Abstract
Streptococcus cristatus ArcA inhibits production of a major adhesin, FimA, in Porphyromonas gingivalis, a primary periodontal pathogen. In this study, we demonstrate the differential expression of arcA in two streptococcal species. The expression level of arcA in streptococci appears to be controlled by both cis and trans elements.
Human dental plaque is a multispecies microbial biofilm that is associated with two common oral diseases: dental caries and periodontal diseases. A characteristic feature of dental plaque development is bacterial cell-cell communication. While interspecies communication in oral microbial communities is frequently based on autoinducer 2 (AI-2) (2, 7, 11, 12), we have recently identified arginine deiminase (ArcA) of Streptococcus cristatus CC5A as critical for intergeneric communication between S. cristatus and Porphyromonas gingivalis, a periodontal pathogen involved in several forms of periodontitis (16). ArcA selectively represses expression of the fimA gene that encodes the major subunit protein of the P. gingivalis long fimbriae, a key virulence factor responsible for colonization and invasion of the organism (6). As a result of ArcA activity, P. gingivalis is unable to form biofilm microcolonies in the presence of S. cristatus (15). Hence, the expression and production of arginine deiminase in S. cristatus can be predicted to play an important role in regulating the development of potentially pathogenic microbial biofilms in the oral cavity. Arginine deiminase is found in many bacteria, including other oral streptococci that are common constituents of oral biofilms (1). The enzyme is involved in the arginine metabolism pathway that converts arginine to ornithine, ammonia, and CO2 (3). Alkali generation in oral biofilms by streptococci is thought to have an important role in neutralizing bacterial organic acids that cause enamel demineralization. Hence, the production of ArcA may protect against dental caries (1). We have shown that arginine deiminase repression of fimA expression in P. gingivalis does not depend on its enzymatic activity (16). Arginine deiminase inhibitors, such as aminoguanidine and l-lysine, that completely inhibit the hydrolytic activity of arginine deiminase, have little effect on the inhibitory activity on fimA expression in P. gingivalis. Furthermore, although many oral streptococci possess arcA, significant inhibitory effects on fimA expression are only observed in S. cristatus (15). Therefore, we hypothesize that the arcA gene is differentially expressed among oral streptococcal strains and that a higher-level expression of arcA in S. cristatus may contribute to the ability of the organism to prevent P. gingivalis biofilm formation and ultimately impact colonization in the oral cavity.
Characterization of the arcA gene of S. cristatus.
Based on our observation that S. cristatus, but not other common oral streptococci, including S. gordonii, can significantly inhibit expression of P. gingivalis fimA (15), we postulated that the arcA gene may either be divergent or differentially expressed among oral streptococci (Table 1). To test this hypothesis, The DNA sequence of CC5A arcA gene was compared to the arcA gene of S. gordonii DL1, a predominant early colonizer of dental plaque. Unlike S. cristatus CC5A, S. gordonii supports and promotes P. gingivalis biofilm formation (8), a development process initiated by the interaction of the P. gingivalis FimA long fimbrial subunit protein with glyceraldehyde 3-phosphate dehydrogenase on the streptococcal surface (10). Alignment of the arcA gene of S. cristatus CC5A and S. gordonii DL1 (AF534569) (5) with BLAST showed 79.4% identity (367 of 1,780) (Fig. 1). However, more variation was detected in upstream regulatory regions (+1 to −126) of the arcA gene, which had only 69% identity between CC5A and DL1 arcA genes. The alignment of the deduced ArcA protein sequences revealed that the identity is 93% (383 of 411) between CC5A and DL1 (data not shown). The variation between the sequences is scattered throughout the protein as amino acid substutions, and there is no hypervariable region.
TABLE 1.
