Abstract
Salmonella enterica serovar Typhi and Typhimurium vaccine candidates elicit significant immune responses in mice by intranasal (i.n.) immunization. Because of the proximity of the cribriform plate of the ethmoid bone, we were concerned that Salmonella bacteria delivered i.n. might access the brain. Accordingly, wild-type and attenuated (by single and double mutations) strains of S. enterica serovars Typhimurium and Typhi were recovered at low numbers initially from the olfactory lobe and then from the brain for 3 to 4 days after i.n. immunization. This was independent of invA gene function. Although the presence of bacteria in blood 1 to 3 h after i.n. inoculation was sometimes observed, this was infrequent compared to the frequency of bacteria detected in brain tissues. In confirmation of recent observations by Wickham et al. (M. E. Wickham, N. F. Brown, J. Provias, B. B. Finlay, and B. K. Coombes, BMC Infect. Dis. 7:65, 2007) that oral inoculation with wild-type S. enterica serovar Typhimurium strains lead to bacteria in blood with subsequent colonization of brain tissues with neurological symptoms of disease, we found similar results by using the i.n. and intraperitoneal (i.p.) routes of inoculation for wild-type but not for attenuated strains of S. enterica serovar Typhimurium. In contrast, a highly modified attenuated S. enterica serovar Typhimurium strain was not present in brain tissues when administered at higher doses by the oral, i.n., and i.p. routes than the wild-type strain even though the presence of bacteria in blood was detectable 1 to 3 h after inoculation by each of the three routes. Our results indicate that i.n. and possibly even oral delivery of live Salmonella vaccines may be unsafe although it is possible to reduce this risk by appropriate genetic modifications.
Attenuated strains of Salmonella have been widely investigated for use as oral vaccines and as live vectors to deliver antigens because of their ability to induce mucosal, humoral, and cell-mediated immune responses (4, 5, 19, 20). Preclinical studies have shown that intranasal (i.n.) immunization of mice with attenuated recombinant Salmonella enterica serovar Typhi and Typhimurium vaccine vectors induced excellent immune responses to the expressed antigens, as well as to Salmonella antigens (10, 15, 22, 23, 26). Since the cribriform plate is a fragile, well-hidden bone in the nasal cavity with numerous perforations that allow passage of the olfactory nerves to the brain, there exists a potential route for Salmonella bacteria to enter the cranial cavity if administered i.n. We therefore evaluated strains of S. enterica serovars Typhimurium and Typhi attenuated by several different means, as well as their wild-type parents, for the ability to reach the brains of BALB/c and Swiss Webster mice after i.n. inoculation and investigated the route of brain access by colonization of the olfactory bulbs versus access through the circulatory system (25, 29).
As reported in the clinical literature, meningitis is occasionally a consequence of presumably oral S. enterica serovar Typhi infection, especially in young children (7, 13, 18, 21). Recently, Wickham et al. (30) reported that oral infection of mice with wild-type S. enterica serovar Typhimurium strains can cause meningoencephalitis. We have confirmed these observations and determined that such infections also arise after inoculation with wild-type strains by the i.n. and i.p routes. Another question of interest is whether substantially modified vaccine strains might still be able to cause infections that result in brain colonization or neurological disease symptoms. We have addressed these issues and report our findings here.
(The findings reported here were briefly communicated earlier [2].)
MATERIALS AND METHODS
Bacterial strains and media.
The S. enterica serovar Typhimurium UK-1 and S. enterica serovar Typhi strains used in this study and their respective genotypes are listed in Table 1. The mutants or wild-type strains were grown with aeration to late log phase in LB broth (1) at 37°C. Buffered saline with gelatin (BSG) (3) was used as a diluent and to suspend bacteria prior to inoculation of mice.
TABLE 1.
