Abstract
Arabinan polymers are major components of the cell wall in Mycobacterium tuberculosis and are involved in maintaining its structure, as well as playing a role in host-pathogen interactions. In particular, lipoarabinomannan (LAM) has multiple immunomodulatory effects. In the nonpathogenic species Mycobacterium smegmatis, EmbC has been identified as a key arabinosyltransferase involved in the incorporation of arabinose into LAM, and an embC mutant is viable but lacks LAM. In contrast, we demonstrate here that in M. tuberculosis, embC is an essential gene under normal growth conditions, suggesting a more crucial role for LAM in the pathogenic mycobacteria. M. tuberculosis EmbC has an activity similar to that of M. smegmatis EmbC, since we were able to complement an embC mutant of M. smegmatis with embCMtb, confirming that it encodes a functional arabinosyltransferase. In addition, we observed that the size of LAM produced in M. smegmatis was dependent on the level of expression of embCMtb. Northern analysis revealed that embC is expressed as part of a polycistronic message encompassing embC and three upstream genes. The promoter region for this transcript was identified and found to be up-regulated in stationary phase but down-regulated during hypoxia-induced nonreplicating persistence. In conclusion, we have identified one of the key genes involved in LAM biosynthesis in M. tuberculosis and confirmed its essential role in this species.
Mycobacteria remain the causative agents of devastating infections. The increasing appearance of multiple and extremely drug-resistant strains poses further threats and underscores the need for novel therapeutic agents. The mycobacterial cell wall contains a number of carbohydrate residues or glycans in the form of unique species-specific glycolipids and lipoglycans, several of which play important roles in the physiology and virulence of these bacteria. Thus, the specific pathways leading to their synthesis are of interest for drug development. Of the 50 or so proposed Mycobacterium tuberculosis glycosyltransferases, approximately 20 have been functionally characterized and, for the most part, are involved in or associated with cell wall biosynthesis (3).
The cell wall of the mycobacteria has some characteristic features of the gram-positive bacteria, in particular, the presence of a complex arabinogalactan (AG) heteropolysaccharide which is covalently attached to the peptidoglycan (17). However, in Mycobacterium and related genera, the nonreducing ends of the AG are the attachment sites for the ester-linked mycolic acids forming the mycolyl AG-peptidoglycan complex (18). In M. tuberculosis, several characteristic lipids are found interspersed within this layer that contribute to host-pathogen interactions; these include a major component of the mycobacterial cell wall lipoarabinomannan (LAM), as well as lipomannan (LM), and the phthiocerol-containing lipids (5, 6). LAM is a key factor in many aspects of the interaction between Mycobacterium species and host cells (7, 34, 36). Mannose-capped LAM produced by M. tuberculosis is involved in the modulation of macrophage and dendritic cell activation and is therefore able to control the host inflammatory response (12, 15, 22).
Arabinans are common constituents of both AG and LAM and dominate the structure of the mycobacterial cell wall; consequently, they have important structural and pathogenic implications (5). Previous work with Mycobacterium smegmatis has demonstrated that the Emb proteins (EmbA, EmbB, and EmbC) are required for the biosynthesis of the arabinan components of AG and LAM (11, 37). The three Emb homologs located adjacently on the chromosomes of both M. tuberculosis and M. smegmatis have 65% identity at the amino acid level and belong to glycosyltransferase superfamily C (4, 14). embA and embB are cotranscribed in M. tuberculosis (2). If their exact biochemical functions remain unknown, it appears that both EmbA and EmbB are dedicated to the biosynthesis of the arabinan portion of AG (11), whereas EmbC is involved in LAM biosynthesis (37), at least in M. smegmatis.
EmbC is involved in LAM biosynthesis in M. smegmatis, where disruption of embC leads to a loss of LAM production, while LM synthesis is unaffected (37). EmbC is a membrane protein with 13-transmembrane helices in the N-terminal domain coupled to an extracytoplasmic domain involved in arabinan chain extension during LAM biosynthesis (4, 33). Previous studies have focused on embC of M. smegmatis. In order to determine the function of M. tuberculosis EmbC (EmbCMtb), we attempted to construct a deletion mutant by gene replacement. We demonstrate here that embC is essential in M. tuberculosis under normal culture conditions. EmbCMtb is a functional arabinosyltransferase, since it is able to restore LAM production in an M. smegmatis embC mutant. We demonstrate that embC is expressed as part of a polycistronic mRNA transcript together with three genes upstream, from a promoter region located upstream of Rv3790. We analyze the expression of the promoter region and show that it is induced during late stationary phase but is down-regulated in a hypoxia-induced nonreplicating state.
MATERIALS AND METHODS
Culture.
M. tuberculosis was grown in Middlebrook liquid medium (7H9-OADC) containing 4.7 g liter−1 Middlebrook 7H9 plus 10% (vol/vol) OADC (oleic acid, albumin, dextrose, catalase) supplement (Becton Dickinson) and 0.05% (wt/vol) Tween 80 or in Middlebrook solid medium (7H10-OADC) containing 19 g liter−1 Middlebrook 7H10 plus 10% (vol/vol) OADC supplement. Dubos medium (Becton Dickinson) supplemented with 10% (vol/vol) Dubos medium albumin (Becton Dickinson) was used for hypoxic cultures. Aerobic liquid cultures of M. tuberculosis were grown statically in 10-ml cultures. Hypoxic cultures were performed with 17 ml medium in 20-mm glass tubes with slow stirring (50 rpm) and a starting optical density at 570 nm of 0.004. We used kanamycin at 20 μg ml−1, hygromycin at 100 μg ml−1, streptomycin at 20 μg ml−1, gentamicin at 10 μg ml−1, 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) at 50 μg ml−1, and sucrose at 5% (wt/vol). M. smegmatis was cultivated in Lemco medium (9).
