Abstract
Otitis media caused by nontypeable Haemophilus influenzae (NTHi) is a common and recurrent bacterial infection of childhood. The structural variability and diversity of H. influenzae lipopolysaccharide (LPS) glycoforms are known to play a significant role in the commensal and disease-causing behavior of this pathogen. In this study, we determined LPS glycoform populations from NTHi strain 1003 during the course of experimental otitis media in the chinchilla model of infection by mass spectrometric techniques. Building on an established structural model of the major LPS glycoforms expressed by this NTHi strain in vitro (M. Månsson, W. Hood, J. Li, J. C. Richards, E. R. Moxon, and E. K. Schweda, Eur. J. Biochem. 269:808-818, 2002), minor isomeric glycoform populations were determined by liquid chromatography multiple-step tandem electrospray mass spectrometry (LC-ESI-MSn). Using capillary electrophoresis ESI-MS (CE-ESI-MS), we determined glycoform profiles for bacteria from direct middle ear fluid (MEF) samples. The LPS glycan profiles were essentially the same when the MEF samples of 7 of 10 animals were passaged on solid medium (chocolate agar). LC-ESI-MSn provided a sensitive method for determining the isomeric distribution of LPS glycoforms in MEF and passaged specimens. To investigate changes in LPS glycoform distribution during the course of infection, MEF samples were analyzed at 2, 5, and 9 days postinfection by CE-ESI-MS following minimal passage on chocolate agar. As previously observed, sialic acid-containing glycoforms were detected during the early stages of infection, but a trend toward more-truncated and less-complex LPS glycoforms that lacked sialic acid was found as disease progressed.
Nontypeable Haemophilus influenzae (NTHi) is a significant cause of otitis media in children, accounting for upwards of 25% of acute otitis media, and is the most-common pathogen recovered from the middle ear of children with recurrent episodes (27). Although immunity against NTHi appears to develop after acute otitis media, protection is strain specific, permitting recurrent episodes due to antigenically distinct isolates (3, 35).
Lipopolysaccharide (LPS) is an essential surface component of H. influenzae and is implicated as a major virulence factor. Extensive structural studies of LPS from H. influenzae by us and others have led to the identification of a conserved glucose-substituted triheptosyl inner-core moiety l-α-d-Hepp-(1→2)-[PEtn→6]-l-α-d-Hepp-(1→3)-[β-d-Glcp-(1→4)]-l-α-d-Hepp linked to lipid A via 3-deoxy-d-manno-oct-2-ulosonic acid (Kdo) 4-phosphate (where PEtn is phosphoethanolamine) (7, 19-26, 28-33, 40-42). This inner-core unit provides the template for the attachment of oligosaccharide and noncarbohydrate substituents. The structures, genetics, and expression of variant LPS glycoforms and their implications for virulence have recently been reviewed (34).
LPS of H. influenzae can mimic host glycolipids and has a propensity for reversible switching of the expression of epitopes (phase variation) which is thought to provide an adaptive mechanism for the bacteria when confronted by the differing microenvironments and immune responses of the host. Our previous studies have focused on the extent of conservation and variability of LPS expression in a representative set of clinical isolates of NTHi obtained from otitis media patients (15, 18, 19-22, 32, 33, 41, 42) and relating this to the role of the molecule in commensal and virulence behavior. The results of several recent studies have shown that NTHi LPS oligosaccharides replaced by terminal sialic acid (N-acetyl neuraminic acid) residues are critical virulence factors in the pathogenesis of otitis media in chinchillas (5) and gerbils (37). This was demonstrated by comparison of the pathogenesis in vivo of wild-type and isogenic sialic acid-deficient mutant NTHi strains. The mechanism by which sialylation of LPS contributes to virulence has been investigated in the chinchilla model of otitis media, in which an important role for interactions with complement was demonstrated (9). Sialylation could also play a role in masking LPS epitopes that are targets for the host immune system (38) or through molecular mimicry of hostlike structures (5, 39). It has also been proposed that the expression of sialylated LPS glycoforms may play a role in pathogenesis by promoting biofilm formation (8, 10, 14, 37). By using a capillary electrophoresis electrospray mass spectrometry (CE-ESI-MS) technique, we determined in the well-defined chinchilla model that virulence afforded by LPS sialylation depends on the capacity of NTHi to scavenge sialic acid from the host (5). However, due to the relatively small number of bacterial cells in the middle ear fluid (MEF), only data for samples taken during the early stages of infection (4 to 6 days after inoculation) were reported. In addition, these direct samples showed high background noise in CE-MS experiments due to tissue and blood contamination and needed particular care in sample preparation and loading onto the CE column.
For this study, we analyzed MEF samples taken after 7 days from 10 animals inoculated with NTHi strain 1003 as the challenge strain. Detailed information on major LPS structures elaborated by strain 1003 was available (Fig. 1) (20), and structural detail on minor LPS glycoforms is provided here. It was possible to detect major glycoforms in direct MEF samples from 7 of the 10 animals. We passaged bacteria from ex vivo samples minimally on solid medium and determined that their LPS glycan profiles were essentially the same as those obtained for direct MEF samples. Following passage, the signal-to-noise ratio in the CE-ESI-MS spectra increased significantly and the amount of LPS material was sufficient to determine the sequence of glycoforms by liquid chromatography multiple-step tandem ESI-MS (LC-ESI-MSn). With this increased sensitivity, CE-MS was employed to monitor changes in glycoform expression during the course of infection in a further series of experiments. A trend toward less-complex glycoforms containing fewer hexose residues was found as disease progressed. This is the first detailed structural description of the glycan pattern of LPS expressed during the course of an infection.
FIG. 1.
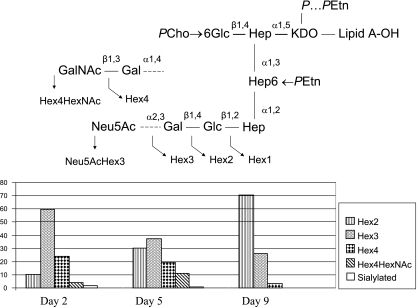
Schematic representation of major LPS glycoforms reported for NTHi strain 1003 (20). A triheptosyl inner-core unit is substituted at the O-4 position of HepI by a β-d-Glcp residue (GlcI) which, in turn, is linked to PCho at O-6. HepIII is chain elongated at O-2 by either a β-d-Glcp residue (Hex2 glycoform), lactose (Hex3 glycoform), or sialyllactose [minor, i.e., α-Neu5Ac-(2→3)-β-d-Galp-(1→4)-β-d-Glcp]. GlcI has been found to carry an O-acetyl substituent at the O-4 position; in some glycoforms, a second O-acetyl group is located at O-3 of HepIII, and a third minor acetylation site was identified at the glucose residue off HepIII. Glycine is linked to HepIII and Kdo. Dashed lines indicate the various truncated Hex2 and Hex3 glycoforms.
MATERIALS AND METHODS
Bacterial strain and culture.
NTHi 1003 was obtained from the Finnish Otitis Media Study Group. It is an isolate from the middle ear and has been described previously (20). For middle ear inoculation, NTHi 1003 was grown to mid-log phase in brain-heart infusion broth supplemented with 10 μg/ml haemin and 2 μg/ml NAD (sBHI) at 37°C (5). Following growth in chinchillas, bacteria from middle ear exudate samples were cultivated on chocolate agar (CA) (Remel, Lenexa, KS) with (25 μg/ml) or without added NeuAc.
Experimental otitis media model.