Bacterial strains used in this studya
| Strain | Relevant genotype | Derivation, source, and/or reference |
|---|---|---|
| S. enterica serovar Typhimurium | ||
| χ3339 | SL1344 hisG | 11 |
| χ3761 | UK-1 wild type | 6 |
| χ4402 | invA::Km | χ3761 |
| χ8132 | Δcya-27 Δcrp-27 | χ3761 |
| χ8768 | ΔphoP233 | χ3761 |
| χ9558 | Δpmi-2426 Δ(gmd-fcl)-26 ΔPfur-81::TT araC PBAD fur ΔPcrp-527::TT araC PBAD crp ΔasdA27::TTaraC PBAD c2 ΔaraE25 ΔaraBAD23 ΔrelA198::araC PBAD lacI TT ΔsopB1925 ΔagfBAC811 | |
| S. enterica serovar Typhi | ||
| χ3744 | ISP1820 cys trp | 27 |
| χ3769 | Ty2 rpoS cys trp | WRAIR |
| χ8110 | ISP1820 Δcrp-pabA40 Δcya-27 cys trp | χ3744 |
| χ8438 | Ty2 RpoS+cys trp | χ3769, 24 |
| χ8542 | Ty2 RpoS+ ΔphoP24 cys trp | χ8438 |
Defined deletion strains were constructed by using suicide vectors and P22 cotransduction according to methods described by Kang et al. (16). Strains were stored in 1% Bacto peptone containing 5% glycerol at −70°C prior to use.
Mice and inoculation methods.
Six- to 7-week-old female BALB/c and Swiss Webster mice (Charles River Laboratories, Wilmington, MA) were maintained in Nalgene filter-covered isolators for 1 week prior to infection. The rodent suite was maintained at 22 to 23°C with 12 h of illumination daily. Bacterial strains were grown as overnight standing cultures that were diluted 1 to 100 in prewarmed LB broth and grown with mild aeration to an optical density at 600 nm of 0.8 to 0.9. Bacteria were sedimented by centrifugation at room temperature and resuspended in BSG to densities appropriate for the inoculation route and dose. Mice were fasted for food and water for 4 h prior to inoculation. Ten microliters of ∼109 CFU of S. enterica serovar Typhimurium or Typhi suspended in BSG was administered i.n. (5 μl per nare) to mice without anesthesia. This method ensures nasal delivery and avoids delivery of bacteria to the lungs, as occurs when using larger volumes and/or anesthesia. In some experiments, lower doses of CFU were administered. Doses of 105 to 109 CFU in 20 μl were used for oral inoculation, and doses of 103 to 106 CFU in 50 μl were used for intraperitoneal (i.p.) inoculation with lower doses of wild-type virulent strains than of attenuated strains. Food and water were returned 30 min after inoculation. Mice were euthanized via asphyxiation with CO2 and necropsied at various times.
Analysis of brain tissues for the presence of bacteria.
All incisions in euthanized mice were made with flame-sterilized scissors on the dorsal side of the mouse directly into the brain cavity. Brain tissues were extracted by slipping flame-sterilized forceps under the brain and gently pulling the brain toward the neck and then out of the brain cavity. The nose and respiratory pathway were left intact and undisturbed. Brain tissues were washed in 1 ml of sterile BSG in 24-well plates. The cerebrum and cerebellum were resuspended in sterile BSG until homogenization. In some studies, the olfactory lobe was separated from the remainder of the brain for separate analysis. The homogenizer (Brinkmann, Westbury, NY) was washed with 5% Amphyl, followed by a wash with 70% ethanol, followed by two washes with distilled H2O. Homogenized tissues were plated onto MacConkey agar-1% lactose plates. Salmonella colonies were white on the MacConkey agar plates. Isolated colonies were further identified by agglutination with Salmonella O antiserum.
Analysis of blood for the presence of bacteria.
Fifty-microliter samples of blood were taken from the saphenous veins of anesthetized mice. These samples were directly plated on MacConkey agar-1% lactose.
RESULTS
Presence of bacteria in brain tissues following i.n. inoculation.