Construction of embC deletion vectors.
The upstream and downstream flanking regions of embC were amplified with primer pairs embC5 (CAA GCT TCA TCG GAT CCA CCA CCT G) plus embC6 (CGG TAC CCA CGG AGG TAG ATG GTA G) and embC7 (GGG TAC CGA TCT GAA CCT AGG AAC G) plus embC8 (GGC GGC CGC GCA AGC ACC GAT GTA TAC) and cloned into pGEMT-Easy (Promega). Restriction sites (underlined) were engineered into the primers. The two fragments were excised as HindIII-KpnI and NotI-KpnI fragments and cloned into p2NIL (25) to make pEMPTY4. The lacZ-sacB-hyg cassette from the marker cassette vector pGOAL19 (25) was excised as a PacI fragment and inserted into pEMPTY4 to make the final deletion delivery vector, pEMPTY6. A second deletion vector was constructed by inverse PCR on pEMPTY4 with primer pair RG embC-1 (CCA TCA CGC GCT CTC CTG C) plus RG embC-2 (CCT TAA CCG CGT CGC CTA C); the lacZ-sacB-hyg cassette from the marker cassette vector pGOAL19 (25) was then inserted to make the final deletion delivery vector, pRG76.
Attempts to construct embC deletion strains.
Plasmids pEMPTY6 and pRG76 were pretreated with UV to promote homologous recombination and electroporated into M. tuberculosis (13). Single crossovers (SCOs) were isolated on hygromycin, kanamycin, and X-Gal and checked by Southern blotting. One SCO of each was streaked onto solid media without antibiotics to allow the second crossover to occur. Double crossovers (DCOs) were isolated on sucrose-X-Gal plates. White colonies were patch tested for kanamycin and hygromycin sensitivity to confirm vector loss. A PCR screen with primers EMBC9 (CCA AGC TTC GCC GCT ACA CGG TGG) and EMBC11 (CTG GGT GAT GTT GCC GTC) was used to distinguish between wild-type (3.6 kb) and deletion (0.3 kb) DCOs.
Construction of an embC merodiploid strain.
The complementation vector pEMPTY25 was constructed by amplifying the embC gene from M. tuberculosis with primers Empathy1 (TTA ATT AAG TTT CGT CGT CGA GGA CAT T) and Empathy7 (TTA ATT AAC AAC CTG TGG CTT CTT CTC C) and subcloning it into pAPA3 (L5 integrating vector with the Ag85a promoter) (24) as a PacI fragment (sites underlined) in the correct orientation for expression from the Ag85a promoter. pEMPTY25 was transformed into SCO strains to generate a merodiploid strain. Generation of DCOs was carried out as before, but with the inclusion of gentamicin. Sucrose-resistant white colonies were screened by PCR. Del-int DCO strains (one deleted copy and one integrated copy of embC) were isolated and confirmed by Southern analysis.
Switching experiments with M. tuberculosis embC delinquent strain.
Integrating vectors carrying embC from M. tuberculosis and M. smegmatis with hygromycin markers were created. pRG603 was constructed by replacing the Gm-Int fragment in pEMPTY25 with the Hyg-Int HindIII cassette from pUC-Hyg-Int (16). embC of M. smegmatis was excised as an NdeI-HindIII fragment from pVEwt (4), blunt ended, and cloned into the PacI site of pAPA3 (carrying the mycobacterial Ag85a promoter) (24) to obtain pRG642. The final plasmid, pRG643, was constructed by replacing the Gm-Int fragment in pRG642 with the Hyg-Int HindIII cassette from pUC-Hyg-Int (16). The switching experiment to replace the integrated pEMTY25 vector with pRG603 and pRG643 was carried out as previously described (27). pRG603 and pRG643 were electroporated into the M. tuberculosis embC delinquent strain, and transformants were isolated by hygromycin selection (for the incoming vector). Transformants were patch tested for gentamicin resistance.
Extraction and analysis of LM and LAM.
M. smegmatis was grown in Lemco broth containing 100 μg ml−1 hygromycin and 20 μg ml−1 kanamycin. After 24 h of culture, cells were harvested, resuspended in 400 μl phenol-water at 1:1, and incubated at 80°C for 2 h. A 100-μl volume of chloroform was added, and 10 μl of the aqueous phase was analyzed with a denaturing nonreducing 16% acrylamide gel, followed by periodic acid-Schiff staining (8, 28).
Analysis of promoter activity.
