Abstract
Porphyromonas gingivalis has been shown to accelerate atherosclerotic lesion development in hyperlipidemic animals. We assessed the potential of a nasal vaccine against P. gingivalis infection for the prevention of atherosclerosis. Apolipoprotein E-deficient spontaneously hyperlipidemic (Apoeshl) mice were nasally immunized with the 40-kDa outer membrane protein (OMP) of P. gingivalis plus cholera toxin (CT) as adjuvant and then challenged intravenously with P. gingivalis strain 381. The animals were euthanized 11 or 14 weeks later. Atheromatous lesions in the proximal aorta of each animal were analyzed histomorphometrically, and the serum concentrations of 40-kDa OMP-specific antibodies and cytokines were determined. The areas of the aortic sinus that were covered with atherosclerotic plaque and the serum levels of inflammatory cytokines and chemokines were increased in Apoeshl mice challenged with P. gingivalis compared to nonchallenged mice. In comparison, nasal immunization with 40-kDa OMP plus CT significantly reduced atherosclerotic plaque accumulation in the aortic sinus and lowered the serum levels of cytokines and chemokines compared to nonimmunized animals. Nasal immunization also induced 40-kDa OMP-specific serum immunoglobulin G (IgG) and saliva IgA antibody responses. These findings suggest that systemic infection with P. gingivalis accelerates atherosclerosis in Apoeshl mice, and 40-kDa OMP plus CT may be an effective nasal vaccine for the reduction of atherosclerosis accelerated by P. gingivalis in the hyperlipidemic mouse model.
Atherosclerotic cardiovascular disease is the leading cause of death in Western societies; however, as many as 50% of patients with atherosclerosis lack currently identified risk factors such as hypertension, hypercholesterolemia, diabetes, and smoking, suggesting the presence of other contributory mechanisms (43, 51). Emerging evidence suggests that infection with specific pathogens is an additional risk factor for atherosclerosis (28).
Periodontitis is a chronic multibacterial infection that affects the tissues that surround and support the teeth and may lead to tooth loss. The global prevalence of periodontal disease is high, and severe forms of chronic periodontitis affect ca. 15% of individuals worldwide (37). Periodontitis tends to appear following an imbalance of the normal oral flora, leading to the emergence of periopathogenic bacteria. As periodontitis progresses, the composition of subgingival bacteria is altered, allowing some species such as the major periodontopathogen Porphyromonas gingivalis to flourish (4).
Cohort and case-control studies have shown that periodontitis is associated with endothelial dysfunction (3), atherosclerosis (7), and an increased risk of myocardial infarction and stroke (47). A recent prospective, randomized study also showed that treatment of periodontitis is associated with alterations in endothelial function (50). In addition to clinical studies, the atherogenic role of periodontal pathogens, such as P. gingivalis, has emerged through seroepidemiological studies (12, 38). In contrast, early cross-sectional and case-control studies that have investigated this association have presented conflicting results (22, 40, 55). Periodontal pathogens such as P. gingivalis promote platelet aggregation (19), foam cell formation (39), and the development of atheromas in experimental models (26, 30). Furthermore, DNA from periodontal pathogens (18), including P. gingivalis, has been detected in atheromatous plaques (25). However, the real significance of this bacterium in the etiology of atherosclerosis is still uncertain.
The colonization of periodontal pathogens on gingival tissues and the agglutination of erythrocytes are critical in the pathogenic process of periodontal disease. The ability of P. gingivalis to adhere to erythrocytes and epithelial cells may be an important virulence determinant in chronic periodontitis. The 40-kDa outer membrane protein (OMP) of P. gingivalis is a key virulence factor for coaggregation (20, 21, 46) and hemagglutination (48). We previously showed that immunoglobulin G (IgG) antibodies (Abs) induced by the nasal administration of the 40-kDa OMP with cholera toxin (CT) as adjuvant inhibited coaggregation by P. gingivalis (35), suggesting that the 40-kDa OMP might be an effective vaccine for the prevention of P. gingivalis infection. Intranasal delivery of vaccines is an attractive mode of immunization. Like the mouth, the nose offers several advantages in terms of vaccine administration, and nasopharynx-associated lymphoid tissue efficiently induces both mucosal and systemic immune responses, resulting in two levels of host protection against infectious diseases (34). Intranasal immunization is also advantageous from a practical point of view because it does not require needles or syringes. In the past decade, several clinical studies have confirmed the generation of local and systemic immunity in humans after nasal immunization against diphtheria, tetanus (2), influenza (16), and Streptococcus mutans (29).
Apolipoprotein E (apoE)-deficient, spontaneously hyperlipidemic (KOR-Apoeshl) mice, an inbred strain created from Japanese wild mice, are deficient in the expression of apoE because of a gross disruption in the apoE gene (32). These mice are hypercholesterolemic and accumulate large amounts of remnant-like particles in the bloodstream, as has been observed in apoE-knockout mice. Several congenic apoE-deficient mice with the genetic background of laboratory mice were developed by transferring the apoE gene mutation from a KOR genetic background (33). An alternative apoE-deficient murine model may be useful given the complexities involved in importing and maintaining genetically modified animals. We used congenic mice with a BALB/c genetic background (BALB/c.KOR-Apoeshl) as an alternative animal model of apoE deficiency to examine the effect of P. gingivalis on the progression of atherosclerosis, as well as the effect of nasal immunization with 40-kDa OMP on atherosclerosis accelerated by P. gingivalis.
MATERIALS AND METHODS
Bacterial strain.
P. gingivalis strain 381 was cultured on anaerobic blood agar plates (Becton Dickinson, Sunnyvale, CA) in a model 1024 anaerobic system (Forma Scientific, Marietta, OH) with 10% H2, 80% N2, and 10% CO2 for 3 to 5 days. Cultures were then inoculated into brain heart infusion broth (Difco Laboratories, Detroit, MI) supplemented with 5 μg of hemin/ml and 0.4 μg of menadione/ml and grown for 2 days until reaching an optical density of 0.8 at 660 nm, corresponding to 109 CFU/ml. The cultured cells were then centrifuged at 8,000 × g for 20 min at 4°C and diluted with phosphate-buffered saline (PBS) for intravenous (i.v.) infection.
