Abstract
Our understanding of the mechanisms used by Mycobacterium tuberculosis to persist in a “dormant” state is essential to the development of therapies effective in sterilizing tissues. Gene expression profiling in model systems has revealed a complex adaptive response thought to endow M. tuberculosis with the capacity to survive several months of combinatorial antibiotic treatment. We show here that this adaptive response may involve remodeling of the peptidoglycan network by substitution of 4→3 cross-links generated by the d,d-transpeptidase activity of penicillin-binding proteins by 3→3 cross-links generated by a transpeptidase of l,d specificity. A candidate gene, previously shown to be upregulated upon nutrient starvation, was found to encode an l,d-transpeptidase active in the formation of 3→3 cross-links. The enzyme, LdtMt1, was inactivated by carbapenems, a class of β-lactam antibiotics that are poorly hydrolyzed by the M. tuberculosis β-lactamases. LdtMt1 and carbapenems may therefore represent a target and a drug family relevant to the eradication of persistent M. tuberculosis.
In spite of a stable decline in the incidence of tuberculosis in countries participating in control surveys, there were an estimated 8.8 million new cases and 1.6 million deaths in 2005 (28). The treatment of Mycobacterium tuberculosis infections requires at least 6 months of antimycobacterial therapy with the use of multiple drugs. This long duration of treatment is justified by the poor efficacy of available antibiotics, including the main drugs isoniazid and rifampin, against the dormant M. tuberculosis bacilli (10, 26) that are thought to persist in particular environments such as the granuloma or caseum (6, 21). In vitro models that mimic the persistent state have been developed based on nutrient starvation (4, 12), oxygen deprivation (25), and exposure to nitric oxide (23). These models showed that nonreplicative and low metabolic states of the bacteria could be responsible for the poor in vivo response to currently available drugs. The adaptive response of M. tuberculosis during the transition from aerobic growth to stationary phase results in the activation of a “dormancy” regulon (4, 22, 24). The regulon includes genes that are likely to play an essential role in the long-term survival of the bacteria and therefore encode potential targets for the development of sterilizing drugs.
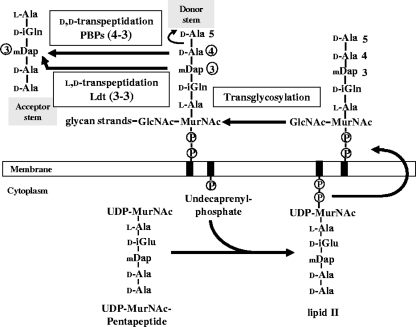
The “dormancy” regulon of M. tuberculosis was not previously reported to include genes involved in peptidoglycan metabolism, although changes in the structure of this cell wall polymer are known to be associated with the transition to the stationary phase in other bacteria. In Escherichia coli, the transition is associated with an increase (1.8 to 5%) in the content of 3→3 cross-links to the detriment of the classical 4→3 cross-links formed by the d,d-transpeptidase activity of penicillin-binding proteins (PBPs) (Fig. 1) (11). We have previously identified the l,d-transpeptidases (Ldt) that catalyze the formation of 3→3 peptidoglycan cross-links as members of a novel family of active-site cysteine peptidases that have various cellular functions (5, 14, 15, 18). In E. coli, these functions include the anchoring of a lipoprotein to the peptidoglycan in addition to the formation of 3→3 cross-links (14, 15). In a mutant of Enterococcus faecium, an l,d-transpeptidase (Ldtfm) is the key enzyme of an adaptive response to β-lactam antibiotics since it bypasses the d,d-transpeptidase activity of PBPs, leading to high-level resistance to the drugs (18, 20).
FIG. 1.
