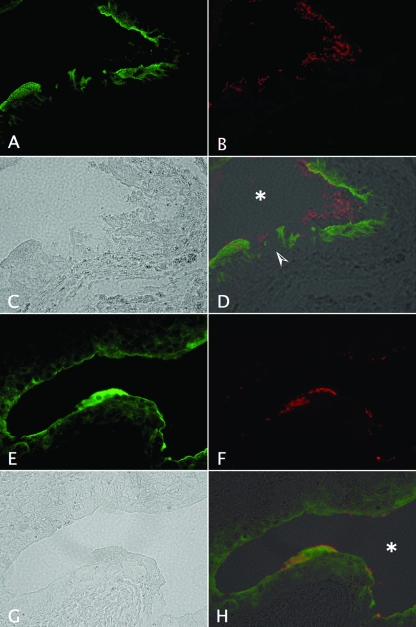
To confirm our hematoxylin and eosin and Giemsa stain observations concerning the impact of Hly produced by CP9 on the uroepithelium after 1 day of infection, we treated unstained bladder sections from mice infected with CP9 and the isogenic CP9ΔhlyA1::cat mutant with antibodies that recognize both the bacteria and the uroplakins, a group of proteins that play a major role in the structure and physiology of the urothelium. We found that at 1 day postinfection, the parental strain had a direct detrimental effect on the structural support indicated by immunoreactivity with antibodies to uroplakins (Fig. 9A). The damage was apparent even in phase-contrast images (Fig. 9C). In contrast, no destructive effect of the CP9ΔhlyA1::cat mutant on the uroplakin-labeled urothelium was discernible (Fig. 9E and G). Lastly, CP9 seemed to be shed into the lumen of the bladder and did not adhere as well to the damaged uroepithelium (Fig. 9B and D) as the closely uroepithelium-associated mutant CP9ΔhlyA1::cat (Fig. 9F and H). Taken together, these immunofluorescence results and histological data are suggestive of the significant role that Hly may play in causing uroepithelial tissue damage and even in the location of the bacteria in infected tissue at 1 day postchallenge.
FIG. 9.
Impact of CP9 and the isogenic CP9ΔhlyA1::cat mutant on bladder uroepithelium as revealed by immunostaining of uroplakins. Female C3H/HeOuJ mice were inoculated via the urethra with 2.5 × 107 CFU of CP9 (A to D) or CP9ΔhlyA1::cat (E to H). Mice were euthanized 1 day later. Bladders were removed, fixed in formalin, embedded in paraffin, processed for immunostaining, and analyzed by immunofluorescence microscopy. The sequence shows samples stained with goat anti-uroplakin III antibody (green) (A and E) and with rabbit anti-E. coli antibody (red) (B and F), as well as phase-contrast (C and G) and merged images (D and H). The arrowhead in panel D indicates the severe damage to the uroplakin-lined urothelium caused by CP9, in contrast to the minimal effect on the uroplakin-stained uroepithelium of the CP9ΔhlyA1::cat-infected bladder in panel H. The asterisks in panels D and H indicate the murine bladder luminal space. Magnification, ×40.
DISCUSSION
In this investigation, we created or used previously generated single, double, or complemented hlyA1 or cnf1 mutants with the UPEC strain CP9 background. We evaluated the relative impact of expression of CNF1 and Hly on bacterial colonization and histopathology in C3H/HeOuJ mouse bladders and kidneys after intraurethral challenge in a model of ascending UTI. The major findings of this study were as follows. First, we observed no statistically significant impact of either Hly or CNF1 on bacterial colonization in the bladder or kidney in single-mutant infection studies or in a mixed infection with CP9 and CP9ΔhlyA1::cat. Second, we found that Hly expression by CP9 caused extensive damage to the uroepithelium and augmented tissue hemorrhage in murine bladders at 1 day after challenge. However, no Hly-evoked uroepithelial damage was noted at day 3 or 5 after CP9 challenge. Third, CNF1 was primarily responsible for the striking submucosal edema that was evident in CP9-infected bladders (compared with cnf1 mutant-challenged bladders) at days 1 and 3 and to a much lesser extent at day 5 after intraurethral inoculation of bacteria. Fourth, both CNF1 and Hly contributed to leukocyte infiltration into the bladder at day 1 after infection, which was apparent from the lower (albeit not statistically significant) histopathology score obtained with the double mutant.
