Abstract
The IcsA (VirG) protein of Shigella flexneri is a polarly localized, outer membrane protein that is essential for virulence. Within host cells, IcsA activates the host actin regulatory protein, neural Wiskott-Aldrich syndrome protein (N-WASP), which in turn recruits the Arp2/3 complex, which nucleates host actin to form F-actin comet tails and initiate bacterial motility. Linker insertion mutagenesis was undertaken to randomly introduce 5-amino-acid in-frame insertions within IcsA. Forty-seven linker insertion mutants were isolated and expressed in S. flexneri ΔicsA strains. Mutants were characterized for IcsA protein production, cell surface expression and localization, intercellular spreading, F-actin comet tail formation, and N-WASP recruitment. Using this approach, we have identified a putative autochaperone region required for IcsA biogenesis, and our data suggest an additional region, not previously identified, is required for N-WASP recruitment.
Shigella flexneri causes bacillary dysentery in humans due to bacterial invasion and colonization of the colonic epithelium that leads to acute mucosal inflammation (42). S. flexneri IcsA (VirG), an essential virulence factor, is a polarly localized, outer membrane (OM) protein that is required for intra- and intercellular spreading throughout the host epithelium (17, 23, 24, 27, 45, 52). Within host cells, IcsA interacts with the host actin regulatory protein, neural Wiskott-Aldrich syndrome protein (N-WASP), which in turn recruits the host Arp2/3 complex, which polymerizes host globular actin into filamentous actin (F-actin) (10, 17, 41). The accumulation of F-actin in “comet tails” at one pole of the bacterium initiates bacterial actin-based motility (ABM) (2, 10, 17).
IcsA is an autotransporter (AT) protein, belonging to the largest family of gram-negative bacterial extracellular proteins, with more than 700 members (40). Like other members of this family, IcsA consists of three major domains: an N-terminal signal sequence (amino acids [aa] 1 to 52), to direct Sec-dependent protein transport across the inner-membrane; and a C-terminal translocation domain (aa 759 to 1102) that enables export of the remaining N-terminal region of IcsA, the passenger domain (aa 53 to 758), across the OM (5, 6, 19, 48). The passenger domain becomes exposed on the outer surface, anchored by the translocation domain, and is responsible for IcsA activity in N-WASP recruitment and ultimately bacterial motility (17, 52).
Within the host cell, N-WASP regulates the host actin cytoskeleton and functions as a link between signaling pathways and de novo actin polymerization, thereby initiating host cell motility and morphological changes (28, 55). IcsA103-433, which contains a series of glycine-rich repeats (GRR) (aa 140 to 307), is sufficient for N-WASP binding in vitro (49, 50). However, in vitro actin polymerization requires aa 53 to 508, and it has been suggested that the residues involved in N-WASP recruitment in vivo are within this region (17, 51, 52). Correspondingly, a Shigella strain expressing IcsAΔ509-729 was reported to recruit N-WASP inside host cells (49). However, this mutant had previously been shown to have a defect in F-actin tail formation, which was attributed to nonpolar IcsA localization (51). Consequently, whether aa 53 to 508 of IcsA are actually sufficient for F-actin comet tail formation inside host cells awaits clear verification. A region encompassing the GRR region (aa 104 to 506) has been shown to interact with the host protein vinculin (51), and another region (aa 320 to 433) interacts with S. flexneri IcsB and the host autophagy protein, Atg5 (37).