Antigen and adjuvant.
The recombinant plasmid containing the 40-kDa OMP gene (pMD125) was kindly provided by Y. Abiko (Nihon University). OMP was purified to homogeneity from a cell suspension prepared by sonication of Escherichia coli K-12 harboring pMD125, as described previously (23). The purity of the preparation was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (data not shown). Further, an LAL Pyrochrome kit (Associates of Cape Cod, Inc., Woods Hole, MA) was used to determine the level of residual endotoxin. A 1-mg portion of the 40-kDa OMP preparation contained <0.4 pg of endotoxin. CT was obtained from List Biologic Laboratories (Campbell, CA).
Mice.
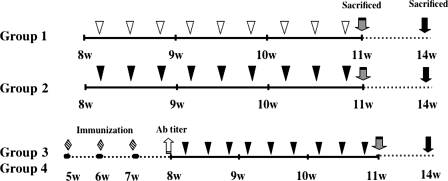
The institutional Animal Care and Use Committee of Nihon University approved all animal protocols. Eight-week-old female BALB/c, apoE-deficient spontaneously hyperlipidemic (c.KOR-Apoeshl) mice (33), obtained from Japan SLC, Inc. (Hamamatsu, Japan), were given regular mouse chow and water ad libitum. The mice were randomly divided into four groups (n = 12 for each group; Fig. 1). The first group was challenged i.v. three times a week for 3 weeks with 0.1 ml of PBS, whereas the second group was challenged i.v. three times a week for 3 weeks with 0.1 ml of live P. gingivalis (108 CFU/mouse; Fig. 1). The third group was immunized intranasally three times a week with 40-kDa OMP plus CT in sterile, pyrogen-free saline prior to the i.v. challenge, whereas the fourth group was immunized intranasally three times a week with CT alone prior to the i.v. challenge (Fig. 1). All mice were monitored daily until sacrifice and appeared healthy throughout the course of the study. Mice from each group were euthanized at 11 or 14 weeks of age.
FIG. 1.
Experimental procedure. Eight-week-old female Apoeshl mice were randomly divided into four groups: group 1 was inoculated with 100 μl of PBS (▿), group 2 was inoculated with 100 μl (108 CFU) of P. gingivalis (▾), group 3 was immunized with 40-kDa OMP plus CT ( ) and inoculated with 100 μl (108 CFU) of P. gingivalis (▾), and group 4 was immunized with CT only (
) and inoculated with 100 μl (108 CFU) of P. gingivalis (▾), and group 4 was immunized with CT only ( ) and inoculated with 100 μl (108 CFU) of P. gingivalis (▾). The immunized animals were nasally vaccinated with 40-kDa OMP plus CT or CT alone once a week for 3 weeks prior to the bacterial challenge. The animals were challenged i.v. with P. gingivalis strain 381 three times a week for 3 weeks. The animals were sacrificed either 24 h or 3 weeks after the final challenge.
) and inoculated with 100 μl (108 CFU) of P. gingivalis (▾). The immunized animals were nasally vaccinated with 40-kDa OMP plus CT or CT alone once a week for 3 weeks prior to the bacterial challenge. The animals were challenged i.v. with P. gingivalis strain 381 three times a week for 3 weeks. The animals were sacrificed either 24 h or 3 weeks after the final challenge.
Quantification of the atherosclerotic lesion area.
Blood was collected into heparinized syringes from the orbital veins of mice anesthetized with Isozol (Nichi Iko, Toyama, Japan). The heart and aortic tree were then perfused through the left ventricle with iced 0.9% PBS for 10 min. The heart was then carefully dissected and removed. The upper half of the heart containing the aortic origin was separated and embedded in Tissue-Tek (Fisher Scientific, Newark, DE) OCT compound in cryomolds, and cryostat sections were prepared (36). Using a modified version of the method of Paigen et al. (36), we examined cryosections of the aortic sinus for atherosclerotic plaque accumulation by oil red-O staining. The lesion area was then quantified by using a microscope interfaced with a charge-coupled device camera and an image analysis system (BX51; Olympus, Tokyo, Japan). Briefly, cross-sectional areas from three images were added to obtain the total lesion area per slide, and the percentage of the aortic lumen that was occupied by lesions per section was calculated. Slide analysis was conducted in a blinded fashion. Finally, the total lesion area and the percentage of the aortic lumen occupied by lesions were averaged over 15 sections per animal and expressed as the mean lesion area and the percentage of the lumen of the proximal aorta occupied by lesions per section per animal.
Measurement of serum cholesterol levels.
The serum was isolated from the blood by centrifugation at 1,200 × g for 10 min after clotting at room temperature. The serum levels of total cholesterol were determined with the use of a quantitative kit (Cholestest; Daiichi Pure Chemicals, Tokyo, Japan).
PCR detection of P. gingivalis.
Whole blood was collected from 11- and 14-week-old mice. Total DNA was isolated by using a QiaAmp kit (Qiagen, Tokyo, Japan). The P. gingivalis 16S gene was then detected by PCR as described previously (6). Genomic DNA extracted from P. gingivalis 381 was also subjected to PCR as a positive control.
Detection of 40-kDa OMP-specific serum IgG, saliva IgA, and interleukin-8 (IL-8).