Oligonucleotide primers for arcA gene
| Primer | Sequence (5′-3′)a | Application |
|---|---|---|
| CC5A-arcA-pThioF | GCGGTACCTATGTCTACACATCCAATTC (KpnI) | For amplification of CC5A arcA codon region |
| CC5A-arcA-pThioR | GCGAGCTCTTAAACTTCTTCACGTTC (SacI) | For amplification of CC5A arcA codon region |
| CARCA | GCGGTACCGCTTACGGAATGTCGTCTCA (KpnI) | For amplification of CC5A arcA promoter-codon region |
| ARCAR | GCCCATGGAGGTATTCTAACTCTGCACG (NcoI) | For amplification of CC5A arcA promoter-codon region |
| DARCA | GCGGTACCTCAGCTATGAGCACAAACAG (KpnI) | For amplification of DL1 arcA promoter-codon region |
| TSR1 | GAAGACGTTCTAGGTAGTCCGGTA | For arcA transcript start site |
| TSR2 | GGAGAAATCAAGGATTCAGCAG | For arcA transcript start site |
| arcA186 F | TCCAATGCCAAACCTTTACT | For real-time-PCR of arcA |
| arcA186 R | ATACGAGTATCTTCTTCACG | For real-time-PCR of arcA |
| arcR268F | GACGCTATGTTTATCAAATA | For real-time-PCR of arcR |
| arcR268R | GAGGGCATCTTCCATATA | For real-time-PCR of arcR |
| ccpA168F | TATCGTCCTAATGCAGT | For real-time-PCR of ccpA |
| ccpA168R | ATCTTCATCGCTGTTTGC | For real-time-PCR of ccpA |
The restriction site for the enzyme in parentheses is indicated by underscoring.
FIG. 1.
Sequence alignment of promoter region of S. cristatus CC5A arcA gene (GenBank accession no. 884757) with S. gordonii DL1 arcA (GenBank accession no. AF534569). The boldfaced ATG is a potential start codon. TSS A (+1) of CC5A arcA is in the same position as the published TSS of S. gordonii DL1 (17). The transcriptional start sites, the potential −10 and −35 positions, and the potential start codons are in boldface. The potential catabolite response elements are shaded.
In order to compare the cis-acting elements of the arcA gene between S. cristatus CC5A and S. gordonii DL1, we first determined the transcriptional start site of the CC5A arcA gene by using FirstChoice RLM-RACE kit as described previously (13). We identified the transcriptional start site (TSS) of CC5A arcA at a location 50 bp upstream of the potential start codon (Fig. 1). This is at the same position in S. gordonii DL1 arcA but consists of a different nucleotide base. Transcription of CC5A arcA starts with adenine (A), whereas transcription of DL1 arcA starts with guanine (G) (17). The putative −10 sequences (TAGAAT) are conserved in the two strains; however, the first two bases of the −35 sequence (AGGTGT) of CC5A arcA are different from the DL1 −35 sequence (TTGTGT). Interestingly, there are also variations in two potential catabolite response elements (CREs), which are known to be important cis elements in the repression of the arcA gene (17). There is one base differing in the upstream CRE from positions −22 to −36 and four bases differing in the downstream CRE from positions −106 to −120 between CC5A and DL1. Promoter activity of arcA in S. gordonii DL1 carrying mutations in the CRE sites was much higher compared to that of the wild-type strain (17). Noticeably, the spacer between two CREs in CC5A is one base longer, and the spacer between the −10 sequence and the TSS in CC5A is one base shorter than in DL1. These different distances may be critical for interaction of the arcA promoter and RNA polymerase or regulatory proteins. Therefore, the sequence variations of the promoter region may contribute to the differential expression of arcA in S. cristatus, which would provide an explanation for the higher inhibitory activity for fimA expression found in S. cristatus.
Expression and function of ArcA in S. cristatus versus S. gordonii.
To compare the activities of the arcA promoter regions of S. cristatus and S. gordonii, arcA mutants of S. cristatus (S. cristatus ARCAE) and S. gordonii (S. gordonii ARCAE) were constructed by using ligation-independent cloning of PCR-mediated mutagenesis as described before (16). arcA complemented strains of S. cristatus ARCAE and S. gordonii ARCAE were then created by using an E. coli-streptococcus shuttle vector (pTet) (16). The coding region of CC5A arcA, along with 382 bp of upstream sequence from the potential start codon, was amplified by PCR with the primers CARCA and ARCAR. The coding region of DL1 arcA, along with 330 bp of upstream sequence from the potential start codon, was amplified by PCR with the primers DARCA and ARCAR. The PCR products were cloned into pTet vector. The recombinant plasmids were introduced by transformation into the arcA-deficient mutant, S. cristatus ARCAE or S. gordonii ARCAE. After transformation, erythromycin- and tetracycline-resistant transconjugants were selected, and plasmid identity was confirmed by PCR analysis. The following strain designations were made: the CC5A arcA complemented S. cristatus ARCAE as S. cristatus cARCAE, the DL1 arcA complemented S. cristatus ARCAE as S. cristatus dARCAE, the CC5A arcA complemented S. gordonii ARCAE as S. gordonii cARCAE, and the DL1 arcA complemented of S. gordonii ARCAE as S. gordonii dARCAE (Table 2).