An experimental chinchilla model of acute otitis media was used (1). All procedures and manipulations were performed using sedation analgesia with a mixture of ketamine and xylazine in accordance with approved IACUC protocols at Boston University Medical Center (5). Two experiments with 10 animals each were carried out. Isolates of NTHi 1003 grown to mid-log phase in sBHI at 37°C were diluted in Hank's balanced salt solution (HBSS), and 50 CFU in 100 μl were inoculated through the left superior bulla of adult chinchillas with a 25-gauge tuberculin needle (5). Forty-eight hours after inoculation with NTHi, tympanometry, otomicroscopy, and middle ear cultures were performed to determine the presence of infection. Direct and indirect ear examinations were performed on days 2, 5, 7, 9, and 16 after inoculation until the cultures of two consecutive middle ear samplings were sterile.
The middle ear cavity was accessed as described previously (1). MEF, when present, was obtained with a 22-gauge angiocatheter connected to an empty tuberculin syringe and used as follows: 10 μl of MEF was diluted 1:10 in HBSS, and serial 10-fold dilutions were plated onto unsupplemented CA (uCA) plates for quantitation. The lower limit of detection of viable organisms in MEF using this dilution series was 100 CFU/ml. For the first set of animals (animals 1 to 10), in separate experiments, viable organisms in MEF were passaged on solid (CA) and liquid (sBHI) media. MEF (10 μl) was streaked onto uCA plates which were incubated overnight at 37°C. The resulting colonies were harvested and suspended in 0.5 ml of Hank's solution, to which phenol was added (2% final concentration). An additional 10 μl of MEF was grown in sBHI broth overnight at 37°C, and 0.5 ml of the liquid culture was placed in 2% phenol and frozen at −80°C for subsequent analysis. Quantitation of cells was not performed on these samples. Phenol (final concentration 2%) was added directly to the remaining MEF aspirate for direct analysis of LPS glycoforms by mass spectrometry. If MEF was absent, the middle ear was flushed with 0.5 to 1 ml of HBSS, phenol was added (2% final concentration), and the specimen was frozen for subsequent analysis. For the second set of animals (animals 11 to 20), a direct culture from the middle ear was obtained with a calcium alginate swab and immediately streaked onto two CA plates, one supplemented with 75 μl of Neu5Ac (10 μg/μl) and the second one without supplemental Neu5Ac. The plates were incubated overnight and then passed to new CA plates four consecutive times. Colonies were harvested after each passage, suspended in 0.5 ml of HBSS to which 2% phenol was added, and flash frozen.
LPS preparation.
LPS from in vitro-grown NTHi 1003 was obtained as previously described (20). Samples of bacteria obtained directly from the middle ear of chinchillas or following passage were frozen in 2% phenol and then processed in an identical manner. LPS was extracted as follows: phenol was removed by low-speed centrifugation and washing with water. The bacterial cell wall was disrupted with proteinase K followed by successive treatments with DNase and RNase to enhance the release of free LPS, which was O-deacylated in situ with anhydrous hydrazine to give O-deacylated LPS (LPS-OH) and analyzed directly by CE-ESI-MS (17).
Preparation of oligosaccharides.
Reduced core oligosaccharide material was obtained following mild-acid hydrolysis of LPS-OH (1% acetic acid [pH 3.1], 100°C, 2 h) and reduction with sodium borohydride (NaBH4, 1 M NH3, 20°C, 16 h). Samples were dephosphorylated with 48% hydrogen fluoride (300 μl 48% aqueous HF, 4°C, 48 h). Methylation was performed with methyl iodide in dimethyl sulfoxide in the presence of lithium methylsulfinylmethanide (4). The methylated compounds were recovered by using a SepPak C18 cartridge and subjected to LC-ESI-MSn.
MS.
CE-ESI-MS was carried out with a Crystal model 310 CE instrument (ATI Unicam, Boston, MA) coupled to an API 3000 mass spectrometer (Perkin-Elmer/Sciex, Concord, Canada) via a MicroIonspray interface as described previously (5). In order to improve the signal-to-noise ratio, the mass resolution was set as “unit” in all CE-MS experiments. LC-ESI-MSn experiments on permethylated oligosaccharide samples were performed in the positive-ion mode on a Waters 2690 high-performance liquid chromatography (HPLC) system (Waters, Milford, MA) coupled to a Finnigan LCQ ion trap MS (Finnigan-MAT, San Jose, CA). The experiments were done on sodium adducts [M + Na]1+, using selective ion monitoring (SIM) or selective reaction monitoring (SRM). A microbore C18 column [150 by 0.5 mm LUNA 5μ C18 (2); Phenomenex, Torrance, CA] was used with an eluent gradient consisting of 0.1 mM NaOAc and 1% HOAc in MeOH as eluent A and 0.1 mM NaOAc and 1% HOAc in H2O. Gradient elution was conducted as follows: 50% A at 0 min, 54% A at 15 min, 100% A at 35 min, 54% A at 55 min, 50% A at 65 to 75 min. The flow rate was 0.018 ml/min.
RESULTS
Sequence analysis of dephosphorylated and permethylated oligosaccharides derived from NTHi strain 1003 by MSn.
To obtain information on previously undetected minor glycoforms and glycoform isomerism, we subjected dephosphorylated and permethylated oligosaccharide material from in vitro-cultured NTHi 1003 to LC-ESI-MSn. This strategy has been applied for profiling glycoform expression in several NTHi strains (12, 32, 41) and was also the method of choice for profiling isomeric LPS glycoforms in material derived from the chinchilla ear (see below). Thus, due to the increased MS response obtained by permethylation in combination with added sodium acetate, several glycoforms were observed in the full-scan MS spectra that were not easily detected in underivatized samples (20). In addition, sequence information was readily obtained, since methyl tagging allows fragment ions generated by the cleavage of a single glycosidic bond and inner fragments resulting from the rupture of two glycosidic linkages to be distinguished. For most glycoforms, the presence of several isomeric compounds was revealed by identifying product ions in MS2 spectra, and when necessary, MS3 experiments were used to confirm structures. The LC-ESI-MS spectrum (positive mode) of dephosphorylated and permethylated oligosaccharide material from in vitro-grown NTHi 1003 (in sBHI broth) showed major sodiated adduct ions [M + Na]+ at m/z 1,467.7 and 1,671.6, corresponding to permethylated Hex2·Hep3·AnKdo-ol (where AnKdo-ol is reduced anhydro-Kdo) and Hex3·Hep3·AnKdo-ol, respectively. Minor ions at m/z 1,263.7 and 1,875.9 corresponded to Hex1·Hep3·AnKdo-ol and Hex4·Hep3·AnKdo-ol, respectively. In order to obtain sequence and branching information, these molecular ions were further fragmented in MS2 and MS3 experiments, and the results are summarized in Fig. 2. The predominant Hex1 glycoform, A1 (90% of Hex1 isomers), was defined by ions at m/z 1,001.6 and 753.5, corresponding to consecutive losses of a t-Hep-Hep unit (where t is terminal) from the parent ion (m/z 1,263.7). A minor Hex1 glycoform, A2, was defined by ions at m/z 797.3 and 737.3, corresponding to losses of t-Hex-Hep and Hep-Kdo, respectively. The Hex2 glycoform B1 was defined by ions at m/z 1,249.5, 1,001.5, and 753.4, resulting from consecutive losses of t-Hex, Hep, and Hep from the parent ion at m/z 1,467.7. This was the only Hex2 glycoform detected when the bacteria were grown in vitro. Two Hex3 glycoforms could be identified. Of these, the major glycoform C1 (90%) was defined by ions at m/z 1,001.5 and 753.4, corresponding to the loss of t-Hex-Hex-Hep and t-Hex-Hex-Hep-Hep, respectively, from the parent ion (m/z 1,671.8). The minor Hex3 glycoform C2 was defined by ions m/z 1,205.6 and 957.4, corresponding to consecutive losses from a t-Hex-Hep-Hep unit. Three Hex4 glycoforms could be identified, of which D1 (10%) was defined by ions at m/z 1,001.5 and 753.4, due to consecutive losses from a t-Hex-Hex-Hex-Hep-Hep unit present in the parent ion (m/z 1,875.9). D2 (45%) was defined by ions at m/z 1,205.6 and 957.5, corresponding to consecutive losses from t-Hex-Hex-Hep-Hep, while the third Hex4 glycoform (D3; 45%) was defined by ions at m/z 1,409.5 and 1,161.5, due to consecutive losses from t-Hex-Hep-Hep. It should be noted that the sequences of the major Hex2 and Hex3 glycoforms B1 and C1 corresponded to those previously published (Fig. 1) but none of the minor isomers were detected or described earlier.