We concluded that Hly production by strain CP9 had no influence on bacterial colonization of the bladder or kidneys of intraurethrally challenged mice based on the results of both single-strain (CP9 versus CP9ΔhlyA1::cat) and mixed-strain (CP9 plus CP9ΔhlyA1::cat) intraurethral infection studies. This conclusion is consistent with several previous reports on the effect of Hly on bacterial persistence at various body sites. Most germane to this study, Haugen and colleagues (30) recently reported that an hlyA mutation in wild-type UPEC strain CFT073 (a strain that does not make CNF1) had no effect on colonization of the urinary tract at 48 h after infection. Additionally, Sheshko et al. found that an hlyA knockout mutation in an E. coli strain used as a probiotic for premature and newborn pigs did not adversely effect colonization of the gut of the animals (60). Lastly, Linggood and Ingram (40) showed that when a mixture of an E. coli strain with an Hly-encoding plasmid and an E. coli strain without the Hly-encoding plasmid was inoculated intraperitoneally into mice, the nonhemolytic isolate colonized as well as the hemolytic parent strain. Together, our results and those of other workers suggest that any putative (30, 60) or demonstrated (this study) damage evoked by Hly does not translate into enhanced fitness of the infecting E. coli strain.
Our observation that colony counts in the urine, bladders, and kidneys of mice 1 day after intraurethral infection with either CP9 or CP9cnf1 were not statistically significantly different is consistent with the results of our previous study (55). Nevertheless, in the previous investigation, we did observe an advantage for the wild type in a mixed infection, although no enrichment for CP9 in competition with CP9cnf1 was evident in the rat acute prostatitis model (54). Overall, we inferred from these data that CNF1 synthesis provides an edge for persistence of a UPEC strain in the mouse bladder but that the effect is not apparent under the normal circumstances of a UTI (i.e., single-strain infections of an otherwise sterile site [the bladder or the kidney]). Based on our colonization studies with the set of CP9 hlyA1 and cnf1 isogenic mutants, we surmised that other UPEC virulence factors, such as fimbrial and afimbrial adhesins (for a review, see reference 7), are largely responsible for maintenance of UPEC proliferation in organs of the urinary tract.
In striking contrast to the lack of an impact on UPEC colonization, Hly and CNF1 each had a marked, but different, major effect on the histopathology of the bladder (Fig. 5 and 6). Of particular note was the readily apparent destruction of the uroplakin-containing uroepithelium of the bladder mediated by Hly in tissue sections from bladders harvested after 1 day of infection (Fig. 9). The fact that this clear-cut Hly-mediated effect in the bladder had not been previously documented may reflect several unique aspects of this investigation. First, we constructed a mutation in the hlyA1 structural gene that is in the hly operon linked to cnf1 and in the operon (of the two operons in CP9) that we had previously shown was primarily responsible for hemolysis and destruction of bladder cells in vitro (61). Thus, we know that in CP9 the hly operon associated with cnf1 is more active than the second, unlinked hly operon. Second, we used a multicopy plasmid to complement the mutant and restored hemolysis in vitro and damage in vivo. Third, we harvested bladders early in the infection (1 day). Fourth, we viewed cross sections of the entire infected bladder at a magnification of ×4 as a first step in the evaluation of histopathology. This lower-power scan yielded a broader field for examination of all features of acute inflammation.
The fact that we first assessed the entire infected bladder section under low magnification made the extent of CNF1-evoked edema much more apparent. Although we certainly emphasized the impact of CNF1 on acute inflammation in our previous investigations (54, 55), we did not focus on submucosal edema as a prominent feature of the CNF1-augmented immune response. The whole-bladder assessments made us better able to evaluate relative levels of submucosal edema evoked by CP9 on the various days after infection. Thus, we feel comfortable with our conclusion that CNF1 imparts similar degrees of pathology on the bladder at days 1 and 3 after infection and, to a much lesser extent, at day 5. Exactly how CNF1 promotes edema is not clear, but edema may reflect the relationship between the mechanism of action of CNF1 (i.e., constitutive activation of small Rho-GTPases) and the capacity of the toxin to stimulate transcription of cytokine genes involved in acute inflammation (48).