The intergenic regions between adjacent genes spanning the sequence from Rv3789 to embC (Rv3793) of M. tuberculosis were amplified with the following primer pairs and cloned as ScaI or SmaI fragments into L5-based integrating vector pSM128 (10) upstream of the promoterless lacZ gene and sequence verified: p3790 (Rv3789-Rv3790), forward primer CCC AGTACT GTC GGA CTC AAC CAC CTC TG and reverse primer CCC AGTACT GTA GCT CCC ACG CTC AAC AT; p3791 (Rv3790-Rv3791), forward primer CAT CCC GGG CTG GAA CAT TCT G and reverse primer G CCC GGG CTC GGA GGT GCC ACC GAG C; Rv3791-Rv3792, forward primer C CCC GGG CGA ACT TCG TCT AC and reverse primer CA CCT CCC GGG CGC GAG CAG; p3792 (Rv3792-embC), forward primer CCC AGT ACT GTT CGC CGC TAC ACG GTG G and reverse primer CCC AGT ACT GTC TTG CCG GTG TTC TGC GAT CC; p3793 (embA-embB), forward primer CCC AGT ACT CGT TGT CGA GGA GGG CGT G and reverse primer CCC AGT ACT CAT GGC GCG CAC CAC GTC G.
Plasmids were electroporated into M. tuberculosis, and streptomycin-resistant transformants were isolated. Three independent transformants for each were selected for promoter activity determinations. Cell extracts were prepared (26), and β-galactosidase assays were performed as previously described (20). Hypoxic cultures were performed with 17 ml medium in 20-mm glass tubes with slow stirring (50 rpm) and a starting optical density at 570 nm of 0.004. Assays were performed in NRP2 phase (14 days old). Oxidative stress was generated by adding 10 mM hydrogen peroxide to the medium and omitting catalase and albumin. Exposure to ethambutol and ofloxacin was performed for 1 h with 1 μg/ml drug.
Isolation of RNA and identification of the embC transcript.
Total RNA was isolated as previously described (2). For Northern analysis, 12 μg total RNA from M. tuberculosis grown in 7H9 medium was separated by gel electrophoresis with a 1% agarose gel and transferred to a positively charged nylon membrane (Amersham Hybond-N+). The embC probe (probe A) was a 325-bp purified PCR fragment amplified with primers Mut test f (CGT CGG GGC CAA CAC CTC CGA CGA C) and Mut test r (CCG AAG GCC GTT GTC CAG CGG). The Rv3790 probe (probe B) was a 1,300-bp purified PCR fragment amplified with N3790f (CGG AGC GAA CCT TTG AAA TTC G) and N3790r (GAC CCG AGG CTT GAA TAA CGC). Labeling and detection were carried out with the AlkPhos Direct kit (Amersham) according to the manufacturer's instructions.
RESULTS
Essentiality of embC in M. tuberculosis.
The role of EmbC in the biosynthesis of LAM in M. smegmatis was previously elucidated by the construction of an embC mutant (11). However, predictions based on saturating transposon mutagenesis suggested that embC might be an essential gene in M. tuberculosis (29). Our previous work had demonstrated that another arabinosyltransferase, EmbA, is essential in M. tuberculosis (2) but not in M. smegmatis (11), confirming major differences between the two species. Therefore, we decided to determine whether we could construct an embC deletion mutant of M. tuberculosis.
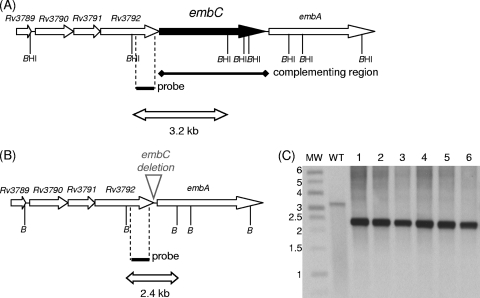
Two deletion vectors were constructed, i.e., pEMPTY4, carrying an unmarked partial deletion of the embC gene, and pRG76, carrying an unmarked complete deletion of the embC gene. Each of these was used in a two-step homologous recombination procedure to attempt to generate DCO embC deletion strains. One SCO strain was generated with each plasmid; DCOs were isolated from these strains and screened by PCR for the presence of either the wild-type or the deletion allele. We screened 100 DCO strains for each deletion vector; all 200 strains carried the wild-type allele, strongly suggesting that the gene is essential. In order to demonstrate this, we constructed a merodiploid strain carrying a second functional copy of embC under the control of the constitutive Ag85a promoter (with plasmid pEMPTY25) in the SCO carrying the complete deletion vector (pRG76). In this background, we were able to isolate both wild-type and deletion DCOs; 10/24 DCOs had the deletion allele. The genotypes of six DCO strains were confirmed by Southern blotting (Fig. 1). Thus, we have confirmed that embC is indeed essential in M. tuberculosis under normal growth conditions.
FIG. 1.
Demonstration of the essentiality of embC in M. tuberculosis. (A) The genetic organization of the wild-type embC region is shown. BamHI sites are indicated (BHI); the probe used for Southern analysis is shown as a solid bar. The region present in the complementing vector is indicated. (B) Map of the deletion. (C) Southern analysis of deletion DCOs isolated in the merodiploid background. Genomic DNA was digested with BamHI and hybridized to the probe. Lane MW, molecular mass marker (sizes are in kilobase pairs). Lane WT, wild-type genomic DNA. Lanes 1 to 6, genomic DNAs from Del-int strains (deletion DCOs with integrated embC).