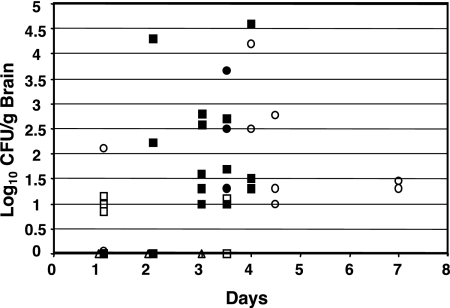
Figure 1 shows data on the recovery of the S. enterica serovar Typhimurium UK-1 and SL1344 wild-type (χ3761 and χ3339) and mutant strains from the brains of mice after i.n. infection. High numbers of χ4402 invA::Km mutant cells were recovered from the brains on days 4 and 4.5, indicating that the inv locus (9) does not play a role in the ability of Salmonella bacteria to colonize the brain. However, the highly attenuated ΔphoP233 mutant strain χ8768 colonized the brains of mice at lower numbers on days 1 and 3.5 compared to the wild-type and invA mutant strains (Fig. 1). Lastly, the avirulent and highly immunogenic UK-1 Δcya-27 Δcrp-27 mutant strain χ8132, in the few animals inoculated, was not observed to colonize the brains of BALB/c mice after i.n. inoculation at days 1, 2, and 3 (Fig. 1).
FIG. 1.
Colonization of brains of BALB/c mice after i.n. inoculation of S. enterica serovar Typhimurium UK-1 and SL1344 and their derivatives. Each symbol represents the log10 number of CFU per gram of brain tissue at various times for individual mice. Strains: χ3761, UK-1 wild type (▪); χ3339, SL1344 wild-type (•); χ8768, ΔphoP233 mutant (□); χ4402, invA::Km mutant (○); χ8132, Δcya-27 Δcrp-27 mutant (▵).
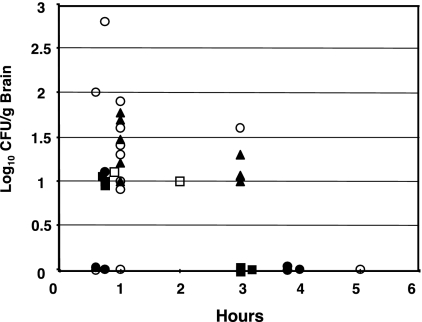
Figure 2 presents data on the recovery of S. enterica serovar Typhi mutants from the brains of mice after i.n. inoculation with 109 CFU of ISP1820, Ty2 (which is RpoS−), and a Ty2 RpoS+ derivative. Isolated colonies were further identified by agglutination with Salmonella Vi antiserum. The Ty2 wild-type strain was recovered in higher numbers at 1 and 3 h after i.n. inoculation than was its RpoS+ derivative χ8438. Therefore, it is evident that the sigma factor RpoS, which regulates virulence in S. enterica serovar Typhimurium (8) and also mediates the stationary-phase expression of many genes, including resistance to low pH, oxidation, and starvation (14), has no appreciable effect on the access of Salmonella bacteria to the brain. After i.n. inoculation with 109 CFU, the χ8542 ΔphoP24 mutant S. enterica serovar Typhi strain was isolated in higher numbers from the brains of mice at 45 min and 1 h than were any of the wild-type strains, indicating that the highly attenuating ΔphoP24 mutation has no apparent ability to diminish the access of S. enterica serovar Typhi to the brain. This observation was in contrast to the reduced brain-colonizing ability of ΔphoP233 mutant S. enterica serovar Typhimurium χ8768 (Fig. 1). ISP1820 and the ISP1820 derivative χ8110, with Δcya and Δ(crp-pabA) attenuating mutations, colonized the brains of mice to a lesser extent than did the phoP mutant strains but was not evaluated a sufficient number of times to allow any conclusions concerning its relative brain-accessing ability. Overall, 102 of 170 mice inoculated i.n. were positive for the presence of Salmonella bacteria in the brain.
FIG. 2.