Two regions of the IcsA passenger domain, polar localization region 1 (aa 1 to 104) and polar localization region 2 (aa 507 to 620), have been shown to be independently responsible for targeting IcsA export to the old cell pole (9, 51). IcsA polarity is thought to enhance unidirectional movement of the bacteria, which has been shown to correlate with an increased frequency of protrusion formation into adjacent host cells (a process that leads to intercellular spreading) (29). Additionally, a cleavage site for the virulence plasmid-encoded, OM, serine protease IcsP (SopA), exists between residues R758 and R759. Cleavage of IcsA by IcsP results in the release of the 95-kDa N-terminal fragment into the extracellular milieu at a low efficiency, such that during exponential growth of the bacteria, 80% of the IcsA molecules remain uncleaved and anchored to the OM (11, 13, 15, 46, 47). The significance of IcsA cleavage by IcsP remains controversial, although it has been suggested that it functions to enhance IcsA polarity (13, 46).
Previous IcsA structure and function studies have relied predominantly on relatively large deletion mutations of IcsA regions (8, 49-51). Such approaches may have resulted in the removal of multiple functional domains, complicating the interpretation of the phenotypes of the icsA mutants in those studies. In previous studies we have provided evidence of masking of IcsA function by lipopolysaccharide O antigen (LPS Oag) (31, 32). LPS is a major constituent of the OM of gram-negative bacteria, consisting of a lipid A, a core polysaccharide component, and an Oag polysaccharide chain (also called O antigen) that extends into the extracellular milieu (4, 21). Hypothetically, truncated IcsA proteins may be more prone to LPS Oag masking, further complicating the assessment of their activity. To better understand the IcsA structure-function relationship, pentapeptide linker insertion mutagenesis of IcsA was undertaken and 47 unique IcsAi mutants were isolated and characterized. In addition to evaluating the abilities of various IcsAi mutants to interact with N-WASP in the presence of native LPS-Oag, we also investigated the influence of LPS Oag on IcsA-N-WASP interactions by assessing the function of these proteins in the absence of LPS Oag. We have identified regions of IcsA required for N-WASP recruitment inside host cells and a putative autochaperone region required for IcsA biogenesis.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The strains and plasmids used in this study are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristicsa | Reference or source |
|---|---|---|
| E. coli K-12 | ||
| DH5α | Cloning host | Gibco-BRL |
| UT5600 | Protease-deficient strain; ΔompT ΔompP; Smr | Laboratory stock |
| S. flexneri | ||
| 2457T | S. flexneri 2a WT | 53 |
| RMA2041 | 2457T ΔicsA::Tcr | 54 |
| RMA2090 | RMA2041(pIcsA) | 54 |
| RMA2043 | RMA2041 ΔrmlD::Kmr | 54 |
| RMA2107 | RMA2043(pIcsA) | 54 |
| Plasmids | ||
| pIcsA | icsA gene cloned into pBR322; Apr; medium copy no.; ColE1 ori | 33 |
| pD10 | Plasmid encoding IcsAWT; Tpr | 51 |
| pD10-virG1 | Plasmid encoding IcsAΔ103-320; Tpr | 51 |
| pD10-virG3 | Plasmid encoding IcsAΔ508-730; Tpr | 51 |
| pD10-virG4 | Plasmid encoding IcsAΔ103-507; Tpr | 51 |
Smr, streptomycin resistant; Tcr, tetracyline resistant; Kmr, kanamycin resistant; Apr, ampicillin resistant; Tpr, trimethroprim resistant.
Growth media and growth conditions.
S. flexneri strains were grown from a Congo red-positive colony. All bacterial strains were routinely cultured in Luria Bertani (LB). Bacteria were grown in media with antibiotics for 16 h with aeration and then subcultured 1:50 and grown to log phase by incubation with aeration for 2 h at 37°C. Where appropriate, media were supplemented with ampicillin (100 μg/ml), chloramphenicol (25 μg/ml), kanamycin (50 μg/ml), tetracycline (50 μg/ml), or trimethroprim (10 μg/ml).
DNA methods.
Escherichia coli K-12 strain DH5α was used for routine cloning, and general cloning techniques and PCR and DNA sequencing were performed as described previously (1, 34).
Antibodies and antisera.
