Abstract
The extracellular depolymerization of arabinopolysaccharides by microorganisms is accomplished by arabinanases, xylanases, and galactanases. Here, we characterize a novel endo-α-1,5-l-arabinanase (EC 3.2.1.99) from Bacillus subtilis, encoded by the yxiA gene (herein renamed abn2) that contributes to arabinan degradation. Functional studies by mutational analysis showed that Abn2, together with previously characterized AbnA, is responsible for the majority of the extracellular arabinan activity in B. subtilis. Abn2 was overproduced in Escherichia coli, purified from the periplasmic fraction, and characterized with respect to substrate specificity and biochemical and physical properties. With linear-α-1,5-l-arabinan as the preferred substrate, the enzyme exhibited an apparent Km of 2.0 mg ml−1 and Vmax of 0.25 mmol min−1 mg−1 at pH 7.0 and 50°C. RNA studies revealed the monocistronic nature of abn2. Two potential transcriptional start sites were identified by primer extension analysis, and both a σA-dependent and a σH-dependent promoter were located. Transcriptional fusion studies revealed that the expression of abn2 is stimulated by arabinan and pectin and repressed by glucose; however, arabinose is not the natural inducer. Additionally, trans-acting factors and cis elements involved in transcription were investigated. Abn2 displayed a control mechanism at a level of gene expression different from that observed with AbnA. These distinct regulatory mechanisms exhibited by two members of extracellular glycoside hydrolase family 43 (GH43) suggest an adaptative strategy of B. subtilis for optimal degradation of arabinopolysaccharides.
Microorganisms are present in all natural environments and play fundamental roles in biogeochemical cycling or in decomposition processes. A key step in the carbon cycle is the degradation of plant cell wall polysaccharides by the concerted action of several glycosyl hydrolases (see references 2 and 35 and references therein). Due to their increased value in several biotechnological processes, namely, the conversion of several hemicellulosic substrates to fermentable sugars and the successive production of fuel alcohol, the characterization of these cellulose- and/or hemicellulose-degrading enzymes has been an important goal for achieving efficient breakdown of plant cell wall polymers (24, 28, 29). The gram-positive bacterium Bacillus subtilis participates in the enzymatic dissolution of plant biomass in the soil. Thus, it is able to synthesize a vast variety of glycoside hydrolases capable of the depolymerization of plant cell wall polysaccharides, such as cellulose, hemicellulose, or pectin (see references 35 and 37 and references therein). l-Arabinose, the second most abundant pentose in nature, is found in significant amounts in homopolysaccharides, branched and debranched arabinans, and heteropolysaccharides, such as arabinoxylans and arabinogalactans. In fact, B. subtilis produces exo- and endoacting arabinases capable of releasing arabinosyl oligomers and l-arabinose from plant cell walls (15, 16, 19, 30, 41).
Previous work by our group studied the transcriptional regulation of three B. subtilis arabinan-degrading genes, abnA, abfA, and abf2(xsa), that are clustered with genes encoding enzymes that further catabolize arabinose (25). These three genes are induced by arabinose and arabinan, repressed by glucose, and subjected to temporal regulation (25). Moreover, we characterized their product, AbnA, as an extracellular endo-α-1,5-l-arabinanase (EC 3.2.1.99), belonging to glycoside hydrolase family 43 (GH43) that hydrolyzed sugar beet arabinan (branched) and linear α-1,5-l-arabinan (19). AbfA and Abf2 are alpha-l-arabinofuranosidases (EC 3.2.1.55) belonging to glycoside hydrolase family 51 (GH51), but they display different substrate specificities: AbfA acted preferentially on (1→5) arabinofuranosyl linkages, and in contrast Abf2 was most active on (1→2) and (1→3) linkages (J. M. Inácio, I. L. Correia, and I. Sá-Nogueira, submitted for publication).
To completely characterize the major enzymes belonging to this B. subtilis hemicellulolytic system directed to arabinose-containing polysaccharides, we performed genetic and functional analysis of the yxiA gene, which encodes a putative arabinanase. Signals that regulate gene expression were identified, together with trans-acting factors and cis elements involved in transcription. Furthermore, the enzyme was overproduced in Escherichia coli and the biochemical properties of recombinant protein were determined. The results indicate that the product of yxiA is an extracellular endo-α-1,5-l-arabinanase; thus, we propose to rename this gene abn2.
MATERIALS AND METHODS
Substrates.
Sugar beet arabinan, debranched arabinan (linear α-1,5-l-arabinan, purity 95%), wheat arabinoxylan, and Red debranched arabinan were purchased from Megazyme International Ireland, and larch wood arabinogalactan, and pectin from apple and p-nitrophenyl-α-l-arabinofuranoside (pNPAf) were purchased from Sigma Chemical Co.
Bacterial strains and growth conditions.
The B. subtilis strains used in this study are listed in Table 1. Escherichia coli DH5α (Gibco BRL) was used for routine molecular cloning work, and E. coli BL21(DE3) pLysS (36) was used as the host for the expression of native and recombinant Abn2. E. coli strains were grown in Luria-Bertani (LB) (22) medium, and kanamycin (20 μg ml−1), chloramphenicol (25 μg ml−1), or IPTG (isopropyl-α-d-thiogalactopyranoside) was added as appropriate. For the transcriptional studies, B. subtilis strains were grown in liquid C minimal medium (25) supplemented with 1% (wt/vol) casein hydrolysate. When necessary 0.4% (wt/vol) l-arabinose, 0.4% (wt/vol) arabinan, 0.4% (wt/vol) xylan, 0.4% (wt/vol) pectin, or 0.4% (wt/vol) d-glucose was added to the cultures. For the determination of arabinanase activity in supernatants, B. subtilis strains were grown in MC complex medium (19). The amyE phenotype was tested by plating on TBAB (tryptose blood agar base) medium (Difco) as described previously by Raposo et al. (25). The transformation of E. coli and B. subtilis strains was performed as described previously (13).
TABLE 1.