The Ab titers in serum and saliva samples were determined by using enzyme-linked immunosorbent assay (ELISA). Briefly, plates were coated with 40-kDa OMP (5 μg/ml) and blocked with PBS containing 2% bovine serum albumin. After blocking, serial dilutions of the serum or saliva samples were added in duplicate at starting dilutions of 1:32 and 1:4, respectively. After incubation, the plates were washed, and peroxidase-labeled goat anti-mouse γ or α heavy-chain-specific Abs (Southern Biotechnology Associates, Birmingham, AL) were added to the appropriate wells. Finally, ABTS [2,2′-azino-bis(3-ethylbanz-thiazoline-6-sulfonic acid)] with H2O2 (Moss, Inc., Pasadena, MD) was added for color development. The endpoint titers were expressed as the reciprocal log2 of the last dilution that gave an optical density of 0.1 greater than background at 414 nm after 15 min of incubation. Each serum sample from blood collected at euthanasia was also screened by using ELISA (R&D Systems, Inc., Minneapolis, MN) for IL-8 (MIP-2).
Cytokine Ab array.
Serum samples from two mice were pooled and then analyzed by using a cytokine Ab array (RayBio Mouse Atherosclerosis Ab array I; Ray Biotech, Inc., Norcross, GA) consisting of 22 different atherosclerosis-related cytokine Abs, which were spotted in duplicate on a membrane. Briefly, the cytokine array membranes were blocked in 2 ml of 1× blocking buffer for 30 min and then incubated with 1 ml of sample at room temperature for 1 to 2 h. The samples were then decanted from each container, and the membranes were washed three times with 2 ml of 1× wash buffer I, followed by two washes with 2 ml of 1× wash buffer II at room temperature with shaking. The membranes were then incubated in a 1:250 dilution of biotin-conjugated primary Ab at room temperature for 1 to 2 h and then washed as described above prior to incubation in a 1:1,000 dilution of horseradish peroxidase-conjugated streptavidin. After incubation in horseradish peroxidase-conjugated streptavidin for 30 to 60 min, the membranes were thoroughly washed and exposed to a peroxide substrate (detection buffers C and D [Ray Biotech, Inc.]) for 5 min in the dark before imaging. Chemiluminescent signals from the bound cytokines were detected by using a Lumino image analyzer (Fuji Film, Tokyo, Japan). Signal intensities were quantified by using an Image Reader LAS-1000 (Fuji Film) and analyzed using Image Gauge (Fuji Film). Biotin-conjugated IgG served as a positive control at six spots, where it was used to identify the membrane orientation and to normalize the results from the different membranes. For each spot, the net optical density was determined by subtracting the background optical level from the total raw optical density. The level of each cytokine was represented as a percentage of the positive control.
Statistical analysis.
The data are presented as the mean ± the standard deviation (SD). One-way analysis of variance followed by Tukey-Kramer multiple comparison was used to assess differences in total atherosclerotic plaque accumulation. A P value of <0.05 was considered significant.
RESULTS
Clinical assessment.
No clinical signs of infection or mortality were noted in any of the animals at any time. There were no significant differences in body weight between the P. gingivalis- and sham-inoculated mice. The heart, kidneys, spleen, liver, and small intestine of each animal showed normal histological structure.
Nasal immunization with 40-kDa OMP stimulates P. gingivalis-specific Ab production.
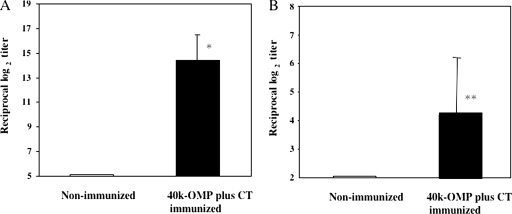
Immunization of mice with the 40-kDa OMP plus CT produced a maximal 40-kDa OMP-specific serum IgG response (Fig. 2A) and a significant anti-40-kDa OMP-specific salivary IgA response (Fig. 2B). In contrast, negligible amounts of 40-kDa OMP-specific serum IgG and salivary IgA Abs were produced by the nonimmunized animals (Fig. 2A and B) and animals that received the adjuvant alone (data not shown).
FIG. 2.
40-kDa OMP-specific Ab responses after nasal vaccination with 40-kDa OMP plus CT. Apoeshl mice were nasally immunized with 10 μg of 40-kDa OMP plus 1 μg of CT on days 0, 7, and 14 (▪) or not immunized (□). Serum and saliva samples were collected on day 21 and assessed for 40-kDa OMP-specific serum IgG (A) and saliva IgA (B) Abs. The results are expressed as the mean ± the SD for six mice per group. *, P < 0.01; **, P < 0.05 (compared to the control group).
Nasal immunization of mice with 40-kDa OMP prevents P. gingivalis-enhanced atherosclerosis.
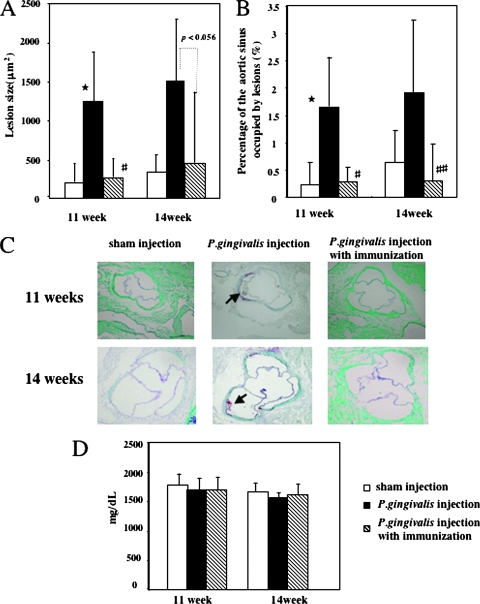
Histomorphological analysis was used to calculate the percentage of the aortic lumen occupied by atheromatous lesions. At 11 weeks, there was a notable increase in atherosclerotic plaque accumulation in the Apoeshl mice inoculated with P. gingivalis compared to the sham control animals (Fig. 3A, 1, 254 ± 655 versus 203 ± 261 μm2, P < 0.01; Fig. 3B, 1.65 ± 0.90 versus 0.23% ± 0.41%, P < 0.01). Lesions were not detected in four of the six sham control mice, whereas all mice in the P. gingivalis-inoculated group had lesions. The size of the affected area increased during the subsequent weeks. At 14 weeks, atherosclerotic plaque accumulation was greater in the P. gingivalis-challenged mice than in the vehicle-control animals (Fig. 3A, 1,509 ± 790 versus 361 ± 225 μm2; Fig. 3B, 1.92 ± 1.33 versus 0.67% ± 0.58%), although this difference was not significant. In contrast, nasal immunization with 40-kDa OMP plus CT reduced atherosclerotic plaque accumulation in the P. gingivalis-infected group at 11 weeks (Fig. 3A, 1, 254 ± 655 versus 271 ± 235 μm2, P < 0.01; Fig. 3B; 1.65 ± 0.90 versus 0.28% ± 0.27%, P < 0.01) and 14 weeks (Fig. 3A, 1, 509 ± 790 versus 458 ± 904 μm2, P < 0.056; Fig. 3B; 1.92 ± 1.33 versus 0.30% ± 0.67%, P < 0.05). The lesions were not detected in two (11 weeks) or four (14 weeks) of six P. gingivalis-infected mice immunized with 40-kDa OMP plus CT. Nasal immunization with CT alone did not reduce atherosclerotic plaque accumulation in the P. gingivalis-infected group (data not shown). Complete attenuation of lesion formation was visually observed in P. gingivalis-infected animals immunized with 40-kDa OMP plus CT. Unchallenged Apoeshl mice had high cholesterol levels, and P. gingivalis-infection or immunization had no effect on the levels (Fig. 3D).