FIG. 2.
Isomeric glycoforms observed in NTHi strain 1003 as identified by tandem ESI-MSn. Glycoform B2 is only indicated in chinchilla sample 25 and evidenced by MS2 spectra showing ions m/z 1,205.4 and 957.3 due to consecutive losses of tHep-Hep. Glycoform E was not observed in vitro but was detected in chinchilla samples 22 to 24, as described in the text.
Analyses of LPS glycoform distribution in samples taken from the chinchilla middle ear by CE-ESI-MS. Investigation of the effect of passage on glycoform distributions.
Ten chinchillas (animals 1 to 10) were inoculated in the left ear with NTHi strain 1003. All animals developed otitis media characterized by inflammatory exudates and recovery of NTHi cells from the middle ear. MEF was taken from the animals on day 7 after inoculation (approximately 107 CFU/ml). MEF as described above was cultured ex vivo both in sBHI broth and on uCA. LPS was obtained by using a micro extraction procedure (17) and directly analyzed by CE-ESI-MS analysis (negative mode) following O-deacylation in situ to give LPS-OH. The data are presented in Table 1. Samples from animals 7, 9, and 10 were contaminated with Staphylococcus aureus, and no ions due to LPS-OH glycoforms could be detected using CE-ESI-MS. Doubly charged ions corresponding to Hex2 glycoforms were detected in all other MEF samples and, in addition, ions corresponding to Hex1, Hex3, or Hex4 glycoforms were detected in the MEF from some of the animals. The spectra for LPS-OH from sBHI-cultured organisms were characterized by weak ion intensities corresponding to a very low abundance or an absence of Hex3 and Hex4 glycoforms, with Hex1 and Hex2 glycoforms representing the major populations observed. The shift toward truncated glycoforms precluded the use of this passage protocol in subsequent analyses. However, the CE-ESI-MS spectra of samples obtained after ex vivo growth on solid medium (uCA) not only showed significantly increased signal-to-noise ratio but, for the majority of animals, the distribution of glycoforms corresponded to that from MEF. This is exemplified by the results obtained for animal 6 (Fig. 3). Thus, doubly charged ions at m/z 1,220/1,282, 1,300, and 1,382 are observed in the spectrum corresponding to LPS-OH from MEF (Fig. 3A), which correspond to Hex2, Hex3, and Hex4 glycoforms with the respective compositions PCho·Hex2·Hep3·PEtn1-2·P·Kdo·lipid A-OH, PCho·Hex3·Hep3·PEtn·P·Kdo·lipid A-OH, and PCho·Hex4·Hep3·PEtn·P·Kdo·lipid A-OH, where PCho is phosphocholine.
TABLE 1.
CE-ESI-MS (negative mode) identification of LPS glycoforms from NTHi strain 1003a
| Sample | Animal | Sample origin | Avg intensity and % of most-abundant peak of ion (m/z) for:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hex1
|
Hex2
|
Hex3
|
Hex4
|
HexNAcHex4
|
||||||||
| PEtn (1,139) | PEtn2 (1,200) | PEtn (1,220) | PEtn2 (1,282) | PEtn (1,300) | PEtn2 (1,363) | PEtn (1,382) | PEtn2 (1,444) | PEtn1 (1,484) | PEtn2 (1,545) | |||
| 1 | 1 | Direct MEF | * | − | * | − | − | − | − | − | − | − |
| 2 | 2 | Direct MEF | − | − | ** | * | − | − | * | − | − | − |
| 3 | 3 | Direct MEF | − | − | ** | − | * | − | ** | − | − | − |
| 4 | 4 | Direct MEF | * | * | * | − | − | − | − | − | − | − |
| 5 | 5 | Direct MEF | − | − | ** | ** | * | − | * | − | − | − |
| 6 | 6 | Direct MEF | − | − | **** | ** | ** | − | * | − | − | − |
| 7 | 7 | Direct MEF | − | − | − | − | − | − | − | − | − | − |
| 8 | 8 | Direct MEF | ** | ** | ** | ** | * | − | − | − | − | − |
| 9 | 9 | Direct MEF | − | − | − | − | − | − | − | − | − | − |
| 10 | 10 | Direct MEF | − | − | − | − | − | − | − | − | − | − |
| 11 | 1 | sBHI | * | * | * | * | − | − | − | − | − | − |
| 12 | 2 | sBHI | * | * | * | * | − | − | − | − | − | − |
| 13 | 3 | sBHI | * | * | * | * | − | − | − | − | − | − |
| 14 | 4 | sBHI | * | * | * | * | − | − | − | − | − | − |
| 15 | 5 | sBHI | − | − | * | * | − | − | − | − | − | − |
| 16 | 6 | sBHI | * | * | * | − | − | − | − | − | − | − |
| 17 | 7 | sBHI | − | − | − | − | − | − | − | − | − | − |
| 18 | 8 | sBHI | * | * | * | * | − | − | − | − | − | − |
| 19 | 9 | sBHI | − | − | − | − | − | − | − | − | − | − |
| 20 | 10 | sBHI | − | − | − | − | − | − | − | − | − | − |
| 21 | 1 | uCA | + | + | +++++ | ++ | + | − | − | − | − | − |
| 22 | 2 | uCA | + | + | +++++ | +++ | + | + | − | − | − | − |
| 23 | 3 | uCA | − | − | +++++ | +++ | + | − | ++ | + | + | − |
| 24 | 4 | uCA | + | + | +++++ | +++ | + | + | − | − | − | − |
| 25 | 5 | uCA | +++++ | ++ | + | − | − | − | − | − | − | − |
| 26 | 6 | uCA | + | + | +++++ | +++ | ++ | + | − | − | − | − |
| 27 | 7 | uCA | − | − | − | − | − | − | − | − | − | − |
| 28 | 8 | uCA | ++++ | +++ | +++++ | +++ | + | − | − | − | − | − |
| 29 | 9 | uCA | − | − | − | − | − | − | − | − | − | − |
| 30 | 10 | uCA | − | − | − | − | − | − | − | − | − | − |
Glycoforms were isolated directly from chinchilla middle ear fluid 7 days after inoculation and following passage on BHI and uCA. *****, the average intensity of five scans is greater than 2,001 cps (counts per second); ****, the average intensity of five scans is between 1,000 and 2,000 cps; ***, the average intensity of five scans is between 501 and 1,000 cps; **, the average intensity of five scans is between 201 and 500 cps; *, the average intensity of five scans is less than 200 cps. +++++, the most-abundant peak; ++++, 80% of the most-abundant peak; +++, 60% of the most-abundant peak; ++, 40% of the most-abundant peak; +, 20% of the most-abundant peak; −, not detected.
FIG. 3.
CE-ESI-MS analysis (negative mode) of LPS-OH isolated from animal 6. The spectra correspond to LPS-OH in MEF (A), LPS-OH isolated after ex vivo growth in sBHI (B), and LPS-OH obtained from cells grown ex vivo on uCA (C).
