Abstract
Helicobacter hepaticus is a gram-negative, spiral-shaped microaerophilic bacterium associated with chronic intestinal infection leading to hepatitis and colonic and hepatic carcinomas in susceptible strains of mice. In the closely related human pathogen Helicobacter pylori, l-proline is a preferred respiratory substrate and is found at significantly high levels in the gastric juice of infected patients. A previous study of the proline catabolic PutA flavoenzymes from H. pylori and H. hepaticus revealed that Helicobacter PutA generates reactive oxygen species during proline oxidation by transferring electrons from reduced flavin to molecular oxygen. We further explored the preference for proline as a respiratory substrate and the potential impact of proline metabolism on the redox environment in Helicobacter species during host infection by disrupting the putA gene in H. hepaticus. The resulting putA knockout mutant strain was characterized by oxidative stress analysis and mouse infection studies. The putA mutant strain of H. hepaticus exhibited increased proline levels and resistance to oxidative stress relative to that of the wild-type strain, consistent with proline's role as an antioxidant. The significant increase in stress resistance was attributed to higher proline content, as no upregulation of antioxidant genes was observed for the putA mutant strain. The wild-type and putA mutant H. hepaticus strains displayed similar levels of infection in mice, but in mice challenged with the putA mutant strain, significantly reduced inflammation was observed, suggesting a role for proline metabolism in H. hepaticus pathogenicity in vivo.
Helicobacter hepaticus is a gram-negative, spiral-shaped microaerophilic bacterium. The organism was first isolated from the liver tissues of mice that had a high prevalence for hepatitis and liver tumors. Subsequently, H. hepaticus was found to be the causative agent of hepatic and intestinal inflammation in mice, leading to hepatic and intestinal tumorigenesis (11-14). Although it is frequently isolated from the liver and associated with hepatitis, like the other enterohepatic Helicobacter species (EHS), H. hepaticus colonizes primarily the lower bowel, where it is associated with persistent typhlocolitis (13, 17, 20, 28, 43, 55, 56). About 59% of mice bred for research purposes have been shown to carry H. hepaticus alone or in combination with other EHS (49). The potential impact of Helicobacter infection of laboratory mice on biomedical research is therefore a major concern. H. hepaticus is also the prototype EHS, which makes it an excellent candidate for understanding the molecular basis of intestinal and hepatic infections associated with EHS in general.
H. hepaticus is closely related to the gastric human pathogen Helicobacter pylori, which is responsible for acute gastric inflammation that can then progress from superficial gastritis to peptic ulceration and gastric cancer. H. pylori is classified by the World Health Organization as a group 1 carcinogen (8, 31-33). One of the important features in the pathogenesis of Helicobacter infection is its ability to establish persistent colonization within the gastrointestinal mucosa. The initial stage of H. pylori colonization is associated with considerable neutrophil exudation along the gastric mucosa (38). The reactive oxygen species (ROS) produced during the neutrophil response are thought to play an important role in the oxidative damage to the host mucosa (42). To combat this oxidative stress, Helicobacter uses a number of different antioxidant systems (52).
The focus of this study was to investigate the role of proline metabolism in oxidative stress and infection by H. hepaticus. Previous studies have implicated proline as having an important role in Helicobacter species' ability to colonize and persist at the host mucosal surfaces. Nagata et al. showed that the gastric juice of patients infected with H. pylori had 10-fold higher levels of the amino acid proline than that of noninfected individuals (40). They also showed that the concentration of l-proline was higher than all the other amino acids in cultured H. pylori cells and that l-proline was a preferred respiratory substrate, along with l-serine (40). These observations suggested that in the gut environment, proline serves as a key energy source for this gastrointestinal pathogen. Using signature-tagged mutagenesis, it has been shown that the high-affinity proline-specific transporter gene putP is one of the 47 genes absolutely essential for gastric colonization by H. pylori (22). There is also evidence that the human serum prolidase enzyme is upregulated during the course of H. pylori infection (2). Prolidase is an iminodipeptidase that releases proline or hydroxyproline from the carboxy terminus of a glycine-proline dipeptide, thereby increasing serum proline levels (51). Taken together, these observations suggest an important role for proline metabolism in H. pylori colonization and pathogenesis.
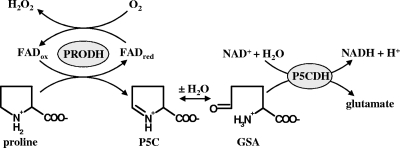
Proline oxidation in gram-negative bacteria is catalyzed by the bifunctional PutA flavoenzyme, which has two catalytic domains in a single polypeptide (19, 25). PutA from H. hepaticus (PutAHh) and H. pylori (PutAHp) share about 64% amino acid sequence identity (26). The first step in proline oxidation is performed by the flavin adenine dinucleotide-dependent PRODH domain (Fig. 1, reaction scheme), which couples the 2e− oxidation of proline to the reduction of the electron chain transport system of the cytoplasmic membrane. The product of the PRODH reaction, Δ1-pyrroline-5-carboxylate (P5C), is subsequently hydrolyzed to glutamate-γ-semialdehyde (GSA), which is then oxidized to glutamate by the NAD+-dependent P5CDH domain of PutA (4). Glutamate that is formed by the oxidation of proline eventually enters the tricarboxylic acid cycle via α-ketoglutarate. In a previous study, PutAHh and PutAHp were shown to transfer electrons to molecular oxygen during catalytic turnover with proline, forming superoxide anion and hydrogen peroxide (H2O2) (Fig. 1) (26). This oxygen reactivity during turnover with proline is essentially absent in the PutA protein from Escherichia coli. The impact of Helicobacter PutA reactivity with oxygen was assessed by oxidative stress studies with an E. coli model system that demonstrated that the enzyme actions of PutAHh and PutAHp are toxic to E. coli (26). Thus, it seemed plausible that proline metabolism in Helicobacter species may lead to changes in the redox environment by the formation of superoxide anion by PutA and the subsequent decrease in the levels of proline, an imino acid that has been shown to act as a potent antioxidant in yeast, plants, and mammalian cells (6, 7, 27, 46).
FIG. 1.
Generation of H2O2 by PutA in H. hepaticus. Shown are the reactions catalyzed by the PRODH and the P5C dehydrogenase (P5CDH) domains of PutA. During the oxidation of proline, the PRODH domain of PutA can catalyze the transfer of electrons from reduced flavin adenine dinucleotide to molecular oxygen, leading to H2O2 formation. The intermediates in the overall conversion of proline to glutamate are P5C and glutamate-γ-semialdehyde (GSA).
To better understand the role of proline metabolism in disease associated with Helicobacter species infection, a putA mutant strain of H. hepaticus was made by cassette mutagenesis of the putA gene. The putA gene knockout strain was compared to the wild-type H. hepaticus strain to assess proline metabolism in oxidative stress and infection. The present study suggests that proline metabolism in H. hepaticus can modify the redox environment in vitro and that it affects pathogenicity in vivo.
MATERIALS AND METHODS
Chemicals, bacterial strains, and culture conditions.