FIG. 3.
Atherosclerotic plaque formation in the aortic sinuses of Apoeshl mice i.v. challenged with P. gingivalis or nasally immunized with 40-kDa OMP prior to the challenge. Histomorphometric analysis of the mean lesion area (A) and the percentage of the aortic sinus occupied by lesions (B) at 11 and 14 weeks. The data represent the mean ± the SD of six mice per group. *, P < 0.01 (compared to the control group). #, P < 0.01; ##, P < 0.05 (compared to the P. gingivalis-challenged group). (C) Oil red-O stained cryosections of the proximal aortas of 11- and 14-week-old mice. The arrow indicates a typical lipid-rich atherosclerotic area stained with oil red-O. (D) Serum analysis (n = 6 mice/group) of total cholesterol levels from unchallenged (□), P. gingivalis-challenged (▪), or immunized (▧) mice.
Amplification of the P. gingivalis ribosomal 16S gene by PCR.
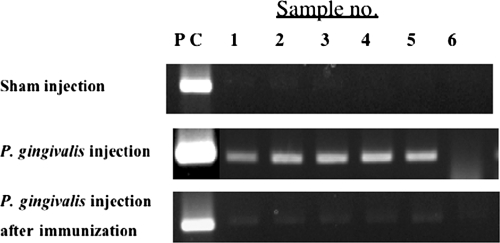
Porphyromonas gingivalis was detected in the blood of P. gingivalis-challenged mice by using PCR at 11 weeks; five of six mice were positive. In contrast, none of the sham-inoculated or immunized mice tested positive for P. gingivalis DNA. This result suggests that P. gingivalis may be eliminated from the blood by nasal immunization with 40-kDa OMP (Fig. 4). PCR detection of P. gingivalis was also attempted on mice at 14 weeks of age. However, we did not detect any PCR positives at this time point. These additional 3 weeks after the last infection may be responsible for decreasing the levels of bacterium in the blood to below the detection threshold of PCR.
FIG. 4.
Detection of P. gingivalis in the blood of Apoeshl mice. Each group of mice was euthanized after the final challenge. Total DNA was isolated from the blood of the mice, and P. gingivalis DNA was detected by PCR using P. gingivalis 16S gene-specific primers.
Cytokine array.
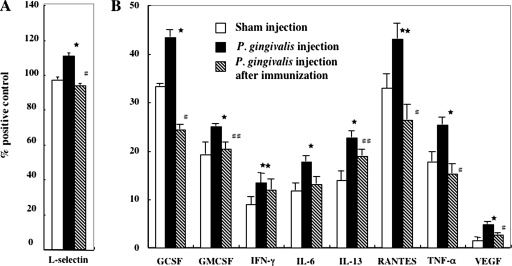
Cytokine array analysis showed that P. gingivalis has a significant inductive effect on the cytokine levels in mice; in particular, the level of a cell adhesion molecule, L-selectin, was elevated at 11 weeks (Fig. 5A; 111 ± 1.5 versus 97 ± 1.5, P < 0.01), whereas granulocyte colony-stimulating factor (43.3 ± 1.5 versus 33.8 ± 0.5, P < 0.01), granulocyte-macrophage colony-stimulating factor (25.1 ± 0.5 versus 19.3 ± 2.5, P < 0.01), gamma interferon (13.4 ± 2.0 versus 8.9 ± 1.7, P < 0.05), IL-6 (17.8 ± 1.1 versus 11.8 ± 1.5, P < 0.01), IL-13 (22.7 ± 1.4 versus 13.9 ± 2.0, P < 0.01), RANTES (for regulated on activation, normal T-cell expressed and secreted) (43.1 ± 3.2 versus 32.9 ± 3.0, P < 0.05), tumor necrosis factor alpha (25.4 ± 1.5 versus 17.8 ± 2.0, P < 0.01), and vascular endothelial growth factor (4.8 ± 0.5 versus 1.6 ± 0.6, P < 0.01) were elevated at 14 weeks compared to the sham-inoculated group (Fig. 5B). In contrast, neither group showed any change in the levels of basic fibroblast growth factor, CD40, exotaxin, IL-2, IL-3, IL-4, IL-5, monocyte chemoattractant protein 1, macrophage colony-stimulating factor, macrophage inflammatory protein 3α, or P-selectin (data not shown). Notably, the rise in the inflammatory cytokine and chemokine levels caused by infection with P. gingivalis was substantially reduced in animals immunized with 40-kDa OMP plus CT (Fig. 5).
FIG. 5.