The signal-to-noise ratio in the spectrum corresponding to LPS-OH isolated after ex vivo growth in liquid sBHI (Fig. 3B) is significantly decreased. However, doubly charged ions at m/z 1,139, 1,200, and 1,220 were detected and correspond to the more truncated glycoform populations with the respective glycoform compositions PCho·Hex·Hep3·PEtn·P·Kdo·lipid A-OH, PCho·Hex·Hep3·PEtn2·P·Kdo·lipid A-OH, and PCho·Hex2·Hep3·PEtn·P·Kdo·lipid A-OH. This pattern was observed in the majority of samples passaged in liquid medium (Table 1). Figure 3C shows the CE-ESI-MS spectrum of LPS-OH obtained from cells grown ex vivo on solid medium (uCA). This spectrum more closely mirrors the glycoform distribution observed in direct MEF samples. Significant doubly charged ions are observed at m/z 1,220/1,282, corresponding to PCho·Hex2·Hep3·PEtn1-2·P·Kdo·lipid A-OH, as well as a less prominent ion at m/z 1,300 and 1,382 corresponding to PCho·Hex3·Hep3·PEtn·P·Kdo·lipid A-OH and PCho·Hex4·Hep3·PEtn·P·Kdo·lipid A-OH, respectively. LPS isolated from bacteria obtained from only two animals (animals 5 and 8) showed Hex1 instead of Hex2 as the predominant glycoform after growth on uCA. In addition, glycoforms with two PEtn residues appeared to increase in abundance in the samples passaged on uCA (Table 1). These observations indicate that LPS glycoform populations obtained following single passage of bacteria derived from MEF on uCA closely represent those presented in bacteria directly from the MEF.
Sequence analysis on dephosporylated and permethylated oligosaccharide material obtained from chinchilla samples.
To accommodate the limited amount of available bacterial material in MEF samples, we applied a micro method involving mild acid hydrolysis of LPS-OH and the reduction and dephosphorylation of the resulting oligosaccharide material in one reaction vial prior to permethylation as outlined by us previously (12). The samples obtained in this way were subjected to LC-ESI-MSn in the positive mode, and to increase sensitivity, we employed SIM and SRM experiments, using information obtained from CE-ESI-MS, on the glycoforms present. Singly charged sodiated ions corresponding to Hex1, Hex2, Hex3, Hex4, and Hex4HexNAc glycoforms have the m/z values 1,264.0, 1,468.0, 1,672.0, 1,876.0, and 2,121.0, respectively, which were used for SIM and SRM analyses in these experiments. Using this sensitive methodology, a Hex4HexNAc glycoform corresponding to the ion at m/z 2,121.0 that was only observed in sample 23 (animal 3) in the LPS-OH (Table 1) was detected in three of the methylated samples (samples 22 to 24; animals 2 to 4) (see below).
In the spectra of MEF samples (samples 3 to 6 and 8) and those passaged in sBHI (samples 11 to 16 and 18), only ions of low signal intensity corresponding to the Hex1 to Hex3 glycoforms detected by CE-ESI-MS were observed, precluding further tandem MS analyses other than MS2. Figure 4 shows MS2 spectra obtained for ions at m/z 1,264.0, 1,468.0, and 1,672.0 of sample 3 (animal 3). Although weak, ions at m/z 753.3 and 1,001.4 in Fig. 4A indicate the presence of the Hex1 glycoform A1. In Fig. 4B, the Hex2 glycoform B1 is evidenced by ions at m/z 1,001.5 and 737.3 and the Hex3 glycoform C1 by ions at m/z 941.4 and 693.3. Spectra of samples obtained after passage on uCA (samples 21 to 26 and 28) correlated well with those from CE-ESI-MS and showed sufficient signal-to-noise ratios for MSn experiments. The presence of isomeric glycoforms in these samples was established by the results of MS2 and MS3 experiments and is summarized in Table 2. Figure 5 shows the total ion chromatogram of the selected ions m/z 1,264, 1,468, 1,672, 1,876, and 2,121 and ion chromatograms of the SRM experiments run on the different glycoforms (A to E) in sample 23. The results of MS2 of the Hex2 glycoforms B1 and B2 identified in samples 23 (animal 3) and 25 (animal 5), respectively, are shown in Fig. 6. In all samples, the predominant Hex1 glycoform was A1, as for the in vitro-grown sample (see above). The Hex2 glycoform B1 was dominant in all samples except in sample 25 (animal 5), in which structure B2 was predominant. B2 is characterized by a disaccharide unit elongating HepI, evident from ions m/z 1,205.4 and 957.3, due to the consecutive loss of a t-Hep-Hep unit from the molecular ion (Fig. 6B). B2 was not observed in the in vitro-grown NTHi 1003. The Hex3 glycoform C1 was the most abundant in all samples. The Hex4 glycoform was only detectable in samples 21 to 24 (animals 1 to 4), and in these, structure D1 predominated. Interestingly, D1 was minor when NTHi 1003 was grown in vitro in sBHI. Only samples 22 to 24 (animals 2 to 4) revealed an ion at m/z 2121.0 corresponding to HexNAc·Hex4·Hep3·AnKdo-ol. MS2 fragmentation on this ion resulted, inter alia, in signals at m/z 1,002.3 and 753.3 defining a structure E in which a HexNAcHex3 unit and a hexose are linked to HepIII and HepI, respectively. Figure 7 shows the elucidation of the Hex4 and Hex4HexNAc glycoforms of sample 23 from animal 3.
FIG. 4.
HPLC-ESI-MS2 spectra (positive mode) obtained for the ions m/z 1,264.0 (A), 1,468.0 (B), and 1,672.0 (C) of permethylated sample 3 (animal 3), taken directly from the MEF.
TABLE 2.
Glycoforms identified by tandem MS2 and MS3 experiments on permethylated oligosaccharide material
| Sample (animal) | Major abundant glycoforms | Minor observed glycoforms |
|---|---|---|
| 21 (1) | A1, B1, C1, D1 | A2, C2, D2, D3 |
| 22 (2) | A1, B1, C1, D1, E | C2 |
| 23 (3) | A1, B1, C1, D1, E | A2 |
| 24 (4) | A1, B1, C1, D1, E | A2, C2 |
| 25 (5) | A1, B2 | B1 |
| 28 (8) | A1, B1, C1 | A2, C2 |
FIG. 5.
Total ion chromatogram of the ions m/z 1,264.0, 1,468.0, 1,672.0, 1,876.0, and 2,121.0 obtained on permethylated oligosaccharide from sample 23 (animal 3) after passage on uCA. The chromatograms of each single ion are shown in insets A (m/z 1,264.0), B (m/z 1,468.0), C (m/z 1,672.0), D (m/z 1,876.0), and E (m/z 2,121.0).
FIG. 6.
HPLC-ESI-MS2 spectra (positive mode) of the Hex2 glycoform (m/z 1,468.0) of sample 23 from animal 3 (A) and sample 25 from animal 5 (B), both cultured ex vivo on uCA.
FIG. 7.
HPLC-ESI-MS2 spectra (positive mode) of the Hex4 (A) and Hex4HexNAc (B) glycoforms (m/z 1,876.0 and 2,121.0) of sample 23 from animal 3. MS3 spectra of the fragment ions at m/z 1,145.4 (Aa) and 1,001.4 (Ab), as well as at m/z 1,391.7 (Ba) and 1,002.3 (Bb), are shown.
Applications to large-scale sample analysis: identification of glycoform profile changes during disease progression.