All chemicals and buffers, unless otherwise noted, were purchased from Fisher Scientific and Sigma-Aldrich, Inc. Restriction endonucleases and T4 DNA ligases were purchased from Fermentas and Promega, respectively. Cloning and genetic manipulations were performed with H. hepaticus strain ATCC 51449T and Escherichia coli DH5α (BRL). H. hepaticus was grown on brucella agar plates (Difco) supplemented with 10% defibrinated sheep blood (BA) without any antibiotics. The H. hepaticus putA mutant strain was grown on BA plates supplemented with kanamycin (50 μg/ml). Both the wild-type and the putA mutant strains were grown in a microaerobic environment (5% CO2 and 1% O2) at 37°C. E. coli was grown aerobically at 37°C in Luria-Bertani medium containing ampicillin (100 μg/ml) or chloramphenicol (34 μg/ml). For analysis of the H. hepaticus population in fecal and tissue matter, samples were plated on BA medium containing amphotericin B (10 μg/ml), vancomycin (10 μg/ml), and cefoperazone (20 μg/ml). The sequences of all the primers used in this study are provided in Table S1 in the supplemental material.
Construction of the H. hepaticus putA mutant strain by insertional mutagenesis.
The putA gene was amplified using H. hepaticus ATCC 51449T genomic DNA as a template and the putA-specific primers (see Table S1 in the supplemental material). The PCR fragment was ligated into a pGEM-T vector (Promega) according to recommendations of the manufacturer to generate the construct pGEM-T::putA. Subsequently, a kanamycin resistance cassette (1.1 kb) was inserted into the putA gene (3.505 kb), using unique AflII and XhoI sites that were incorporated within the putA gene by QuikChange (Stratagene) site-directed mutagenesis at bp 745 (AflII primer) and 2728 (XhoI primer), respectively. The resulting construct containing the kanamycin insertion (pGEM-T::putA::Kan) was introduced into H. hepaticus ATCC 51449T by electrotransformation (2.5-kV pulse; Gene Pulser; Bio-Rad). Transformants were then plated on kanamycin-BA plates to isolate the putA mutant strain. Insertion of the kanamycin cassette into the putA gene of H. hepaticus was confirmed by PCR using the putA-specific primers described above. PCR conditions were 30 s at 95°C, 1 min at 52°C, and 4 min at 72°C for 30 cycles.
Growth of the wild-type and putA knockout mutant strains.
The impact of disrupting the putA gene and proline catabolism on cell growth, proline content, and antioxidant gene expression was assessed by comparing the wild-type and the putA mutant H. hepaticus strains grown on BA plates under microaerobic conditions. The wild-type and putA mutant strains of H. hepaticus grown on BA plates were suspended and diluted in brucella broth medium to ∼1.0 × 109 cells/ml, assuming that an optical density at 600 nm (OD600) of 1.0 is ∼1.6 × 109 cells/ml, as previously described (37). Cells (3.0 × 108) were then plated on fresh BA plates and grown for 0, 24, 48, 96, 144, and 192 h. The number of cells at 0 h was 3.0 × 108 cells/ml. Cell numbers from 24 to 192 h were monitored by resuspending cells from the BA plates at different time points in brucella broth medium to a final volume of 1 ml and estimating the number of cells, assuming that an OD600 of 1.0 is ∼1.6 × 109 cells/ml (37). The cell suspensions were used for determining proline content and for analysis of PutA, superoxide dismutase (SOD), and catalase expression as described below. Measurements at 0 h were performed with cell suspensions before the cells were plated on BA plates.
Quantitation of proline in the wild-type and putA mutant strains.
The cell suspensions as described above were used for determining proline content at 0, 24, 48, 96, 144, and 192 h of growth. Approximately 3.0 × 108 cells from each time point were pelleted by centrifugation, resuspended in 0.5 ml of sterile water, and lysed by boiling for 10 min. The amount of proline was quantitated by PRODH activity assays using the E. coli PRODH domain construct, EcPutA86-630, which was purified as previously described (59). Briefly, proline:dichlorophenolindophenol oxidoreductase assays were performed in 50 mM Tris buffer (pH 7.5) as previously described, except for the use of EcPutA86-630 (100 μg) and 100 μl of cell extract (60). A standard curve of EcPutA86-630 PRODH activity from 0 to 0.5 mM proline in the assay was used to determine the amount of proline in the total cell lysates.
Western blotting analysis.
Antiserum directed against purified recombinant PutAHh was prepared by Proteintech Inc. Recombinant PutAHh was purified as previously described (26). Cells (∼3 × 108) were lysed by gentle sonication for 1 min (15-s pulse on, 30-s pulse off) in ice-cold phosphate-buffered saline (PBS) buffer containing 0.2% Triton, unless otherwise indicated, and the lysates were centrifuged for 10 min at 16,000 × g at 4°C. Total protein concentration in the soluble portion of the cell lysates was determined by using bicinchoninic acid (Pierce) and bovine serum albumin as a standard. Samples of 20 μg or 50 μg of total protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electroblotted onto a polyvinylidene difluoride immunoblotting membrane (0.2-μm pore size; Bio-Rad) as previously described (27). The polyvinylidene difluoride membranes were then blocked with 5% milk and incubated with PutAHh antiserum (1:20,000 dilution). Immunoreactive bands were detected by using a monoclonal anti-Flag-fluorescein isothiocyanate conjugate (Sigma-Aldrich) and were visualized by using a LI-COR Odyssey imager as previously described (27). PutA enzymes from other bacterial sources such as E. coli were not recognized by the PutAHh antiserum.
Oxidative stress studies.
Oxidative stress was induced by using three different cytotoxic agents and was evaluated by disk assays (54). Proline-mediated stress was also studied by the same method using 1 M proline. Sterile filter paper disks, 7.5 mm in diameter, containing 10 μl of 1 M hydrogen peroxide (H2O2), 0.2 M tert-butyl hydroperoxide (tBH), and 50 mM paraquat were placed on BA plates (100 by 15 mm) that had been previously streaked for confluent growth with either the putA mutant or wild-type cells. The plates were then placed in a 2% O2 incubator. Following a 48-h incubation period, the clear zones or inhibition zones surrounding the disks were measured. The zone of inhibition represents the distance from the edge of the disk to where growth begins. No growth inhibition was seen with disks containing only H2O. The mean zone inhibition diameter for the wild-type and the putA mutant H. hepaticus strains with each oxidant was obtained from three separate experiments (six samples per experiment). The statistical significance of the data was assessed using Student's t test (two-tailed). Differences observed between the parent and the mutant strains were considered statistically significant at a P value of <0.05.
RNA isolation and RT-PCR.
Expression of the putA, the neutrophil-activating protein (napA), and the proline transporter (putP) genes in the wild-type and the putA mutant H. hepaticus strains was analyzed using cells grown on BA plates under microaerobic conditions. Expression profiles of SOD (sodF), catalase (katA), alkyl hydroperoxide reductase TsaA (tsaA), and thiol peroxidase (tpx) were assessed in the wild-type and putA mutant H. hepaticus strains, using ∼3 × 108 cells harvested at 0, 24, 48, 96, 144, and 192 h of growth on BA plates, as described above. For RNA isolation, cells were lysed in 50 mM Tris-HCl (pH 8.0) with 1 mM EDTA and 50 mM NaCl supplemented with 1.5% sodium dodecyl sulfate for 5 min at 95°C. Total RNA was phenol extracted and precipitated and then dissolved in diethylpyrocarbonate-treated water. The concentration of the isolated RNA was then determined at 260 nm. The collected RNA was reverse transcribed to synthesize total cDNA in the presence of 1 mM of each deoxynucleoside triphosphate, 25 U of murine leukemia virus (MuLV) reverse transcriptase (RT), 1 U of RNase inhibitor, and 2.5 μM of random hexamers in a final volume of 20 μl. All reagents were from Invitrogen. Reactions were carried out at 42°C for 30 min in an MJ mini-thermal cycler unit (Bio-Rad), followed by a 10-min step at 95°C to denature the enzyme and then a cooling step to 4°C. The resulting cDNA was then used as a template for PCR amplification. The cytolethal distending toxin (CdtB) gene product coding for subunit B of the cdtB gene was used as the loading control. Primers used for PCR amplifications are listed in Table S1 in the supplemental material. PCR conditions were 30 s at 95°C, 1 min at 52°C, and 2 min at 72°C for 30 cycles. For analysis of sodF, katA, tsaA, and tpx, reactions were terminated at 20 cycles to ensure that the amplifications were still in the exponential phase. PCR products were visualized by ethidium bromide staining and imaged using a Bio-Rad Gel Doc system.