Affinity-purified rabbit polyclonal anti-IcsA antiserum was produced in our laboratory as described previously (53). The polyclonal rabbit anti-N-WASP antibody was initially a generous gift from Hiroaki Miki. Subsequent stocks of comparable polyclonal rabbit anti-N-WASP antibodies were made in our laboratory according to the method described by Fukuoka et al. (16). The vector encoding a glutathione S-transferase-tagged carboxyl-terminal region of N-WASP (VCA region; amino acids 388 to 501), used to produce purified protein for the production of antiserum, was kindly provided by Hiroaki Miki. The anti-N-WASP antibody was used at 1:100. The polyclonal anti-Shigella LPS (3, 4) was from Denka Seiken Co. (Japan) and was used at 1:100.
Linker insertion mutagenesis using the Mutation Generation System (Finnzymes).
Linker insertion mutagenesis of IcsA was performed using the Mutation Generation System (Finnzymes) according to the manufacturer's instructions. Briefly, an in vitro transposition reaction was carried out to randomly introduce an artificial entranceposon (M1-Cmr) into pIcsA (33, 54), which encodes wild-type (WT) IcsA (IcsAWT). The entranceposons were removed from the mutated plasmids using the NotI restriction enzyme, and the digested plasmids were then religated to leave an in-frame insertion of 15 bp encoding 5 aa. Plasmids were screened initially by PCR using the NotI miniprimer (Finnzymes) (a primer complementary to 10 bp of the insertion) and primer 2156IcsAF (see Table S2 in the supplemental material), which is complementary to a region 100 bp upstream of the icsA gene in pIcsA, to identify those which contained insertions within the IcsA passenger domain. The location of each insertion and the encoded amino acids for each of these plasmids were determined by DNA sequencing using primers 2156IcsAF, IcsA549F, IcsA2381R, 2170IcsAR (see Table S2 in the supplemental material).
Preparation of whole-cell lysates.
The equivalent of 5 × 108 bacteria were pelleted by centrifugation (3,300 × g for 6 min at 4°C and resuspended in 100 μl of 2× sample buffer (26).
TCA precipitation of culture supernatants.
The equivalent of 5 × 109 bacteria were pelleted by centrifugation at 2,200 ×g for 10 min at 4°C. The supernatants were collected, supplemented with 5% ice-cold trichloroacetic acid (TCA), and incubated on ice for 1 h. The TCA precipitate was pelleted by centrifugation at 40 000 × g for 30 min at 4°C. The supernatant was removed and the pellet centrifuged again for 5 min as before to remove the remaining supernatant. The protein pellet was washed with ice-cold acetone and centrifuged for another 5 min and the acetone carefully removed. The pellet was air-dried and then resuspended in 100 μl of 1× sample buffer (26).
Western transfer and detection.
Proteins were separated on 7.5% or 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred to a nitrocellulose membrane. The membrane was blocked for 1 h in TTBS (Tris-buffered saline, 0.05% Tween 20) containing 5% skim milk and incubated with the desired primary antibody in the same buffer overnight. After three 10-min washes in TTBS, the membrane was incubated with horseradish peroxidase-conjugated goat antirabbit or a horseradish peroxidase-conjugated goat antimouse secondary antibodies (Biomediq DPC) for 2 h and washed three times in TTBS and then three times in Tris-buffered saline. The membrane was incubated with chemiluminescence blotting substrate (Roche) for 1 min. Chemiluminescence was detected by exposure of the membrane to X-ray film (Agfa), and the film was developed using a Curix 60 automatic X-ray film processor (Agfa).
Indirect immunofluorescence (IF) of whole bacteria.