Schematic representation of peptidoglycan synthesis. The nucleotide UDP-MurNAc-pentapeptide is formed in the cytoplasm by sequential addition of l-Ala, d-Glu, meso-diaminopimelic acid (mDap), and the dipeptide d-Ala-d-Ala. The membrane steps of peptidoglycan synthesis involve the transfer of the phospho-MurNAc-pentapeptide moiety of the nucleotide to the lipid carrier (undecaprenyl-phosphate) and the addition of the second sugar (GlcNAc). The complete precursor linked to the lipid carrier (lipid II) is translocated through the membrane and polymerized by glycosyltransferases (formation of glycan strands) and by the d,d-transpeptidase activity of PBPs (formation of the cross-links). These enzymes cleave the d-Ala4-d-Ala5 bond of a pentapeptide donor and link the carbonyl of d-Ala4 to the side chain amine of mDap at the third position of an acceptor stem (4→3 cross-links). β-Lactam antibiotics are structural analogues of the d-Ala4-d-Ala5 extremity of the precursors and act as suicide substrates of the d,d-transpeptidases. l,d-Transpeptidases cleave the mDap3-d-Ala4 bond of a tetrapeptide donor and link the carbonyl of mDap3 to the acceptor stem (3→3 cross-links).
Examination of the microarray data published by Betts et al. showed that an M. tuberculosis gene encoding a member of the active-site cysteine peptidase family, referred to as Rv0116c, of unknown function, was upregulated 17-fold under nutrient starvation (4). We have therefore investigated here the structure of peptidoglycan from M. tuberculosis to evaluate whether formation of 3→3 peptidoglycan cross-links could be part of the adaptive response to the stationary phase. We have also produced a soluble form of the Rv0116c gene product in E. coli to analyze the catalytic activity of the purified protein and its interaction with β-lactam antibiotics.
MATERIALS AND METHODS
Growth conditions, purification, and peptidoglycan structure analysis.
M. tuberculosis H37Rv was grown at 37°C without shaking in Dubos broth (Difco) supplemented with 10% (vol/vol) of OADC medium (Becton Dickinson), which contains oleic acid, bovine serum albumin, fraction V-glucose, and catalase. A 10-day preculture of 25 ml was used to inoculate 250 ml of the growth medium. After 3 weeks of incubation, bacteria were collected by centrifugation, resuspended in 25 ml of 10 mM phosphate buffer (pH 7.0), and inactivated by heat (96°C for 30 min). A second inactivation step was performed by adding 8% (vol/vol) sodium dodecyl sulfate, followed by incubation for 30 min at 96°C. Bacteria were collected by centrifugation and disrupted with glass beads (150 to 212 μm; 5 g/5 ml [wt/vol]) for 16 h at 4°C in a cell disintegrator (The Mickle Laboratory Engineering Co., Gromshall, United Kingdom). The peptidoglycan was collected by centrifugation (15,000 × g for 15 min at 4°C), extracted with 8% boiling sodium dodecyl sulfate, and washed three times with water. The peptidoglycan was treated with proteases and digested with mutanolysin and lysozyme, as previously described for purification of the peptidoglycan from enterococci (2). The resulting muropeptides were treated with ammonium hydroxide to cleave the ether link internal to MurNAc (2) or with sodium borohydride to reduce MurNAc into muramitol (20). Peptidoglycan fragments were purified by reversed-phase high-pressure liquid chromatography (rp-HPLC) and analyzed by mass spectrometry (2).
Production and purification of recombinant LdtMt1.
A portion of the ldtMt1 gene, previously designated Rv0116C (http://www.ncbi.nlm.nih.gov/), was amplified with primers 5′-TTCCATGGCGCCACTCCAACCGATCC-3′ and 5′-TTGGATCCGCCGACCACCTCAATGGGA-3′. The PCR product was digested with NcoI plus BamHI (underlined) and cloned into pET2818 (18). The resulting plasmid encoded a fusion protein consisting of a methionine specified by the ATG initiation codon of pET2818, residues 32 to 251 of LdtMt1, and a C-terminal polyhistidine tag with the sequence GSH6. E. coli BL21(DE3) harboring pREP4GroESL (1) and pET2818ΩldtMt1 was grown at 37°C to an optical density at 600 nm of 0.6 in three liters of brain heart infusion broth containing ampicillin (150 μg/ml). IPTG (isopropyl-β-d-thiogalactopyranoside) was added to a final concentration of 0.5 mM, and incubation was continued for 17 h at 16°C. LdtMt1 was purified from a clarified lysate by affinity chromatography on Ni2+-nitrilotriacetate-agarose resin (Qiagen GmbH, Hilden, Germany), followed by anion-exchange chromatography (MonoQ HR5/5; Amersham Pharmacia Biotech) with an NaCl gradient in 50 mM Tris-HCl (pH 8.5). An additional size-exclusion chromatography was performed on a Superdex HR10/30 column (Amersham Pharmacia Biotech) equilibrated with 50 mM Tris-HCl (pH 7.5) containing 300 mM NaCl at a flow rate of 0.5 ml/min. The protein was concentrated by ultrafiltration (Amicon Ultra-4 centrifugal filter devices; Millipore) and stored at −20°C in the same buffer supplemented with 20% glycerol.