TABLE 2.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| Strains | ||
| S. cristatus CC5A | Wild type | Lab collection |
| S. gordonii DL1 | Wild type | Lab collection |
| S. cristatus ARCE | Derivative of S. cristatus CC5A containing an insertional mutation in the arcA gene, Emr | 16 |
| S. gordonii ARCE | Derivative of S. gordonii DL1 containing an insertional mutation in the arcA gene, Emr | This study |
| S. cristatus cARCE | Complemented strain of S. cristatus ARCE with the CC5A arcA gene, Tetr Emr. | This study |
| S. cristatus dARCE | Complemented strain of S. cristatus ARCE with the DL1 arcA gene, Tetr Emr | This study |
| S. gordonii dARCE | Complemented strain of S. gordonii ARCE with the DL1 arcA gene, Tetr Emr | This study |
| S. gordonii cARCE | Complemented strain of S. gordonii ARCE with the CC5A arcA gene, Tetr Emr | This study |
| P. gingivalis 33277 | Type strain from the ATCC | Lab collection |
| P. gingivalis UPF | Derivative of P. gingivalis 33277 containing fimA::lacZ gene fusion in its chromosomal DNA, Emr | 14 |
| E. coli DH5α | F− φ80dlacZΔ(lacZYA-argF)U169 endA1 supE44 recA1 relA1 | BRL |
| Plasmids | ||
| pVA3000 | Suicide vector for Bacteroides; Emr, 5.3 kb | 9 |
| pTet | Shuttle plasmid with tetracycline resistance that replicates both in E. coli and in streptococci | This study |
| pCRII-TOTO | Linearized plasmid with single 3′ dT residues; Kmr Amr. | Invitrogen |
| pTOTO-ARCA | pCRII-TOTO carrying arcA gene of CC5A (2,030 bp). | This study |
| pTet-cARCA | pTet plasmid carrying the CC5A arcA gene (1,928 bp), Tetr | This study |
| pTet-dARCA | pTet plasmid carrying the DL1 arcA gene, Tetr | This study |
Kmr, Tetr, Emr, and Amr indicate resistance to kanamycin, tetracycline, erythromycin, and ampicillin, respectively.
The expression levels of the arcA gene in wild-type CC5A and DL1 and the complemented strains were compared by using real-time PCR analysis. Streptococcal strains were grown to late exponential phase (optical density at 600 nm, 1.0 to 1.2) in 4 ml of Trypticase-peptone broth. Bacteria were harvested by centrifugation at 10,000 rpm, and resuspended in 300 μl of distilled H2O and 900 μl of TRIzol (Invitrogen). The cells were disrupted by using Mini-Beadbeater 3110BX (BioSpec Products, Inc.). RNA was extracted with chloroform and precipitated with ethanol. Gene expression was measured by using a QuantiTect Sybr Green RT-PCR kit (Qiagen) and the iCycler iQ real-time detection system (Bio-Rad Laboratories, Inc.) as described previously (13). A significant difference in expression of the arcA gene between the wild-type strains, S. cristatus CC5A, and S. gordonii DL1 was observed. The expression of the arcA gene was 13-fold greater in CC5A than in DL1 (Table 3). Introduction of the CC5A arcA or DL1 arcA into the S. cristatus ARCAE restored the expression level of arcA, but with different efficiency. Thus, the mRNA level of the CC5A arcA gene was sixfold higher than that of the DL1 arcA gene in S. cristatus. Similar results were observed when S. gordonii ARCAE was complemented with the CC5A arcA gene versus the DL1 arcA gene, suggesting that the cis-controlling element plays a critical role in the regulation of arcA expression. Moreover, trans elements appear to also contribute to expression of arcA, since higher expression of arcA was observed in the S. cristatus arcA mutant complemented with CC5A arcA than in the S. gordonii arcA mutant complemented with CC5A arcA (Table 3).
TABLE 3.