Log-phase bacteria were pelleted by centrifugation (2,200 × g, 10 min, 4°C) and the supernatant discarded. Bacteria were fixed in formalin (3.7% paraformaldehyde in 0.85% saline) for 15 min at room temperature. Sterile coverslips were placed in 24-well trays and incubated with 10% poly-l-lysine in phosphate-buffered saline (PBS) for 1 min. The poly-l-lysine was aspirated, and formalin fixed bacteria were centrifuged onto poly-l-lysine-coated coverslips at 1,000 × g for 5 min. Bacteria were incubated with the desired primary antibody diluted 1:100 in PBS with 10% fetal calf serum (FCS). Bacteria were washed three times in PBS and incubated with either Alexa 488-conjugated donkey antirabbit or Alexa 488-conjugated donkey antimouse secondary antibodies (Molecular Probes) diluted 1:100 in PBS with 10% FCS.
Plaque assays.
Plaque assays were performed with HeLa cells using a modification of the method described by Oaks et al. (35). HeLa cells were seeded to 60-mm-diameter, six-well trays at 1 × 106 in minimal essential medium-10% FCS with penicillin and streptomycin. Cells were grown to confluence overnight and washed twice with Dulbecco's PBS (D-PBS) and once in Dulbecco's modified Eagle medium (DMEM) prior to inoculation. Log-phase bacteria were diluted to 1:100 and 1:300 in DMEM, and 0.2 ml was added to each well. Trays were incubated at 37°C in a humidified CO2 (5%) incubator, and the trays were rocked gently every 15 min to ensure that the inoculum was spread evenly across the monolayer. At 90 min postinfection, the inoculum was carefully aspirated and 4 ml of the first overlay (DMEM, 5% FCS, 20 μg/ml of gentamicin, 0.5% agarose [Seakem ME]) was added to each well. The second overlay (DMEM, 5% FCS, 20 μg/ml of gentamicin, 0.5% agarose, 0.1% Neutral Red solution [Gibco BRL]) was added at either 24 h or 48 h postinfection and plaque formation observed 6 to 8 h later. Plaques were in general visible without staining at 48 h.
Trypsin accessibility assay.
Limited proteolysis was performed as described by Oliver et al. (39) with modifications. The equivalent of 5 × 109 log-phase bacteria were pelleted by centrifugation, the supernatant was discarded, and the pellet was resuspended in 150 μl of PBS. Bacterial suspensions were supplemented with 0.1 μg/ml of Trypsin from bovine pancreas (no. 109819; Roche) and incubated at room temperature to allow proteolysis. Aliquots were taken at several time points (0 min, 5 min, and 10 min) and supplemented with 1 mM phenylmethylsulphonylfluoride (Sigma) to inhibit Trypsin and further proteolysis. An equal volume of 2× sample buffer (26) was added to each sample, all of which were then heated at 100°C for 5 min prior to SDS-PAGE and western immunoblotting.
Infection of tissue culture monolayers with S. flexneri and IF labeling.
Infection of tissue culture cells and IF staining were performed as recently described (31). Briefly, 5 × 108 log-phase bacteria were pelleted by centrifugation (3,300 × g, 6 min) and resuspended at 109 bacteria/ml in D-PBS. One hundred microliters of bacterial suspension was then centrifuged onto HeLa cells grown to semiconfluence on sterile glass coverslips. After 1 h of incubation at 37°C in 5% CO2, the infected cells were washed three times with D-PBS and incubated with 0.5 ml minimal essential medium containing 40 μg/ml of gentamicin for a further 1.5 h. Infected cells were washed a further three times in D-PBS and then fixed for 15 min in formalin, incubated with 50 mM NH4Cl in D-PBS for 10 min, and then permeabilized with 0.1% Triton X-100 in H2O for 5 min. After blocking in 10% FCS in PBS, the infected cells were incubated at 37°C for 30 min with the desired primary antibody. After washing in PBS, coverslips were incubated with either Alexa 594-conjugated donkey antirabbit or Alexa 594-conjugated donkey antimouse secondary antibodies (Molecular probes) (1:100), as required. F-actin was visualized by staining with fluorescein isothiocyanate (FITC) phalloidin (0.1 μg/ml; Sigma), and 4′,6′-diamidino-2-phenylindole (DAPI) (0.1 μg/ml, Sigma) was used to counterstain bacteria and cellular nuclei as required.