l,d-Transpeptidase assays.
The disaccharide-tetrapeptide containing amidated meso-diaminopimelic acid (GlcNAc-MurNAc-l-Ala1-d-iGln2-mesoDapNH23-d-Ala4) was purified from C. jeikeium strain CIP103337, and the concentration was determined by amino acid analysis after acid hydrolysis (2, 3). In vitro formation of muropeptide dimers was tested in 10 μl of 50 mM Tris-HCl (pH 7.5) containing 300 mM NaCl, 11 μM LdtMt1, and 280 μM disaccharide-tetrapeptide. The reaction mixture was incubated for 2 h at 37°C and treated with ammonium hydroxide, and the resulting lactoyl-peptides were analyzed by nanoelectrospray tandem mass spectrometry using N2 as the collision gas (2).
Inhibition of LdtMt1 by β-lactams.
LdtMt1 (12.5 μM) was preincubated for 20 min at 37°C with ampicillin (Bristol-Myers), ceftriaxone (Roche Applied Science), and imipenem (Merck Sharpe and Dhome-Chibret) in 50 mM Tris-HCl (pH 7.5) containing 300 mM NaCl (buffer A). The l,d-transpeptidation reaction was started by the addition of the disaccharide-tetrapeptide (final concentration 280 μM) and allowed to proceed for 2 h at 37°C. The reaction products were detected by mass spectrometry (19).
The formation of enzyme-drug adducts was tested by incubating LdtMt1 (12.5 μM) with β-lactams (125 μM) for 1 h at 37°C in buffer A. The reaction mixture was dialyzed against water for 30 min, and the average mass of proteins and protein-β-lactam adducts was determined as described previously (19).
RESULTS
Cross-links generated by l,d-transpeptidation are predominant in the peptidoglycan from M. tuberculosis in stationary phase after growth in rich medium.
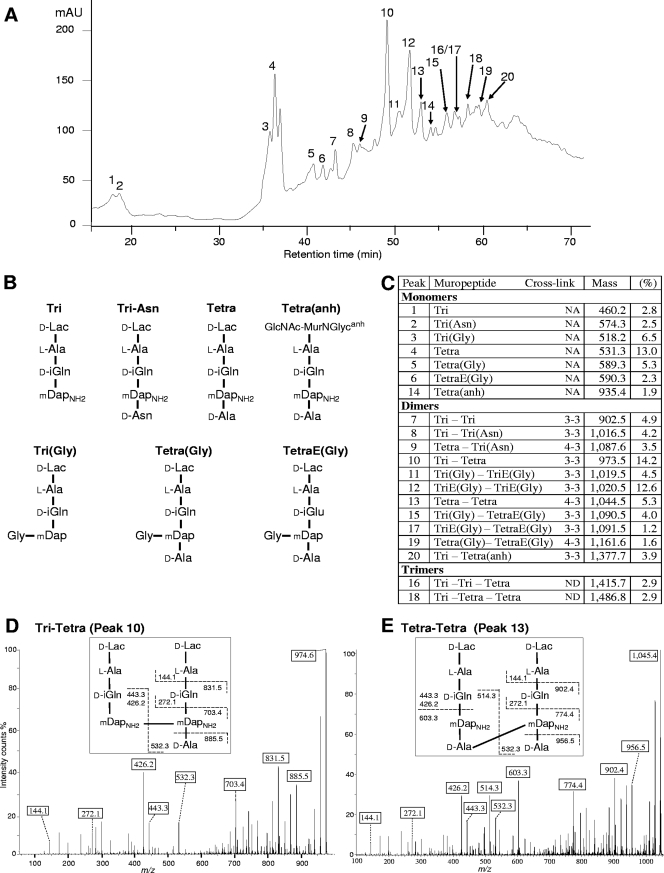
The peptidoglycan of M. tuberculosis H37Rv was analyzed by rp-HPLC (Fig. 2A) and mass spectrometry (Fig. 2B and C) to evaluate the contribution of d,d- and l,d-transpeptidases to the formation of cross-links. Peptidoglycan dimers contained both mDap3→mDap3 cross-links generated by l,d-transpeptidation (Fig. 2D) and d-Ala4→mDap3 cross-links generated by d,d-transpeptidation (Fig. 2E). A comprehensive analysis of the dimers indicated that the majority (80%) of the cross-links were generated by l,d-transpeptidation (Fig. 2 and data not shown). Such a high content in 3→3 cross-links has never been reported in wild-type isolates of eubacteria, revealing that the l,d-transpeptidation reaction is likely to have an essential role in the adaptation of M. tuberculosis to the stationary phase.
FIG. 2.