Colonization of brains of BALB/c mice after i.n. inoculation of S. enterica serovar Typhi ISP1820, Ty2, and their derivatives. Each symbol represents the log10 number of CFU per gram of brain tissue at various times for individual mice. Strains: χ3744, ISP1820 wild type (▪); χ3769, Ty2 RpoS− (▴); χ8438, Ty2 RpoS+ (•); χ8110, Δcrp-pabA40 Δcya-27 mutant (□); χ8542, ΔphoP24 mutant (○).
Initial colonization of the olfactory bulb.
In a separate series of experiments, we separated the olfactory bulb from the remainder of the brain prior to the homogenization and enumeration of bacteria. In an analysis of 96 mice inoculated with doses ranging from 107 to 109 CFU, 27 had detectable Salmonella bacteria in olfactory lobe tissue and only 18 had Salmonella bacteria in the remainder of the brain tissue (Table 2). In no case did we detect Salmonella bacteria in brain tissue in the absence of detection of Salmonella bacteria in the olfactory lobe of the same mouse. This study provides support for the belief that Salmonella bacteria access the brain following i.n. inoculation by traversing the olfactory nerve through the cribriform plate to the olfactory bulb.
TABLE 2.
Colonization of olfactory bulbs and the cerebrum by S. enterica serovar Typhimurium χ3761
| Sampling time or parameter | No. of olfactory bulbs positive/total | No. of cerebra positive/total | Breed of mouse |
|---|---|---|---|
| Day 1 | 2/12a | 1/12 | BALB/c inbred |
| 3/12 | 0/12 | BALB/c inbred | |
| 1/12 | 1/12 | Swiss Webster outbred | |
| Avg no. of CFU | 4.2 × 101 | 1.3 × 102 | |
| Day 2 | 5/12 | 4/12 | BALB/c inbred |
| 0/12 | 1/12 | Swiss Webster outbred | |
| Avg no. of CFU | 8.4 × 102 | 6.5 × 102 | |
| Day 3 | 6/12 | 2/12 | BALB/c inbred |
| 2/12 | 2/12 | Swiss Webster outbred | |
| 6.0 × 102 | 6.4 × 102 | ||
| Day 6 | 8/12 | 7/12 | BALB/c inbred |
| Avg no. of CFU | 7.3 × 103 | 4.6 × 105 | |
| Total | 27/96 | 18/96 |
Each value shown is the number positive/total, unless otherwise noted.
Potential for blood being the conduit for Salmonella bacteria accessing the brain.
In several i.n. infections with 109 CFU, we failed to detect any Salmonella bacteria in 100-μl samples of blood analyzed 1 and 3 days after inoculation. However, since oral inoculation of mice with wild-type S. enterica serovar Typhimurium can lead to bacteria in blood (29) very soon after infection and blood-borne S. enterica serovar Typhimurium can infect brain tissue (25), we investigated whether i.n. inoculation could lead to the presence of bacteria in blood soon after inoculation. As shown by the data in Table 3, both wild-type S. enterica serovar Typhimurium and a highly attenuated strain can be detected in blood 1 and 3 h after infection by the oral, i.n., and i.p. routes. In addition, all three routes of infection lead to the presence of bacteria in brain tissues 24 and 72 h after infection by the wild-type strain but not after infection by the highly attenuated mutant χ9558(pYA4088). In these studies, we did not investigate the behavior of attenuated strains having a single attenuating mutation, as was done for the studies reported in Fig. 1, but presume we might have found bacteria in blood and bacteria in olfactory bulb and brain tissues. However, in numerous studies, we have never observed neurological symptoms as described below when inoculating 109 CFU of a diversity of attenuated S. enterica serovar Typhimurium strains via the oral route of inoculation. It should be reiterated that we were scrupulous in avoiding blood contamination of brain tissues during their removal and also washed tissues with buffer prior to their homogenization. Nevertheless, it appears that wild-type and attenuated Salmonella strains can access brain tissue by trafficking up the olfactory nerve through the cribriform plate to the olfactory bulb and then to the brain or, in the case of wild-type strains, at least, by traversing through blood to penetrate the blood-brain barrier to access brain tissues.