Microscopy.
Coverslips were mounted on glass slides with Mowiol 4-88 (Calbiochem) containing 20 μg/ml p-phenylenediamine (Sigma) and examined with an Olympus IX-70 microscope with phase-contrast optics using a 100× oil immersion objective and a 1.5× enlarger as required. Fluorescence and phase-contrast images were false color merged using the Metamorph software program (version 6.3r7; Molecular Devices).
Quantitation of F-actin tail formation and N-WASP recruitment.
Quantification of F-actin comet tails is made difficult by the fact that their numbers naturally vary greatly between cells. Therefore, the quantitation method used by Frischknecht et al. (14) was adopted, in which the presence of a single actin tail within an infected cell is scored as positive. N-WASP recruitment was also quantitated in this manner. Initially, for each strain, 20 cells were observed and scored as described above in two independent experiments. For strains expressing the mutant IcsAi56, IcsAi193, IcsAi288, IcsAi312, or IcsAi502, where the frequency of F-actin tail formation was comparable to that of the WT despite obvious intercellular spreading defects, 100 cells were scored in three independent experiments.
RESULTS
Linker insertion mutagenesis of IcsA passenger domain.
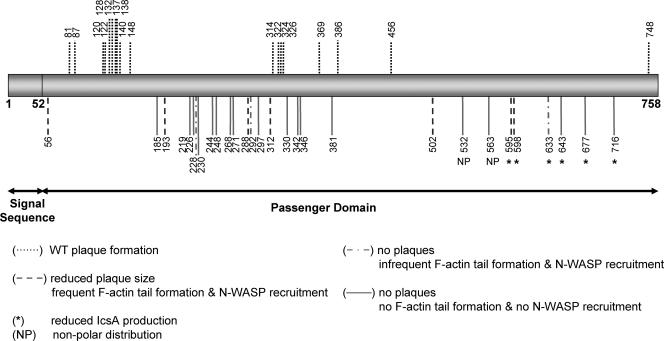
Pentapeptide linker insertion mutagenesis of IcsA was performed using the Mutation Generation System (Finnzymes) as described in Materials and Methods. Forty-seven plasmids were obtained that harbored unique insertions within the IcsA passenger domain (Fig. 1; also see Table S1 in the supplemental material). Plasmid pIcsA and those encoding the IcsA linker insertion mutants (IcsAi) were electroporated into an S. flexneri ΔicsA strain (RMA2041; Table 1) to enable characterization of IcsAi production and function.
FIG. 1.
Schematic locations of the 5 amino acid insertions in 47 mutant IcsAi proteins and the corresponding phenotypes. The location of the linker insertion for each of the 47 IcsAi mutants is indicated with a vertical line.
Effects of linker insertions on IcsA production and secretion.