Structure of the peptidoglycan of M. tuberculosis. (A) rp-HPLC profile of peptidoglycan fragments obtained by digestion of the peptidoglycan of strain H37Rv with muramidases and treatment with ammonium hydroxide. mAU, absorbance unit × 103 at 210 nm. (B) Structure of monomers. d-iGln, d-iso-glutamine; d-iGlu, d-iso-glutamic acid; d-Lac, d-lactate; GlcNAc-MurNGlycanh, N-acetylglucosamine-anhydro-N-glycolylmuramic acid; mDap, meso-diaminopimelic acid; mDapNH2, mDap with an amidated ɛ carboxyl group. (C) Peptidoglycan composition. The relative abundance (%) was calculated by integration of the absorbance. “Mass” refers to observed monoisotopic mass. NA, not applicable; ND, not determined. E, d-iGlu. (D) Sequencing of a dimer generated by l,d-transpeptidation. Tandem mass spectrometry was performed on the [M+H]+ ion at m/z 974.6 (peak 10). Boxes indicate ions generated by cleavage at single peptide bonds. (E) Sequencing of a dimer generated by d,d-transpeptidation (peak 13; [M+H]+ ion at m/z 1,045.4).
High-resolution mass spectrometry analysis of the peptidoglycan of M. tuberculosis H37Rv confirmed several features previously identified in disaccharide-peptide monomers (reference 16 and references cited herein). The stem peptide contained l-Ala at the first position and predominantly d-iGln at the second position, which was occupied to a lesser extent by d-iGlu due to the absence of amidation of the α-carboxylate (Fig. 2B and C). Likewise, the ɛ-carboxylate of mDap at the third position was mostly amidated. Gly residues linked to the ɛ-amine of mDap were also detected and formed cross-bridges in muropeptide dimers. The presence of Gly has been previously reported in the peptidoglycan of M. tuberculosis, but the position of this residue was not determined (16). d-Ala, mostly present at the fourth position, was replaced by Asn, presumably of the D configuration, in a minority of the stem peptides. Since the latter amino acid was abundant in the culture medium, its presence in muropeptides could result from the exchange of d-Ala with d-Asn in the peptidoglycan due to an l,d-transpeptidation reaction, as previously discussed for E. faecium (9, 18).
In order to analyze the sugar moiety of muropeptides, peptidoglycan fragments were reduced with sodium borohydride (data not shown) in place of the ammonium hydroxide treatment. This analysis confirmed the presence of N-glycolylmuramic acid (MurNGlyc) or N-acetylmuramic acid (MurNAc) linked to N-acetylglucosamine or glucosamine (17). Anhydro forms of disaccharide peptides were also detected, indicative of the terminal unit of the glycan chains. These forms were not modified by treatment with ammonium hydroxide (Fig. 2C).