Plasmids, B. subtilis strains, and oligonucleotides used in this study
| Plasmid, strain, or oligonucleotide | Relevant construction, genotype, or sequence (5′→3′)a | Source, reference, or transformation type |
|---|---|---|
| Plasmids | ||
| pET30a(+) | Expression vector allowing N- or C-terminal His6 tag insertion; T7 promoter, kan | Novagen |
| pSN32 | Promoterless lacZ preceded by rbsspoVG and MCS, cat, flanked by amyE-5′ and amyE-3′, bla | 23 |
| pMS38 | pLITMUS38 derivate encompassing a chloramphenicol resistance gene, cat bla | 42 |
| pZI38 | pSN32 carrying a 942-bp fragment of the abn2(yxiA) promoter region in MCS | This work |
| pZI39 | pET30a(+) containing the abn2(yxiA) encoding region in MCS | This work |
| pZI41 | pMS38 with the cat gene flanked by abn2(yxiA) 5′ and 3′ fragments | This work |
| pZI44 | Same as pZI38 bearing a single-base-pair substitution position +25 C→A | This work |
| pGP211 | pBSK+ derivative that contains the spc gene inserted into the hprK gene | 9 |
| Strains | ||
| 168T+ | Prototroph | 25 |
| QB5223 | trpC2 ptsH1 | 20 |
| QB7097 | trpC2 crh::spc | 20 |
| WLN29 | trpC2 aroG932 ccpA::Tn917 | 11 |
| IQB215 | araR::km | 33 |
| IQB413 | abnA::km | 25 |
| IQB483 | amyE::[abn2(yxiA)-lacZ cat] | pZI38→168T+b |
| IQB484 | amyE::[abn2(yxiA)-lacZ cat] araR::km | pZI38→IQB215b |
| IQB485 | abn2(yxiA)::cat | pZI41→168T+b |
| IQB486 | abn2(yxiA)::cat abnA::km | pZI41→IQB413b |
| IQB487 | amyE::[abn2(yxiA)-lacZ cat] ΔccpA::Tn917 | WLN29→IQB483 |
| IQB488 | amyE::[abn2(yxiA)-lacZ cat] ptsH1 | pZI38→QB5223b |
| IQB489 | amyE::[abn2(yxiA)-lacZ cat] crh::spc | QB7097→IQB483 |
| IQB490 | amyE::[abn2(yxiA)-lacZ cat] ptsH1 crh::spc | QB7097→IQB488 |
| IQB491 | amyE::[abn2(yxiA)-lacZ cat] ptsK::spc | pGP211→IQB483b |
| IQB492 | amyE::[abn2(yxiA)-lacZ cat creC→A] | pZI44→168T+b |
| Oligonucleotidesc | ||
| ARA193 | −598AGGAGAATTCACAGCCG−582 | |
| ARA194 | +358GAGTATCGGATCCCGCC+341 | |
| ARA196 | +1792GCTTTTCATGTAAGTCGG+1775 | |
| ARA237 | +50GGCGAATTGTTCATATGTTCAACCG+74 | |
| ARA238 | +1485CGCTTCTCCCTCGAGTTTAGATCCC+1461 | |
| ARA257 | +1GGTATCTTCATAGAGAAATGTAAGAGTTTAAAATTTAATAAAAAAGAGAGG+51 | |
| ARA258 | +51CCTCTCTTTTTTATTAAATTTTAAACTCTTACATTTCTCTATGAAGATACC+1 | |
| ARA321 | +98GCAAGAAAACATACACGGAACAATCGG+72 |
The arrows indicate transformation and point from donor DNA to the recipient strain. MCS, multiple-cloning site.
Transformation was carried out with linearized plasmid DNA.
The number in the primers refers to the position of the sequence relative to the transcription start site of abn2 gene. Restriction sites and the substituted base pairs are underlined in the oligonucleotide sequence.
DNA manipulation and sequencing.
DNA manipulations were carried out as described previously by Sambrook et al. (31). Restriction enzymes were purchased from Fermentas and used according to the manufacturer's instructions. DNA was eluted from agarose gels by using the GFX gel band purification kit (GE Healthcare). DNA sequencing was performed with the ABI PRIS BigDye terminator ready reaction cycle sequencing kit (Applied Biosystems). PCR amplifications were carried out using high-fidelity Pfu Turbo DNA polymerase (Stratagene), and the resulting products were purified by the QIAquick PCR purification kit (Qiagen).
Construction of plasmids and strains.
For the construction of pZI39 (harboring a recombinant abn2 allele, bearing a C-terminal His6-tag under the control of T7 inducible promoter), the coding sequence of abn2 was amplified by PCR with the primers ARA237 and ARA238 by using chromosomal DNA of wild-type strain B. subtilis 168T+ as the template. These primers introduced unique restriction sites NdeI and XhoI at the 5′ and 3′ ends, respectively. The resulting 1,568-bp DNA fragment was digested with NdeI-XhoI and cloned into the same sites of pET30a(+) (Novagen). Plasmid pZI43, encoding the native Abn2, was constructed by subcloning the 1,568-bp DNA fragment amplified by PCR as described above by using the primers ARA237 and ARA196 and was digested with NdeI into pET30a(+)NdeI-EcoRV. A 375-bp XbaI-AatII DNA fragment from pZI39 was inserted between the same sites of pMS38 (42), yielding pZI40. Plasmid pZI41, obtained by subcloning a 392-bp SmaI-XhoI DNA fragment from pZI39 into pZI40 EcoRI (fill-in)-XhoI, was used for the deletion of abn2 in B. subtilis. Plasmid pZI38, bearing the promoter region of abn2 fused to the E. coli lacZ gene, is a derivative of pSN32 (23). To construct pZI38, a DNA fragment from the abn2 promoter region amplified by PCR as described above with the primers ARA193 (EcoRI) and ARA194 (BamHI), was digested with EcoRI and BamHI and cloned into pSN32 (EcoRI-BamHI). To create a single-nucleotide substitution in cre abn2 (C→A) the QuikChange method (Stratagene) was used to amplify the DNA template pZI38 with overlapping oligonucleotides ARA257 and ARA258, yielding pZI44. Linearized plasmid DNA from pZI38 and pZI44, carrying the different promoter-lacZ transcriptional fusions, was used to transform B. subtilis strains (Table 1) and the fusions integrated into the chromosome via double recombination with the amyE gene back and front sequences. This event led to the disruption of the amyE locus and was confirmed as described above.
β-Galactosidase activity assays.
Strains of B. subtilis harboring the transcriptional lacZ fusions were grown as described above. Samples of cell culture were collected 2 h (exponential growth phase) after induction (t2) and 4 h (late exponential growth phase) after induction (t4), and the level of β-galactosidase activity was determined as described previously (25). The ratio of β-galactosidase activity from cultures grown in the presence and absence of glucose was taken as a measure of glucose repression (glucose repression index).
RNA preparation, Northern blotting, and primer extension analysis.
B. subtilis strains were grown as described above, and cells were harvested 2 h after induction. Total RNA was prepared as described previously by Igo and Losick (12). For Northern blot analysis, 10 μg of total RNA was run on a 1.2% (wt/vol) agarose formaldehyde denaturing gel and transferred to positively charged nylon membranes HybondN+ (Amersham) according to standard procedures (31). Size determination was carried out using an RNA ladder (6 to 0.2 kb; Fermentas). A DNA fragment of 1,568 bp used as abn2 probe was obtained by PCR amplification of chromosomal DNA with the primers ARA237 and ARA238. The DNA probe was labeled with the Megaprime DNA labeling system (Amersham) and [α-32P]dCTP (3,000 Ci/mmol [Amersham]). Primer extension analysis was performed essentially as described previously by Sambrook et al. (31). The primer ARA321, complementary to the abn2 sequence (Table 1), was end labeled with [γ-32P]ATP (3,000 Ci/mmol) by using T4 polynucleotide kinase (Fermentas). A total of 2.5 pmol of the labeled primer was mixed with 100 μg of RNA, denatured by heating to 85°C for 10 min, and annealed by incubation at 45°C overnight. The extension reaction was conducted for 2 h at 37°C by using 50 U of avian Moloney murine leukemia virus reverse transcriptase (RevertAid; Fermentas). An analysis of the extended products was carried out on 6% (wt/vol) polyacrylamide urea gels.