TABLE 3.
S. enterica serovar Typhimurium entry into the blood and brains of BALB/c mice depending on the route of inoculationa
| Strain and no. of CFU (route) | Presence in blood
|
Presence in brain
|
||
|---|---|---|---|---|
| 1 hpib | 3 hpi | 24 hpi | 72 hpi | |
| Wild-type χ3761 | ||||
| 0.7 × 109-1.2 × 109 (oral) | 4/12c | 1/12 | 2/12 | 4/12 |
| 1.1 × 106-1.2 × 106 (i.n.) | 2/6 | 0/6 | 2/6 | 4/6 |
| 7.0 × 108 (i.n.) | 0/6 | 0/6 | 1/3 | 2/3 |
| 1.1 × 103-1.2 × 103 (i.p.) | 5/6 | 6/6 | 0/6 | 6/6 |
| 7.0 × 108 (i.p.) | 6/6 | 6/6 | 3/3 | Dead |
| Attenuated strain χ9558(pYA4088) | ||||
| 0.62 × 109-1.3 × 109 (oral) | 3/6 | 1/6 | 0/6 | 0/6 |
| 0.62 × 108-1.3 × 108 (i.n.) | 3/6 | 2/6 | 0/6 | 0/6 |
| 0.62 × 106-1.3 × 106 (i.p.) | 4/6 | 2/6 | 0/6 | 0/6 |
The methods used are described in Materials and Methods.
hpi, hours postinoculation.
Each value shown is the number positive/total.
Neurological disease symptoms in mice caused by S. enterica serovar Typhimurium infection.
Mice infected by the oral, i.n., and i.p. routes with doses of the wild-type S. enterica serovar Typhimurium strain χ3761 that were near the 50% lethal dose for those routes of infection occasionally developed signs of neurological disease. For example, 4 of 25 BALB/c mice that ultimately died or were euthanized after the administration of oral doses of 1.2 × 104, 1.2 × 105, and 1.2 × 106 CFU of χ3761 (10 mice per dose) showed neurological signs of illness (twirling, rolling, jumping, loss of motor control, etc.) and had high numbers of bacteria in brain tissues, as well as in the Peyer's patches, mesenteric lymph nodes, spleen, and liver. In this study, four of nine mice euthanized 10 days after inoculation also had bacteria cultured from brain tissues but at a lower mean titer (4 × 103 CFU) than the mice euthanized with neurological symptoms (1.3 × 104 CFU). These results confirm and extend the observations of Wickham et al. (30) that wild-type strains of S. enterica serovar Typhimurium can cause meningoencephalitis when infection is by diverse routes, including the i.n. route.
Neurological symptoms have so far been undetected in mice inoculated by any route at doses up to those used for the studies in Table 3 with the attenuated vaccine strain χ9558(pYA4088). This strain, although able to access blood, also has so far been undetected in brain tissues independent of the route of inoculation.