We concluded that the LPS from MEF passaged on uCA was comparable to that from direct samples. Based on this premise, we used this approach to evaluate changes in LPS glycoform expression during the course of otitis media in the chinchilla. Ten chinchillas (animals 11 to 20) were each inoculated with ca. 50 CFU of NTHi 1003 into the middle ear bilaterally. All animals developed otitis media characterized by inflammatory exudates and large numbers of organisms (105 to 107 CFU/ml) that were evident by 2 days after inoculation and persisted for more than 16 days. Exudates from the middle ears of chinchillas 2, 5, and 9 days after inoculation were passaged on CA plates with or without Neu5Ac, and the LPS was analyzed by CE-ESI-MS following O-deacylation. Interestingly, there was no significant difference in glycoform distribution during four consecutive passages or between growths irrespective of whether sialic acid was added to the medium or not. This is illustrated with the data from animal 11 at day 5 (Fig. 8). The results, averaged over the four passes with and without sialic acid (eight growths), are presented for the 10 animals in Table 3. The extracted mass spectra for animal 11 following the first passage (in the presence of sialic acid) are presented in Fig. 9. After day 2, the glycoform distribution ranged from Hex2 to Hex4, of which the Hex3 glycoform predominated. This was evident from prominent doubly charged ions at m/z 1,301 and 1,363 corresponding to PCho·Hex3·Hep3·PEtn·P·Kdo·lipid A-OH and PCho·Hex3·Hep3·PEtn2·P·Kdo·lipid A-OH, respectively. After 5 days (Fig. 9B), the doubly charged ions corresponding to the Hex2 glycoform (m/z, 1,220 and 1,282) were significantly more prominent. In addition, ions corresponding to the Hex4HexNAc glycoform were also present at m/z 1,484 and 1,545. This was the only animal in which the presence of this glycoform was detectable (Table 3). After 9 days, the Hex2 glycoform was the most prominent, while ions corresponding to the Hex4HexNAc glycoform were no longer detectable (Fig. 9C). The trend toward lower-hexose-containing (truncated) glycoforms observed during the course of infection is illustrated for animal 11 in Fig. 10. This trend was observed for 8 out of the 10 animals in this study (Table 3). In five of these animals, the occurrence of Hex1 glycoforms became significant by day 9 even though they only represented on average a small portion (<3%) of the glycoform population at day 2. This was particularly pronounced in animals 15 (Hex1; ∼80%) and 17 (Hex1; ∼94%), and tandem MS experiments on LPS-OH from animal 15 at day 9 indicated that the Hex1 glycoform A1 was predominant (Fig. 11). In three animals (animals 12, 16, and 19), Hex2 glycoforms persisted, showing no apparent trend toward further truncation (Table 3).
FIG. 8.
Histogram showing relation of glycoform compositions from bacteria isolated from animal 11 at day 5 following one to four passages on uCA and on CA supplemented with sialic acid. +, present; −, absent.
TABLE 3.
Comparison of glycoform compositions during the course of infection in the chinchilla infection model of otitis mediaa
| Animal | Day | % Expression in glycoform profile
|
||||
|---|---|---|---|---|---|---|
| Hex1 | Hex2 | Hex3 | Hex4 | Hex4HexNAc | ||
| 11 | 2 | − | 10.5 | 59.5 | 24.0 | − |
| 5 | − | 30.1 | 37.2 | 19.4 | 13.3 | |
| 9 | − | 70.3 | 26.3 | 3.4 | − | |
| 12 | 2 | 2.5 | 85.4 | 12.1 | − | − |
| 5 | 1.3 | 90.8 | 7.9 | − | − | |
| 9 | ||||||
| 13 | 2 | 0.5 | 54.3 | 45.2 | − | − |
| 5 | 1.0 | 90.6 | 8.4 | − | − | |
| 9 | 0.5 | 93.7 | 5.8 | − | − | |
| 14 | 2 | 0.5 | 88.9 | 10.6 | − | − |
| 5 | 17.4 | 74.5 | 8.1 | − | − | |
| 9 | 17.5 | 74.5 | 8.0 | − | − | |
| 15 | 2 | 0.5 | 83.5 | 16.0 | − | − |
| 5 | 0.2 | 86.5 | 13.3 | − | − | |
| 9 | 79.8 | 16.8 | 3.4 | − | − | |
| 16 | 2 | − | 88.7 | 11.3 | − | − |
| 5 | − | 89.7 | 10.3 | − | − | |
| 9 | − | 89.2 | 10.8 | − | − | |
| 17 | 2 | 2.3 | 92.4 | 6.3 | − | − |
| 5 | 20.3 | 76.3 | 3.4 | − | − | |
| 9 | 93.5 | 6.5 | − | − | − | |
| 18 | 2 | 1.8 | 93.5 | 4.3 | − | − |
| 5 | 1.1 | 95.3 | 3.6 | − | − | |
| 9 | 17.0 | 79.2 | 3.8 | − | − | |
| 19 | 2 | − | 90.0 | 10.0 | − | − |
| 5 | − | 90.7 | 9.3 | − | − | |
| 9 | − | 90.8 | 9.2 | − | − | |
| 20 | 2 | 0.2 | 83.8 | 16.0 | − | − |
| 5 | 0.2 | 86.8 | 13.0 | − | − | |
| 9 | 41.5 | 50.7 | 7.8 | − | − | |
Values were determined from signal areas of ESI-MS results for molecular ions at indicated time points. Data are the averages of measurements taken after each of four passes on uCA and four passes on CA supplemented with sialic acid (i.e., a total of eight passes). No sample was available for animal 12 on day 9. −, not detected.
FIG. 9.
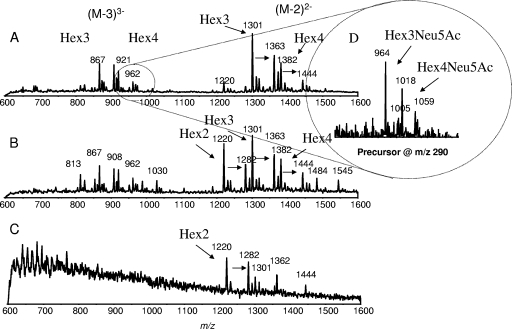
CE-ESI-MS analysis (negative mode) of LPS-OH isolated from animal 11 after 2 days (A), 5 days (B), and 9 days (C). (D) Precursor ion spectrum (negative mode) using m/z 290 as the fragment ion for identification of sialylated components after 2 days.
FIG. 10.
Histogram showing changes in LPS glycoform populations of bacteria isolated from animal 11 after 2, 5, and 9 days following passage on uCA. Data were obtained by CE-ESI-MS analysis of LPS-OH (see Fig. 9). Glycoforms are depicted in structural model of NTHi 1003 LPS.
FIG. 11.
CE-ESI-MS and tandem MSn analysis (positive mode) of LPS-OH from animal 15 showing extracted mass spectrum (A), tandem MS spectrum of ion at m/z 1,141 ([M + 2]2+) (B), and MS3 spectrum of ion at m/z 1,328 ([M + 1]+) derived from m/z 1,141 (C). Assignments of major fragment ions are indicated.
Sialylated glycoforms were not evident in the full-scan mass spectra of any of the samples, but could be detected by precursor ion monitoring for ions giving rise to the fragment ion at m/z 290 (Neu5Ac). Sialic acid-containing glycoforms were only present in LPS-OH samples obtained at 2 and 5 days; no sialic acid was detectable in the samples obtained at day 9. In addition to the expected sialyllactose Hex3 LPS glycoform (Fig. 1) as evidenced by the triply charged ions at m/z 964 (Kdo-P) and 1,005 (Kdo-PPEtn), a pair of triply charged ions at m/z 1,018 and 1,059 indicated a sialylated Hex4 glycoform (Fig. 9D). This probably arises from the sialylation of a disaccharide extension (sialyllactose) from HepIII in the Hex4 glycoform D2 (cf. Fig. 2). NTHi strain 1003, when grown in vitro, has been shown to express glycoforms having sialyllactose linked to HepIII (Fig. 1), but only in very low abundance. The expression of sialic acid-containing glycoforms was at a low frequency in the passaged exudate samples, and after 5 days of growth in the middle ear, sialic acid-containing glycoforms represented only 1 to 2% of the total LPS populations.