M. tuberculosis embC encodes an arabinosyltransferase involved in LAM biosynthesis.
M. tuberculosis EmbC was previously identified as an arabinosyltransferase on the basis of sequence similarity to M. smegmatis embC (4, 37). We tested the ability of EmbCMtb to complement the arabinosyltransferase activity of EmbCMsm by using the previously constructed embC disruption strain of M. smegmatis (11). In this strain, lack of EmbC activity results in an inability to synthesize LAM.
We cloned EmbCMtb into two expression vectors under the control of mycobacterial promoters of differing strengths, i.e., pVV16, a multicopy extrachromosomal vector with the strong Hsp60 promoter (pMTembC), and pAPA3, an L5 mycobacteriophage-derived integrating vector with the weaker Ag85a promoter (pEMPTY25). We transformed each plasmid into the M. smegmatis embC mutant and looked at complementation of LAM biosynthesis (11). As a positive control, we used pVV16 carrying M. smegmatis embC (pMSembC). LM and LAM were extracted from these strains and analyzed (Fig. 2). We confirmed that the embC mutant did not produce any LAM. The strain complemented with EmbCMsm produced a larger LAM than the wild type, as previously noted (4). M. smegmatis ΔembC complemented with either vector expressing EmbCMtb was able to synthesize LAM, confirming that EmbCMtb is an arabinosyltransferase with activity similar to that of its ortholog in M. smegmatis. Interestingly, the size of LAM in the complemented strains was dependent on the level of expression of embC, with a larger LAM being produced in the strain expressing EmbCMtb to a higher level (pMTembC). In addition, the strain complemented by EmbCMsm produced a larger LAM than the one complemented by EmbCMtb, even when it was expressed from the same promoter.
FIG. 2.
Analysis of LAM/LM from M. smegmatis mutants. LAM/LM was extracted from M. smegmatis and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. (A) Wild-type strain. (B) ΔembC strain with pVV16. (C) ΔembC strain with pMSembC (Phsp60-EmbCMsm). (D) ΔembC strain with pEMPTY25 (PAg85a-EmbCMtb). (E) ΔembC strain with pMTembC (Phsp60-EmbCMth). MW, molecular mass marker (masses are in kilodaltons).
Functional complementation of M. tuberculosis EmbC by M. smegmatis EmbC.
embC is essential in M. tuberculosis, unlike in M. smegmatis. However, M. tuberculosis embC can complement the LAM− phenotype of the M. smegmatis embC mutant. Although there is 74% amino acid identity between the EmbC proteins from M. tuberculosis and M. smegmatis, it is possible that the M. tuberculosis protein could have additional functions not found in the M. smegmatis protein. In order to address this, we determined whether M. smegmatis embC could functionally complement the M. tuberculosis embC deletion. We used gene switching to replace the resident integrated vector carrying M. tuberculosis embC (pEMPTY25) with an alternate integrating vector carrying M. smegmatis embC (pRG643) in the Del-int strain. Gene switching is based on the high-efficiency replacement of resident L5-based integrating vectors in M. tuberculosis with incoming vectors carrying alternative selection markers (27). Replacement of pEMPTY25 (embCMtb) with pRG643 (embCMsm) in the strain carrying the chromosomal deletion (embCΔ) was achieved at a high efficiency of 1.5 × 103 transformants/μg DNA, comparable to switching with the control vector (embCMtb), with an efficiency of 2.7 × 103 transformants/μg DNA. We confirmed that both plasmids carried functional copies of embC, as assessed by the complementation of the M. smegmatis embC mutant (data not shown). Thus, we confirmed that we were able to generate a strain of M. tuberculosis whose only functional copy of embC was derived from M. smegmatis and that the M. smegmatis gene was able to complement the function of the M. tuberculosis gene.
Identification of the promoter region for embC.
The genomic organization of embC in M. tuberculosis is shown in Fig. 1. Previous studies suggested that embC could be expressed from a promoter located immediately upstream (11) or from a polycistronic message encompassing the embCAB region (35). In order to identify the promoter for embC, we looked at the expression of M. tuberculosis embC in its native host. The genetic organization and spacing suggest that embC is part of an operon with the upstream genes Rv3790, Rv3791, and Rv3792, since there are no intergenic regions. embC is likely to be the last gene in the operon, since there is an intergenic region of 86 bp downstream of embC containing a functional promoter (2).
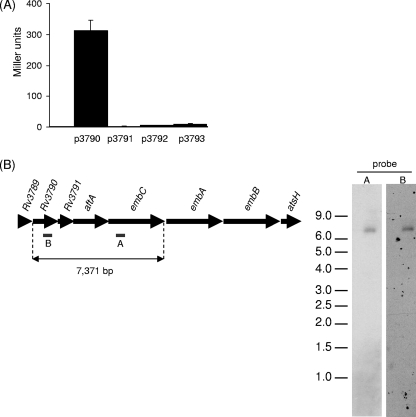
In order to identify the promoter of embC, we cloned the regions upstream of embC, Rv3790, Rv3791, and Rv3792 into pSM128, creating transcriptional fusions with the β-galactosidase gene (10), and assayed promoter activity in M. tuberculosis. The region preceding Rv3790 had a strong promoter activity during exponential phase (312 ± 35 Miller units) (Fig. 3A). None of the other regions had significant activity, even after drug (ethambutol or ofloxacin) treatment (data not shown). This strongly suggests that embC is part of an operon starting with Rv3790.