Production and purification of recombinant arabinanase.
E. coli BL21(DE3) pLysS cells harboring pZI39 were grown at 37°C and 160 rpm in 1 liter of LB with the appropriate antibiotic selection. When the optical density at 600 nm reached 0.6, the expression of Abn2 was induced by the addition of 1 mM IPTG. The culture was grown for an additional 4 h at 37°C and 160 rpm. Cells were harvested by centrifugation at 4°C and 8,000 × g for 10 min. All subsequent steps were carried out at 4°C. The periplasmic protein fraction was prepared by osmotic shock as described previously (19). The periplasmic protein fraction was loaded onto a 1-ml HisTrap column (Amersham Pharmacia Biotech). The bounded proteins were eluted by discontinuous imidazole gradient, and the fractions containing Abn2 that were more than 95% pure were dialyzed overnight against storage buffer (20 mM Na-phosphate buffer, pH 7.4, 50 mM NaCl, 10% glycerol) and then frozen in liquid nitrogen and kept at −80°C until further use. The analysis of production, the homogeneity, and the molecular mass of the enzymes were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), using broad-range molecular weight markers (Bio-Rad) as the standards. The degree of purification was determined by densitometric analysis of Coomassie blue-stained SDS-PAGE gels. The protein content was determined by using the Bradford reagent (Bio-Rad) with bovine serum albumin as the standard. Recombinant Abn2-His6 was subjected to N-terminal microsequencing on a Procise 491 HT protein sequencer.
Biochemical characterization.
The source of the enzyme was supernatants of B. subtilis cultures or purified arabinanase, Abn2-His6. The enzyme activity was determined as described previously by Leal and Sá-Nogueira (19). The reducing sugar content after hydrolysis of the polysaccharides was determined by the Nelson-Somogyi method, with l-arabinose as the standard. One unit of activity was defined as the amount of enzyme that produces 1 μmol of arabinose equivalents per minute. α-l-Arabinofuranosidase activity was determined by using pNPAf as the substrate, as previously reported (19). Temperature and pH for maximum enzymatic activity of Abn2-His6 were tested at temperatures ranging from 30°C to 80°C and buffers ranging from pH 4.0 to 8.0, as described previously (19). Thermal stability of the enzyme was estimated by incubation of appropriate dilutions of the enzyme in PC buffer (200 mM phosphate-100 mM citrate), pH 7.0, at 50°C. Samples were removed after 5, 10, 20, and 30 min and kept on ice for 10 min, and residual enzyme activity was determined at an optimum pH and temperature, by using linear α-1,5-l-arabinan 0.5% (wt/vol) as the substrate. Enzymatic activity was also determined in the presence of 1 mM EDTA by using the same conditions. The kinetic parameters, apparent Km and Vmax values, were determined from the Lineweaver-Burk plot method at an optimum pH and temperature by using linear α-1,5-l-arabinan as the substrate at concentrations ranging from 1 mg ml−1 to 10 mg ml−1.
Nucleotide sequence accession number.
The nucleotide sequence of the abn2 gene from the B. subtilis strain 168T+ reported in this paper has been submitted to GenBank under accession number EU373814.
RESULTS
Functional analysis of Abn2.
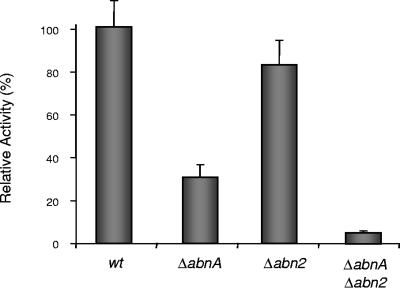
In previous studies, we showed that the supernatant of cultures of a B. subtilis abnA-null mutant still retained about 30% of the capacity to hydrolyze linear α-1,5-l-arabinan relative to the capacity of the wild type (19). This observation gave us an indication that B. subtilis secretes other enzymes capable of hydrolyzing this substrate. A likely candidate responsible for the remaining arabinanase activity is the product of the abn2 gene, a hypothetical arabinanase displaying 27% identity to AbnA (18). To test this hypothesis, we constructed single-Δabn2 and double-Δabn2 ΔabnA B. subtilis null mutants by insertion-deletion mutations and the arabinanase activity was measured in the supernatant of cultures of wild-type and mutant strains grown in the presence of branched arabinan. The results showed an almost complete loss of this activity in the Δabn2 ΔabnA double mutant (Fig. 1), indicating that AbnA and Abn2 are the two major enzymes responsible for extracellular arabinanase activity in B. subtilis.
FIG. 1.
Comparison of the relative arabinanase activities of B. subtilis wild-type (wt) and abn2- and abnA-null mutant strains. The wild-type strain 168T+ and mutant strains IQB413 (ΔabnA), IQB485 (Δabn2), and IQB486 (ΔabnA Δabn2) were grown for 24 h at 37°C in MC complex medium supplemented with branched arabinan 0.4% (wt/vol). Arabinanase activity, measured in the supernatant of liquid cultures, was determined by the ability to release arabinose from linear α-1,5-l-arabinan 0.2% (wt/vol) at pH 6.6 and 37°C, as described in Materials and Methods. The activity measured in the supernatant of the wild-type strain was defined as 100% and corresponds to 10.8 ± 1.4 mU ml−1/optical density at 600 nm. Values represent the average of three independent experiments (averages ± standard deviations [error bars]) each performed in triplicate.
abn2 transcription is stimulated by arabinan and pectin and is repressed by glucose.
To study the expression of the abn2 gene, an abn2′-lacZ transcriptional fusion was integrated at the amyE locus of the B. subtilis wild-type chromosome (Table 1). The level of accumulated β-galactosidase activity in the resulting strain IQB483 was examined in the absence of sugars and in the presence of potential inducers, arabinose, arabinan (branched), and pectin. Samples were collected at t2 and t4, which correspond to the exponential growth phase and the early postexponential (transitional) phase, respectively (14). The expression from the abn2′-lacZ fusion in the absence of sugars is very low, but during exponential growth (t2), expression is stimulated by arabinan and pectin at levels of about 9- and 14-fold, respectively (Table 2). Additionally, a small increment of expression was observed at the early postexponential phase (t4), especially in the presence of pectin, suggesting temporal regulation. No significant increase in abn2′-lacZ expression was observed in the presence of arabinose. To further identify potential inducers, we examined the effect of galacturonic acid (a major component of pectin), rhamnose, galactose, and xylose (also present in pectins) on abn2′-lacZ expression. All sugars failed to trigger abn2′-lacZ fusion expression (data not shown). To determine whether the transcription of abn2 was sensitive to glucose, the strain was grown in the presence of arabinan plus glucose. The results showed that glucose caused a 17- and 44-fold repression of the abn2′-lacZ fusion expression during the exponential growth phase and the early postexponential phase, respectively (Table 2).
TABLE 2.