LdtMt1 catalyzes formation of 3→3 peptidoglycan cross-links in vitro.
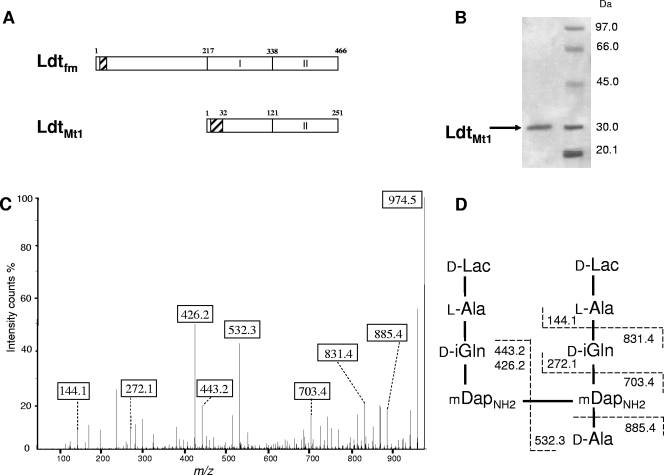
LdtMt1 was identified as a homologue of the l,d-transpeptidase of E. faecium (Fig. 3A) that is overproduced by M. tuberculosis under nutrient starvation (4; see also the introduction). A soluble fragment of LdtMt1 was produced in E. coli, purified (Fig. 3B) and tested for in vitro cross-linking activity using as the substrate the disaccharide-tetrapeptide monomer isolated from the peptidoglycan of Corynebacterium jeikeium, which has the same structure as the predominant monomer of M. tuberculosis (unpublished data). The products of the reaction were treated with ammonium hydroxide to cleave the ether link internal to MurNAc, and the resulting lactoyl-peptides were sequenced by tandem mass spectrometry. The fragmentation pattern (Fig. 3C and D) demonstrated the in vitro formation of mDap3→mDap3 cross-links by LdtMt1. The formation of dimers was not observed with disaccharide-pentapeptide ending in d-Ala-d-Ala (data not shown), indicating that LdtMt1 catalyzes peptidoglycan cross-linking exclusively with tetrapeptide-containing donors, as previously reported for the l,d-transpeptidase from E. faecium (18).
FIG. 3.
Characterization of LdtMt1 from M. tuberculosis. (A) Domain composition of l,d-transpeptidases from E. faecium (Ldtfm) and M. tuberculosis (LdtMt1). Residues 121 to 251 of LdtMt1 are related (29% identity) to the catalytic domain of Ldtfm (domain II, positions 338 to 466). Hatched boxes represent hydrophobic regions that could act as a membrane anchor for Ldtfm and as a signal peptide for LdtMt1. (B) Purification of a soluble fragment of LdtMt1 produced in E. coli. (C) Analysis of a dimer formed in vitro by LdtMt1. Fragmentation was performed on the [M+H]+ ion at m/z 974.5. Boxes indicate ions generated by cleavage at single peptide bonds. (D) Structure of the dimer and inferred fragmentation pattern.
Inactivation of LdtMt1 by formation of adducts with β-lactams.
To investigate inhibition of the l,d-transpeptidase activity of LdtMt1 by β-lactams, the formation of dimers containing mDap3→mDap3 cross-links was tested in the presence of various drug concentrations (data not shown). LdtMt1 was not inhibited by ampicillin up to the highest tested concentration of 5.7 mM. The expanded-spectrum cephalosporin ceftriaxone was active against LdtMt1, although a high drug concentration (360 μM) was required for full inhibition of enzyme activity. In contrast, the carbapenem imipenem abolished formation of mDap3→mDap3 cross-links at a drug to enzyme molar ratio of 5.