FIG. 3.
Identification of the promoter for embC. (A) Promoter activities of p3791, p3792, and p3793 in M. tuberculosis. The regions upstream of Rv3790, Rv3791, Rv3792, and embC were cloned into the pSM128 reporter vector. β-Galactosidase activity from 10-ml static cultures was determined after 10 days. Results are the mean ± standard deviation of three individual transformants, each assayed in duplicate and are given in nanomoles of O-nitrophenyl-β-d-galactopyranoside produced per minute per milligram of total protein. (B) Northern blot analysis. Twelve micrograms of total RNA from M. tuberculosis grown in 7H9 medium was separated on a 1% agarose gel and transferred to a positively charged nylon membrane. The blot was hybridized to the two probes indicated, probe A for the embC transcript and probe B for the Rv3790 transcript.
In order to determine if embC was expressed as part of a polycistronic message, we conducted a Northern blot assay with two different probes, one to embC and one to Rv3790 (Fig. 3B). A single transcript was identified with the embC probe with an approximate size of 7.4 kb. This corresponds to the length of a transcript spanning the sequence from Rv3790 to embC. No other, smaller, transcripts were identified. A single transcript of the same size was detected with the Rv3790 probe, confirming that both genes are present on the same transcript. Thus, embC is expressed from a single promoter located upstream of Rv3790. Reverse transcription-PCR on the junctions of each gene pair confirmed that a polycistronic message was present (data not shown). Thus, embC is transcribed independently from embA and embB but is part of a polycistronic message with Rv3790, Rv3791, and Rv3792.
Activity of PembC.
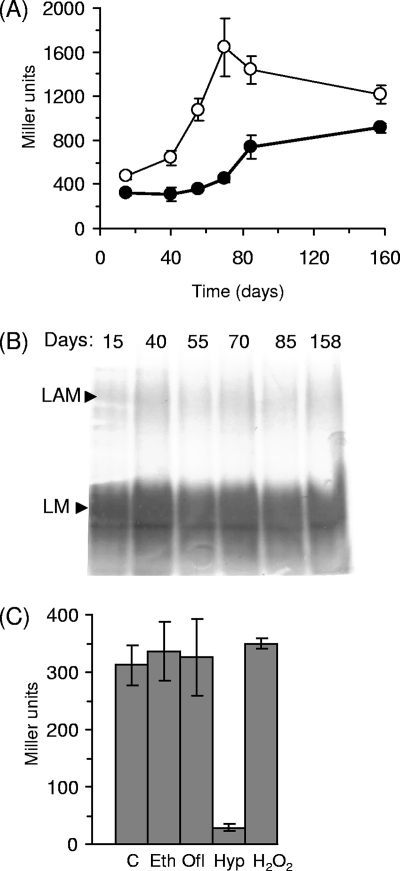
We looked at the expression of embC, as assessed by promoter activity from P3790 (PembC) during different growth phases to determine if there was any regulation. We measured PembC activity in M. tuberculosis over 158 days in liquid and solid media. PembC was more active in cells grown on solid medium than in cells grown in liquid medium. In both media, significant induction occurred after extended growth periods. In the liquid medium, promoter activity was constant during the first 40 days but was increased about threefold to reach 800 Miller units. Promoter activity steadily increased on the solid medium, peaking at 1,600 Miller units before slowly decreasing to 1,200 Miller units (Fig. 4A).
FIG. 4.
PembC activity and LAM production in M. tuberculosis. (A) Promoter activity assays. M. tuberculosis transformants carrying PembC were grown in 10-ml static cultures (filled symbols) or on 7H10-OADC plates (open symbols), and β-galactosidase activity was measured. Results are the mean ± standard deviation of three individual transformants assayed in duplicate and are given in nanomoles of O-nitrophenyl-β-d-galactopyranoside produced per minute per milligram of total protein. (B) LM/LAM profile during growth. LM and LAM were extracted from liquid cultures after 15, 40, 55, 70, 85, and 158 days of static culture and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. (C) Response of PembC to stress conditions and drug treatment. PembC activity was measured in M. tuberculosis after exposure to ethambutol (Eth; 0.5 μg ml−1), ofloxacin (Ofl; 0.2 μg ml−1), or hydrogen peroxide (H2O2; 10 mM for 30 min) or after 2 weeks under hypoxic conditions (Hyp). Results are the mean ± standard deviation of three individual transformants assayed in duplicate and are given in nanomoles of O-nitrophenyl-β-d-galactopyranoside produced per minute per milligram of total protein. C, control.
Since we had seen that the size of LAM in M. smegmatis was dependent on the expression level of embC (Fig. 2), we predicted that the induction of the promoter driving embC in stationary phase could result in an increase in the size of LAM in M. tuberculosis. Thus, we analyzed LM and LAM profiles during extended growth of M. tuberculosis in liquid medium. However, we did not see any significant increase in the size of LAM or the LM/LAM ratio (Fig. 4B).