Cytokine profiles from P. gingivalis challenged or nasally immunized serum samples collected after 11 (A) and 14 (B) weeks. The level of each cytokine is presented as the percentage of the positive control. The data represent the mean ± the SD of three independent experiments of three pooled samples. ★, P < 0.01; ★★, P < 0.05 (compared to the control group). #, P < 0.01; ##, P < 0.05 (compared to the P. gingivalis-challenged group). Abbreviations: GCSF, granulocyte colony-stimulating factor; GMCSF, granulocyte-macrophage colony-stimulating factor; IFN-γ, gamma interferon; TNF-α, tumor necrosis factor alpha; VEGF, vascular endothelial growth factor.
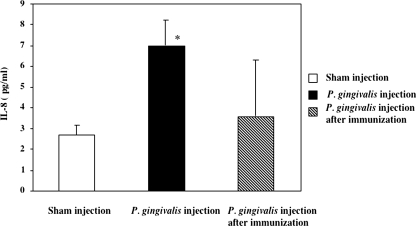
Serum level of IL-8.
The association between inflammation and the development and progression of atherosclerosis suggests that serum markers such as IL-8 may be useful in predicting an individual's risk of coronary heart disease (45). Therefore, we tested the serum level of IL-8 in each group of mice at 11 and 14 weeks by using ELISA. No significant differences were observed between the groups at 24 h after the final challenge (data not shown); however, 3 weeks after the final challenge, mice challenged with P. gingivalis had by far the highest levels of IL-8 (Fig. 6). The amount of IL-8 detected in the group inoculated with P. gingivalis was 2.8 times higher than that in the sham-inoculated group. In contrast, no increase in IL-8 was observed in the sera of the unchallenged or nasally immunized animals. This finding suggests that nasal immunization with 40-kDa OMP plus CT inhibits the host inflammatory response and reduces atheroma development.
FIG. 6.
Serum IL-8 levels in P. gingivalis-challenged or nasally immunized serum samples collected after 14 weeks. The data represent the mean ± the SD for six mice per group. *, P < 0.01 (compared to the control group).
DISCUSSION
A recent mice study has demonstrated that the i.v. inoculation of mice with P. gingivalis accelerates atherosclerotic development (30). In addition, Lalla et al. (26) induced periodontal infection in mice via oral inoculation with P. gingivalis and later recovered P. gingivalis DNA from the aortic tissue that showed signs of accelerated early atherosclerosis. Furthermore, certain strains of P. gingivalis are capable of infecting macrophages and enhancing foam cell formation in the vascular wall, adding further credence to the purported ability of P. gingivalis to initiate or exacerbate the atherosclerotic process (9, 14). These findings prompted us to investigate the potential for a vaccine directed against the infectious pathogens responsible for triggering a systemic inflammatory response leading to the promotion of atherosclerosis.
Nasal immunization with 40-kDa OMP admixed with CT protected mice against P. gingivalis-accelerated atherosclerosis. Previous studies showed that a monoclonal antibody (MAb) to 40-kDa OMP inhibits coaggregation by P. gingivalis and promotes complement-mediated bactericidal and opsonic activity for the phagocytosis of P. gingivalis (1, 21, 46). Furthermore, rats that are transcutaneously immunized with a 40-kDa OMP vaccine have significantly diminished P. gingivalis-induced abscess formation (24). Therefore, the development of a mucosal 40-kDa OMP vaccine for humans may be a significant milestone in the search for an effective vaccine against P. gingivalis infection.
We further explored the potential of the 40-kDa OMP by using it as a model system to study the protective capability of the protein against the systemic inflammatory response initiated and/or exacerbated by P. gingivalis. Nasal immunization with the 40-kDa OMP induced a significant 40-kDa OMP-specific IgG Ab response in serum and mucosal IgA Abs in saliva. Thus, the 40-kDa OMP-specific serum IgG response (35) induced by the nasal vaccine might have a protective effect against P. gingivalis-accelerated atherosclerosis.
Atherosclerosis has a strong inflammatory component (27, 44), and epidemiological evidence suggests that increased levels of systemic inflammation are predictive of cardiovascular events (41, 42). Cytokine secretion by endothelial cells and other cells are decisive factors in the growth and propagation of atherosclerotic lesions (13, 17, 49, 53, 54). These proinflammatory cytokines and a cell adhesion molecule have a broad range of actions, including the induction of other cytokines; the activation of neutrophils, endothelial cells, monocytes, and adipocytes; and the induction of procoagulant changes in the endothelium, leading to the expression of adhesion molecules, which attract monocytes, lymphocytes, and neutrophils into the developing lesion. L-selectin, granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, gamma interferon, IL-6, IL-13, RANTES, tumor necrosis factor alpha, and vascular endothelial growth factor were significantly upregulated in the serum of P. gingivalis-challenged animals, whereas the levels of these cytokines and L-selectin in immunized animals were equal to those in the controls. These data raise the possibility that proinflammatory cytokines and L-selectin may be involved in atherosclerosis accelerated with P. gingivalis. Furthermore, these data suggest the possibility that the 40-kDa OMP vaccine could control the progress of inflammation induced by P. gingivalis. Interestingly, the observed upregulation of these markers at 14 weeks was not prominent at 11 weeks (data not shown), suggesting a time lag between the first exposure to the bacterial agent and amplification of the host response, possibly through the activation or enhancement of macrophage activity (52). It is conceivable that the time lag following stimulation with P. gingivalis caused the expression of systemic proinflammatory factors, raising the overall systemic inflammatory response, thereby predisposing the animals to atherosclerosis. Indeed, using IL-1R- and IL-6-deficient mice, recent studies have indicated the role of inflammatory cytokines, which affect the progression of atherosclerotic plaques, in P. gingivalis-enhanced atherosclerosis (10, 31). For example, atherosclerotic lesions of proximal aortas were substantially reduced in ApoE+/−-IL-1R−/− mice challenged with P. gingivalis, which indicates that IL-1 plays a crucial role in bacterium-enhanced atherogenesis (10). IL-8 and its corresponding receptor CXCR2 are also involved in the early stages of atherosclerosis (5, 8). Our data suggest that the increased serum level of IL-8 associated with P. gingivalis-infection may be an important factor in the pathogenesis of infectious agent-induced atherosclerosis (11).