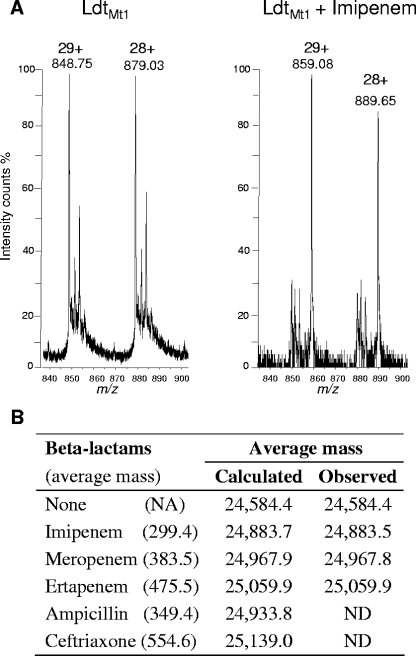
To gain insight into the mechanism of LdtMt1 inhibition by β-lactams, binding of the drugs to the enzyme was tested by electrospray mass spectrometry. Incubation of LdtMt1 with imipenem resulted in the formation of an adduct with an average mass matching the addition of imipenem (Fig. 4). Adducts matching increments of the average mass of other carbapenems, meropenem and ertapenem, were also detected (Fig. 4B). No adduct was detected for ampicillin and ceftriaxone.
FIG. 4.
Formation of adducts between LdtMt1 and β-lactams. (A) LdtMt1 (12.5 μM) was incubated without antibiotic (left) or with 125 μM imipenem (right). Proteins and adducts were detected by electrospray mass spectrometry. Peaks at m/z 848.75 and 879.03 correspond to the [M+29H]29+ and [M+28H]28+ ions of the native protein, respectively (deduced average mass of 24,584.4). Peaks at m/z 859.08 and 889.65 correspond to the [M+29H]29+ and [M+28H]28+ ions of the LdtMt1-imipenem adduct (deduced average mass of 24,883.5). (B) Formation of adducts between LdtMt1 and various β-lactams. NA, not applicable; ND, not detected.
DISCUSSION
The new structure of M. tuberculosis peptidoglycan from a stationary-phase culture reported here revealed an unusually high content (80%) of 3→3 cross-links generated by l,d-transpeptidation (Fig. 1 and 2). These cross-links are likely in participate in the adaptation to the stationary phase since the cross-links are predominantly formed by the d,d-transpeptidase activity of the PBPs during the exponential phase of growth (11, 27). The shift from 4→3 to 3→3 cross-links may have at least two selective advantages. First, l,d-transpeptidases are the only enzymes able to catalyze the formation of new cross-links in the absence of de novo synthesis of precursors since the mature peptidoglycan is devoid of pentapeptide stems required for the d,d-transpeptidation reaction (Fig. 1 and 2) (11). Second, modification of the cross-links may render the peptidoglycan resistant to the hydrolytic activity of endopeptidases.
The adaptative response to the stationary phase involving formation of 3→3 cross-links is likely to result from increased synthesis of LdtMt1 (Rv0116c) since the gene encoding this enzyme is turned on 17-fold during nutrient starvation (4) and we have directly shown that the purified enzyme catalyzes peptidoglycan cross-linking in vitro (Fig. 3). LdtMt1 (Rv0116c) is therefore a potential target to develop drugs against persistent M. tuberculosis. Strikingly, we have shown that the carbapenems act as a suicide substrate of LdtMt1, leading to irreversible inactivation of the enzyme (Fig. 4). Among drugs of this class of β-lactams, imipenem has already been shown to be a poor substrate of the β-lactamases produced by M. tuberculosis (7, 8, 13). Carbapenems are therefore potentially useful adjuvant drugs for the eradication of persistent M. tuberculosis.
Acknowledgments
This study was supported by the Fondation pour la Recherche Médicale (Equipe FRM 2006, DEQ200661107918).
Footnotes
Published ahead of print on 11 April 2008.