We also looked at PembC activity under conditions of stress exposure, antibiotic treatment, and nonreplicating persistence. PembC was clearly down-regulated (10-fold) in the hypoxia-induced nonreplicating state (Fig. 4C). PembC activity was also assayed in response to oxidative stress generated by hydrogen peroxide exposure in M. tuberculosis. No change in promoter activity was seen (Fig. 4C). Previous work suggested that embC is up-regulated in response to ethambutol treatment (23). We assayed PembC activity in M. tuberculosis cultivated with ethambutol and ofloxacin (at 0.5 times the MIC). No induction of the promoter activity was seen in response to ethambutol or ofloxacin treatment, revealing that expression of embC is not controlled in response to these drugs (Fig. 4C).
DISCUSSION
We have demonstrated that embC is an essential gene in the pathogenic species M. tuberculosis, in contrast to its dispensability in the nonpathogenic species M. smegmatis. M. smegmatis EmbC was able to complement the function of M. tuberculosis EmbC. This suggests that the essentiality of embC comes from a more crucial role for LAM in the biology of the pathogenic species rather that an additional, uncharacterized role for M. tuberculosis EmbC. A large number of studies have shown that LAM is a potent immune modulator which affects many processes, including phagocytosis, cytokine induction, and dendritic cell activity. However, most of these functions have been determined with the isolated LAM molecule in vitro, and the role of LAM in the context of the whole organism and in vivo settings is much more restricted. We demonstrate here, for the first time, that LAM plays a critical role in the physiology of the bacterium itself. To date, the functionality of LAM in the bacterial cell has not been determined. Aside from maintaining structural integrity, it could also be involved in defense against stress, such as reactive oxygen intermediates. The complementation of the LAM− phenotype of the M. smegmatis embC mutant by embC from M. tuberculosis confirms that it has a similar arabinosyltransferase activity. In addition, the essentiality of embC, together with its level of expression, makes it a potentially interesting drug target, especially since the Emb proteins appear to be unique to the Actinomycetales and are not found in eukaryotes.
Our results demonstrate that embC is transcribed as part of a polycistronic mRNA in M. tuberculosis. We have previously shown that embA and embB are coexpressed on a transcript of a different size, further reinforcing our conclusion that embCAB is not a bona fide operon (2). It is cotranscribed with Rv3790 and Rv3791, both of which are involved in the biosynthesis of the arabinose donor decaprenylarabinose (19), and AftA (Rv3792), which attaches the first arabinose unit from the decaprenylarabinose carrier to AG (1). Thus, this operon is dedicated to the biosynthesis of the cell wall arabinans for both AG and LAM. Our data also confirm our previous observation that embC is not cotranscribed with embA and embB (2), allowing for differential expression. In this light, it is interesting that no up-regulation of the embAB promoter was seen in stationary phase, in contrast to that seen with the embC promoter, possibly reflecting a differential requirement for AG. Both promoters are down-regulated in hypoxia, when cell division ceases, reflecting a lack of requirement for novel cell wall biosynthesis.
It is interesting that the embC promoter region is up-regulated in late stationary phase in aerobic culture but turned off in a hypoxia-induced nonreplicating state. In contrast to previous work measuring mRNA (23), we saw no induction of embC in response to ethambutol as assessed by promoter activity. It is possible that mRNA stability is affected under these conditions, and an increase in expression of the protein cannot be ruled out. However, the previously reported up-regulation was only 1.96-fold for embC and 1.33-fold for embB, indicating that only minor changes were seen, despite the use of a sensitive real-time PCR technique (23). Regulation of embC expression has also been seen in M. smegmatis (32), where it can be controlled by the M. tuberculosis regulatory protein EmbR. EmbR itself is a substrate of multiple serine/threonine kinases (pknA, pknB, and pknH) and a phosphatase (31) and could form part of a complex network of control. However, care needs to be taken when interpreting data from M. smegmatis, since the promoter activity of PembC is significantly different in this species than in its native host (data not shown).
Our results and previous work (4) suggest that the overexpression of embC results in the production of larger LAM species, at least in M. smegmatis. However, the analysis of LAM during stationary phase, where embC was up-regulated, did not reveal any increase in the size (or amount) of LAM compared to exponential-phase cells of M. tuberculosis. This was surprising, but we cannot exclude the possibility that there is a higher turnover of LAM under these conditions. There are reports of arabinomannan (AM) in culture supernatants of M. tuberculosis (21, 30); AM has a structure very similar to that of LAM and could represent a processed form of LAM which is secreted into the supernatant. However, we were unable to detect LAM or AM in the supernatant, making it unlikely that it accumulates under these conditions. Alternatively, it may be that overexpression of embC does not lead to increased LAM in M. tuberculosis and that this phenomenon is specific to M. smegmatis.
Acknowledgments
This work was funded by Wellcome Trust grant 074612 awarded to T.P. and National Institutes of Health grant AI 37319 awarded to D.C.
We are grateful to Stefan Berg for valuable discussion.
Footnotes
Published ahead of print on 18 April 2008.