Mouse infection.
All animal studies were performed according to NIH guidelines for proper care and handling of animals. Mice were housed and maintained in the animal research facility at the University of Nebraska—Lincoln. Inbred male A/J mice certified to be free of Helicobacter were purchased at 6 weeks of age from Jackson Laboratory, Bar Harbor, ME. The animals were fed autoclaved mouse chow and given water and bedding for the duration of the study, and cage changes were performed under a laminar flow hood. Animals were housed in groups of five per microisolator cage, with cycles of 12 h of light and 12 h of darkness.
H. hepaticus reference strain 3B1/Hh-1 (ATCC 51449T) was propagated on Trypticase soy agar supplemented with 5% (vol/vol) sheep blood (TSA-BA; Remel, Lenexa, KS) incubated at 37°C under microaerobic conditions (Mitsubishi Gas Chemical Co. Inc., New York, NY). The reference and putA mutant strains were grown on TSA-BA plates and harvested by centrifugation after being washed in PBS (pH 7.2). The OD600 of the inoculum was adjusted to 0.32 (approximately 5 × 108 cells/ml) (34). Ten mice were inoculated intraperitoneally with a volume of 0.5 ml of bacterial suspension of the wild-type or the putA mutant cells. Ten control mice were sham inoculated with 0.5 ml of sterile PBS. Fecal pellets were sampled from the cages after weeks 1 and 3. Twenty-one days postinoculation, the mice were euthanized by carbon monoxide overdose.
Qualitative analysis of liver, cecum, and fecal specimens.
Approximately 200 mg of fecal pellets collected from the cages at 21 days postinoculation were homogenized in 5 ml of sterile PBS. The suspension (200 μl) was plated on TSA-BA plates containing amphotericin B (10 μg/ml), vancomycin (10 μg/ml), and cefoperazone (20 μg/ml). The plates were incubated under a microaerobic atmosphere (1% O2, 5% CO2) at 37°C for 72 to 96 h. Tissues from euthanized mice at 21 days postinoculation were harvested for qualitative, quantitative, and histopathological analyses. Sections of the liver (left, right, caudate, and part of the median lobe) and cecum (the entire tissue after a sample was removed for histopathology) were homogenized by using a Tissue Tearor (Biospec Products, Inc.), and 200 μl of each tissue suspension was plated onto TSA-BA plates with antibiotics to determine the presence of H. hepaticus. The presence of H. hepaticus in tissues and suspensions was also assessed by real-time PCR as described below.
Quantitative analysis of liver and cecum.
Because H. hepaticus grows as a continuously spreading lawn, counting bacterial colonies is not possible. Instead, real-time PCR was used to estimate H. hepaticus populations in different tissues as previously described (13, 15, 37, 58). DNA was extracted from 25 mg of homogenized liver and cecum tissue, using a DNeasy tissue kit (Qiagen). Real-time PCR (iCycler Thermal Cycler) was performed using 6-carboxyfluorescein-labeled H. hepaticus-specific cdtB primers (see Table S1 in the supplemental material), which were designed using the Plexor primer design tool from Promega. A BLAST search against known genomes was used to verify that the cdtB primers and the cdtB gene probe (coding for subunit B of the cytolethal distending toxin) were highly specific for H. hepaticus. Real-time PCR analysis was performed by using a 25-μl mixture containing 12.5 μl of Supermix (Promega), 200 nM concentrations each of the forward and reverse primers, and 5 μl of tissue DNA (equivalent to 1.0 mg of tissue). Conditions for real-time PCR were 1 cycle at 95°C for 3 min and 40 cycles each of 95°C for 30 s and 65°C for 30 s. Real-time PCR generated an amplified product of 110 bp. A standard curve (not shown) was generated from H. hepaticus genomic DNA samples ranging from 102 to 107 fg, from which the number of genome copies was estimated for each sample as described previously (37).
Histopathological studies.
Tissue specimens consisting of the median lobe of the liver, the cecum, the proximal colon, and the spleen were fixed overnight in 10% buffered formalin, processed, and embedded in paraffin. Sections (5 μm thick) were stained with hematoxylin-eosin stain and semiquantitatively scored for histopathological changes as previously described (37). Portal inflammation was scored as 0 (no inflammation), 1 (mild infiltrate in minority of portal tracts), and 2 (mild to moderate infiltrate in almost all portal tracts). Lobular inflammation and/or hepatocytic necrosis was graded as 0 (absence), 1 (mild), and 2 (moderate). Biliary lesions and oval cell changes were scored as 0 (no lesions) and 1 (occasional or mild cholangitis). The histopathological scores of tissues from mice infected with the wild-type the and putA mutant H. hepaticus strains were analyzed by the nonparametric Mann-Whitney test, using SPSS statistical software, with statistical significance set at a P value of <0.05.
RESULTS
Disruption of the putA gene.
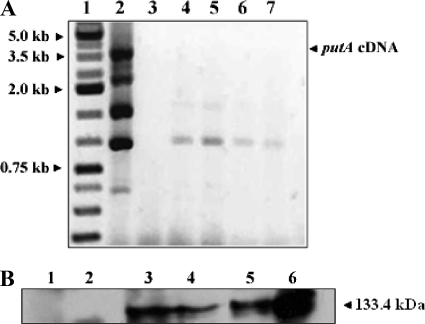
The putA gene in H. hepaticus was disrupted by the insertion of a kanamycin cassette. Insertion of the cassette was confirmed by a comparison of the ∼4.6-kb PCR product for the putA mutant with the ∼3.5-kb product for the wild-type strain. Disruption of the gene was also confirmed at the transcript and protein levels by RT-PCR and by Western blotting analysis, respectively (Fig. 2). RT-PCR targeting the putA cDNA did not give any product for the putA mutant strain, whereas significant product was observed with the wild-type strain. Figure 2 shows an ∼3.5-kb band from wild-type H. hepaticus, indicating expression of the full-length putA gene. Additional smaller-sized bands were also observed with the wild-type H. hepaticus strain and were most likely due to off-target priming events within the cDNA of putA. Western blotting analysis was performed using antibodies raised against purified full-length H. hepaticus PutA. Immunoreactive bands of the expected size (133.4 kDa) were not observed for the mutant strain, but a 133-kDa band corresponding to PutAHh was detected with the wild-type strain. To establish that there were no polar effects, the expression levels of the neighboring upstream putP and downstream napA genes were tested in both the wild-type and the mutant strains by RT-PCR. The putP gene encodes a high-affinity proline transporter, while the napA gene encodes the neutrophil-activating protein. Both strains showed similar levels of expression of the putP and napA genes, indicating that these genes were not disrupted by the kanamycin cassette mutagenesis of the putA gene (data not shown).