REFERENCES
- 1.Amrein, K. E., B. Takacs, M. Stieger, J. Molnos, N. A. Flint, and P. Burn. 1995. Purification and characterization of recombinant human p50csk protein-tyrosine kinase from an Escherichia coli expression system overproducing the bacterial chaperones GroES and GroEL. Proc. Natl. Acad. Sci. USA 921048-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arbeloa, A., J. E. Hugonnet, A. C. Sentilhes, N. Josseaume, L. Dubost, C. Monsempes, D. Blanot, J. P. Brouard, and M. Arthur. 2004. Synthesis of mosaic peptidoglycan cross-bridges by hybrid peptidoglycan assembly pathways in gram-positive bacteria. J. Biol. Chem. 27941546-41556. [DOI] [PubMed] [Google Scholar]
- 3.Auger, G., J. van Heijenoort, D. Mengin-Lecreulx, and D. Blanot. 2003. A MurG assay which utilizes a synthetic analogue of lipid I. FEMS Microbiol. Lett. 219115-119. [DOI] [PubMed] [Google Scholar]
- 4.Betts, J. C., P. T. Lukey, L. C. Robb, R. A. McAdam, and K. Duncan. 2002. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol. Microbiol. 43717-731. [DOI] [PubMed] [Google Scholar]
- 5.Biarrotte-Sorin, S., J. E. Hugonnet, V. Delfosse, J. L. Mainardi, L. Gutmann, M. Arthur, and C. Mayer. 2006. Crystal structure of a novel beta-lactam-insensitive peptidoglycan transpeptidase. J. Mol. Biol. 359533-538. [DOI] [PubMed] [Google Scholar]
- 6.Bishai, W. 2000. Lipid lunch for persistent pathogen. Nature 406683-685. [DOI] [PubMed] [Google Scholar]
- 7.Chambers, H. F., D. Moreau, D. Yajko, C. Miick, C. Wagner, C. Hackbarth, S. Kocagoz, E. Rosenberg, W. K. Hadley, and H. Nikaido. 1995. Can penicillins and other beta-lactam antibiotics be used to treat tuberculosis? Antimicrob. Agents Chemother. 392620-2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chambers, H. F., J. Turner, G. F. Schecter, M. Kawamura, and P. C. Hopewell. 2005. Imipenem for treatment of tuberculosis in mice and humans. Antimicrob. Agents Chemother. 492816-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cremniter, J., J. L. Mainardi, N. Josseaume, J. C. Quincampoix, L. Dubost, J. E. Hugonnet, A. Marie, L. Gutmann, L. B. Rice, and M. Arthur. 2006. Novel mechanism of resistance to glycopeptide antibiotics in Enterococcus faecium. J. Biol. Chem. 28132254-32262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dick, T. 2001. Dormant tubercle bacilli: the key to more effective TB chemotherapy? J. Antimicrob. Chemother. 47117-118. [DOI] [PubMed] [Google Scholar]
- 11.Goffin, C., and J. M. Ghuysen. 2002. Biochemistry and comparative genomics of SxxK superfamily acyltransferases offer a clue to the mycobacterial paradox: presence of penicillin-susceptible target proteins versus lack of efficiency of penicillin as therapeutic agent. Microbiol. Mol. Biol. Rev. 66702-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hampshire, T., S. Soneji, J. Bacon, B. W. James, J. Hinds, K. Laing, R. A. Stabler, P. D. Marsh, and P. D. Butcher. 2004. Stationary phase gene expression of Mycobacterium tuberculosis following a progressive nutrient depletion: a model for persistent organisms? Tuberculosis 84228-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hugonnet, J. E., and J. S. Blanchard. 2007. Irreversible inhibition of the Mycobacterium tuberculosis beta-lactamase by clavulanate. Biochemistry 4611998-12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magnet, S., A. Arbeloa, J. L. Mainardi, J. E. Hugonnet, M. Fourgeaud, L. Dubost, A. Marie, V. Delfosse, C. Mayer, L. B. Rice, and M. Arthur. 2007. Specificity of l,d-transpeptidases from gram-positive bacteria producing different peptidoglycan chemotypes. J. Biol. Chem. 28213151-13159. [DOI] [PubMed] [Google Scholar]