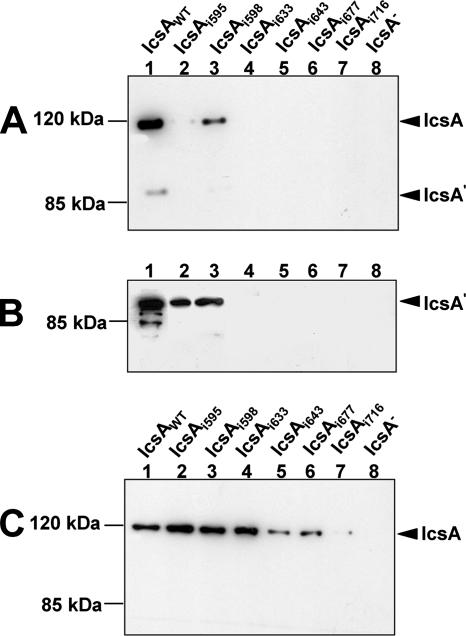
To investigate the effects on protein production arising from the 5-aa linker insertion in each IcsAi mutant, SDS-PAGE and Western immunoblotting with IcsA antibody were performed on whole-cell lysates. In 41 of 47 mutants, IcsA protein levels were comparable to those of the WT (Table 2). The mutant proteins IcsAi595, IcsAi598, IcsAi633, IcsAi643, IcsAi677, and IcsAi716 demonstrated aberrant production in S. flexneri compared to that of IcsAWT. The mutants IcsAi595 and IcsAi598 were produced at very low levels compared to IcsAWT (Fig. 2A), while IcsAi633, IcsAi643, IcsAi677, and IcsAi716 could be detected only with higher concentrations of anti-IcsA antibody (data not shown). For these mutants, along with IcsAi595 and IcsAi598, the amount of protein in the culture supernatant was also evaluated to determine if reduction of the full-length, OM forms of these proteins was possibly due to increased cleavage and secretion of these proteins into the supernatant. Cleaved forms of IcsAi595 and IcsAi598 were observed in the supernatant, although at lower levels than IcsAWT, and the cleaved forms of IcsAi633, IcsAi643, IcsAi677, and IcsAi716 could not be detected (Fig. 2B). Notably, all six mutants possessed insertions immediately upstream of, or within, a region of IcsA (aa 634 to 735) that shares similarity to the putative autochaperone domain of the Bordetella pertussis BrkA autotransporter (39). Deletion of this region in BrkA made the protein susceptible to degradation by OM proteases and trypsin (39). Therefore, we expressed IcsAi595, IcsAi598, IcsAi633, IcsAi643, IcsAi677, and IcsAi716 in E. coli strain UT5600 (Table 1), which is deficient in the OM proteases ompT and ompP, to determine if production of these mutants could be restored. Mutants IcsAi595, IcsAi598, and IcsAi633 were produced at levels comparable to IcsAWT in E. coli strain UT5600 (Fig. 2C). However, production of IcsAi643, IcsAi677, and IcsAi716 was still greatly reduced in this background (Fig. 2C).
TABLE 2.
Phenotypes of S. flexneri strains expressing IcsAi mutants
| Proteina | IcsAi productionb | IcsAi localizationc | Plaque formationd | N-WASP recruitmente | F-actin tailsf |
|---|---|---|---|---|---|
| IcsAWT | +++ | P | +++ | +++ | +++ |
| IcsAi56 | +++ | P | ++ | +++ | +++ |
| IcsAi81 | +++ | P | +++ | NT | NT |
| IcsAi87 | +++ | P | +++ | NT | NT |
| IcsAi120 | +++ | P | +++ | NT | NT |
| IcsAi122 | +++ | P | +++ | NT | NT |
| IcsAi128 | +++ | P | +++ | NT | NT |
| IcsAi132 | +++ | P | +++ | NT | NT |
| IcsAi137 | +++ | P | +++ | NT | NT |
| IcsAi138 | +++ | P | +++ | NT | NT |
| IcsAi140 | +++ | P | +++ | NT | NT |
| IcsAi148 | +++ | P | +++ | NT | NT |
| IcsAi185 | +++ | P | − | − | − |
| IcsAi193 | +++ | P | + | +++ | +++ |
| IcsAi219 | +++ | P | − | − | − |
| IcsAi226 | +++ | P | − | − | − |
| IcsAi228 | +++ | P | − | − | + |
| IcsAi230 | +++ | P | − | − | − |
| IcsAi244 | +++ | P | − | − | − |
| IcsAi248 | +++ | P | − | − | − |
| IcsAi268 | +++ | P | − | − | − |