REFERENCES
- 1.Alderwick, L. J., M. Seidel, H. Sahm, G. S. Besra, and L. Eggeling. 2006. Identification of a novel arabinofuranosyltransferase (AftA) involved in cell wall arabinan biosynthesis in Mycobacterium tuberculosis. J. Biol. Chem. 28115653-15661. [DOI] [PubMed] [Google Scholar]
- 2.Amin, A. G., R. Goude, L. Shi, J. Zhang, D. Chatterjee, and T. Parish. 2008. EmbA is an essential arabinosyltransferase in Mycobacterium tuberculosis. Microbiology 154240-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berg, S., D. Kaur, M. Jackson, and P. J. Brennan. 2007. The glycosyltransferases of Mycobacterium tuberculosis—roles in the synthesis of arabinogalactan, lipoarabinomannan, and other glycoconjugates. Glycobiology 1735R-56R. [DOI] [PubMed] [Google Scholar]
- 4.Berg, S., J. Starbuck, J. B. Torrelles, V. D. Vissa, D. C. Crick, D. Chatterjee, and P. J. Brennan. 2005. Roles of conserved proline and glycosyltransferase motifs of EmbC in biosynthesis of lipoarabinomannan. J. Biol. Chem. 2805651-5663. [DOI] [PubMed] [Google Scholar]
- 5.Brennan, P. J. 2003. Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis. Tuberculosis (Edinburgh) 8391-97. [DOI] [PubMed] [Google Scholar]
- 6.Briken, V., S. A. Porcelli, G. S. Besra, and L. Kremer. 2004. Mycobacterial lipoarabinomannan and related lipoglycans: from biogenesis to modulation of the immune response. Mol. Microbiol. 53391-403. [DOI] [PubMed] [Google Scholar]
- 7.Chan, J., X. D. Fan, S. W. Hunter, P. J. Brennan, and B. R. Bloom. 1991. Lipoarabinomannan, a possible virulence factor involved in persistence of Mycobacterium tuberculosis within macrophages. Infect. Immun. 591755-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chatterjee, D., K. Lowell, B. Rivoire, M. R. McNeil, and P. J. Brennan. 1992. Lipoarabinomannan of Mycobacterium tuberculosis. Capping with mannosyl residues in some strains. J. Biol. Chem. 2676234-6239. [PubMed] [Google Scholar]
- 9.Clarke, P. H., and P. M. Meadow. 1959. Evidence for the occurrence of permeases for tricarboxylic acid cycle intermediates in Pseudomonas aeruginosa. J. Gen. Microbiol. 20144-155. [DOI] [PubMed] [Google Scholar]
- 10.Dussurget, O., J. Timm, M. Gomez, B. Gold, S. Yu, S. Z. Sabol, R. K. Holmes, W. R. Jacobs, Jr., and I. Smith. 1999. Transcriptional control of the iron-responsive fxbA gene by the mycobacterial regulator IdeR. J. Bacteriol. 1813402-3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Escuyer, V. E., M. A. Lety, J. B. Torrelles, K. H. Khoo, J. B. Tang, C. D. Rithner, C. Frehel, M. R. McNeil, P. J. Brennan, and D. Chatterjee. 2001. The role of the embA and embB gene products in the biosynthesis of the terminal hexaarabinofuranosyl motif of Mycobacterium smegmatis arabinogalactan. J. Biol. Chem. 27648854-48862. [DOI] [PubMed] [Google Scholar]
- 12.Geijtenbeek, T. B., S. J. Van Vliet, E. A. Koppel, M. Sanchez-Hernandez, C. M. Vandenbroucke-Grauls, B. Appelmelk, and Y. Van Kooyk. 2003. Mycobacteria target DC-SIGN to suppress dendritic cell function. J. Exp. Med. 1977-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinds, J., E. Mahenthiralingam, K. E. Kempsell, K. Duncan, R. W. Stokes, T. Parish, and N. G. Stoker. 1999. Enhanced gene replacement in mycobacteria. Microbiology 145(Pt. 3)519-527. [DOI] [PubMed] [Google Scholar]
- 14.Liu, J., and A. Mushegian. 2003. Three monophyletic superfamilies account for the majority of the known glycosyltransferases. Protein Sci. 121418-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maeda, N., J. Nigou, J. L. Herrmann, M. Jackson, A. Amara, P. H. Lagrange, G. Puzo, B. Gicquel, and O. Neyrolles. 2003. The cell surface receptor DC-SIGN discriminates between Mycobacterium species through selective recognition of the mannose caps on lipoarabinomannan. J. Biol. Chem. 2785513-5516. [DOI] [PubMed] [Google Scholar]
- 16.Mahenthiralingam, E., B. I. Marklund, L. A. Brooks, D. A. Smith, G. J. Bancroft, and R. W. Stokes. 1998. Site-directed mutagenesis of the 19-kilodalton lipoprotein antigen reveals no essential role for the protein in the growth and virulence of Mycobacterium intracellulare. Infect. Immun. 663626-3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McNeil, M., M. Daffe, and P. J. Brennan. 1990. Evidence for the nature of the link between the arabinogalactan and peptidoglycan of mycobacterial cell walls. J. Biol. Chem. 26518200-18206. [PubMed] [Google Scholar]
- 18.McNeil, M., M. Daffe, and P. J. Brennan. 1991. Location of the mycolyl ester substituents in the cell walls of mycobacteria. J. Biol. Chem. 26613217-13223. [PubMed] [Google Scholar]