FIG. 2.
RT-PCR and Western blotting analyses confirm the disruption of the putA gene in H. hepaticus. (A) RT-PCR performed with putA gene-specific primers. Lanes: 1, kb ladder; 2, wild-type H. hepaticus strain; 3, empty; 4 to 7, different colonies screened for putA deletion in H. hepaticus. (B) Western blotting analysis of protein extracts from the wild-type and putA H. hepaticus strains. Two different amounts of total protein were loaded for each cell lysate sample. Lanes: 1 and 2, putA mutant cell lysates (20 μg and 50 μg, respectively); 3 and 4, wild-type cell lysates (50 μg and 20 μg, respectively); 5 and 6, purified recombinant PutAHh (20 μg and 50 μg, respectively).
Increased oxidative stress and proline toxicity resistance of the putA mutant strain.
We characterized the in vitro oxidative stress response for the wild-type and the putA mutant strains by performing paper disk assays with hydrogen peroxide (1 M), tBH (0.2 M), and paraquat (0.05 M). The putA mutant strain exhibited a zone-of-inhibition diameter with H2O2 that was 2-fold smaller than that of the wild-type strain (Table 1). With tBH and paraquat, the putA mutant strain had a 3-fold smaller zone-of-inhibition diameter than the wild-type strain (Table 1). Thus, the mutant strain exhibited significantly higher resistance to oxidative stress than the parental wild-type strain. Because PutA has been shown to produce ROS in vitro, we determined whether the addition of proline also leads to stress and cell death. After a wide range of proline concentrations was tested, 1 M proline was used on the filter disks for these experiments. The wild-type strain showed a high sensitivity to proline, with a zone-of-inhibition diameter similar to that observed with H2O2 stress. In contrast, proline did not have any effect on the putA mutant strain, as no inhibition zone was observed. These observations agree with our previous results in which the overexpression of PutAHh in E. coli proved to be toxic to the cells when it was supplemented with proline (5 mM) (26). Here, it is shown that the enzymatic action of PutAHh is also toxic to H. hepaticus when proline levels are elevated.
TABLE 1.
Disk oxidative stress assaysa
| H. hepaticus strain | Mean inhibition zone diam (mm) ± SD
|
||||
|---|---|---|---|---|---|
| Water | H2O2 (1 M) | Paraquat (0.05 M) | tBH (0.2 M) | Proline (1 M) | |
| Wild type | None | 2 ± 0.4 | 1.8 ± 0.6 | 3.5 ± 0.5 | 1.5 ± 0.5 |
| putA mutant | None | 1 ± 0.3 | 0.7 ± 0.2 | 0.5 ± 0.1 | None |
Zones of inhibition were measured from filter paper disks treated with 10 μl of the indicated compounds. Water was used as the control, which did not result in any zones of growth inhibition. Results are the means ± standard deviations (SD) from three separate experiments. The inhibition zones for the putA mutant strain with H2O2, paraquat, and tBH are significantly lower than those for the parental wild-type strain at the 95% level of confidence (P < 0.05), based on Student's t test.
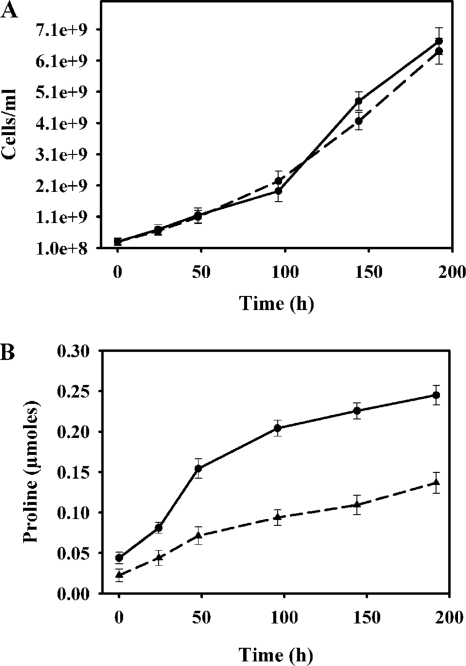
Increased levels of proline in the putA mutant strain.
Cells were grown on BA plates for up to 192 h. No significant differences were observed between the growth rates of the wild-type and those of the putA mutant strains (Fig. 3A). Proline content in the cells was measured at different time points during growth. The mutant strain had higher levels of intracellular proline than the wild-type strain under normal growth conditions (Fig. 3B). The proline levels were about 2-fold higher in the mutant strain than in the wild-type strain throughout the 192-h growth period. This result is in agreement with the mutant strain's lack of ability to catabolize proline in the absence of PutA. As proline has been suggested to be an antioxidant with radical scavenging properties (1), the higher levels of proline in the putA mutant strain may explain the increased stress resistance observed with the mutants, along with the lack of PutA-generated ROS.
FIG. 3.
(A) Growth curves for the wild-type (solid curve) and the putA mutant (dashed curve) H. hepaticus strains incubated on BA plates for 0, 24, 48, 96, 144, and 192 h. Cells were resuspended in brucella medium for OD measurements at each time point. (B) Proline content for the wild-type and putA mutant H. hepaticus strains at 0, 24, 48, 96, 144, and 192 h of growth on BA plates. Approximately 3 ×108 cells were used for determining proline content at each time point.
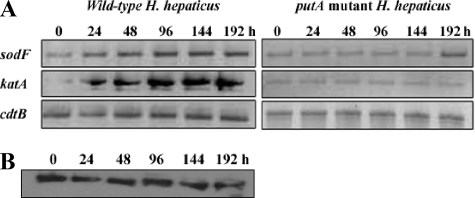
Antioxidant gene expression levels.
To better understand the reason for the increased resistance to oxidative stress observed with the putA mutant strain, mRNA levels of two main antioxidant genes, sodF and katA, were determined for the wild-type and for the putA mutant strains. Under normal growth conditions, the transcript levels of sodF and katA were significantly higher in the wild-type strain than in the putA mutant strain over the entire 192-h growth period (Fig. 4A). Transcript levels of the tsaA (alkyl hydroperoxide reductase) and tpx (thiol peroxidase) genes were also checked, but no significant differences were observed between the expression of the wild-type and that of the putA mutant strains. The expression of PutA was confirmed in wild-type H. hepaticus during the same growth period used for monitoring SOD and catalase expression. Western analysis shows that PutA protein levels appear constant from 0 to 192 h (Fig. 4B), consistent with the correlation between PutA expression and higher SOD and catalase levels in the wild-type H. hepaticus strain. Thus, SOD and catalase expression may be required to protect cells from ROS generated by PutA during turnover with proline.
FIG. 4.
(A) Expression levels of the antioxidant genes sodF and katA were analyzed by RT-PCR in the wild-type (left panel) and the putA mutant (right panel) H. hepaticus strains in cells grown for 0, 24, 48, 96, 144, and 192 h. (B) Western blotting analysis of PutA expression in the wild-type H. hepaticus cells grown for 0, 24, 48, 96, 144, and 192 h.
Analysis of H. hepaticus colonization in liver, cecum, and feces in mice.