Quantification of arcA expressiona
| Strain | Mean expression of arcA ± SD |
|---|---|
| S. cristatus CC5A | 20.24 ± 1.76 |
| S. gordonii DL1 | 1.45 ± 0.43 |
| S. cristatus ARCAE | 0.005 ± 0.001 |
| S. gordonii ARCAE | 0.002 ± 0.00 |
| S. cristatus cARCAE | 26.27 ± 1.18 |
| S. cristatus dARCAE | 4.05 ± 1.36 |
| S. gordonii dARCAE | 0.67 ± 0.01 |
| S. gordonii cARCAE | 2.20 ± 0.14 |
Transcript levels were measured by real-time PCR, and the relative expression of arcA was normalized by using 23S rRNA. The results shown are means from four independent experiments.
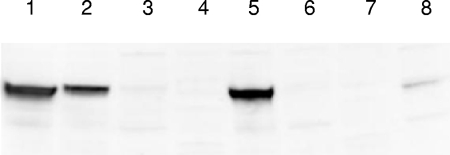
We also examined production of the arcA gene product in these streptococcal strains by using Western blot analysis. As shown in Fig. 2, ArcA production was consistent with the expression of arcA mRNA in CC5A versus DL1. The level of ArcA protein in S. cristatus CC5A was higher compared to that in S. gordonii DL1. Interestingly, the ArcA protein was not detected in the S. cristatus arcA mutant complemented with the DL1 arcA gene, despite the fact that a higher mRNA level was found in S. cristatus dARCAE than in S. gordonii DL1. This finding suggests that the cis-controlling element of arcA gene may also be involved in species specific posttranscriptional regulation.
FIG. 2.
Western blot analysis of ArcA production with polyclonal antibodies to the CC5A ArcA. Shown are 14-h cultures of streptococcal strains grown in Trypticase peptone broth. Cell extracts (5 μg) from streptococcal cells were used. Lane 1, S. cristatus CC5A; lane 2, S. gordonii DL1; lane 3, S. cristatus ARCE (arcA mutant); lane 4, S. gordonii ARCE (arcA mutant); lane 5, S. cristatus cARCE (S. cristatus ARCE complemented with CC5A arcA gene); lane 6, S. cristatus dARCE (S. cristatus ARCE complemented with DL1 arcA gene); lane 7, S. gordonii dARCE (S. gordonii ARCE complemented with DL1 arcA gene); lane 8, S. gordonii cARCE (S. gordonii ARCE complemented with CC5A arcA gene).
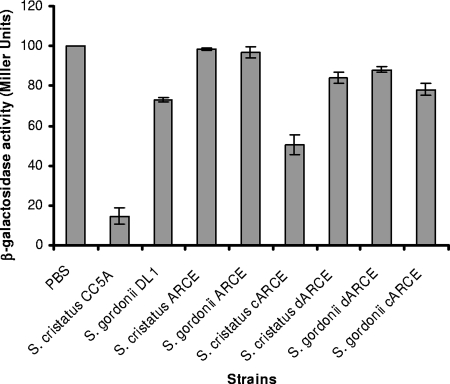
To assess the communication-dependent properties of ArcA, the engineered strains were examined for their ability to repress expression of fimA in P. gingivalis by using a β-galactosidase assay (15). Streptococcal extracts (50 μg) were mixed with 105 cells of P. gingivalis UPF that contains a chromosomal fimA promoter-lacZ reporter construct and spotted onto a Trypticase soy broth blood agar plate for 48 h. Expression of the lacZ gene under control of the fimA promoter was measured by the standard spectrophotometric β-galactosidase assay with ONPG (o-nitrophenyl-β-d-galactopyranoside) as the substrate, as described previously (14). The CC5A extract decreased expression of β-galactosidase in P. gingivalis 10-fold (Fig. 3). A 50% downregulation of fimA expression in P. gingivalis was observed in the presence of the extract from the CC5A arcA complemented strain of S. cristatus. However, ArcA activity was not rescued in S. cristatus ARCE by the DL1 arcA, neither in S. gordonii ARCE by the CC5A arcA and the DL1 arcA. Only a 20% decrease in fimA was detected when P. gingivalis was grown in the presence of S. gordonii DL1 extract (Fig. 3). These data corroborate the protein expression data and support the concept that both cis and trans elements contribute to regulation expression of arcA in oral streptococci.
FIG. 3.
Determination of the expression level of fimA in P. gingivalis in response to streptococcal strains. P. gingivalis UPF carrying a fimA promoter-lacZ fusion gene was tested for β-galactosidase activity in the presence or absence of the surface extracts (50 μg) isolated from the S. cristatus or S. gordonii strains. Standard errors are indicated (n = 3). Means with different letters are significantly different (P < 0.05; two-way analysis of variance and Student Newman-Keuls test), and means with the same letter are not significantly different.