DISCUSSION
In evaluating the ability of S. enterica serovars Typhimurium and Typhi to access the brain after i.n. inoculation, the number of bacteria present in the brains of individual mice was highly variable. The number of S. enterica serovar Typhi CFU per gram of brain tissue was lower than that of S. enterica serovar Typhimurium, and S. enterica serovar Typhimurium was isolated from the brain at later time points than S. enterica serovar Typhi. These observations can be attributed to the host specificity of S. enterica serovar Typhi such that mice are able to survive high doses of S. enterica serovar Typhi administered by various routes whereas this bacterium causes a systemic infection and typhoid fever in humans. In contrast, S. enterica serovar Typhimurium causes gastroenteritis in humans but causes a systemic infection similar to human typhoid fever in mice. Lastly, despite the means of attenuation by single or double mutations, S. enterica serovar Typhimurium and S. enterica serovar Typhi were able to access the brains of BALB/c mice and persist for a number of days. Based on our results, it is evident that i.n. inoculation of Salmonella bacteria into BALB/c and Swiss Webster mice permits the entry of both wild-type virulent and attenuated (by single and double mutations) Salmonella strains into the brain. This has previously been shown for intravenous (25) and oral (30) infections with wild-type virulent S. enterica serovar Typhimurium strains, and we have extended this to i.p. infection in this study. Although the number of Salmonella CFU per gram of brain tissue in our studies was variable and low, all of the S. enterica serovar Typhimurium and Typhi mutant and wild-type strains used, with the exception of strains χ8132 and χ9558, were capable of colonizing the brains of BALB/c mice. Moreover, all of the serotypes of Salmonella have been associated with occasionally causing bacterial meningitis and have been isolated from the cerebrospinal fluid of humans (7, 21). Meningitis is an unusual complication of typhoid fever, with the majority of cases involving newborns and young infants and being associated with significant mortality (7, 13, 18). However, 5 to 35% of adult typhoid fever patients have signs and symptoms associated with the central nervous system such as seizure or acute psychotic behavior, confusion, and dizziness, usually occurring within the first few days of fever (28). In addition, a significant percentage of the patients who survive Salmonella meningitis suffer permanent neurological deficits (17). The results reported by Wickham et al. (30) and those in this report reveal that multiple routes of infection with wild-type S. enterica serovar Typhimurium can all lead to meningitis in mice. By the intravenous, i.p., and oral inoculation routes, it appears that bacteria enter the circulatory system and somehow pass the blood-brain barrier to colonize brain tissues. In several i.n. infections with 109 CFU of both wild-type and attenuated S. enterica serovar Typhimurium strains, we failed to detect any Salmonella bacteria in 100-μl samples of blood analyzed 1 and 3 days after inoculation. However, as shown by the data in Table 3, we did detect both wild-type and attenuated strains in blood 1 and 3 h after i.n. inoculation. Thus, although we observed the sequential presence of Salmonella bacteria on olfactory bulbs before their presence in higher brain tissues (Table 2), it remains possible that brain colonization following i.n. inoculation could be by either hematogenous or nonhematogenous routes.
Since the i.n. route of inoculation is an attractive route for vaccination due to the induction of excellent mucosal and systemic immune responses, it should continue to be used to evaluate the immunogenicity of antigens presented by host-adapted live attenuated bacterial vaccine candidates in various animal species such as mice. However, further evaluation of the safety of i.n. inoculation in human clinical trials remains to be discussed and further research is needed to evaluate new means of attenuating live bacterial vaccines to preclude colonization of the brain. Also of importance is a more careful analysis of orally administered attenuated vaccine strains to demonstrate that they fail to access brain tissues even though neurological symptoms are absent. In this regard, we have so far not observed such colonization of brain tissues or induction of neurological symptoms with an extensively modified attenuated S. enterica serovar Typhimurium strain being developed as safe for more disease-susceptible ages, physiological states, and genotypes of mice (12). This strain, χ9558(pYA4088), is very immunogenic, and its safety properties and excellent attributes as an antigen delivery vector will be the subject of other publications from our group.
Acknowledgments
We thank Shelley Haydel and Josephine Clark-Curtiss for critical review of the manuscript and Daniel Piatchek and Jack Diani at WU and Jacki Kilbourne at ASU for assistance with the animal experiments.
This research project was supported by grants from the National Institutes of Health (R01 DE06669 and R01 AI024533) and the Bill and Melinda Gates Foundation (37863).
Editor: F. C. Fang
Footnotes
Published ahead of print on 12 May 2008.
REFERENCES
- 1.Bertani, G. 1951. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 62293-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bollen, W. S., B. Gunn, M. Lay, and R. Curtiss III. 2003. Colonization of the brain following intranasal inoculation with Salmonella, abstr. E-088, p. 265-266. Abstr. 103rd Gen. Meet. Am. Soc. Microbiol. American Society for Microbiology, Washington, DC.