A/J mice were inoculated with H. hepaticus wild-type and putA mutant strains. A/J mice inoculated with sterile PBS served as negative controls. Twenty-one days after inoculation, liver and cecum homogenates, as well as fecal suspensions, from each mouse were collected and plated on BA plates. No growth was observed for plates with homogenates from the control mice. Growth was observed with homogenates from both the putA mutant and the wild-type strains, indicating colonization of H. hepaticus in the liver and cecum. Real-time PCR of the H. hepaticus cdtB gene was also used to quantify the colonization of the wild-type and mutant strains. A standard plot was used to estimate the genome copy numbers based on quantitation of the cdtB gene. Similar genome copy numbers were observed in the livers and ceca from mice inoculated with either the wild-type or the putA mutant strains (data not shown). There was no PCR product detected in the tissues obtained from the negative-control mice. The average genome copy among all samples obtained from mice inoculated with either the H. hepaticus wild type or the mutant strain were in the range of 102 (liver) to 103 (cecum). Thus, there was no significant difference between the genome copy number of the wild type and that of the putA mutant strain. This strongly suggests that PutA expression is not important for colonization of H. hepaticus in the liver or the cecum.
Histopathology results.
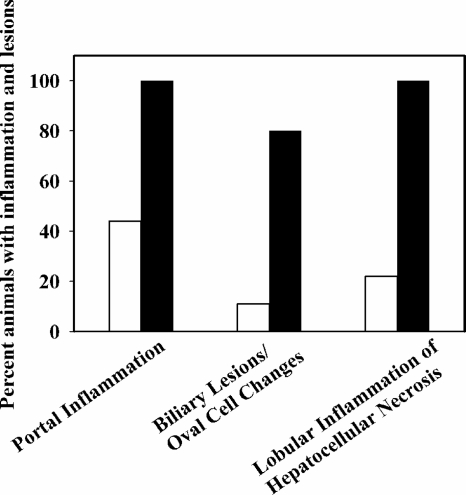
The pathological changes associated with H. hepaticus infection in mice are very well documented (37, 39). The histopathologies of the liver, colon, spleen, and cecum were examined in the control mice (10 animals), the mice inoculated with wild-type H. hepaticus (10 animals), and the mice inoculated with the putA mutant strain (9 animals). No significant gross changes were seen in the livers, intestines, and other visceral organs of the control mice, the mice infected with the H. hepaticus wild type, and the putA strains at necropsy. In the liver, as in every vascularized tissue, cell death (necrosis) evokes an inflammatory reaction which is also the hallmark of H. hepaticus infection. Figure 5 summarizes the histopathology scoring results of liver samples from mice in each group. The control mice did not show any evidence of liver infection, as no portal or lobular inflammation or biliary lesions were present. All the mice that were inoculated with the wild-type H. hepaticus strain clearly showed portal inflammation. Mild inflammation was observed in 8/10 animals, and moderate inflammation was seen in 2/10 mice. In contrast, significantly less inflammation (P value, 0.003) was found in mice challenged with the putA mutant strain than in mice infected with wild-type H. hepaticus. Only 4/9 mice infected with the putA mutant strain exhibited mild portal inflammation, and none of the mice was scored for moderate inflammation. Mild to moderate lobular inflammation or hepatocellular necrosis was present in the 10 mice inoculated with the wild-type H. hepaticus strain, while only 2/9 mice inoculated with the putA mutant strain exhibited mild lobular inflammation. Biliary lesions and oval cell changes were also seen in 8/10 mice inoculated with the wild-type strain, whereas only 1/9 mice inoculated with the putA mutant strain had similar changes. The differences observed between the lobular inflammation (P value, 0.007) and biliary lesions/oval changes (P value, 0.007) in mice infected with the wild-type strain and those of mice infected with the putA mutant H. hepaticus strain are considered statistically significant according to the Mann-Whitney test. Inflammation was not observed in the cecum, colon, or spleen from any of the mice.
FIG. 5.
Scoring results of histopathological parameters of livers of wild-type H. hepaticus-infected mice (10 animals) and those of putA mutant H. hepaticus-infected mice (9 animals). Liver tissues from mice inoculated with the wild-type H. hepaticus strain (solid bars) and the putA mutant H. hepaticus strain (open bars), stained with hematoxylin-eosin, were graded semiquantitatively for different histological parameters as described in Materials and Methods. Shown here are the percentages of animals showing portal and lobular inflammation (mild or moderate) and biliary lesions/oval changes.
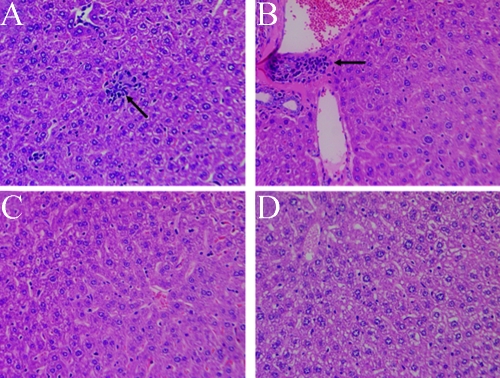
Figure 6 shows photomicrographs of a representative liver section of mouse tissue from each group (those inoculated with the wild-type strain, those with the putA mutant strain, and those that were sham inoculated). The pattern shown in Fig. 6 was observed consistently in liver tissues obtained from different animals within the three groups. This clearly indicates the importance of proline catabolism in H. hepaticus-induced inflammation.
FIG. 6.
Representative photomicrographs of liver sections taken on day 21 postinoculation from mice inoculated with the wild-type H. hepaticus or with the putA mutant H. hepaticus strain and from tissue from sham-inoculated control mice (hematoxylin-eosin stain; magnification, ×20). (A) Wild-type H. hepaticus strain-infected mouse tissue displays mild hepatocellular necrosis with inflammatory cell infiltration (arrow). (B) Wild-type H. hepaticus strain-infected mouse tissue exhibits mild biliary hyperplasia (arrow). (C) Mild portal inflammation and no evidence for tissue necrosis are seen in the liver of a mouse inoculated with the H. hepaticus putA mutant strain. (D) Control mouse liver shows an absence of hepatic damage or inflammation.
DISCUSSION
To combat oxidative stress and persist in the gastric mucosa successfully, Helicobacter species are endowed with a very effective antioxidant system (23, 47, 52, 53). Helicobacter spp. are also capable of producing ROS for signaling and regulating the redox environment at the site of infection (52, 53). Proline metabolism has been associated with a number of different ecological niches, with proline having multifaceted roles in abiotic stress protection and energy utilization (16, 24, 27, 50). In humans, the proline oxidative pathway has been shown to play an important role in cellular redox homeostasis. The upregulation of human PRODH by p53 produces ROS, which leads to apoptosis and is thought to be an important cancer-preventing mechanism (10, 18, 29, 30, 35, 36, 41, 45). Paradoxically, proline biosynthesis has been shown to be upregulated in cells exposed to oxidative stress and in certain cancer cell lines (27, 44). High levels of proline appear to support cell proliferation and enable proline to function as an antioxidant (27, 44). One of the mechanisms by which proline protects cells from oxidative stress is through maintaining reduced glutathione levels in the cell (27).