The kinetics of the influence of Hly on the bladder uroepithelium differed from the kinetics of the influence of CNF1 even though the two toxins are reported to be coexpressed in vitro from wild-type UPEC strain J96 (37). Indeed, histological observations revealed only clear Hly-mediated histological damage at 1 day after infection. There are at least four possible explanations for these findings. First, Hly may not be made throughout the course of the infection, a possibility supported by comments of Mobley et al. (46), while CNF1 continues to be synthesized. Second, both Hly and CNF1 may be made only early in infection, but Hly may act quickly (as a membrane-active toxin) and then degrade, while CNF1 may act slowly (as a toxin with an intracellular target) and then remain stable throughout the course of the infection. Third, both Hly and CNF1 may be produced throughout the course of infection, but Hly may be fairly quickly neutralized by preexisting antibodies known to be present in some human sera (18, 59). Fourth, the uroepithelial cells that replace the cells exfoliated during the first 24 h of infection may be resistant to Hly. Our measurements of the kinetics of alpha-hemolytic and CNF1 activities expressed by the wild-type CP9 strain in vitro most strongly support the first explanation. Nevertheless, the actual expression patterns of the toxins in vivo remain to be determined.
In summary, in this report we addressed the relative roles that CNF1 and Hly play in the pathogenesis of UPEC-conferred UTIs. Although in previous reports workers associated Hly production by UPEC strains with pyelonephritis (46, 63, 64), only a few studies focused on the biological and histological impact of this toxin in cystitis. In this investigation, we demonstrated for the first time (to our knowledge) that Hly plays a major role in evoking damage in the uroepithelium and in inducing hemorrhage in the bladder during the early stages of E. coli-mediated cystitis in the mouse. We also extended our previous discovery that CNF1 stimulates acute inflammation in the bladder by revealing the critical function of the toxin in the elicitation of submucosa edema.
Acknowledgments
We gratefully acknowledge Anastasia L. Sowers for her assistance with the catheterization technique. We thank Farhang Alem, Humberto Carvalho, Steve Darnell, and Dianne McLeod for technical assistance. We are also grateful to Angela Melton-Celsa for assistance with the histology sections and to Cara Olsen for the statistical analysis.
National Institutes of Health grant AI38281 supported this research.
Editor: V. J. DiRita
Footnotes
Published ahead of print on 28 April 2008.
REFERENCES
- 1.Aktories, K. 1997. Rho proteins: targets for bacterial toxins. Trends Microbiol. 5282-288. [DOI] [PubMed] [Google Scholar]
- 2.Alonso, P., J. Blanco, M. Blanco, and E. A. Gonzalez. 1987. Frequent production of toxins by Escherichia coli strains isolated from human urinary tract infections: relation with haemagglutination. FEMS Microbiol. Lett. 48391-396. [Google Scholar]
- 3.Andreu, A., A. E. Stapleton, C. Fennell, H. A. Lockman, M. Xercavins, F. Fernandez, and W. E. Stamm. 1997. Urovirulence determinants in Escherichia coli strains causing prostatitis. J. Infect. Dis. 176464-469. [DOI] [PubMed] [Google Scholar]
- 4.Blanco, J., M. P. Alonso, E. A. Gonzalez, M. Blanco, and J. I. Garabal. 1990. Virulence factors of bacteraemic Escherichia coli with particular reference to production of cytotoxic necrotising factor (CNF) by P-fimbriate strains. J. Med. Microbiol. 31175-183. [DOI] [PubMed] [Google Scholar]