- 3.Curtiss, R., III. 1965. Chromosomal aberrations associated with mutations to bacteriophage resistance in Escherichia coli. J. Bacteriol. 8928-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curtiss, R., III. 1990. Attenuated Salmonella strains as live vectors for the expression of foreign antigens, p. 715-740. In G. C. Woodrow and M. M. Levine (ed.), New generation vaccines. Marcel Dekker, New York, NY.
- 5.Curtiss, R., III., T. Doggett, A. Nayak, and J. Srinivasan. 1996. Strategies for the use of live recombinant avirulent bacterial vaccines for mucosal immunization, p. 499-511. In H. Kiyono and M. F. Kagnoff (ed.), Essentials of mucosal immunology. Academic Press, San Diego, CA.
- 6.Curtiss, R., III., S. B. Porter, M. Munson, S. A. Tinge, J. O. Hassan, C. Gentry-Weeks, and S. M. Kelly. 1991. Nonrecombinant and recombinant avirulent Salmonella live vaccines for poultry, p. 169-198. In L. C. Blankenship, J. S. Bailey, N. A. Cox, S. E. Craven, R. J. Meinersmann, and N. J. Stern (ed.), Colonization control of human bacterial enteropathogens in poultry. Academic Press, Inc., New York, NY.
- 7.Denis, F., S. Badiane, J. P. Chiron, A. Sow, and I. D. Mar. 1977. Salmonella meningitis in infants. Lancet 1910. [DOI] [PubMed] [Google Scholar]
- 8.Fang, F. C., S. J. Libby, N. A. Buchmeier, P. C. Loewen, J. Switala, J. Harwood, and D. G. Guiney. 1992. The alternative sigma factor katF (rpoS) regulates Salmonella virulence. Proc. Natl. Acad. Sci. USA 8911978-11982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galán, J. E., and R. Curtiss III. 1989. Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc. Natl. Acad. Sci. USA 866383-6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galen, J. E., O. G. Gomez-Duarte, G. A. Losonsky, J. L. Halpern, C. S. Lauderbaugh, S. Kaintuck, M. K. Reymann, and M. M. Levine. 1997. A murine model of intranasal immunization to assess the immunogenicity of attenuated Salmonella typhi live vector vaccines in stimulating serum antibody responses to expressed foreign antigens. Vaccine 15700-708. [DOI] [PubMed] [Google Scholar]
- 11.Gulig, P. A., and R. Curtiss III. 1987. Plasmid-associated virulence of Salmonella typhimurium. Infect. Immun. 552891-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gunn, B., X. Zhang, S. Y. Wanda, and R. Curtiss III. 2007. Safety of recombinant attenuated Salmonella vaccines (RASV) in infant mice, abstr. E-063, p. 278. Abstr. 107th Gen. Meet. Am. Soc. Microbiol. American Society for Microbiology, Washington, DC.