Finally, although several possible mechanisms may be involved in the acceleration of atherosclerosis by P. gingivalis (15) as a model organism, the prevention of P. gingivalis infection may be an effective way to reduce the induction of atherosclerosis, in addition to periodontitis. Thus, the prevention of periodontitis might be relevant not only for oral but for systemic health as well. In conclusion, our results demonstrate that atherosclerosis and inflammation are accelerated in Apoeshl mice after P. gingivalis infection and that these can be prevented by nasal immunization with 40-kDa OMP plus CT. However, whether this is relevant to humans remains to be established. Furthermore, our results indicate that the early activation of inflammatory mediators in response to an infectious challenge may be associated with pathogen-accelerated atherosclerosis, suggesting that immunization may be appropriate to control pathogen-accelerated atherosclerosis.
Acknowledgments
This study was supported by Grants-In-Aid for Scientific Research (grants 19390537 and 18592270) from the Japan Society for the Promotion of Science; by the Academic Frontier Project for Private Universities; by a matching fund subsidy from the Ministry of Education, Culture, Sports, Science, and Technology (2007-2011); and by funding for General Individual Research from Nihon University (07-094 and 07-095).
Editor: R. P. Morrison
Footnotes
Published ahead of print on 21 April 2008.
REFERENCES
- 1.Abiko, Y., N. Ogura, U. Matsuda, K. Yanagi, and H. Takiguchi. 1997. A human monoclonal antibody which inhibits the coaggregation activity of Porphyromonas gingivalis. Infect. Immun. 653966-3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aggerbeck, H., S. Gizurarson, J. Wantzin, and I. Heron. 1997. Intranasal booster vaccination against diphtheria and tetanus in man. Vaccine 15307-316. [DOI] [PubMed] [Google Scholar]
- 3.Amar, S., N. Gokce, S. Morgan, M. Loukideli, T. E. Van Dyke, and J. A. Vita. 2003. Periodontal disease is associated with brachial artery endothelial dysfunction and systemic inflammation. Arterioscler. Thromb. Vasc. Biol. 231245-1249. [DOI] [PubMed] [Google Scholar]
- 4.Anonymous. 1996. Consensus report. Periodontal diseases: pathogenesis and microbial factors. Ann. Periodontol. 1926-932. [DOI] [PubMed] [Google Scholar]
- 5.Apostolopoulos, J., P. Davenport, and P. G. Tipping. 1996. Interleukin-8 production by macrophages from atheromatous plaques. Arterioscler. Thromb. Vasc. Biol. 161007-1012. [DOI] [PubMed] [Google Scholar]
- 6.Ashimoto, A., C. Chen, I. Bakker, and J. Slots. 1996. Polymerase chain reaction detection of 8 putative periodontal pathogens in subgingival plaque of gingivitis and advanced periodontitis lesions. Oral Microbiol. Immunol. 11266-273. [DOI] [PubMed] [Google Scholar]
- 7.Beck, J. D., J. R. Elter, G. Heiss, D. Couper, S. M. Mauriello, and S. Offenbacher. 2001. Relationship of periodontal disease to carotid artery intima-media wall thickness: the Atherosclerosis Risk in Communities (ARIC) study. Arterioscler. Thromb. Vasc. Biol. 211816-1822. [DOI] [PubMed] [Google Scholar]
- 8.Boisvert, W. A., R. Santiago, L. K. Curtiss, and R. A. Terkeltaub. 1998. A leukocyte homologue of the IL-8 receptor CXCR2 mediates the accumulation of macrophages in atherosclerotic lesions of LDL receptor-deficient mice. J. Clin. Investig. 101353-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brodala, N., E. P. Merricks, D. A. Bellinger, D. Damrongsri, S. Offenbacher, J. Beck, P. Madianos, D. Sotres, Y. L. Chang, G. Koch, and T. C. Nichols. 2005. Porphyromonas gingivalis bacteremia induces coronary and aortic atherosclerosis in normocholesterolemic and hypercholesteromic pigs. Arterioscler. Thromb. Vasc. Biol. 251446-1451. [DOI] [PubMed] [Google Scholar]
- 10.Chi, H., E. Messas, R. A. Levine, D. T. Graves, and S. Amar. 2004. Interleukin-1 receptor signaling mediates atherosclerosis associated with bacterial exposure and/or a high-fat diet in a murine apolipoprotein E heterozygote model: pharmacotherapeutic implications. Circulation 1101678-1685. [DOI] [PubMed] [Google Scholar]
- 11.Chou, H., H. Yumoto, M. Davey, Y. Takahashi, T. Miyamoto, F. C. Gibson III, and C. A. Genco. 2005. Porphyromonas gingivalis fimbria-dependent activation of inflammatory genes in human aortic endothelial cells. Infect. Immun. 735367-5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ford, P. J., E. Gemmell, P. Timms, A. Chan, F. M. Preston, and G. J. Seymour. 2007. Anti-P. gingivalis response correlates with atherosclerosis. J. Dent. Res. 135-40. [DOI] [PubMed] [Google Scholar]
- 13.Galkina, E., A. Kadl, J. Sanders, D. Varughese, I. J. Sarembock, and K. Ley. 2006. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J. Exp. Med. 2031273-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giacona, M. B., P. N. Papapanou, I. B. Lamster, L. L. Rong, V. D. Agati, A. M. Schmidt, and E. Lalla. 2004. Porphyromonas gingivalis induces its uptake by human macrophages and promotes foam cell formation in vitro. FEMS. Microbiol. Lett. 24195-101. [DOI] [PubMed] [Google Scholar]