- 5.Blanco, J., M. Blanco, M. P. Alonso, J. E. Blanco, E. A. Gonzalez, and J. I. Garabal. 1992. Characteristics of haemolytic Escherichia coli with particular reference to production of cytotoxic necrotizing factor type 1 (CNF1). Res. Microbiol. 143869-878. [DOI] [PubMed] [Google Scholar]
- 6.Blum, G., V. Falbo, A. Caprioli, and J. Hacker. 1995. Gene clusters encoding the cytotoxic necrotizing factor type 1, Prs-fimbriae and α-hemolysin form the pathogenicity island II of the uropathogenic Escherichia coli strain J96. FEMS Microbiol. Lett. 126189-195. [DOI] [PubMed] [Google Scholar]
- 7.Bower, J. M., D. S. Eto, and M. A. Mulvey. 2005. Covert operations of uropathogenic Escherichia coli within the urinary tract. Traffic 618-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caprioli, A., G. Donelli, V. Falbo, R. Possenti, L. G. Roda, G. Roscetti, and F. M. Ruggeri. 1984. A cell division-active protein from E. coli. Biochem. Biophys. Res. Commun. 118587-593. [DOI] [PubMed] [Google Scholar]
- 9.Caprioli, A., V. Falbo, L. G. Roda, F. M. Ruggeri, and C. Zona. 1983. Partial purification and characterization of an Escherichia coli toxic factor that induces morphological cell alterations. Infect. Immun. 391300-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caprioli, A., V. Falbo, F. M. Ruggeri, L. Baldassarri, R. Bisicchia, G. Ippolito, E. Romoli, and G. Donelli. 1987. Cytotoxic necrotizing factor production by hemolytic strains of Escherichia coli causing extraintestinal infections. J. Clin. Microbiol. 25146-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chassin, C., J. M. Goujon, S. Darche, L. du Merle, M. Bens, F. Cluzeaud, C. Werts, E. Ogier-Denis, C. Le Bouguénec, D. Buzoni-Gatel, and A. Vandewalle. 2006. Renal collecting duct epithelial cells react to pyelonephritis-associated Escherichia coli by activating distinct TLR4-dependent and -independent inflammatory pathways. J. Immunol. 1774773-4784. [DOI] [PubMed] [Google Scholar]
- 12.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 976640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis, J. M., S. B. Rasmussen, and A. D. O'Brien. 2005. Cytotoxic necrotizing factor type 1 production by uropathogenic Escherichia coli modulates polymorphonuclear leukocyte function. Infect. Immun. 735301-5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Rycke, J., P. Mazars, J. P. Nougayrede, C. Tasca, M. Boury, F. Herault, A. Valette, and E. Oswald. 1996. Mitotic block and delayed lethality in HeLa epithelial cells exposed to Escherichia coli BM2-1 producing cytotoxic necrotizing factor type 1. Infect. Immun. 641694-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Rycke, J., E. Oswald, and R. Boivin. 1989. An in vivo assay for the detection of cytotoxic strains of Escherichia coli. Ann. Rech. Vet. 2039-46. [PubMed] [Google Scholar]
- 16.De Rycke, J., L. Phan-Thanh, and S. Bernard. 1989. Immunochemical identification and biological characterization of cytotoxic necrotizing factor from Escherichia coli. J. Clin. Microbiol. 27983-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donnenberg, M. S., and R. A. Welch. 1996. Virulence determinants of uropathogenic Escherichia coli, p. 135-174. In H. L. T. Mobley and J. W. Warren (ed.), Urinary tract infections: molecular pathogenesis and clinical management. American Society for Microbiology, Washington, DC.