Expression from abn2′-lacZ transcriptional fusion in the wild-type and araR-null mutant
| Strain (promoter fusion)a | Sugar | β-Galactosidase activity (Miller units) at:b
|
|
|---|---|---|---|
| t2 | t4 | ||
| IQB483 (abn2′-lacZ) | No sugar | 1.3 ± 0.2 | 1.4 ± 0.1 |
| Arabinose | 1.4 ± 0.0 | 0.9 ± 0.1 | |
| Arabinan | 11.0 ± 0.5 | 12.7 ± 0.8 | |
| Pectin | 18.3 ± 1.6 | 29.8 ± 2.0 | |
| Arabinan plus glucose | 0.6 ± 0.0 | 0.2 ± 0.0 | |
| IQB484 (abn2′-lacZ) | No sugar | 1.1 ± 0.1 | 1.1 ± 0.1 |
| (ΔaraR) | Arabinan | 6.8 ± 0.8 | 12.1 ± 0.6 |
The strains containing abn2′-lacZ fusion were grown on C minimal medium supplemented with casein hydrolysate in the absence or presence of the different sugars. Samples were analyzed 2 h and 4 h after the addition of sugars.
The levels of accumulated β-galactosidase activity represent the average ± standard deviations of three independent experiments each performed with duplicate measurements.
abn2 is monocistronic, and its expression is driven from two different promoters.
Transcriptional studies by mRNA analysis were performed. Total RNA isolated from the wild-type and abn2-null mutant strains grown for 2 h in the absence or presence of the inducers arabinan and pectin was annealed to a DNA probe for abn2. An arabinan- and pectin-inducible abn2 transcript of 1.6 kb was detected (Fig. 2A). Additionally, a 0.6-kb transcript was visible with RNA from cells of the abn2-null mutant strain, corresponding to the 5′ end of the gene present in the abn2 locus of the insertion-deletion mutant (see Materials and Methods), confirming the specificity of the transcript. The extent of abn2 mRNA signal matched the expected size (1.6 kb) and revealed its monocistronic nature. Primer extension analysis of total RNA isolated from cells grown both in the absence and presence of arabinan detected two distinct messages. One transcript displayed a 5′ end that corresponds to a G residue located 63 bp upstream from the initiation ATG codon (Fig. 2B), and the second message corresponds to an A residue 185 bp upstream from the initiation ATG codon (Fig. 2C). The potential −35 and −10 regions (TGAATA-17 bp-TTTAAT) of the first transcription start site resembled the consensus sequence for recognition by B. subtilis σA-containing RNA polymerase (TTGACA-17 bp-TATAAT) (Fig. 2D). Centered at −35 and −10 bp upstream from the second transcription start site are two sequences, GAAGGAGAA and GTTGAAC, respectively, which are similar to the consensus sequence for recognition of σH, RNAGGAWWW-(11-12 bp)-RNNGAAT (R, A, or G; W, A, or T; and N, any base [10]) (Fig. 2D). The detection of abn2-specific extension products in RNA extracted from cells grown in the absence of sugar correlates well to the result observed by Northern blot analysis.
FIG. 2.
Analysis of abn2 mRNA. (A) Northern blot analysis of abn2-specific transcripts. Total RNA (10 μg), extracted from the wild-type (WT) strain (grown in the absence of sugars [−], in the presence of arabinan [ABN], and in the presence of pectin [PEC]), was run on an 1.2% (wt/vol) agarose-formaldehyde denaturing gel. Ten micrograms of total RNA extracted from strain IQB485 (Δabn2) grown in the absence of sugars was also analyzed. The RNA ladder used as molecular size markers is indicated. abn2-specific transcripts are shown by an arrow. (B and C) Mapping of the transcriptional start site of the abn2 gene. Radiolabeled oligonucleotide ARA321 (Table 1), complementary to the abn2 sequence was hybridized and used to direct cDNA synthesis from total RNA isolated from exponentially growing cells in the absence (−) or presence of arabinan (ABN). After extension, the products were analyzed by gel electrophoresis, together with a set of dideoxynucleotide chain termination sequencing reactions, by using the same primer and plasmid pZI38 as for the template. Arrows and asterisks indicate the positions of the abn2-specific primer extension products and deduced start sites of transcription, an A residue in the σH-dependent promoter (B) and a G residue in the σA-dependent promoter (C). (D) Promoter region of the abn2 gene. The nucleotide sequence of the abn2 nontranscribed strand is shown in the 5′-to-3′ direction. The transcription start sites (+1 σH) and (+1 σA) defined by primer extension and the −35 and −10 of each promoter are indicated below the nucleotide sequence. The putative ribosome-binding site (rbs) is represented, and the cre sequence is shaded. The single-nucleotide change (C→A) introduced in the site-directed experiment is indicated below the cre abn2 sequence. The predicted primary structure of Abn2 is given in a single-letter code above the nucleotide sequence. Bold indicates position +1, arrows indicate direction of transcription, and underlines indicate −35 and −10 regions.
trans-Acting factors and cis elements involved in abn2 transcription.
Previous work by our group showed that in the absence of the inducer arabinose, AraR, the key regulator of arabinose utilization, negatively controls the expression of the abnA, abfA, and abf2(xsa) genes, encoding other B. subtilis arabinan-degrading enzymes (25). Although abn2′-lacZ expression did not respond to the presence of arabinose (see above), we investigated the role of AraR in the control of abn2 transcription. The level of accumulated β-galactosidase activity in an araR-null mutant background (strain IQB484 [Table 1]) was examined, and the results, compared to those of the wild-type strain, suggested no involvement of AraR in the transcriptional control of the abn2 gene (Table 2). The expression of abn2, however, is subjected to glucose repression (Table 2). In B. subtilis, the master regulator of carbon catabolite repression (CCR) is CcpA, a global regulator that binds to cres (catabolite responsive elements) located upstream of, in the promoter region of, or within the coding regions of target genes. This interaction is modulated by the phosphorylation of HPr or Crh, an HPr-like protein (reviewed in references 5 and 40). Thus, to identify trans-acting factors that participate in the CCR of abn2, we examined the expression of the abn2′-lacZ transcriptional fusion in ccpA, ptsH1, crh, or ptsK mutant backgrounds. The level of accumulated β-galactosidase activity of the resulting strains was determined in the absence or presence of the inducer arabinan and in repressing conditions (arabinan plus glucose). The disruption of the ccpA gene led to an almost complete loss of CCR by glucose of expression from the transcriptional fusion (strain IQB487) (Table 3). The ptsH1 mutation (HPr Ser46 to Ala), which impaired HPr phosphorylation (6), had a small impact on the CCR of abn2′-lacZ expression (strain IQB488) (Table 3) at t2 but showed a more relevant effect at t4. The disruption of the crh gene caused no effect on glucose repression (strain IQB489) (Table 3). In the ptsH1 crh double mutant (strain IQB490) (Table 3), the glucose repression index of abn2′-lacZ expression appeared to be slightly smaller than that observed in the single ptsH1 mutant (strain IQB488) (Table 3), but this effect could be due to the prevention of induction by arabinan rather than to the relief of glucose repression (strain IQB490, Table 3). However, the disruption of the ptsK gene, encoding a bifunctional HPr kinase/phosphorylase, which reversibly phosphorylates HPr and Crh (26), causes a complete loss of glucose repression (strain IQB491) (Table 3). These results indicated that CcpA plays the major role in the CCR (by glucose) of the gene and suggested no or a small contribution by Crh in this phenomenon. Additionally, a potential cre sequence was identified in the promoter region of an abn2 gene, cre abn2 (Fig. 2D). To assess the functionality of this cis element, we introduced a single-base-pair substitution (C→A) that destroyed the central symmetry of cre abn2 (Fig. 2D). The mutant abn2′-lacZ transcriptional fusion was analyzed as described above. Strain IQB492 (Table 3), bearing the mutant cre, displayed at t4 a relief in glucose repression relative to the case for the wild type (strain IQB483), suggesting that cre abn2 is a cis element involved in CCR by glucose of the abn2 gene.