- 13.Henderson, L. L. 1948. Salmonella meningitis: report of three cases and review of one hundred and forty-four cases from the literature. Am. J. Dis. Child. 75351-375. [DOI] [PubMed] [Google Scholar]
- 14.Hengge-Aronis, R. 2000. A role for the sigma S subunit of RNA polymerase in the regulation of bacterial virulence. Adv. Exp. Med. Biol. 48585-93. [DOI] [PubMed] [Google Scholar]
- 15.Hopkins, S., J. P. Kraehenbuhl, F. Schödel, A. Potts, D. Peterson, P. de Grandi, and D. Nardelli-Haefliger. 1995. A recombinant Salmonella typhimurium vaccine induces local immunity by four different routes of immunization. Infect. Immun. 633279-3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang, H. Y., C. M. Dozois, S. A. Tinge, T. H. Lee, and R. Curtiss III. 2002. Transduction-mediated transfer of unmarked deletion and point mutations through use of counterselectable suicide vectors. J. Bacteriol. 184307-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karim, M., and N. Islam. 2002. Salmonella meningitis: report of three cases in adults and literature review. Infection 30104-108. [DOI] [PubMed] [Google Scholar]
- 18.Kauffman, C. A., and R. J. St Hilaire. 1979. Salmonella meningitis. Occurrence in an adult. Arch. Neurol. 36578-580. [DOI] [PubMed] [Google Scholar]
- 19.Medina, E., and C. A. Guzman. 2001. Use of live bacterial vaccine vectors for antigen delivery: potential and limitations. Vaccine 191573-1580. [DOI] [PubMed] [Google Scholar]
- 20.Mielcarek, N., S. Alonso, and C. Locht. 2001. Nasal vaccination using live bacterial vectors. Adv. Drug Delivery Rev. 5155-69. [DOI] [PubMed] [Google Scholar]
- 21.Olsen, S. J., R. Bishop, F. W. Brenner, T. H. Roels, N. Bean, R. V. Tauxe, and L. Slutsker. 2001. The changing epidemiology of Salmonella: trends in serotypes isolated from humans in the United States, 1987-1997. J. Infect. Dis. 183753-761. [DOI] [PubMed] [Google Scholar]
- 22.Pasetti, M. F., T. E. Pickett, M. M. Levine, and M. B. Sztein. 2000. A comparison of immunogenicity and in vivo distribution of Salmonella enterica serovar Typhi and Typhimurium live vector vaccines delivered by mucosal routes in the murine model. Vaccine 183208-3213. [DOI] [PubMed] [Google Scholar]
- 23.Pasetti, M. F., R. Salerno-Goncalves, and M. B. Sztein. 2002. Salmonella enterica serovar Typhi live vector vaccines delivered intranasally elicit regional and systemic specific CD8+ major histocompatibility class I-restricted cytotoxic T lymphocytes. Infect. Immun. 704009-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santander, J., S. Y. Wanda, C. A. Nickerson, and R. Curtiss III. 2007. Role of RpoS in fine-tuning the synthesis of Vi capsular polysaccharide in Salmonella enterica serotype Typhi. Infect. Immun. 751382-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sebastiani, G., V. Blais, V. Sancho, S. N. Vogel, M. M. Stevenson, P. Gros, J. M. Lapointe, S. Rivest, and D. Malo. 2002. Host immune response to Salmonella enterica serovar Typhimurium infection in mice derived from wild strains. Infect. Immun. 701997-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Srinivasan, J., A. Nayak, R. Curtiss III, and S. Rubino. 1995. Effect of the route of immunization using recombinant Salmonella on mucosal and humoral immune responses, p. 273-280. In R. M. Chanock, F. Brown, H. S. Ginsberg, and E. Norrby (ed.), Vaccines 95. Cold Spring Harbor Laboratory Press, Plainview, NY.
- 27.Tacket, C. O., D. M. Hone, R. Curtiss III, S. M. Kelly, G. Losonsky, L. Guers, A. M. Harris, R. Edelman, and M. M. Levine. 1992. Comparison of the safety and immunogenicity of ΔaroC ΔaroD and Δcya Δcrp Salmonella typhi strains in adult volunteers. Infect. Immun. 60536-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uysal, H., A. Karademir, M. Kilinc, and O. Erturk. 2001. Salmonella encephalopathy with seizure and frontal intermittent rhythmic delta activity. Infection 29103-106. [DOI] [PubMed] [Google Scholar]
- 29.Vazquez-Torres, A., J. Jones-Carson, A. J. Bäumler, S. Falkow, R. Valdivia, W. Brown, M. Le, R. Berggren, W. T. Parks, and F. C. Fang. 1999. Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature 401804-808. [DOI] [PubMed] [Google Scholar]
- 30.Wickham, M. E., N. F. Brown, J. Provias, B. B. Finlay, and B. K. Coombes. 2007. Oral infection of mice with Salmonella enterica serovar Typhimurium causes meningitis and infection of the brain. BMC Infect. Dis. 765. [DOI] [PMC free article] [PubMed] [Google Scholar]