- 18.Emödy, L., I. Batai, Jr., M. Kerenyi, J. Szekely, Jr., and L. Polyak. 1982. Anti-Escherichia coli alpha-hemolysin in control and patient sera. Lancet ii986. [DOI] [PubMed] [Google Scholar]
- 19.Falbo, V., M. Famiglietti, and A. Caprioli. 1992. Gene block encoding production of cytotoxic necrotizing factor 1 and hemolysin in Escherichia coli isolates from extraintestinal infections. Infect. Immun. 602182-2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Falbo, V., T. Pace, L. Picci, E. Pizzi, and A. Caprioli. 1993. Isolation and nucleotide sequence of the gene encoding cytotoxic necrotizing factor 1 of Escherichia coli. Infect. Immun. 614909-4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falzano, L., P. Filippini, S. Travaglione, A. G. Miraglia, A. Fabbri, and C. Fiorentini. 2006. Escherichia coli cytotoxic necrotizing factor 1 blocks cell cycle G2/M transition in uroepithelial cells. Infect. Immun. 743765-3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flatau, G., E. Lemichez, M. Gauthier, P. Chardin, S. Paris, C. Fiorentini, and P. Boquet. 1997. Toxin-induced activation of the G protein p21 Rho by deamidation of glutamine. Nature 387729-733. [DOI] [PubMed] [Google Scholar]
- 23.Foxman, B., R. Barlow, H. D'Arcy, B. Gillespie, and J. D. Sobel. 2000. Urinary tract infection: self-reported incidence and associated costs. Ann. Epidemiol. 10509-515. [DOI] [PubMed] [Google Scholar]
- 24.Giamboi-Miraglia, A., S. Travaglione, P. Filippini, A. Fabbri, C. Fiorentini, and L. Falzano. 2007. A multinucleating Escherichia coli cytotoxin perturbs cell cycle in cultured epithelial cells. Toxicol. In Vitro 21235-239. [DOI] [PubMed] [Google Scholar]
- 25.Hacker, J., and J. B. Kaper. 2000. Pathogenicity islands and the evolution of microbes. Annu. Rev. Microbiol. 54641-679. [DOI] [PubMed] [Google Scholar]
- 26.Hagberg, L., I. Engberg, R. Freter, J. Lam, S. Olling, and C. Svanborg-Edén. 1983. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect. Immun. 40273-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall, A. 1992. Ras-related GTPases and the cytoskeleton. Mol. Biol. Cell 3475-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall, A. 1998. Rho GTPases and the actin cytoskeleton. Science 279509-514. [DOI] [PubMed] [Google Scholar]
- 29.Harper, J. R., and T. J. Silhavy. 2001. Germ warfare: the mechanisms of virulence factor delivery, p. 43-74. In E. A. Groisman (ed.), Principles of bacterial pathogenesis. Academic Press, San Diego, CA.
- 30.Haugen, B. J., S. Pellett, P. Redford, H. L. Hamilton, P. L. Roesch, and R. A. Welch. 2007. In vivo gene expression analysis identifies genes required for enhanced colonization of the mouse urinary tract by uropathogenic Escherichia coli strain CFT073 dsdA. Infect. Immun. 75278-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson, J. R. 1991. Virulence factors in Escherichia coli urinary tract infection. Clin. Microbiol. Rev. 480-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson, J. R., and J. J. Brown. 1996. Defining inoculation conditions for the mouse model of ascending urinary tract infection that avoid immediate vesicoureteral reflux yet produce renal and bladder infection. J. Infect. Dis. 173746-749. [DOI] [PubMed] [Google Scholar]
- 33.Johnson, J. R., T. A. Russo, F. Scheutz, J. J. Brown, L. Zhang, K. Palin, C. Rode, C. Bloch, C. F. Marrs, and B. Foxman. 1997. Discovery of disseminated J96-like strains of uropathogenic Escherichia coli O4:H5 containing genes for both PapG(J96) (class I) and PrsG(J96) (class III) Gal(alpha1-4)Gal-binding adhesins. J. Infect. Dis. 175983-988. [DOI] [PubMed] [Google Scholar]
- 34.Koronakis, V., J. Eswaran, and C. Hughes. 2003. The type I export mechanism, p. 71-79. In D. L. Burns, J. T. Barbieri, B. H. Iglewski, and R. Rappuoli (ed.), Bacterial protein toxins. ASM Press, Washington, DC.