TABLE 3.
Effect of the ccpA, ptsH1, crh, and ptsK mutations on glucose repression of the abn2 gene
| Straina promoter fusion | Time | β-Galactosidase activity (Miller units) under the indicated conditionb
|
Glucose repression indexc | ||
|---|---|---|---|---|---|
| −Abn | +Abn | +Abn +Glc | |||
| IQB483 (WT) | t2 | 1.3 ± 0.2 | 11.0 ± 0.5 | 0.6 ± 0.0 | 17.3 |
| t4 | 1.4 ± 0.1 | 12.7 ± 0.8 | 0.2 ± 0.0 | 44.9 | |
| IQB487 (ccpA) | t2 | 10.8 ± 0.5 | 22.6 ± 4.0 | 8.7 ± 0.4 | 2.6 |
| t4 | 9.6 ± 1.2 | 22.2 ± 1.1 | 9.1 ± 1.1 | 2.4 | |
| IQB488 (ptsH1) | t2 | 0.8 ± 0.0 | 8.9 ± 0.6 | 0.6 ± 0.0 | 15.3 |
| t4 | 1.0 ± 0.1 | 11.3 ± 0.7 | 0.6 ± 0.0 | 18.4 | |
| IQB489 (crh) | t2 | 1.4 ± 0.0 | 14.2 ± 0.5 | 0.6 ± 0.0 | 22.6 |
| t4 | 1.8 ± 0.1 | 13.5 ± 1.0 | 0.3 ± 0.0 | 44.9 | |
| IQB490 (ptsH1 crh) | t2 | 1.4 ± 0.6 | 5.2 ± 0.6 | 1.0 ± 0.0 | 5.0 |
| t4 | 0.8 ± 0.1 | 6.1 ± 0.8 | 0.3 ± 0.0 | 17.9 | |
| IQB491 (ptsK) | t2 | 1.3 ± 0.1 | 16.3 ± 0.3 | 7.9 ± 0.9 | 2.0 |
| t4 | 1.8 ± 0.1 | 12.3 ± 1.3 | 9.6 ± 1.5 | 1.2 | |
| IQB492 (cre mutC→A) | t2 | 1.1 ± 0.1 | 10.6 ± 0.3 | 1.0 ± 0.1 | 10.4 |
| t4 | 1.0 ± 0.1 | 9.2 ± 0.3 | 0.7 ± 0.1 | 13.3 | |
The strains contained abn2′-lacZ fusions and were grown on C minimal medium supplemented with casein hydrolysate in the absence of sugar (−Abn), in the presence of branched arabinan (+Abn), and in the presence of branched arabinan plus glucose (+Abn +Glc). Samples were analyzed 2 h (t2) and 4 h (t4) after the addition of sugars.
The levels of accumulated β-galactosidase activity represent the average ± standard deviations of three independent experiments each performed with duplicate measurements.
The glucose repression index was calculated as the ratio of the level of expression (in Miller units) obtained in the presence of branched arabinan (+Abn) to the value determined in the presence of branched arabinan plus glucose (+Abn +Glc).
Production, purification, and characterization of Abn2.
Abn2 from B. subtilis 168T+ has a potential signal peptide with a type I signal peptidase cleavage site and was shown to be extracellular in B. subtilis strain 168 (1, 39). The deduced molecular mass for the full-length form of B. subtilis 168T+ Abn2 is 52,380 Da (469 amino acids), with a theoretically isoelectric point of 7.37. We cloned the full-length abn2 coding region in the expression vector pET30a(+) (Novagen), which allows the insertion of a His6-tag at the C terminus, under the control of a T7 promoter. The resulting plasmid, pZI39, was introduced into E. coli BL21(DE3) pLysS (36) for protein overproduction. Cells were grown in the presence and absence of the inducer IPTG, and the periplasmatic protein fraction was prepared by osmotic shock as described in Materials and Methods. In the SDS-PAGE analysis, a protein of about 46 kDa was detected in the periplasmic fraction of IPTG-induced cells (Fig. 3A), suggesting that recombinant Abn2 is recognized by the translocation machinery of E. coli. The recombinant enzyme was purified from the periplasmic fraction to more than 98% homogeneity by Ni-nitrilotriacetic acid agarose affinity chromatography (Fig. 3B). The N-terminal amino acid sequence was determined by microsequencing and corresponded to residues 27 to 31 (QKPIF) of the deduced primary sequence, indicating that processing of the Abn2 signal peptide in E. coli is identical to that predicted for signal peptidase I of B. subtilis (1).
FIG. 3.
Production and purification of Abn2. (A) Analysis of the periplasmic protein fraction (10 μl) of induced (+) and noninduced (−) IPTG cultures of E. coli Bl21(DE3) pLysS harboring pET30a (control) and pZI39 (Abn2-His6). (B) Analysis of purified recombinant Abn2 (0.8 μg). The proteins were separated by SDS-PAGE 12.5% gels and stained with Coomassie blue. Abn2-His6 is indicated by an arrowhead. The sizes, in kilodaltons, of the broad range molecular mass markers (Bio-Rad) are indicated.
Biochemical properties of Abn2.
The enzymatic characteristics of purified recombinant Abn2-His6 arabinanase were determined and are summarized in Table 4. Abn2 displayed catalytic properties typical of an endo-α-1,5-l-arabinanase. Specificity was assayed with different substrates, and the enzyme was found to be active toward linear α-1,5-l-arabinan, branched sugar beet arabinan, and pectin from apple, but showed no activity toward larchwood arabinogalactan, wheat arabinoxylan, and pNPAf. The catalytic activity of Abn2 against branched sugar beet arabinan is lower than that observed for linear-α-1,5-l-arabinan. Moreover, the arabinanase was also able to hydrolyze Red debranched arabinan (data not shown), indicating that the enzyme acts in an endo fashion because dye molecules attached to arabinose residues prevent the release of arabinosyl residues from the nonreducing end (21). The effects of pH and temperature on the activity of Abn2 were determined, and the enzyme was most active at pH 7.0 and 50°C. The thermal stability data showed that Abn2 remained fully active after 30 min of preincubation at 50°C; however, after preincubation at 60°C, the residual activity was only 15%. Kinetic studies in the presence of linear α-1,5-l-arabinan as the substrate at optimum temperature and pH allowed the determination of the Michaelis-Menten parameters (Table 4). The enzyme had an apparent Km of 2.0 mg ml−1 and a Vmax of 0.25 mmol min−1 mg−1. The effect of the addition of different metals was not analyzed, but the addition of EDTA did not affect the activity, suggesting that no metals are needed for enzymatic reaction.