DISCUSSION
A structural model for LPS glycoforms expressed by NTHi strain 1003 cultured under in vitro growth conditions in liquid medium (sBHI) has been previously established by nuclear magnetic resonance and ESI-MS on LPS-OH and the major core oligosaccharide material obtained following mild-acid hydrolysis of LPS (20). Here we have analyzed dephosphorylated and permethylated oligosaccharide, derived from the previously investigated NTHi 1003 LPS (obtained after growth in liquid medium), by LC-ESI-MSn to obtain information on undetected minor glycoforms and glycoform isomerism. We found Hex1 to Hex4 glycoforms, of which the major Hex2 and Hex3 (B1 and C1; Fig. 2) glycoforms corresponded to those described earlier (Fig. 1). Of particular interest were glycoforms C2, D2, and D3 in which chain elongation from GlcI is indicated. Chain elongation from GlcI in H. influenzae LPS has been found to be initiated by the phase-variable gene lex2B (11). The lex2 locus comprises lex2A and lex2B, of which lex2B encodes a glycosyltransferase. Sequence polymorphisms in lex2B between various NTHi strains is proposed to direct the addition of either a β-glucose or a β-galactose to GlcI (P. Hermant, M. E. Deadman, M. K. R. Engskog, E. R. Moxon, E. K. H. Schweda, and D. W. Hood, unpublished data). From the sequence of lex2 in NTHi strain 1003 (data not shown), we propose that the sugar linked to GlcI is a β-d-Glcp. This would suggest that the next sugar linked to this glucose in D3 is a β-d-Galp, as previously observed in other NTHi strains (34). Analysis of dephosphorylated and permethylated oligosaccharides derived from chinchilla samples by LC-ESI-MSn enabled us to identify structures B2 and E which were not previously identified (20) or detected here in the in vitro-grown sample. B2 is proposed to have a β-d-Glcp-(1→4)-β-d-Glcp-(1→ unit linked to HepI, based on the proposed function of lex2 in this strain (as discussed above). In all strains investigated so far, when a β-d-Glcp residue is linked to HepIII, this glucose shows further extension at O-4 by β-d-GalNAcp-(1→3)-α-d-Galp-(1→4)-β-d-Galp-(1→ or α-Neu5Ac-(2→8)-α-Neu5Ac-(2→3)-β-d-Galp-(1→ and/or truncated versions of these (34). The genes lgtC and lgtD are responsible for the addition of α-d-Galp and β-d-GalNAcp, respectively, to build the terminal β-d-GalNAcp-(1→3)-α-d-Galp-(1→ unit (13). The glycosyltransferase LgtC competes with a sialyltransferase, Lic3A, in adding either an α-d-Galp or α-Neu5Ac, respectively, to the lactose. The genes lgtD and lgtC are both present in NTHi strain 1003, which is consistent with the prediction that a globotetraose unit is linked to HepIII in structure E.
Using sensitive CE-ESI-MS techniques, we have previously structurally characterized LPS glycoforms from ex vivo samples. We profiled LPS oligosaccharide glycoforms during experimental infection in the chinchilla model of otitis media by analyzing MEF samples taken from the animals (5). CE coupled to ESI-MS is a particularly sensitive method for profiling LPS glycoforms on O-deacylated samples. LC-ESI-MSn of dephosphorylated and permethylated oligosaccharide material provides sequence and branching details of the various LPS glycoforms. However, due to the limited number of bacterial cells (not more than 107 per ml) in the MEF, direct samples in general gave weak signals upon analysis and showed high background noise due to the significant amount of host material present. This makes it difficult to detect minor glycoforms or isomeric glycoform distributions by CE-ESI-MS and LC-ESI-MS. Thus, the CE-ESI-MS spectrum of the MEF sample taken after 7 days from animal 3 (sample 3) revealed ions corresponding to Hex2 to Hex4 glycoforms (Table 1); however, the signal-to-noise ratio is weak. This was also reflected in the analysis of glycoform isomerism by LC-ESI-MSn on dephosphorylated and permethylated oligosaccharide material derived from this MEF sample (Fig. 4). Glycoforms A1, B1, and C1 were deduced from the MS2 spectra; however, no minor glycoforms were detected and the signal-to-noise ratio was too low to give informative MS3 spectra. When bacteria from MEF samples were passaged by a single culture on uCA, the LPS yield was increased and the resulting spectra showed a significantly higher signal-to-noise ratio, enabling the detection of a Hex4HexNAc glycoform from animal 3 (Fig. 7). The results of LC-ESI-MSn experiments allowed us to determine the sequence of this glycoform using MS3 experiments. This trend of a comparable distribution of glycoforms in MEF and single-passaged samples was observed in 5 of 7 samples and, thereby, provided a useful procedure for evaluating LPS glycoform profiles during the course of pathogenesis under sample-limiting conditions.
We applied this strategy in a second set of animal experiments in which MEF samples were taken at day 2, day 5, and day 9 following middle ear inoculation with NTHi strain 1003, and the LPS-OH was analyzed by CE-ESI-MS following passage on uCA or CA supplemented with sialic acid. In this experiment, all animals developed otitis media characterized by inflammatory exudates that were evident by 2 days after inoculation and persisted for more than 16 days. It was particularly noteworthy that a trend toward more truncated and less complex glycoforms was observed during the course of infection. This finding, together with a reduction in sialylated glycoforms during the course of the infection, deserves further comment. A critical factor in interpreting both of these findings concerns the sampling of organisms from middle ear exudate or washings. As noted, the numbers of ex vivo organisms obtained per sample were sufficiently small as to place constraints on the analysis of LPS glycoforms even using the most-sensitive, state-of-the-art analytical methods. Nonetheless, even taking into account possible alterations in the LPS phenotype resulting from minimal passaging of ex vivo organisms in vitro, we conclude that the results are likely to be robust, providing that the ex vivo organisms are representative of the biomass present in the middle ear. However, this is not certain because there could be many factors that result in biased sampling of NTHi organisms. The bacteria analyzed were those present in exudates or amenable to the washing technique, and in the case of organisms analyzed after minimal passage, only LPS glycoforms from viable organisms would be analyzed. Other factors to be considered are sequestration of infecting organisms through intracellular residence within the lining cells of the middle ear or recalcitrance to sampling through the formation of biofilms (2) or entrapment of organisms in inflammatory debris, such as neutrophil NETS (6). A previous study has emphasized the heterogeneity of phenotypes during the course of experimental infection of the middle ear of chinchillas (16).
The trend toward more truncated glycoforms during the course of the infection might be considered a counterintuitive finding in that evidence from in vitro assays suggests that the more truncated the LPS glycoforms, the more susceptible would these bacteria be to innate host defense clearance mechanisms. In particular, we have previously provided evidence that sialylated LPS glycoforms are required for virulence of NTHi (5), at least at some stage in the first several days of the experimental infection. At the very least, an explanation for our findings must include the possibility that the dynamics of the infection, with respect to the prevalent LPS phenotypes present early and later in the infection, are complex. For example, acquired immunity could select for organisms with truncated LPS glycoforms if the outer-core LPS structures were targeted by antibodies (36). The timing of the acquired immune response (occurring notionally at about 1 week into the infection) is consistent with our observations. In this scenario, the avoidance of clearance by innate immune mechanisms, especially complement-dependent lysis or opsonophagocytosis, would be a feature of the early stages of the pathogenesis, necessary but not sufficient to facilitate the initiation of disease.
Another whole perspective on the observed phenotype of the LPS glycoforms is one that considers the metabolic adaptation of the bacteria to the host environment when large numbers of organisms are present within the middle ear. We know nothing of the relative rates of clearance and replication at different stages of the infection, although mean numbers of bacteria, based on exudates/washings, suggest a steady state for a period of many days until the infection begins to resolve and bacterial numbers decline. However, it seems reasonable that the organisms responsible for maintaining active infection may undergo major changes in their metabolic profile as a consequence of available nutrients and their response to the stresses mediated by the host's plethora of innate and acquired immune responses (8). The biosynthesis of truncated LPS glycoforms could therefore represent a metabolic consequence or even an adaptive response, perhaps an indication of an economy of carbon and energy utilization.