- 35.Lally, E. T., R. B. Hill, I. R. Kieba, and J. Korostoff. 1999. The interaction between RTX toxins and target cells. Trends Microbiol. 7356-361. [DOI] [PubMed] [Google Scholar]
- 36.Landraud, L., M. Gauthier, T. Fosse, and P. Boquet. 2000. Frequency of Escherichia coli strains producing the cytotoxic necrotizing factor (CNF1) in nosocomial urinary tract infections. Lett. Appl. Microbiol. 30213-216. [DOI] [PubMed] [Google Scholar]
- 37.Landraud, L., M. Gibert, M. R. Popoff, P. Boquet, and M. Gauthier. 2003. Expression of cnf1 by Escherichia coli J96 involves a large upstream DNA region including the hlyCABD operon, and is regulated by the RfaH protein. Mol. Microbiol. 471653-1667. [DOI] [PubMed] [Google Scholar]
- 38.Lerm, M., G. Schmidt, U. M. Goehring, J. Schirmer, and K. Aktories. 1999. Identification of the region of Rho involved in substrate recognition by Escherichia coli cytotoxic necrotizing factor 1 (CNF1). J. Biol. Chem. 27428999-29004. [DOI] [PubMed] [Google Scholar]
- 39.Lerm, M., J. Selzer, A. Hoffmeyer, U. R. Rapp, K. Aktories, and G. Schmidt. 1999. Deamidation of Cdc42 and Rac by Escherichia coli cytotoxic necrotizing factor 1: activation of c-Jun N-terminal kinase in HeLa cells. Infect. Immun. 67496-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Linggood, M. A., and P. L. Ingram. 1982. The role of alpha haemolysin in the virulence of Escherichia coli for mice. J. Med. Microbiol. 1523-30. [DOI] [PubMed] [Google Scholar]
- 41.Martinez, J. J., and S. J. Hultgren. 2002. Requirement of Rho-family GTPases in the invasion of type 1-piliated uropathogenic Escherichia coli. Cell. Microbiol. 419-28. [DOI] [PubMed] [Google Scholar]
- 42.Martinez, J. J., M. A. Mulvey, J. D. Schilling, J. S. Pinkner, and S. J. Hultgren. 2000. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J. 192803-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meysick, K. C., M. Mills, and A. D. O'Brien. 2001. Epitope mapping of monoclonal antibodies capable of neutralizing cytotoxic necrotizing factor type 1 of uropathogenic Escherichia coli. Infect. Immun. 692066-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mills, M., K. C. Meysick, and A. D. O'Brien. 2000. Cytotoxic necrotizing factor type 1 of uropathogenic Escherichia coli kills cultured human uroepithelial 5637 cells by an apoptotic mechanism. Infect. Immun. 685869-5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitsumori, K., A. Terai, S. Yamamoto, S. Ishitoya, and O. Yoshida. 1999. Virulence characteristics of Escherichia coli in acute bacterial prostatitis. J. Infect. Dis. 1801378-1381. [DOI] [PubMed] [Google Scholar]
- 46.Mobley, H. L., D. M. Green, A. L. Trifillis, D. E. Johnson, G. R. Chippendale, C. V. Lockatell, B. D. Jones, and J. W. Warren. 1990. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect. Immun. 581281-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mulvey, M. A., Y. S. Lopez-Boado, C. L. Wilson, R. Roth, W. C. Parks, J. Heuser, and S. J. Hultgren. 1998. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 2821494-1497. [DOI] [PubMed] [Google Scholar]
- 48.Munro, P., G. Flatau, A. Doye, L. Boyer, O. Oregioni, J. L. Mege, L. Landraud, and E. Lemichez. 2004. Activation and proteasomal degradation of Rho GTPases by cytotoxic necrotizing factor-1 elicit a controlled inflammatory response. J. Biol. Chem. 27935849-35857. [DOI] [PubMed] [Google Scholar]
- 49.National Institutes of Health. 1985. Guide for the care and use of laboratory animals. National Institutes of Health publication no. 85-23. National Institutes of Health, Bethesda, MD.