Differential expression of arcA regulatory proteins.
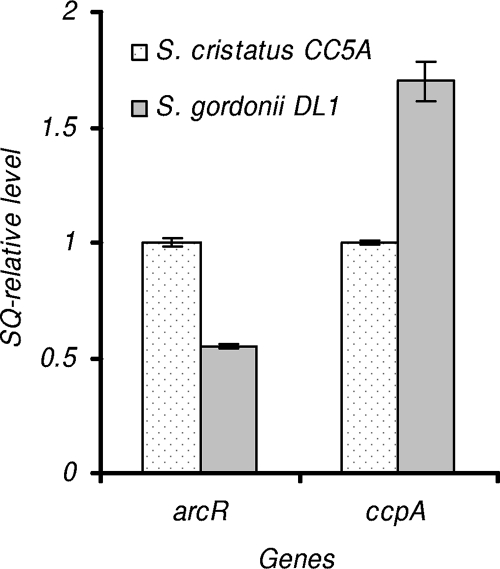
One possible mechanism for the differential expression of arcA in S. cristatus versus S. gordonii is differential expression levels of regulatory proteins that control arcA expression. At least two regulatory proteins are known to be involved in regulation of arcA expression in S. gordonii DL1. One is ArcR, an activator of arcA gene. The arcR gene belongs to the arc operon, and an ArcR-deficient derivative exhibits a lower level of arcA expression than does the wild-type strain (5). A 27-bp region spinning from positions −122 to −96 of arcA appears to be ArcR binding site in S. gordonii DL1 (17). Unlike ArcR that activates arcA expression, catabolite control protein A (CcpA) acts as repressor of arcA expression. Expression of arcA in S. gordonii ccpA deficient was significantly increased when the bacteria were grown with glucose (4). ArcA repression is mediated by CcpA binding to one or both CREs (17). We compared expression levels of arcR and ccpA in S. cristatus CC5A and S. gordonii DL1 by using real-time PCR. Interestingly, the expression levels of arcR and ccpA were significantly distinct in these two strains. Expression of arcR was about twofold higher in CC5A than that in DL1. In contrast, ccpA expression is obviously lower in CC5A, and as much as 70% higher expression of ccpA was observed in DL1 (Fig. 4). These results demonstrate a correlation between the expression of arcA and that of its activator (ArcR) and repressor (CcpA).
FIG. 4.
Expression of arcR and ccpA in S. cristatus CC5A and S. gordonii DL1. Gene expression analysis was examined by using real-time PCR. The transcript level of each gene in S. gordonii DL1 is indicated relative to the expression level of these genes in S. cristatus CC5A as 1 U. The relative abundance of each indicated transcript was also normalized by 23S rRNA. The results presented are the average starting quantities of four independent experiments.
In conclusion, the arcA gene encoding arginine deiminase is a key element in inhibiting production of the P. gingivalis FimA protein, which is the major subunit of the long fimbrial adhesin of the organism. Our earlier study indicated that the developmental pathway of P. gingivalis biofilms may also include two steps, from a monolayer to microcolonies (13). The FimA protein is required for the initial adherence, and the role of minor (short) fimbriae is in the development of the biofilm structure. The enhanced ability of S. cristatus, in comparison to other oral streptococci, in the downregulation of FimA and in the prevention of P. gingivalis biofilm formation, is related to the elevated expression of arcA due to differences in the cis elements of arcA and in the expression of regulatory proteins of arcA. Our findings provide a mechanistic basis for the differing roles of S. cristatus and S. gordonii in the highly orchestrated development of the dental plaque biofilm. Inhibition of FimA production in P. gingivalis requires a higher expression of arcA, which is observed in S. cristatus CC5A. S. cristatus, therefore, does not support the development of a potentially pathogenic biofilm. In contrast, the limited production of ArcA in S. gordonii allows P. gingivalis to make contact through its long fimbrial adhesins. In combination with other adhesins such as Ssp, and other signaling molecules such as AI-2, S. gordonii thus functions as a “recruiter” to attract P. gingivalis. Understanding the molecular basis of the expression of arginine deiminase in streptococci in greater detail may provide new opportunities for controlling P. gingivalis-associated periodontitis.
Acknowledgments
This study was supported by Public Health Service grants DE014699 (H.X.) and DE12505 (R.J.L.) from the National Institute of Dental and Craniofacial Research.