Sialic acid is a critical virulence factor in the pathogenesis of otitis media in the chinchilla. We have previously shown that 6 days after inoculation, NTHi strain 486 ions due to sialylated glycoforms were only just detectable (5). The same observation could be made here when Neu5Ac-containing glycoforms were only detected in the early phase of infection (Fig. 9 and 10). In NTHi strain 1003, the lactose extension in the major Hex3 glycoform (C1) is required as the acceptor for the addition of Neu5Ac by Lic3A. The observed trend toward more truncated Hex2 and Hex1 glycoforms during the course of infection (day 5 to day 9) is consistent with the absence of sialylated glycoforms at day 9. This suggests that sialic acid is necessary for the initiation of infection in the middle ear, but not necessarily for continuation once established.
These findings could have consequences for preventative strategies, for example, LPS-based vaccines. If truncated glycoforms were to provide a mechanism of escape from antibodies elicited by outer-core LPS structures, then targeting inner-core LPS glycoforms might prove a safer strategy. However, this might not be required, since a vaccine-induced response involving antibodies to more-extended LPS glycoforms at the early and critical stages of initiating middle ear infection might prove effective, a view supported by the requirement for sialylated glycoforms at some early stage of the infection.
Acknowledgments
These studies were supported in part by a grant from the Swedish Research Council to E.K.H.S. and NIH NIDCD award DC005855 to R.G. The Medical Research Council, United Kingdom, provided support to D.W.H. and E.R.M.
The provision of NTHi strain 1003 by the Otitis Media Study Group (National Public Health Institute, Finland) is gratefully acknowledged. We thank Stephen Pelton for helpful discussion and critical reading of the manuscript. We also thank Adèle Martin and Frank St. Michael for valuable technical assistance in the preparation of LPS-OH samples.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 5 May 2008.
REFERENCES
- 1.Babl, F. E., S. I. Pelton, and Z. Li. 2002. Experimental acute otitis media due to nontypeable Haemophilus influenzae: comparison of high and low azithromycin doses with placebo. Antimicrob. Agents Chemother. 462194-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakaletz, L. O. 2007. Bacterial biofilms in otitis media: evidence and relevance. Pediatr. Infect. Dis. J. 26S17-S19. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein, J. M., H. S. Faden, B. G. Loos, T. F. Murphy, and P. L. Ogra. 1992. Recurrent otitis media with non-typeable Haemophilus influenzae: the role of serum bactericidal antibody. Int. J. Pediatr. Otorhinolaryngol. 231-13. [DOI] [PubMed] [Google Scholar]
- 4.Blakeney, A. B., and B. A. Stone. 1985. Methylation of carbohydrates with lithium methylsulphinyl carbanion. Carbohydr. Res. 140319-324. [Google Scholar]
- 5.Bouchet, V., D. W. Hood, J. Li, J. R. Brisson, G. A. Randle, A. Martin, Z. Li, R. Goldstein, E. K. Schweda, S. I. Pelton, J. C. Richards, and E. R. Moxon. 2003. Host-derived sialic acid is incorporated into Haemophilus influenzae lipopolysaccharide and is a major virulence factor in experimental otitis media. Proc. Natl. Acad. Sci. USA 1008898-8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brinkmann, V., and A. Zychlinsky. 2007. Beneficial suicide: why neutrophils die to make NETs. Nat. Rev. Microbiol. 5577-582. [DOI] [PubMed] [Google Scholar]
- 7.Cox, A. D., H. Masoud, P. Thibault, J. R. Brisson, M. van der Zwan, M. B. Perry, and J. C. Richards. 2001. Structural analysis of the lipopolysaccharide from the nontypable Haemophilus influenzae strain SB 33. Eur. J. Biochem. 2685278-5286. [DOI] [PubMed] [Google Scholar]
- 8.Erwin, A. L., and A. L. Smith. 2007. Nontypeable Haemophilus influenzae: understanding virulence and commensal behavior. Trends Microbiol. 15355-362. [DOI] [PubMed] [Google Scholar]
- 9.Figueira, M. A., S. Ram, R. Goldstein, D. W. Hood, E. R. Moxon, and S. I. Pelton. 2007. Role of complement in defense of the middle ear revealed by restoring the virulence of nontypeable Haemophilus influenzae siaB mutants. Infect. Immun. 75325-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greiner, L. L., H. Watanabe, N. J. Phillips, J. Shao, A. Morgan, A. Zaleski, B. W. Gibson, and M. A. Apicella. 2004. Nontypeable Haemophilus influenzae strain 2019 produces a biofilm containing N-acetylneuraminic acid that may mimic sialylated O-linked glycans. Infect. Immun. 724249-4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffin, R., A. D. Cox, K. Makepeace, J. C. Richards, E. R. Moxon, and D. W. Hood. 2003. The role of lex2 in lipopolysaccharide biosynthesis in Haemophilus influenzae strains RM7004 and RM153. Microbiology 1493165-3175. [DOI] [PubMed] [Google Scholar]
- 12.Gulin, S., E. Pupo, E. K. Schweda, and E. Hardy. 2003. Linking mass spectrometry and slab-polyacrylamide gel electrophoresis by passive elution of lipopolysaccharides from reverse-stained gels: analysis of gel-purified lipopolysaccharides from Haemophilus influenzae strain Rd. Anal. Chem. 754918-4924. [DOI] [PubMed] [Google Scholar]
- 13.Hood, D. W., A. D. Cox, W. W. Wakarchuk, M. Schur, E. K. Schweda, S. L. Walsh, M. E. Deadman, A. Martin, E. R. Moxon, and J. C. Richards. 2001. Genetic basis for expression of the major globotetraose-containing lipopolysaccharide from H. influenzae strain Rd (RM118). Glycobiology 11957-967. [DOI] [PubMed] [Google Scholar]
- 14.Jurcisek, J., L. Greiner, H. Watanabe, A. Zaleski, M. A. Apicella, and L. O. Bakaletz. 2005. Role of sialic acid and complex carbohydrate biosynthesis in biofilm formation by nontypeable Haemophilus influenzae in the chinchilla middle ear. Infect. Immun. 733210-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Landerholm, M. K., J. Li, J. C. Richards, D. W. Hood, E. R. Moxon, and E. K. Schweda. 2004. Characterization of novel structural features in the lipopolysaccharide of nondisease associated nontypeable Haemophilus influenzae. Eur. J. Biochem. 271941-953. [DOI] [PubMed] [Google Scholar]
- 16.Leroy, M., H. Cabral, M. Figueira, V. Bouchet, H. Huot, S. Ram, S. I. Pelton, and R. Goldstein. 2007. Multiple consecutive lavage samplings reveal greater burden of disease and provide direct access to the nontypeable Haemophilus influenzae biofilm in experimental otitis media. Infect. Immun. 754158-4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li, J., P. Thibault, A. Martin, J. C. Richards, W. W. Wakarchuk, and W. van der Wilp. 1998. Development of an on-line preconcentration method for the analysis of pathogenic lipopolysaccharides using capillary electrophoresis-electrospray mass spectrometry. Application to small colony isolates. J. Chromatogr. A 817325-336. [DOI] [PubMed] [Google Scholar]