- 15.Gibson, F. C., III, H. Yumoto, Y. Takahashi, H. H. Chou, and C. A. Genco. 2006. Innate immune signaling and Porphyromonas gingivalis-accelerated atherosclerosis. J. Dent. Res. 85106-121. [DOI] [PubMed] [Google Scholar]
- 16.Gluck, U., J. O. Gebbers, and R. Gluck. 1999. Phase I evaluation of intranasal virosomal influenza vaccine with and without Escherichia coli heat-labile toxin in adult volunteers. J. Virol. 737780-7786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haghighat, A., D. Weiss, M. K. Whalm, D. P. Cowan, and W. R. Taylor. 2007. Granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor exacerbate atherosclerosis in apolipoprotein E-deficient mice. Circulation 1152049-2054. [DOI] [PubMed] [Google Scholar]
- 18.Haraszthy, V. I., J. J. Zambon, M. Trevisan, M. Zeid, and R. J. Genco. 2000. Identification of periodontal pathogens in atheromatous plaques. J. Periodontol. 711554-1560. [DOI] [PubMed] [Google Scholar]
- 19.Herzberg, M. C., and M. W. Weyer. 1998. Dental plaque, platelets, and cardiovascular diseases. Ann. Periodontol. 3151-160. [DOI] [PubMed] [Google Scholar]
- 20.Hiratsuka, K., W. Yoshida, M. Hayakawa, H. Takiguchi, and Y. Abiko. 1996. Polymerase chain reaction and an outer membrane protein gene probe for the detection of Porphyromonas gingivalis. FEMS. Microbiol. Lett. 138167-172. [DOI] [PubMed] [Google Scholar]
- 21.Hiratsuka, K., Y. Abiko, M. Hayakawa, T. Ito, H. Sasahara, and H. Takiguchi. 1992. Role of Porphyromonas gingivalis 40-kDa outer membrane protein in the aggregation of P. gingivalis vesicles and Actinomyces viscosus. Arch. Oral Biol. 37717-724. [DOI] [PubMed] [Google Scholar]
- 22.Hujoel, P. P., M. Drangsholt, C. Spiekerman, and T. A. DeRouen. 2000. Periodontal disease and coronary heart disease risk. JAMA 2841406-1410. [DOI] [PubMed] [Google Scholar]
- 23.Kawamoto, Y., M. Hayakawa, and Y. Abiko. 1991. Purification and immunochemical characterization of a recombinant outer membrane protein from Bacteroides gingivalis. Int. J. Biochem. 231053-1061. [DOI] [PubMed] [Google Scholar]
- 24.Koizumi, Y., T. Kurita-Ochiai, and M. Yamamoto. 2008. Transcutaneous immunization with an outer membrane protein of Porphyromonas gingivalis without adjuvant elicits marked antibody responses. Oral Microbiol. Immunol. 23131-138. [DOI] [PubMed] [Google Scholar]
- 25.Kozarov, E. V., B. R. Dorn, C. E. Shelburne, W. A. Dunn, Jr., and A. Progulske-Fox. 2005. Human atherosclerotic plaque contains viable invasive Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. Arterioscler. Thromb. Vasc. Biol. 2517-18. [DOI] [PubMed] [Google Scholar]
- 26.Lalla, E., I. B. Lamster, M. A. Hofmann, L. Bucciarelli, A. P. Jerud, S. Tucker, Y. Lu, P. N. Papapanou, and A. M. Schmidt. 2003. Oral infection with a periodontal pathogen accelerates early atherosclerosis in apolipoprotein E-null mice. Arterioscler. Thromb. Vasc. Biol. 231405-1411. [DOI] [PubMed] [Google Scholar]
- 27.Libby, P. 2000. Coronary artery injury and the biology of atherosclerosis: inflammation, thrombosis, and stabilization. Am. J. Cardiol. 863J-9J. [DOI] [PubMed] [Google Scholar]
- 28.Libby, P. 2002. Inflammation in atherosclerosis. Nature 420868-874. [DOI] [PubMed] [Google Scholar]
- 29.Li, F., S. M. Michalek, A. P. Dasanayake, Y. Li, K. Kirk, and N. K. Childers. 2003. Intranasal immunization of humans with Streptococcus mutans antigens. Oral Microbiol. Immunol. 18271-277. [DOI] [PubMed] [Google Scholar]
- 30.Li, L., E. Messas, E. L. Batista, L. A. Levine, and S. Amar. 2002. Porphyromonas gingivalis infection accelerates the progression of atherosclerosis in a heterozygous apolipoprotein E-deficient murine model. Circulation 105861-867. [DOI] [PubMed] [Google Scholar]
- 31.Madan, M., B. Bishayi, M. Hoge, and S. Amar. 2008. Atheroprotective role of interleukin-6 in diet- and/or pathogen-associated atherosclerosis using an ApoE heterozygote murine model. Atherosclerosis 197504-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsushima, Y., S. Hayashi, and M. Tachibana. 1999. Spontaneously hyperlipidemic (SHL) mice: Japanese wild mice with apolipoprotein E deficiency. Mamm. Genome 10352-357. [DOI] [PubMed] [Google Scholar]
- 33.Matsushima, Y., T. Sakurai, A. Ohoka, T. Ohnuki, N. Tada, Y. Asoh, and M. Tachibana. 2001. Four strains of spontaneously hyperlipidemic (SHL) mice: phenotypic distinctions determined by genetic backgrounds. J. Atheroscler. Thromb. 871-79. [DOI] [PubMed] [Google Scholar]
- 34.McGhee, J. R., and H. Kiyono. 1999. The mucosal immune system, p. 909-930. In W. E. Paul (ed.), Fundamental immunology. Lippincott-Raven Publishers, Philadelphia, PA.