The gut of patients infected with H. pylori has been shown to have high levels of proline, and in separate studies, proline supplementation was observed to be critical for H. pylori proliferation (40, 50). These interesting observations led us to characterize the PutA proteins from H. pylori and from H. hepaticus. We found that similar to human PRODH, PutA from Helicobacter species generates superoxide anion during turnover with proline and molecular oxygen (26). The oxidase activities of PutAHh and PutAHp were considered unusual, since PutA homologs from other gram-negative bacteria exhibited very minimal oxidase activity. The oxidase activities of PutAHh and PutAHp proved to be toxic to E. coli and severely inhibited cell survival (26). Thus, in addition to proline's role as an energy substrate, it seemed plausible that proline metabolism may have a dual effect on the redox environment in H. hepaticus, with PutA activity generating ROS and proline accumulation providing protection against oxidative stress. Therefore, it was of interest to test the pathophysiological roles of PutA and proline metabolism in H. hepaticus.
To explore the impact of proline metabolism on H. hepaticus physiology and infection, a putA mutant strain of H. hepaticus was generated. The mutant strain was found to accumulate more proline and was significantly more resistant to oxidative stress than the wild-type strain. The ability of the mutant strain to survive high concentrations of oxidants indicates that the absence of PutA causes a change in the redox environment which is favorable for cell survival. The main reason for the increased resistance to oxidative stress exhibited by the putA mutant strain may be due to the hydroxy radical-scavenging properties of proline (27). Alternatively, expression levels of the antioxidant enzymes may be higher in the putA mutant strain. We found the opposite trend, however, as the wild-type strain exhibited higher expression levels of SOD and catalase than the putA mutant strain. Thus, proline appears to serve as a cytoprotectant in H. hepaticus, a role which has not been demonstrated previously in this pathogen.
High levels of proline were also shown to be toxic to the H. hepaticus wild-type strain but had no lethal effect on the putA mutant strain. These results provide evidence that PutA activity causes proline toxicity and cell death in the H. hepaticus wild-type strain. The toxic effects of PutA may be mediated by ROS formation or by production of the intermediate P5C, which has been shown to induce apoptosis in mammalian cells (35). As reported in a previous study, Helicobacter most likely resides in ecological niches that have elevated proline levels (40); therefore, it must balance the beneficial properties of proline metabolism with the harmful prooxidant effects of PutA activity. The unexpected increase in the sodF and katA gene expression in the wild-type cells relative to that in the putA mutant strain under normal growth conditions suggests that H. hepaticus uses SOD and catalase to combat the adverse effects of PutA activity. Because earlier studies with H. pylori have shown that proline is an important respiratory substrate, H. hepaticus may use SOD and catalase to control the toxic side effects of PutA activity while benefiting from the energetic and growth features of proline metabolism.
Analysis of tissue samples showed no significant differences between colonization of the liver and cecum of mice inoculated with the H. hepaticus wild type and that of mice inoculated with the putA mutant strain, indicating that the disruption of the putA gene does not affect the colonization ability of H. hepaticus. Histopathology examination of the liver tissue from mice infected with the putA mutant strain, however, exhibited significantly less inflammation than liver samples from mice inoculated with the wild-type strain. This suggests that although PutA may not be critical for colonization of the host, PutA and proline metabolism help to promote the pathogenesis of H. hepaticus. Inflammation observed with the liver samples is thought to be due primarily to neutrophil activation and ROS generation around the site of H. hepaticus infection (53). Apparently, the lack of PutA activity and proline utilization diminishes ROS-induced tissue damage and perhaps host neutrophil activation. The accumulation of proline in the H. hepaticus putA mutant may result in lower ROS levels, thereby minimizing or slowing down the inflammatory response. This would be consistent with the increased oxidative stress resistance of the putA mutant strain. Conversely, PutA activity in the wild-type strain would help accelerate or exacerbate inflammation and the pathogenesis of H. hepaticus infection.
Due to the dual effects of proline as a ROS scavenger and as a prooxidant via PutA, proline metabolism is well positioned to modulate inflammatory responses and the carcinogenesis of H. hepaticus infection. Future work will focus on discovering whether proline metabolism invokes certain signaling pathways involved in oxidative stress response and inflammation. For example, virulence factors such as catalase and NapA, an iron-binding protein, are upregulated in H. pylori by oxidative stress to help protect H. pylori and enable chronic inflammation to persist (5, 9, 21). The higher levels of catalase expression observed with the wild-type H. hepaticus strain than with the putA mutant strain suggest that proline oxidation via PutA generates redox signals in the cell that help maintain catalase expression and perhaps virulence. Additional experiments need to be performed to determine whether disrupting the putA gene attenuates the expression of other putative virulence factors. Proline transport will also be explored to distinguish between intracellular and extracellular pools of proline. Proline uptake has been shown to play a crucial role in the survival of the human pathogen Staphylococcus aureus, as deletion of the putP gene encoding the high-affinity proline transporter causes marked attenuation in several animal models of infection (3, 48, 57).
Supplementary Material
Acknowledgments
We thank Rasika Jinadasa for technical assistance and Cheryl Bailey for assistance with real-time PCR.
This work was supported by a contribution from the University of Nebraska Agricultural Research Division, supported in part by funds provided through the Hatch Act. Additional support was provided by the Layman Foundation, NIH grant GM061068, NIH pilot grant 5P-20-RR017675-03, and NIH grant P20 RR-017675-02 from the National Center for Research Resources.