TABLE 4.
Activity of Abn2 against arabinose-containing substrates and biochemical propertiesc
| Substrate | Enzymatic activity (U mg−1)a |
|---|---|
| Linear α-1,5-l-arabinan | 72.8 ± 1.2 |
| Sugar beet arabinan (branched) | 51.3 ± 2.4 |
| Pectin (apple) | 10.0 ± 1.6 |
| Larch wood arabinogalactan | NAb |
| Wheat arabinoxylan | NAb |
| p-Nitrophenyl-α-l-arabinofuranoside | NAb |
Activity was assayed by incubating purified recombinant Abn2 with substrates at 37°C, pH 6.6. The data shown are the averages ± standard deviations of three independent experiments, each performed in duplicate measurements.
NA, no detectable activity.
For Abn2 (YxiA), the Mr was 46 kDa, the optimum pH at 37°C was 7.0, and the optimum temperature at pH 6.6 was 50°C. The Km of 2.0 ± 0.24 (mg ml−1) and Vmax of 0.25 ± 0.012 (mmol min−1 mg−1) were determined at 50°C, pH 7.0, by using linear α-1,5-l-arabinan as substrate. These data are the averages ± standard deviations of three independent experiments, each performed in duplicate measurements.
DISCUSSION
B. subtilis participates in the enzymatic hydrolysis of the plant cell walls and synthesizes at least four enzymes, an endoarabinanase (AbnA), two intracellular arabinofuranosidases (AbfA and Abf2[Xsa]), and an extracellular arabinoxylan arabinofuranohydrolase (XynD, not active on arabinan), capable of releasing arabinosyl oligomers and l-arabinose from arabinose-containing polysaccharides (3, 15, 16, 19, 30, 41; J. M. Inácio et al., submitted). In this study, for a complete characterization of an enzymatic system involved in arabinan depolymerization, we report the characterization and functional analysis of a novel endoarabinanase from B. subtilis, Abn2. Abn2 from strain 168T+ displays 99% amino acid identity to YxiA from B. subtilis 168 and is very similar to putative family 43 glycoside hydrolases from different bacteria: YxiA from Bacillus amyloliquefaciens FZB42 (82% identity; NC_009725.1), YxiA from Bacillus licheniformis DSM 13 (71% identity; NC_006270.2), putative beta-xylosidase from Bacillus clausii KSM-K16 13 (62% identity; NC_006582.1), hypothetical protein BH1878 from Bacillus halodurans C-125 (60% identity; NC_002570.2), and a putative protein from Thermotoga petrophila RKU-1 (57% identity; NC_009486.1). Additionally, Abn2 displays 27% identity to AbnA from B. subtilis 168T+, an extracellular endoarabinanase previously characterized by our group (18). Both AbnA and Abn2 are responsible for the majority of extracellular endoarabinanase activity in B. subtilis, because no activity was detected in the supernatant of the ΔabnA Δabn2 double-null mutant in the conditions tested (Fig. 1).
Abn2 overproduced in E. coli was purified from the periplasmic fraction, and N-terminal sequencing confirmed that the recombinant protein was correctly processed by the cellular sorting and translocation machinery of E. coli. Substrate specificity analysis indicated that Abn2 is an endo-α-1,5-l-arabinanase, active toward linear α-1,5-l-arabinan, sugar beet arabinan, and pectin from apple (Table 4). The biochemical properties of Abn2 resemble those of AbnA and other purified endo-α-1,5-l-arabinanases from other bacteria and fungi (19, 27, 38; reviewed in reference 2). The temperature for Abn2 maximum activity (50°C) was lower than that for AbnA (60°C); however, Abn2 appeared to be a more thermostable enzyme at its optimal temperature and in the absence of substrate than AbnA was (19).
Although Abn2 and AbnA have similar biochemical and physical properties, regulation of the corresponding genes is quite distinct. Both genes, abnA and abn2, are monocistronic but the control of gene expression at the transcriptional level is accomplished by different mechanisms. The expression of abnA is driven by a σA-dependent promoter, is induced by arabinose and arabinan, and is strictly dependent on AraR, the key regulator of the arabinose regulon (25). Arabinose is the effector molecule that modulates AraR binding to DNA (23). In this work, we showed that abn2 is transcribed by both σA-dependent and a σH-like promoters (Fig. 2). This situation is not unusual in B. subtilis, where some of the genes that are transcribed by a σH-recognized promoter are also under the control of σA-dependent promoters, such as the fumarase (citG) gene (4, 8, 10). The expression of abn2 is stimulated by arabinan and pectin, but arabinose failed to increase transcription (Table 2). Accordingly, a disruption of the araR gene did not affect expression from a transcriptional abn2′-lacZ fusion, indicating that AraR is not involved in the regulation of abn2 expression. Sugar beet arabinan is a homopolysaccharide mainly composed of l-arabinose (Fig. 4); however, pectin from apple is a heteropolysaccharide constituted mainly of a backbone of α-(1,4)-d-galacturonic acid residues with alternating α-(1,2)-l-rhamnosyl residues, and other sugars attached in side chains, such as, arabinans, d-galactose, and d-xylose (2). Thus, in addition to arabinose, all monosaccharides present in pectin, galacturonic acid, rhamnose, galactose, and xylose were tested as potential inducers, but failed to stimulate abn2′-lacZ expression. These observations lead us to hypothesize that arabinan- and pectin-mediated induction of abn2 expression is controlled by a yet-unidentified regulator (or regulators) which responds to arabino-oligomers, such as arabinobiose, arabinotriose, and/or mixed oligomers, the true inducers. Additionally, abn2 expression is subjected to catabolite repression by glucose. The results obtained here by transcriptional fusion analysis and site-directed mutagenesis identified trans-acting factors and cis-acting elements involved in this phenomenon (Table 3). CcpA is the major regulator of abn2 glucose repression, which acts most probably via binding to at least one cis element, cre abn2, in the promoter region (Fig. 2D). The individual contribution of the coeffectors, HPr and Crh, to the mechanism is not discernible. On one hand, the results suggest that Crh is not involved in glucose repression. On the other hand, the impact of both the ptsH1 mutation (HPr Ser46 to Ala) and the ptsH1 crh double mutant is very small (Table 3). Nevertheless, the inactivation of PtsK, a bifunctional HPr kinase/phosphorylase, which reversibly phosphorylates HPr and Crh (26), caused a complete loss of glucose repression (Table 3). These observations suggest that in the conditions tested (presence of arabinan plus glucose) at least HPr(Ser-P) acts as coeffector in glucose repression. Recently, we showed that CCR by glucose of abnA expression, tested in the presence of arabinose plus glucose, is accomplished by both CcpA-HPr(Ser-P) or CcpA-Crh(Ser-P) complexes (14). In contrast, distinct contributions of HPr and Crh to CCR by glucose of abfA and abf2(xsa) expression were observed, suggesting that HPr dependency occurs during exponential growth and transition phases, while Crh dependency is detected mainly at transition phase (14).