Footnotes
Published ahead of print on 11 April 2008.
REFERENCES
- 1.Burne, R. A., and R. E. Marquis. 2000. Alkali production by oral bacteria and protection against dental caries. FEMS Microbiol. Lett. 1931-6. [DOI] [PubMed] [Google Scholar]
- 2.Chung, W. O., Y. Park, R. J. Lamont, R. McNab, B. Barbieri, and D. R. Demuth. 2001. Signaling system in Porphyromonas gingivalis based on a LuxS protein. J. Bacteriol. 1833903-3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cunin, R., N. Glansdorff, A. Pierard, and V. Stalon. 1986. Biosynthesis and metabolism of arginine in bacteria. Microbiol. Rev. 50314-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong, Y., Y. Y. Chen, and R. A. Burne. 2004. Control of expression of the arginine deiminase operon of Streptococcus gordonii by CcpA and Flp. J. Bacteriol. 1862511-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong, Y., Y. Y. Chen, J. A. Snyder, and R. A. Burne. 2002. Isolation and molecular analysis of the gene cluster for the arginine deiminase system from Streptococcus gordonii DL1. Appl. Environ. Microbiol. 685549-5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goulbourne, P. A., and R. P. Ellen. 1991. Evidence that Porphyromonas (Bacteroides) gingivalis fimbriae function in adhesion to Actinomyces viscosus. J. Bacteriol. 1735266-5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.James, D., H. Shao, R. J. Lamont, and D. R. Demuth. 2006. The Actinobacillus actinomycetemcomitans ribose binding protein RbsB interacts with cognate and heterologous autoinducer 2 signals. Infect. Immun. 744021-4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuboniwa, M., G. D. Tribble, C. E. James, A. O. Kilic, L. Tao, M. C. Herzberg, S. Shizukuishi, and R. J. Lamont. 2006. Streptococcus gordonii utilizes several distinct gene functions to recruit Porphyromonas gingivalis into a mixed community. Mol. Microbiol. 60121-139. [DOI] [PubMed] [Google Scholar]
- 9.Lee, S. W., J. D. Hillman, and A. Progulske-Fox. 1996. The hemagglutinin genes hagB and hagC of Porphyromonas gingivalis are transcribed in vivo as shown by use of a new expression vector. Infect. Immun. 644802-4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maeda, K., H. Nagata, A. Nonaka, K. Kataoka, M. Tanaka, and S. Shizukuishi. 2004. Oral streptococcal glyceraldehyde-3-phosphate dehydrogenase mediates interaction with Porphyromonas gingivalis fimbriae. Microbes Infect. 61163-1170. [DOI] [PubMed] [Google Scholar]
- 11.Merritt, J., J. Kreth, W. Shi, and F. Qi. 2005. LuxS controls bacteriocin production in Streptococcus mutans through a novel regulatory component. Mol. Microbiol. 57960-969. [DOI] [PubMed] [Google Scholar]
- 12.Rickard, A. H., R. J. Palmer, Jr., D. S. Blehert, S. R. Campagna, M. F. Semmelhack, P. G. Egland, B. L. Bassler, and P. E. Kolenbrander. 2006. Autoinducer 2: a concentration-dependent signal for mutualistic bacterial biofilm growth. Mol. Microbiol. 601446-1456. [DOI] [PubMed] [Google Scholar]
- 13.Wu, J., X. Lin, and H. Xie. 2007. Porphyromonas gingivalis short fimbriae are regulated by a FimS/FimR two-component system. FEMS Microbiol. Lett. 271214-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie, H., S. Cai, and R. J. Lamont. 1997. Environmental regulation of fimbrial gene expression in Porphyromonas gingivalis. Infect. Immun. 652265-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie, H., G. S. Cook, J. W. Costerton, G. Bruce, T. M. Rose, and R. J. Lamont. 2000. Intergeneric communication in dental plaque biofilms. J. Bacteriol. 1827067-7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie, H., X. Lin, B. Y. Wang, J. Wu, and R. J. Lamont. 2007. Identification of a signalling molecule involved in bacterial intergeneric communication. Microbiology 1533228-3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng, L., Y. Dong, and R. A. Burne. 2006. Characterization of cis-acting sites controlling arginine deiminase gene expression in Streptococcus gordonii. J. Bacteriol. 188941-949. [DOI] [PMC free article] [PubMed] [Google Scholar]