The content of this report is the sole responsibility of the authors and does not necessarily represent the official views of the NIH.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 5 May 2008.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Alia, P. Mohanty, and J. Matysik. 2001. Effect of proline on the production of singlet oxygen. Amino Acids 21195-200. [DOI] [PubMed] [Google Scholar]
- 2.Aslan, M., Y. Nazligul, M. Horoz, C. Bolukbas, F. F. Bolukbas, N. Aksoy, H. Celik, and O. Erel. 2007. Serum prolidase activity and oxidative status in Helicobacter pylori infection. Clin. Biochem. 4037-40. [DOI] [PubMed] [Google Scholar]
- 3.Bayer, A. S., S. N. Coulter, C. K. Stover, and W. R. Schwan. 1999. Impact of the high-affinity proline permease gene (putP) on the virulence of Staphylococcus aureus in experimental endocarditis. Infect. Immun. 67740-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker, D. F., and E. A. Thomas. 2001. Redox properties of the PutA protein from Escherichia coli and the influence of the flavin redox state on PutA-DNA interactions. Biochemistry 404714-4722. [DOI] [PubMed] [Google Scholar]
- 5.Boonjakuakul, J. K., D. R. Canfield, and J. V. Solnick. 2005. Comparison of Helicobacter pylori virulence gene expression in vitro and in the rhesus macaque. Infect. Immun. 734895-4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, C., and M. B. Dickman. 2005. Proline suppresses apoptosis in the fungal pathogen of Colletotrichum trifolii. Proc. Natl. Acad. Sci. USA 1023459-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, C., S. Wanduragala, D. F. Becker, and M. Dickman. 2006. A tomato QM-like protein protects Saccharomyces cerevisiae cells against oxidative stress by regulating intracellular proline levels. Appl. Environ. Microbiol. 724001-4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng, N., J. S. Xie, M. Y. Zhang, C. Shu, and D. X. Zhu. 2003. A specific anti-Helicobacter pylori agent NE2001: synthesis and its effect on the growth of H. pylori. Bioorg. Med. Chem. Lett. 132703-2707. [DOI] [PubMed] [Google Scholar]
- 9.Cooksley, C., P. J. Jenks, A. Green, A. Cockayne, R. P. Logan, and K. R. Hardie. 2003. NapA protects Helicobacter pylori from oxidative stress damage, and its production is influenced by the ferric uptake regulator. J. Med. Microbiol. 52461-469. [DOI] [PubMed] [Google Scholar]
- 10.Donald, S. P., X. Y. Sun, C. A. Hu, J. Yu, J. M. Mei, D. Valle, and J. M. Phang. 2001. Proline oxidase, encoded by p53-induced gene-6, catalyzes the generation of proline-dependent reactive oxygen species. Cancer Res. 611810-1815. [PubMed] [Google Scholar]
- 11.Foltz, C. J., J. G. Fox, R. Cahill, J. C. Murphy, L. Yan, B. Shames, and D. B. Schauer. 1998. Spontaneous inflammatory bowel disease in multiple mutant mouse lines: association with colonization by Helicobacter hepaticus. Helicobacter 369-78. [DOI] [PubMed] [Google Scholar]
- 12.Fox, J. G., F. E. Dewhirst, J. G. Tully, B. J. Paster, L. Yan, N. S. Taylor, M. J. Collins, Jr., P. L. Gorelick, and J. M. Ward. 1994. Helicobacter hepaticus sp. nov., a microaerophilic bacterium isolated from livers and intestinal mucosal scrapings from mice. J. Clin. Microbiol. 321238-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox, J. G., X. Li, L. Yan, R. J. Cahill, R. Hurley, R. Lewis, and J. C. Murphy. 1996. Chronic proliferative hepatitis in A/JCr mice associated with persistent Helicobacter hepaticus infection: a model of helicobacter-induced carcinogenesis. Infect. Immun. 641548-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox, J. G., L. Yan, B. Shames, J. Campbell, J. C. Murphy, and X. Li. 1996. Persistent hepatitis and enterocolitis in germfree mice infected with Helicobacter hepaticus. Infect. Immun. 643673-3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ge, Z., D. A. White, M. T. Whary, and J. G. Fox. 2001. Fluorogenic PCR-based quantitative detection of a murine pathogen, Helicobacter hepaticus. J. Clin. Microbiol. 392598-2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hagedorn, C. H., and J. M. Phang. 1986. Catalytic transfer of hydride ions from NADPH to oxygen by the interconversion of proline to delta-pyrroline-5-carboxylate. Arch. Biochem. Biophys. 248166-174. [DOI] [PubMed] [Google Scholar]
- 17.Hailey, J. R., J. K. Haseman, J. R. Bucher, A. E. Radovsky, D. E. Malarkey, R. T. Miller, A. Nyska, and R. R. Maronpot. 1998. Impact of Helicobacter hepaticus infection in B6C3F1 mice from twelve National Toxicology Program two-year carcinogenesis studies. Toxicol. Pathol. 26602-611. [DOI] [PubMed] [Google Scholar]
- 18.Hu, C. A., S. P. Donald, J. Yu, W. W. Lin, Z. Liu, G. Steel, C. Obie, D. Valle, and J. M. Phang. 2007. Overexpression of proline oxidase induces proline-dependent and mitochondria-mediated apoptosis. Mol. Cell. Biochem. 29585-92. [DOI] [PubMed] [Google Scholar]
- 19.Jeffery, C. J. 2004. Molecular mechanisms for multitasking: recent crystal structures of moonlighting proteins. Curr. Opin. Struct. Biol. 14663-668. [DOI] [PubMed] [Google Scholar]
- 20.Josyula, S., H. A. Schut, B. A. Diwan, M. R. Anver, and L. M. Anderson. 2000. Age-related alterations in 32P-postlabeled DNA adducts in livers of mice infected with the tumorigenic bacterial pathogen, Helicobacter hepaticus. Int. J. Oncol. 17811-818. [DOI] [PubMed] [Google Scholar]
- 21.Kaakoush, N. O., Z. Kovach, and G. L. Mendz. 2007. Potential role of thiol:disulfide oxidoreductases in the pathogenesis of Helicobacter pylori. FEMS Immunol. Med. Microbiol. 50177-183. [DOI] [PubMed] [Google Scholar]
- 22.Kavermann, H., B. P. Burns, K. Angermuller, S. Odenbreit, W. Fischer, K. Melchers, and R. Haas. 2003. Identification and characterization of Helicobacter pylori genes essential for gastric colonization. J. Exp. Med. 197813-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim, H. 2005. Oxidative stress in Helicobacter pylori-induced gastric cell injury. Inflammopharmacology 1363-74. [DOI] [PubMed] [Google Scholar]
- 24.Kohl, D. H., K. R. Schubert, M. B. Carter, C. H. Hagedorn, and G. Shearer. 1988. Proline metabolism in N2-fixing root nodules: energy transfer and regulation of purine synthesis. Proc. Natl. Acad. Sci. USA 852036-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krishnan, N., and D. F. Becker. 2005. Characterization of a bifunctional PutA homologue from Bradyrhizobium japonicum and identification of an active site residue that modulates proline reduction of the flavin adenine dinucleotide cofactor. Biochemistry 449130-9139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krishnan, N., and D. F. Becker. 2006. Oxygen reactivity of PutA from Helicobacter species and proline-linked oxidative stress. J. Bacteriol. 1881227-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krishnan, N., M. B. Dickman, and D. F. Becker. 2008. Proline modulates the intracellular redox environment and protects mammalian cells against oxidative stress. Free Radic. Biol. Med. 44671-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li, X., J. G. Fox, M. T. Whary, L. Yan, B. Shames, and Z. Zhao. 1998. SCID/NCr mice naturally infected with Helicobacter hepaticus develop progressive hepatitis, proliferative typhlitis, and colitis. Infect. Immun. 665477-5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu, Y., G. L. Borchert, A. Surazynski, C. A. Hu, and J. M. Phang. 2006. Proline oxidase activates both intrinsic and extrinsic pathways for apoptosis: the role of ROS/superoxides, NFAT and MEK/ERK signaling. Oncogene 255640-5647. [DOI] [PubMed] [Google Scholar]