- 19.Mikusová, K., H. Huang, T. Yagi, M. Holsters, D. Vereecke, W. D'Haeze, M. S. Scherman, P. J. Brennan, M. R. McNeil, and D. C. Crick. 2005. Decaprenylphosphoryl arabinofuranose, the donor of the d-arabinofuranosyl residues of mycobacterial arabinan, is formed via a two-step epimerization of decaprenylphosphoryl ribose. J. Bacteriol. 1878020-8025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller, J. H. 1972. Experiments in molecular genetics, p. 352-355. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 21.Misaki, A., I. Azuma, and Y. Yamamura. 1977. Structural and immunochemical studies on d-arabino-d-mannans and d-mannans of Mycobacterium tuberculosis and other Mycobacterium species. J. Biochem. (Tokyo) 821759-1770. [DOI] [PubMed] [Google Scholar]
- 22.Nigou, J., C. Zelle-Rieser, M. Gilleron, M. Thurnher, and G. Puzo. 2001. Mannosylated lipoarabinomannans inhibit IL-12 production by human dendritic cells: evidence for a negative signal delivered through the mannose receptor. J. Immunol. 1667477-7485. [DOI] [PubMed] [Google Scholar]
- 23.Papavinasasundaram, K. G., B. Chan, J. H. Chung, M. J. Colston, E. O. Davis, and Y. Av-Gay. 2005. Deletion of the Mycobacterium tuberculosis pknH gene confers a higher bacillary load during the chronic phase of infection in BALB/c mice. J. Bacteriol. 1875751-5760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parish, T., G. Roberts, F. Laval, M. Schaeffer, M. Daffe, and K. Duncan. 2007. Functional complementation of the essential gene fabG1 of Mycobacterium tuberculosis by Mycobacterium smegmatis fabG but not Escherichia coli fabG. J. Bacteriol. 1893721-3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parish, T., and N. G. Stoker. 2000. Use of a flexible cassette method to generate a double unmarked Mycobacterium tuberculosis tlyA plcABC mutant by gene replacement. Microbiology 146(Pt. 8)1969-1975. [DOI] [PubMed] [Google Scholar]
- 26.Parish, T., and P. R. Wheeler. 1998. Preparation of cell-free extracts from mycobacteria. Methods Mol. Biol. 10177-89. [DOI] [PubMed] [Google Scholar]
- 27.Pashley, C. A., and T. Parish. 2003. Efficient switching of mycobacteriophage L5-based integrating plasmids in Mycobacterium tuberculosis. FEMS Microbiol. Lett. 229211-215. [DOI] [PubMed] [Google Scholar]
- 28.Prinzis, S., D. Chatterjee, and P. J. Brennan. 1993. Structure and antigenicity of lipoarabinomannan from Mycobacterium bovis BCG. J. Gen. Microbiol. 1392649-2658. [DOI] [PubMed] [Google Scholar]
- 29.Sassetti, C. M., D. H. Boyd, and E. J. Rubin. 2003. Genes required for mycobacterial growth defined by high density mutagenesis. Mol. Microbiol. 4877-84. [DOI] [PubMed] [Google Scholar]
- 30.Schwebach, J. R., A. Casadevall, R. Schneerson, Z. Dai, X. Wang, J. B. Robbins, and A. Glatman-Freedman. 2001. Expression of a Mycobacterium tuberculosis arabinomannan antigen in vitro and in vivo. Infect. Immun. 695671-5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma, K., M. Gupta, A. Krupa, N. Srinivasan, and Y. Singh. 2006. EmbR, a regulatory protein with ATPase activity, is a substrate of multiple serine/threonine kinases and phosphatase in Mycobacterium tuberculosis. FEBS J. 2732711-2721. [DOI] [PubMed] [Google Scholar]
- 32.Sharma, K., M. Gupta, M. Pathak, N. Gupta, A. Koul, S. Sarangi, R. Baweja, and Y. Singh. 2006. Transcriptional control of the mycobacterial embCAB operon by PknH through a regulatory protein, EmbR, in vivo. J. Bacteriol. 1882936-2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi, L., S. Berg, A. Lee, J. S. Spencer, J. Zhang, V. Vissa, M. R. McNeil, K. H. Khoo, and D. Chatterjee. 2006. The carboxy terminus of EmbC from Mycobacterium smegmatis mediates chain length extension of the arabinan in lipoarabinomannan. J. Biol. Chem. 28119512-19526. [DOI] [PubMed] [Google Scholar]
- 34.Stokes, R. W., and D. P. Speert. 1995. Lipoarabinomannan inhibits nonopsonic binding of Mycobacterium tuberculosis to murine macrophages. J. Immunol. 1551361-1369. [PubMed] [Google Scholar]
- 35.Telenti, A., W. J. Philipp, S. Sreevatsan, C. Bernasconi, K. E. Stockbauer, B. Wieles, J. M. Musser, and W. R. Jacobs, Jr. 1997. The emb operon, a gene cluster of Mycobacterium tuberculosis involved in resistance to ethambutol. Nat. Med. 3567-570. [DOI] [PubMed] [Google Scholar]
- 36.Underhill, D. M., A. Ozinsky, K. D. Smith, and A. Aderem. 1999. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc. Natl. Acad. Sci. USA 9614459-14463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang, N., J. B. Torrelles, M. R. McNeil, V. E. Escuyer, K. H. Khoo, P. J. Brennan, and D. Chatterjee. 2003. The Emb proteins of mycobacteria direct arabinosylation of lipoarabinomannan and arabinogalactan via an N-terminal recognition region and a C-terminal synthetic region. Mol. Microbiol. 5069-76. [DOI] [PubMed] [Google Scholar]