FIG. 4.
Model for the degradation of arabinan by B. subtilis. The homopolysaccharide is degraded by two major extracellular endoarabinanases (GH43), AbnA and Abn2. The resulting products, arabinose, and arabino-oligosaccharides, are transported by different systems. Arabinose enters the cell mainly through the AraE permease (34), and the uptake of arabinose oligomers occurs most likely via AraNPQ, an ABC-type transporter (32). These latter products are further digested by the concerted action of two GH51 intracellular arabinofuranosidases, AbfA and Abf2 (J. M. Inácio et al., submitted). The AraE permease is also responsible for the transport of xylose and galactose into the cell (17). In the absence of arabinose (effector molecule) or arabinan, AraR negatively controls the ara genes, including abnA, abfA, and abf2. The transcriptional control of abn2 is most probably achieved by a yet-unidentified regulator (?), which responds to the presence of arabinan (and pectin) via arabino-oligomers, such as arabinobiose, arabinotriose, and/or mixed oligomers, the potential effector molecules.
Although many polysaccharolytic glycoside hydrolases have been purified from both fungi and bacteria, including several Bacillus spp., information on the regulation at the molecular level of hemicellulolytic genes is limited (7, 24, 35). The results presented here lead to the first full characterization at the molecular level of a bacterial hemycellulolytic enzymatic system devoted to arabinan degradation. These observations, together with previous results, allow us to propose the following model for arabinan depolymerization (Fig. 4). The extracellular homopolysaccharide is attacked by two major GH43 family endoarabinanases, AbnA and Abn2. The resulting products, arabinose and arabinose oligomers, are transported by specific transport systems, namely the AraE permease (arabinose [34]) and AraNPQ, an ABC-type transporter similar to transporters of malto-oligosaccharides and multiple sugars (arabinose and/or arabinose oligomers [32; J. M. Inácio et al., submitted]). Once inside the cell, arabinose oligomers, displaying (1→5), (1→2), and (1→3) linkages, are further catabolized by the concerted action of the two GH51 family α-l-arabinofuranosidases AbfA and Abf2 (J. M. Inácio et al., submitted), releasing arabinose. On one hand, at the level of gene expression, in the absence of arabinose and arabinan, the transcription factor AraR represses and tightly controls the transcription of the genes encoding the two intracellular arabinofuranosidases, abfA and abf2(xsa), and the genes of the specific transporters, araE and araNPQ (23, 25). On the other hand, AraR exerts a more flexible negative regulation on abnA transcription and the expression of the abn2 gene is not under the control of AraR. The transcriptional control of abn2 gene expression is most likely achieved by an unidentified regulator, which responds to the presence of arabinan (and pectin) via arabino-oligomers, such as arabinobiose, arabinotriose, and/or mixed oligomers, the potential effector molecules. Though Abn2 and AbnA display similar biochemical properties and substrate specificities, their presence in B. subtilis seems to be nonredundant. Their distinct regulatory mechanisms of gene expression may represent an adaptative strategy of B. subtilis for optimal degradation of arabino-polysaccharides, which warrants the extracellular presence of active endoarabinanases in response to different environmental signals and/or cellular growth stages.
Acknowledgments
This work was partially supported by grant no. POCI/AGR/60236/2004 from Fundação para a Ciência e Tecnologia (FCT) and FEDER to I.D.S.-N. and by fellowship SFRH/BD/18238/2004 from FCT to J.M.I.
Footnotes
Published ahead of print on 11 April 2008.
REFERENCES
- 1.Antelmann, H., H. Tjalsma, B. Voigt, S. Ohlmeier, S. Bron, J. M. van Dijl, and M. Hecker. 2001. A proteomic view on genome-based signal peptide predictions. Genome Res. 111484-1502. [DOI] [PubMed] [Google Scholar]
- 2.Beldman, G., H. A. Schols, S. M. Pitson, M. J. F. Searl-van Leeuwen, and A. G. Voragen. 1997. Arabinans and arabinan degrading enzymes. Adv. Macromol. Carbohydr. Res. 11-64. [Google Scholar]
- 3.Bourgois, T. M., V. van Craeyveld, S. van Campenhout, C. M. Courtin, J. A. Delcour, J. Robben, and G. Volckaert. 2007. Recombinant expression and characterization of XynD from Bacillus subtilis subsp. subtilis ATCC 6051: a GH 43 arabinoxylan arabinofuranohydrolase. Appl. Microbiol. Biotechnol. 751309-1317. [DOI] [PubMed] [Google Scholar]
- 4.Britton, R. A., P. Eichenberger, J. E. Gonzalez-Pastor, P. Fawcett, R. Monson, R. Losick, and A. D. Grossman. 2002. Genome-wide analysis of the stationary-phase sigma factor (sigma-H) regulon of Bacillus subtilis. J. Bacteriol. 1844881-4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deutscher, J., C. Francke, and P. W. Postma. 2006. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol. Mol. Biol. Rev. 70939-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deutscher, J., J. Reizer, C. Fischer, A. Galinier, M. H. Saier, Jr., and M. Steinmetz. 1994. Loss of protein kinase-catalyzed phosphorylation of HPr, a phosphocarrier protein of the phosphotransferase system, by mutation of the ptsH gene confers catabolite repression resistence to several catabolite genes of Bacillus subtilis. J. Bacteriol. 1763336-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Vries, R. P. 2003. Regulation of Aspergillus genes encoding plant cell wall polysaccharide-degrading enzymes; relevance for industrial production. Appl. Microbial. Biotechnol. 6110-20. [DOI] [PubMed] [Google Scholar]
- 8.Feavers, I. M., V. Price, and A. Moir. 1988. The regulation of the fumarase (citG) gene of Bacillus subtilis 168. Mol. Gen. Genet. 211465-471. [DOI] [PubMed] [Google Scholar]
- 9.Hanson, K. G., K. Steinhauer, J. Reizer, W. Hillen, and J. Stulke. 2002. HPr kinase/phosphatase of Bacillus subtilus: expression of the gene and effects of mutations on enzyme activity, growth and carbon catabolite repression. Microbiology 1481805-1811. [DOI] [PubMed] [Google Scholar]
- 10.Helmann, J. D., and C. P. Moran, Jr. 2002. RNA polymerase and sigma factors, p. 289-312. In A. L. Sonenshein, J. Hoch, and R. Losick (ed.) Bacillus subtilis and its closet relatives: from genes to cells. ASM Press, Washington, DC.