- 30.Liu, Y. M., G. L. Borchert, S. P. Donald, A. Surazynski, C. A. Hu, C. J. Weydert, L. W. Oberley, and J. M. Phang. 2005. MnSOD inhibits proline oxidase-induced apoptosis in colorectal cancer cells. Carcinogenesis 261335-1342. [DOI] [PubMed] [Google Scholar]
- 31.Loogna, P., L. Franzen, P. Sipponen, and L. Domellof. 2002. Cyclooxygenase-2 and Bcl-2 expression in the stomach mucosa of Wistar rats exposed to Helicobacter pylori, N′-methyl-N′-nitro-N-nitrosoguanidine and bile. Virchows Arch. 44177-84. [DOI] [PubMed] [Google Scholar]
- 32.Loogna, P., L. Franzen, P. Sipponen, and L. Domellof. 2001. Effects of Helicobacter pylori and bile on N-methyl-N′-nitro-N′-nitrosoguanidine exposed antral mucosa of C57BU6 mice. Virchows Arch. 439661-667. [DOI] [PubMed] [Google Scholar]
- 33.Loogna, P., L. Franzen, P. Sipponen, and L. Domellof. 2001. Helicobacter pylori, N-methyl-N′-nitro-N′-nitrosoguanidine, and bile modulate gastric cell kinetics in experimental cancer. Virchows Arch. 439653-660. [DOI] [PubMed] [Google Scholar]
- 34.Maier, R. J., J. Olson, and A. Olczak. 2003. Hydrogen-oxidizing capabilities of Helicobacter hepaticus and in vivo availability of the substrate. J. Bacteriol. 1852680-2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maxwell, S. A., and G. E. Davis. 2000. Differential gene expression in p53-mediated apoptosis-resistant vs. apoptosis-sensitive tumor cell lines. Proc. Natl. Acad. Sci. USA 9713009-13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maxwell, S. A., and A. Rivera. 2003. Proline oxidase induces apoptosis in tumor cells, and its expression is frequently absent or reduced in renal carcinomas. J. Biol. Chem. 2789784-9789. [DOI] [PubMed] [Google Scholar]
- 37.Mehta, N. S., S. Benoit, J. V. Mysore, R. S. Sousa, and R. J. Maier. 2005. Helicobacter hepaticus hydrogenase mutants are deficient in hydrogen-supported amino acid uptake and in causing liver lesions in A/J. mice. Infect. Immun. 735311-5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mooney, C., J. Keenan, D. Munster, I. Wilson, R. Allardyce, P. Bagshaw, B. Chapman, and V. Chadwick. 1991. Neutrophil activation by Helicobacter pylori. Gut 32853-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Myles, M. H., R. S. Livingston, B. A. Livingston, J. M. Criley, and C. L. Franklin. 2003. Analysis of gene expression in ceca of Helicobacter hepaticus-infected A/JCr mice before and after development of typhlitis. Infect. Immun. 713885-3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagata, K., Y. Nagata, T. Sato, M. A. Fujino, K. Nakajima, and T. Tamura. 2003. l-Serine, d- and l-proline and alanine as respiratory substrates of Helicobacter pylori: correlation between in vitro and in vivo amino acid levels. Microbiology 1492023-2030. [DOI] [PubMed] [Google Scholar]
- 41.Pandhare, J., S. K. Cooper, and J. M. Phang. 2006. Proline oxidase, a proapoptotic gene, is induced by troglitazone: evidence for both peroxisome proliferator-activated receptor gamma-dependent and -independent mechanisms. J. Biol. Chem. 2812044-2052. [DOI] [PubMed] [Google Scholar]
- 42.Rautelin, H., B. Blomberg, H. Fredlund, G. Jarnerot, and D. Danielsson. 1993. Incidence of Helicobacter pylori strains activating neutrophils in patients with peptic ulcer disease. Gut 34599-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rice, J. M. 1995. Helicobacter hepaticus, a recently recognized bacterial pathogen, associated with chronic hepatitis and hepatocellular neoplasia in laboratory mice. Emerg. Infect. Dis. 1129-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richardson, A. D., C. Yang, A. Osterman, and J. W. Smith. 19 September 2007. Central carbon metabolism in the progression of mammary carcinoma. Breast Cancer Res. Treat. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 45.Rivera, A., and S. A. Maxwell. 2005. The p53-induced gene-6 (proline oxidase) mediates apoptosis through a calcineurin-dependent pathway. J. Biol. Chem. 28029346-29354. [DOI] [PubMed] [Google Scholar]
- 46.Saradhi, P. P., Alia, A. S. Arora, and K. V. S. K. Prasad. 1995. Proline accumulates in plants exposed to UV radiation and protects them against UV induced peroxidation. Biochem. Biophys. Res. Commun. 2091-5. [DOI] [PubMed] [Google Scholar]
- 47.Sato, D., A. Yanaka, T. Shibahara, H. Matsui, A. Nakahara, T. Yanagawa, E. Warabi, T. Ishii, and I. Hyodo. 2008. Peroxiredoxin I protects gastric mucosa from oxidative injury induced by H. pylori infection. J. Gastroenterol. Hepatol. 23652-659. [DOI] [PubMed] [Google Scholar]
- 48.Schwan, W. R., K. J. Wetzel, T. S. Gomez, M. A. Stiles, B. D. Beitlich, and S. Grunwald. 2004. Low-proline environments impair growth, proline transport and in vivo survival of Staphylococcus aureus strain-specific putP mutants. Microbiology 1501055-1061. [DOI] [PubMed] [Google Scholar]
- 49.Shames, B., J. G. Fox, F. Dewhirst, L. Yan, Z. Shen, and N. S. Taylor. 1995. Identification of widespread Helicobacter hepaticus infection in feces in commercial mouse colonies by culture and PCR assay. J. Clin. Microbiol. 332968-2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Amsterdam, K., and A. van der Ende. 2004. Nutrients released by gastric epithelial cells enhance Helicobacter pylori growth. Helicobacter 9614-621. [DOI] [PubMed] [Google Scholar]
- 51.Walter, R., W. H. Simmons, and T. Yoshimoto. 1980. Proline specific endo- and exopeptidases. Mol. Cell. Biochem. 30111-127. [DOI] [PubMed] [Google Scholar]
- 52.Wang, G., P. Alamuri, and R. J. Maier. 2006. The diverse antioxidant systems of Helicobacter pylori. Mol. Microbiol. 61847-860. [DOI] [PubMed] [Google Scholar]
- 53.Wang, G., Y. Hong, A. Olczak, S. E. Maier, and R. J. Maier. 2006. Dual roles of Helicobacter pylori NapA in inducing and combating oxidative stress. Infect. Immun. 746839-6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang, G., and R. J. Maier. 2004. An NADPH quinone reductase of Helicobacter pylori plays an important role in oxidative stress resistance and host colonization. Infect. Immun. 721391-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ward, J. M., M. R. Anver, D. C. Haines, and R. E. Benveniste. 1994. Chronic active hepatitis in mice caused by Helicobacter hepaticus. Am. J. Pathol. 145959-968. [PMC free article] [PubMed] [Google Scholar]
- 56.Ward, J. M., J. G. Fox, M. R. Anver, D. C. Haines, C. V. George, M. J. Collins, Jr., P. L. Gorelick, K. Nagashima, M. A. Gonda, R. V. Gilden, and et al. 1994. Chronic active hepatitis and associated liver tumors in mice caused by a persistent bacterial infection with a novel Helicobacter species. J. Natl. Cancer Inst. 861222-1227. [DOI] [PubMed] [Google Scholar]
- 57.Wengender, P. A., and K. J. Miller. 1995. Identification of a PutP proline permease gene homolog from Staphylococcus aureus by expression cloning of the high-affinity proline transport system in Escherichia coli. Appl. Environ. Microbiol. 61252-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Young, V. B., K. A. Knox, J. S. Pratt, J. S. Cortez, L. S. Mansfield, A. B. Rogers, J. G. Fox, and D. B. Schauer. 2004. In vitro and in vivo characterization of Helicobacter hepaticus cytolethal distending toxin mutants. Infect. Immun. 722521-2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu, W., and D. F. Becker. 2005. Exploring the proline-dependent conformational change in the multifunctional PutA flavoprotein by tryptophan fluorescence spectroscopy. Biochemistry 4412297-12306. [DOI] [PubMed] [Google Scholar]
- 60.Zhu, W., and D. F. Becker. 2003. Flavin redox state triggers conformational changes in the PutA protein from Escherichia coli. Biochemistry 425469-5477. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.