- 35.Namikoshi, J., S. Otake, S. Maeba, M. Hayakawa, Y. Abiko, and M. Yamamoto. 2003. Specific antibodies induced by nasally administered 40-kDa outer membrane protein of Porphyromonas gingivalis inhibits coaggregation activity of P. gingivalis. Vaccine 22250-256. [DOI] [PubMed] [Google Scholar]
- 36.Paigen, B., A. Morrow, P. A. Holmes, D. Mitchell, and R. A. Williams. 1987. Quantitative assessment of atherosclerotic lesions in mice. Atherosclerosis 68231-240. [DOI] [PubMed] [Google Scholar]
- 37.Papapanou, P. N. 1996. Periodontal diseases: epidemiology. Ann. Periodontol. 11-36. [DOI] [PubMed] [Google Scholar]
- 38.Pussinen, P. J., P. Jousilahti, G. Alfthan, T. Palosuo, S. Asikainen, and V. Salomaa. 2003. Antibodies to periodontal pathogens are associated with coronary heart disease. Arterioscler. Thromb. Vasc. Biol. 231250-1254. [DOI] [PubMed] [Google Scholar]
- 39.Qi, M., H. Miyakawa, and H. K. Kuramitsu. 2003. Porphyromonas gingivalis induces murine macrophage foam cell formation. Microb. Pathog. 35259-267. [DOI] [PubMed] [Google Scholar]
- 40.Ridker, P. M. 2002. On evolutionary biology, inflammation, infection, and the cause of atherosclerosis. Circulation 1052-4. [PubMed] [Google Scholar]
- 41.Ridker, P. M., M. Cushman, M. J. Stampfer, R. P. Tracy, and C. H. Hennekens. 1997. Inflammation, aspirin, and the risk of future myocarditis infarction among apparently healthy men. N. Engl. J. Med. 336973-979. [DOI] [PubMed] [Google Scholar]
- 42.Ridker, P. M., N. Rifai, M. J. Stampfer, and C. H. Hennekens. 2000. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 1011767-1772. [DOI] [PubMed] [Google Scholar]
- 43.Ross, R. 1999. Atherosclerosis: an inflammatory disease. N. Engl. J. Med. 340115-126. [DOI] [PubMed] [Google Scholar]
- 44.Ross, R. 1999. Atherosclerosis is an inflammatory disease. Am. Heart J. 138S419-S420. [DOI] [PubMed] [Google Scholar]
- 45.Rothenbacher, D., S. Muller-Scholge, C. Herder, W. Koening, and H. Kolb. 2006. Differential expression of chemokines, risk of stable coronary heart disease, and correlation with established cardiovascular risk markers. Arterioscler. Thromb. Vasc. Biol. 26194-199. [DOI] [PubMed] [Google Scholar]
- 46.Saito, S., K. Hiratsuka, M. Hayakawa, H. Takiguchi, and Y. Abiko. 1997. Inhibition of a Porphyromonas gingivalis colonizing factor between Actinomyces viscosus ATCC 19246 by monoclonal antibodies against recombinant 40-kDa outer-membrane protein. Gen. Pharmacol. 28675-680. [DOI] [PubMed] [Google Scholar]
- 47.Scannaperco, F. A., R. B. Bush, and S. Paju. 2003. Associations between periodontal disease and risk for atherosclerosis, cardiovascular disease, and stroke: a systemic review. Ann. Periodontol 838-53. [DOI] [PubMed] [Google Scholar]
- 48.Shibata, Y., K. Hiratsuka, M. Hayakawa, T. Shiroza, H. Takiguchi, Y. Nagatsuka, and Y. Abiko. 2003. A 35-kDa co-aggregation factor is a hemin binding protein in Porphyromonas gingivalis. Biochem. Biophys. Res. Commun. 300351-356. [DOI] [PubMed] [Google Scholar]
- 49.Tellides, G., D. A. Tereb, N. C. Kirkiles-Smith, R. W. Kim, J. H. Wilson, J. S. Schechner, M. I. Lorber, and J. S. Pober. 2000. Interferon-gamma elicits arteriosclerosis in the absence of leukocytes. Nature 403207-211. [DOI] [PubMed] [Google Scholar]
- 50.Tonetti, M. S., F. D'Aiuto, L. Nibali, A. Donald, C. Storry, M. Parkar, J. Suvan, A. D. Hingorani, P. Vallance, and J. Deanfield. 2007. Treatment of periodontitis and endothelial function. N. Engl. J. Med. 356911-920. [DOI] [PubMed] [Google Scholar]
- 51.Vita, J. A., and J. Loscalzo. 2002. Shouldering the risk factor burden: infection, atherosclerosis, and the vascular endothelium. Circulation 106164-166. [DOI] [PubMed] [Google Scholar]
- 52.Vogel, S. N., L. L. Weedon, R. N. Moore, and D. L. Rosenstreich. 1982. BCG-induced enhancement of endotoxin sensitivity in C3H/HeJ mice. II. T-cell modulation of macrophage sensitivity to LPS in vitro. Immunobiology 160479-493. [DOI] [PubMed] [Google Scholar]
- 53.Von der Thusen, J. H., J. Kuiper, T. J. C. Van Berkel, and E. A. L. Biessen. 2003. Interleukins in atherosclerosis: molecular pathways and therapeutic potential. Pharmacol. Rev. 55133-166. [DOI] [PubMed] [Google Scholar]
- 54.Wu, H., S. Ghosh, X. D. Perrard, L. Feng, G. E. Garcia, J. L. Perrard, J. F. Sweeney, L. E. Peterson, L. Chan, C. W. Smith, and C. M. Ballantyne. 2007. T-cell accumulation and regulated on activation, normal T-cell expressed and secreted upregulation in adipose tissue in obesity. Circulation 1151029-1038. [DOI] [PubMed] [Google Scholar]
- 55.Wu, T., M. Trevisan, R. J. Genco, J. P. Dorn, K. L. Falkner, and C. T. Sempos. 2000. Periodontal disease and risk of cerebrovascular disease: the first national health and nutrition examination survey and its follow-up study. Arch. Intern. Med. 1602749-2755. [DOI] [PubMed] [Google Scholar]