- 50.Nobes, C. D., and A. Hall. 1995. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 8153-62. [DOI] [PubMed] [Google Scholar]
- 51.Real, J. M., P. Munro, C. Buisson-Touati, E. Lemichez, P. Boquet, and L. Landraud. 2007. Specificity of immunomodulator secretion in urinary samples in response to infection by alpha-hemolysin and CNF1 bearing uropathogenic Escherichia coli. Cytokine 3722-25. [DOI] [PubMed] [Google Scholar]
- 52.Ridley, A. J., and A. Hall. 1992. The small GTP-binding protein Rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70389-399. [DOI] [PubMed] [Google Scholar]
- 53.Ridley, A. J., H. F. Paterson, C. L. Johnston, D. Diekmann, and A. Hall. 1992. The small GTP-binding protein Rac regulates growth factor-induced membrane ruffling. Cell 70401-410. [DOI] [PubMed] [Google Scholar]
- 54.Rippere-Lampe, K. E., M. Lang, H. Ceri, M. Olson, H. A. Lockman, and A. D. O'Brien. 2001. Cytotoxic necrotizing factor type 1-positive Escherichia coli causes increased inflammation and tissue damage to the prostate in a rat prostatitis model. Infect. Immun. 696515-6519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rippere-Lampe, K. E., A. D. O'Brien, R. Conran, and H. A. Lockman. 2001. Mutation of the gene encoding cytotoxic necrotizing factor type 1 (cnf1) attenuates the virulence of uropathogenic Escherichia coli. Infect. Immun. 693954-3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Russo, T. A., J. E. Guenther, S. Wenderoth, and M. M. Frank. 1993. Generation of isogenic K54 capsule-deficient Escherichia coli strains through TnphoA-mediated gene disruption. Mol. Microbiol. 9357-364. [DOI] [PubMed] [Google Scholar]
- 57.Schmidt, G., P. Sehr, M. Wilm, J. Selzer, M. Mann, and K. Aktories. 1997. Gln 63 of Rho is deamidated by Escherichia coli cytotoxic necrotizing factor-1. Nature 387725-729. [DOI] [PubMed] [Google Scholar]
- 58.Schmidt, H., and M. Hensel. 2004. Pathogenicity islands in bacterial pathogenesis. Clin. Microbiol. Rev. 1714-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seetharama, S., S. J. Cavalieri, and I. S. Snyder. 1988. Immune response to Escherichia coli alpha-hemolysin in patients. J. Clin. Microbiol. 26850-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sheshko, V., J. Hejnova, Z. Rehakova, J. Sinkora, M. Faldyna, P. Alexa, J. Felsberg, R. Nemcova, A. Bomba, and P. Sebo. 2006. HlyA knock out yields a safer Escherichia coli A0 34/86 variant with unaffected colonization capacity in piglets. FEMS Immunol. Med. Microbiol. 48257-266. [DOI] [PubMed] [Google Scholar]
- 61.Smith, Y. C., K. K. Grande, S. B. Rasmussen, and A. D. O'Brien. 2006. Novel three-dimensional organoid model for evaluation of the interaction of uropathogenic Escherichia coli with terminally differentiated human urothelial cells. Infect. Immun. 74750-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Swenson, D. L., N. O. Bukanov, D. E. Berg, and R. A. Welch. 1996. Two pathogenicity islands in uropathogenic Escherichia coli J96: cosmid cloning and sample sequencing. Infect. Immun. 643736-3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Waalwijk, C., D. M. MacLaren, and J. de Graaff. 1983. In vivo function of hemolysin in the nephropathogenicity of Escherichia coli. Infect. Immun. 42245-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Warren, J. W., H. L. Mobley, J. R. Hebel, and A. L. Trifillis. 1995. Cytolethality of hemolytic Escherichia coli to primary human renal proximal tubular cell cultures obtained from different donors. Urology 45706-710. [DOI] [PubMed] [Google Scholar]
- 65.Welch, R. A. 1991. Pore-forming cytolysins of gram-negative bacteria. Mol. Microbiol. 5521-528. [DOI] [PubMed] [Google Scholar]
- 66.Yamamoto, S., T. Tsukamoto, A. Terai, H. Kurazono, Y. Takeda, and O. Yoshida. 1995. Distribution of virulence factors in Escherichia coli isolated from urine of cystitis patients. Microbiol. Immunol. 39401-404. [DOI] [PubMed] [Google Scholar]