- 18.Lundström, S. L., B. Twelkmeyer, M. K. Sagemark, J. Li, J. C. Richards, D. W. Hood, E. R. Moxon, and E. K. Schweda. 2007. Novel globoside-like oligosaccharide expression patterns in nontypeable Haemophilus influenzae lipopolysaccharide. FEBS J. 2744886-4903. [DOI] [PubMed] [Google Scholar]
- 19.Månsson, M., S. H. Bauer, D. W. Hood, J. C. Richards, E. R. Moxon, and E. K. Schweda. 2001. A new structural type for Haemophilus influenzae lipopolysaccharide. Structural analysis of the lipopolysaccharide from nontypeable Haemophilus influenzae strain 486. Eur. J. Biochem. 2682148-2159. [DOI] [PubMed] [Google Scholar]
- 20.Månsson, M., D. W. Hood, J. Li, J. C. Richards, E. R. Moxon, and E. K. Schweda. 2002. Structural analysis of the lipopolysaccharide from nontypeable Haemophilus influenzae strain 1003. Eur. J. Biochem. 269808-818. [DOI] [PubMed] [Google Scholar]
- 21.Månsson, M., D. W. Hood, E. R. Moxon, and E. K. Schweda. 2003. Structural characterization of a novel branching pattern in the lipopolysaccharide from nontypeable Haemophilus influenzae. Eur. J. Biochem. 2702979-2991. [DOI] [PubMed] [Google Scholar]
- 22.Månsson, M., D. W. Hood, E. R. Moxon, and E. K. Schweda. 2003. Structural diversity in lipopolysaccharide expression in nontypeable Haemophilus influenzae. Identification of L-glycero-D-manno-heptose in the outer-core region in three clinical isolates. Eur. J. Biochem. 270610-624. [DOI] [PubMed] [Google Scholar]
- 23.Masoud, H., A. Martin, P. Thibault, E. R. Moxon, and J. C. Richards. 2003. Structure of extended lipopolysaccharide glycoforms containing two globotriose units in Haemophilus influenzae serotype b strain RM7004. Biochemistry 424463-4475. [DOI] [PubMed] [Google Scholar]
- 24.Masoud, H., E. R. Moxon, A. Martin, D. Krajcarski, and J. C. Richards. 1997. Structure of the variable and conserved lipopolysaccharide oligosaccharide epitopes expressed by Haemophilus influenzae serotype b strain Eagan. Biochemistry 362091-2103. [DOI] [PubMed] [Google Scholar]
- 25.Phillips, N. J., M. A. Apicella, J. M. Griffiss, and B. W. Gibson. 1992. Structural characterization of the cell surface lipooligosaccharides from a nontypable strain of Haemophilus influenzae. Biochemistry 314515-4526. [DOI] [PubMed] [Google Scholar]
- 26.Phillips, N. J., M. A. Apicella, J. M. Griffiss, and B. W. Gibson. 1993. Structural studies of the lipooligosaccharides from Haemophilus influenzae type b strain A2. Biochemistry 322003-2012. [DOI] [PubMed] [Google Scholar]
- 27.Pichichero, M. E. 2006. Pathogen shifts and changing cure rates for otitis media and tonsillopharyngitis. Clin. Pediatr. 45493-502. [DOI] [PubMed] [Google Scholar]
- 28.Rahman, M. M., X. X. Gu, C. M. Tsai, V. S. Kolli, and R. W. Carlson. 1999. The structural heterogeneity of the lipooligosaccharide (LOS) expressed by pathogenic non-typeable Haemophilus influenzae strain NTHi 9274. Glycobiology 91371-1380. [DOI] [PubMed] [Google Scholar]
- 29.Risberg, A., H. Masoud, A. Martin, J. C. Richards, E. R. Moxon, and E. K. Schweda. 1999. Structural analysis of the lipopolysaccharide oligosaccharide epitopes expressed by a capsule-deficient strain of Haemophilus influenzae Rd. Eur. J. Biochem. 261171-180. [DOI] [PubMed] [Google Scholar]
- 30.Schweda, E. K., O. E. Hegedus, S. Borrelli, A. A. Lindberg, J. N. Weiser, D. J. Maskell, and E. R. Moxon. 1993. Structural studies of the saccharide part of the cell envelope lipopolysaccharide from Haemophilus influenzae strain AH1-3 (lic3+). Carbohydr. Res. 246319-330. [DOI] [PubMed] [Google Scholar]
- 31.Schweda, E. K., P. E. Jansson, E. R. Moxon, and A. A. Lindberg. 1995. Structural studies of the saccharide part of the cell envelope lipooligosaccharide from Haemophilus influenzae strain galEgalK. Carbohydr. Res. 272213-224. [DOI] [PubMed] [Google Scholar]
- 32.Schweda, E. K., M. K. Landerholm, J. Li, E. Richard Moxon, and J. C. Richards. 2003. Structural profiling of lipopolysaccharide glycoforms expressed by non-typeable Haemophilus influenzae: phenotypic similarities between NTHi strain 162 and the genome strain Rd. Carbohydr. Res. 3382731-2744. [DOI] [PubMed] [Google Scholar]
- 33.Schweda, E. K., J. Li, E. R. Moxon, and J. C. Richards. 2002. Structural analysis of lipopolysaccharide oligosaccharide epitopes expressed by non-typeable Haemophilus influenzae strain 176. Carbohydr. Res. 337409-420. [DOI] [PubMed] [Google Scholar]
- 34.Schweda, E. K., J. C. Richards, D. W. Hood, and E. R. Moxon. 2007. Expression and structural diversity of the lipopolysaccharide of Haemophilus influenzae: implication in virulence. Int. J. Med. Microbiol. 297297-306. [DOI] [PubMed] [Google Scholar]
- 35.Shurin, P. A., S. I. Pelton, I. B. Tager, and D. L. Kasper. 1980. Bactericidal antibody and susceptibility to otitis media caused by nontypable strains of Haemophilus influenzae. J. Pediatr. 97364-369. [DOI] [PubMed] [Google Scholar]
- 36.Sun, J., J. Chen, Z. Cheng, J. B. Robbins, J. F. Battey, and X. X. Gu. 2000. Biological activities of antibodies elicited by lipooligosaccharide based-conjugate vaccines of nontypeable Haemophilus influenzae in an otitis media model. Vaccine 181264-1272. [DOI] [PubMed] [Google Scholar]
- 37.Swords, W. E., M. L. Moore, L. Godzicki, G. Bukofzer, M. J. Mitten, and J. VonCannon. 2004. Sialylation of lipooligosaccharides promotes biofilm formation by nontypeable Haemophilus influenzae. Infect. Immun. 72106-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vimr, E., and C. Lichtensteiger. 2002. To sialylate, or not to sialylate: that is the question. Trends Microbiol. 10254-257. [DOI] [PubMed] [Google Scholar]
- 39.Weiser, J. N., and N. Pan. 1998. Adaptation of Haemophilus influenzae to acquired and innate humoral immunity based on phase variation of lipopolysaccharide. Mol. Microbiol. 30767-775. [DOI] [PubMed] [Google Scholar]
- 40.Yildirim, H. H., D. W. Hood, E. R. Moxon, and E. K. Schweda. 2003. Structural analysis of lipopolysaccharides from Haemophilus influenzae serotype f. Structural diversity observed in three strains. Eur. J. Biochem. 2703153-3167. [DOI] [PubMed] [Google Scholar]
- 41.Yildirim, H. H., J. Li, J. C. Richards, D. W. Hood, E. R. Moxon, and E. K. Schweda. 2005. An alternate pattern for globoside oligosaccharide expression in Haemophilus influenzae lipopolysaccharide: structural diversity in nontypeable strain 1124. Biochemistry 445207-5224. [DOI] [PubMed] [Google Scholar]
- 42.Yildirim, H. H., J. Li, J. C. Richards, D. W. Hood, E. R. Moxon, and E. K. Schweda. 2005. Complex O-acetylation in non-typeable Haemophilus influenzae lipopolysaccharide: evidence for a novel site of O-acetylation. Carbohydr. Res. 3402598-2611. [DOI] [PubMed] [Google Scholar]