- 11.Henkin, T. M., F. J. Grundy, W. L. Nicholson, and G. H. Chambliss. 1991. Catabolite repression of alpha-amylase gene expression in Bacillus subtilis involves a trans-acting gene product homologous to the Escherichia coli Lacl and GalR repressors. Mol. Microbiol. 5575-584. [DOI] [PubMed] [Google Scholar]
- 12.Igo, M. M., and R. Losick. 1986. Regulation of promoter that is utilized by minor forms of RNA polymerase holoenzyme in Bacillus subtilis. J. Mol. Biol. 191615-624. [DOI] [PubMed] [Google Scholar]
- 13.Inácio, J. M., C. Costa, and I. Sá-Nogueira. 2003. Distinct molecular mechanisms involved in carbon catabolite repression of the arabinose regulon in Bacillus subtilis. Microbiology 1492345-2355. [DOI] [PubMed] [Google Scholar]
- 14.Inácio, J. M., and I. Sá-Nogueira. 2007. trans-Acting and cis elements involved in glucose repression of arabinan degradation in Bacillus subtilis. J. Bacteriol. 1898371-8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaji, A., and T. Saheki. 1975. Endo-arabanase from Bacillus subtilis F-11. Biochim. Biophys. Acta 410354-360. [DOI] [PubMed] [Google Scholar]
- 16.Kaneko, S., M. Sano, and I. Kusakabe. 1994. Purification and some properties of alpha-l-arabinofuranosidase from Bacillus subtilis 3-6. Appl. Environ. Microbiol. 603425-3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krispin, O., and R. Allmansberger. 1998. The Bacillus subtilis AraE protein displays a broad substrate specificity for several different sugars. J. Bacteriol. 1803250-3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunst, F., N. Ogasawara, I. Moszer, A. M. Albertini, G. Alloni, V. Azevedo, et al. 1997. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature 390249-256. [DOI] [PubMed] [Google Scholar]
- 19.Leal, T. F., and I. Sá-Nogueira. 2004. Purification, characterization and functional analyses of an endo-arabinanase (AbnA) from Bacillus subtilis. FEMS Microbiol. Lett. 24141-48. [DOI] [PubMed] [Google Scholar]
- 20.Martin-Verstraete, I., J. Stülke, A. Klier, and G. Rapoport. 1995. Two different mechanisms mediate catabolite repression of the Bacillus subtilis levanase operon. J. Bacteriol. 1776919-6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCleary, B. V. 1988. Novel and selective substrates for the assay of endo-arabinanase, p. 291-300. In G. O. Phillips, D. J. Wedlock, and P. A. Williams (ed.), Gums and stabilisers for the food industry. IRL Press, Oxford, United Kingdom.
- 22.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 23.Mota, L. J., P. Tavares, and I. Sá-Nogueira. 1999. Mode of action of AraR, the key regulator of l-arabinose metabolism in Bacillus subtilis. Mol. Microbiol. 33476-489. [DOI] [PubMed] [Google Scholar]
- 24.Numan, M. T., and N. B. Bhosle. 2006. Alpha-l-arabinofuranosidases: the potential applications in biotechnology. J. Ind. Microbiol. Biotechnol. 33247-260. [DOI] [PubMed] [Google Scholar]
- 25.Raposo, M. P., J. M. Inácio, L. J. Mota, and I. Sá-Nogueira. 2004. Transcriptional regulation of arabinan-degrading genes in Bacillus subtilis. J. Bacteriol. 1861287-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reizer, J., C. Hoischen, F. Titgemeyer, C. Rivolta, R. Rabus, J. Stülke, D. Karamata, M. H. Saier, Jr., and W. Hillen. 1998. A novel protein kinase that controls carbon catabolite repression in bacteria. Mol. Microbiol. 271157-1169. [DOI] [PubMed] [Google Scholar]
- 27.Rombouts, J. M., A. G. J. Voragen, M. J. F. Searle-van Leeuwen, C. C. J. M. Gerards, H. A. Schols, and W. Pilnik. 1988. The arabinases of Aspergillus niger—purification and characterization of two alpha-l-arabinofuranosidases and an endo-1,5-l-arabinanase. Carbohydr. Polym. 925-47. [Google Scholar]
- 28.Saha, B. C. 2000. Alpha-l-arabinofuranosidases: biochemistry, molecular biology and application in biotechnology. Biotechnol. Adv. 18403-423. [DOI] [PubMed] [Google Scholar]
- 29.Saha, B. C. 2003. Hemicellulose bioconversion. J. Ind. Microbiol. Biotechnol. 30279-291. [DOI] [PubMed] [Google Scholar]
- 30.Sakai, T., and T. Sakamoto. 1990. Purification and some properties of a protopectin-solubilizing enzyme that has potent activity on sugar beet protopectin. Agric. Biol. Chem. 54879-889. [Google Scholar]
- 31.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 32.Sá-Nogueira, I., T. V. Nogueira, S. Soares, and H. Lencastre. 1997. The l-arabinose (ara) operon of Bacillus subtilis: nucleotide sequence, genetic organization and expression. Microbiology 143957-969. [DOI] [PubMed] [Google Scholar]
- 33.Sá-Nogueira, I., and L. J. Mota. 1997. Negative regulation of l-arabinose metabolism in Bacillus subtilis: characterization of the araR (araC) gene. J. Bacteriol. 1791598-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sá-Nogueira, I., and S. S. Ramos. 1997. Cloning, functional analysis, and transcriptional regulation of the Bacillus subtilis araE gene involved in l-arabinose utilization. J. Bacteriol. 1797705-7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shallom, D., and Y. Shoham. 2003. Microbial hemicellulases. Curr. Opin. Microbiol. 6219-228. [DOI] [PubMed] [Google Scholar]
- 36.Studier, F. W., A. H. Rosenberg, J. J. Dunn, and J. W. Dubendorff. 1990. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 18560-89. [DOI] [PubMed] [Google Scholar]
- 37.Stülke, J., and W. Hillen. 2000. Regulation of carbon catabolism in Bacillus subtilis. Annu. Rev. Microbiol. 54849-880. [DOI] [PubMed] [Google Scholar]
- 38.Takao, M., K. Akiyama, and T. Sakai. 2002. Purification and characterization of thermostable endo-1,5-alpha-l-arabinase from a strain of Bacillus thermodenitrificans. Appl. Environ. Microbiol. 681639-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tjalsma, H., H. Antelmann, J. D. Jongbloed, P. G. Braun, E. Darmon, R. Dorenbos, J.-Y. F. Dubois, H. Westers, G. Zanen, W. J. Quax, O. P. Kuipers, S. Bron, M. Hecker, and J. M. van Dijl. 2004. Proteomics of protein secretion by Bacillus subtilis: separating the “secrets” of the secretome. Microbiol. Mol. Biol. Rev. 68207-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Warner, J. B., and J. S. Lolkema. 2003. CcpA-dependent carbon catabolite repression in bacteria. Microbiol. Mol. Biol. Rev. 67475-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weinstein, L., and P. Albersheim. 1979. Structure of plant cell walls. IX. Purification and partial characterization of a wall-degrading endo-arabanase and an arabinosidase from Bacillus subtilis. Plant Physiol. 63425-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zilhão, R., M. Serrano, R. Isticato, E. Ricca, C. P. Moran, Jr., and A. O. Henriques. 2004. Interactions among CotB, CotG, and CotH during assembly of the Bacillus subtilis spore coat. J. Bacteriol. 1861110-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]