- 15.Magnet, S., S. Bellais, L. Dubost, M. Fourgeaud, J. L. Mainardi, S. Petit-Frere, A. Marie, D. Mengin-Lecreulx, M. Arthur, and L. Gutmann. 2007. Identification of the l,d-transpeptidases responsible for attachment of the Braun lipoprotein to Escherichia coli peptidoglycan. J. Bacteriol. 1893927-3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahapatra, S., D. C. Crick, M. R. McNeil, and P. J. Brennan. 2008. Unique structural features of the peptidoglycan of Mycobacterium leprae. J. Bacteriol. 190655-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahapatra, S., H. Scherman, P. J. Brennan, and D. C. Crick. 2005. N glycosylation of the nucleotide precursors of peptidoglycan biosynthesis of Mycobacterium spp. is altered by drug treatment. J. Bacteriol. 1872341-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mainardi, J. L., M. Fourgeaud, J. E. Hugonnet, L. Dubost, J. P. Brouard, J. Ouazzani, L. B. Rice, L. Gutmann, and M. Arthur. 2005. A novel peptidoglycan cross-linking enzyme for a beta-lactam-resistant transpeptidation pathway. J. Biol. Chem. 28038146-38152. [DOI] [PubMed] [Google Scholar]
- 19.Mainardi, J. L., J. E. Hugonnet, F. Rusconi, M. Fourgeaud, L. Dubost, A. N. Moumi, V. Delfosse, C. Mayer, L. Gutmann, L. B. Rice, and M. Arthur. 2007. Unexpected inhibition of peptidoglycan l,d-transpeptidase from Enterococcus faecium by the beta-lactam imipenem. J. Biol. Chem. 28230414-30422. [DOI] [PubMed] [Google Scholar]
- 20.Mainardi, J. L., R. Legrand, M. Arthur, B. Schoot, J. van Heijenoort, and L. Gutmann. 2000. Novel mechanism of beta-lactam resistance due to bypass of d,d-transpeptidation in Enterococcus faecium. J. Biol. Chem. 27516490-16496. [DOI] [PubMed] [Google Scholar]
- 21.Manabe, Y. C., and W. R. Bishai. 2000. Latent Mycobacterium tuberculosis—persistence, patience, and winning by waiting. Nat. Med. 61327-1329. [DOI] [PubMed] [Google Scholar]
- 22.Sherman, D. R., M. Voskuil, D. Schnappinger, R. Liao, M. I. Harrell, and G. K. Schoolnik. 2001. Regulation of the Mycobacterium tuberculosis hypoxic response gene encoding alpha-crystallin. Proc. Natl. Acad. Sci. USA 987534-7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voskuil, M. I., D. Schnappinger, K. C. Visconti, M. I. Harrell, G. M. Dolganov, D. R. Sherman, and G. K. Schoolnik. 2003. Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J. Exp. Med. 198705-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Voskuil, M. I., K. C. Visconti, and G. K. Schoolnik. 2004. Mycobacterium tuberculosis gene expression during adaptation to stationary phase and low-oxygen dormancy. Tuberculosis 84218-227. [DOI] [PubMed] [Google Scholar]
- 25.Wayne, L. G., and L. G. Hayes. 1996. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect. Immun. 642062-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wayne, L. G., and C. D. Sohaskey. 2001. Nonreplicating persistence of Mycobacterium tuberculosis. Annu. Rev. Microbiol. 55139-163. [DOI] [PubMed] [Google Scholar]
- 27.Wietzerbin, J., B. C. Das, J. F. Petit, E. Lederer, M. Leyh-Bouille, and J. M. Ghuysen. 1974. Occurrence of d-alanyl-(d)-meso-diaminopimelic acid and meso-diaminopimelyl-meso-diaminopimelic acid interpeptide linkages in the peptidoglycan of mycobacteria. Biochemistry 133471-3476. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization. 2007. Tuberculosis control: surveillance, planning, and financing. Document WHO/HTM/TB/2007.376. World Health Organization, Geneva, Switzerland.