| IcsAi271 | +++ | P | − | − | − |
| IcsAi288 | +++ | P | + | ++ | ++ |
| IcsAi292 | +++ | P | − | + | +/− |
| IcsAi297 | +++ | P | − | − | − |
| IcsAi312 | +++ | P | + | +++ | ++ |
| IcsAi314 | +++ | P | +++ | NT | NT |
| IcsAi322 | +++ | P | +++ | NT | NT |
| IcsAi324 | +++ | P | +++ | NT | NT |
| IcsAi326 | +++ | P | +++ | NT | NT |
| IcsAi330a | +++ | P | − | − | − |
| IcsAi330b | +++ | P | − | − | − |
| IcsAi342 | +++ | P | − | − | − |
| IcsAi346 | +++ | P | − | − | +/− |
| IcsAi369 | +++ | P | +++ | NT | NT |
| IcsAi381 | +++ | P | − | − | − |
| IcsAi386 | +++ | P | +++ | NT | NT |
| IcsAi456 | +++ | P | +++ | NT | NT |
| IcsAi502 | +++ | P | + | ++ | ++ |
| IcsAi532 | +++ | NP | − | − | − |
| IcsAi563 | +++ | NP | − | − | − |
| IcsAi595 | ++ | P | ++ | +++ | +++ |
| IcsAi598 | ++ | P | ++ | +++ | +++ |
| IcsAi633 | + | NT | − | + | + |
| IcsAi643 | + | NT | − | − | − |
| IcsAi677 | + | NT | − | − | − |
| IcsAi716 | + | NT | − | − | − |
| IcsAi748 | +++ | P | +++ | NT | NT |
Mutants with altered function are in boldface, and mutants with WT function are in plain type.
The “+++,” “++,” and “+” symbols indicate relative band intensities of Western immunoblots of whole-cell lysates.
NP, nonpolar; P, polar.
+++, WT plaques; ++, small plaques; +, foci; −, no plaques.
+++, WT N-WASP recruitment/F-actin comet tail formation; ++, 20 to 90% reduction in N-WASP recruitment/F-actin tail formation relative to WT; +, >90% reduction in N-WASP recruitment/F-actin tail formation; +/−, F-actin capping; −, N-WASP/F-actin tail formation not detected; NT, not tested. Quantitated as detailed in Materials and Methods.
FIG. 2.
Western blot analyses of IcsAi mutant production and secretion. (A) Whole-cell lysates from log-phase S. flexneri ΔicsA strains expressing IcsAWT or IcsAi mutants. (B) Tricholoroacetic acid-precipitated culture supernatants from log-phase S. flexneri ΔicsA strains expressing IcsAWT or IcsAi mutants. (C) Whole-cell lysates from E. coli UT5600 strains expressing IcsAWT or IcsAi mutants. All samples were electrophoresed on a 7.5% SDS-PAGE gel prior to Western blot analysis with anti-IcsA antibodies. The 116-kDa band corresponds to full-length IcsA, and the 95-kDa band corresponds to the cleaved form (IcsA′). Samples represent 1 × 108 cells or the culture supernatant equivalent of 1 × 109 cells.
Trypsin accessibility of IcsAi mutants.
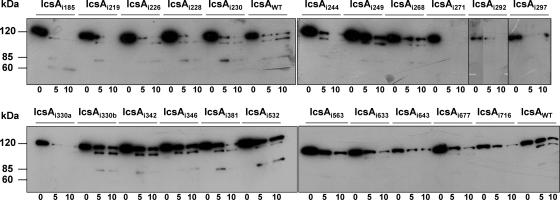
Despite linker insertions being small (5 aa) and in-frame, these mutations could potentially disrupt the overall conformation of the protein, and this may account for the loss of function demonstrated by some IcsAi mutants. In order to evaluate this possibility, in situ limited proteolysis with trypsin was performed on 22 IcsAi mutants that were, as detailed later, negative for plaque formation on a tissue culture monolayer (Table 2). To avoid intrinsic protein degradation by endogenous proteases, these studies were performed in an E. coli OM protease-deficient background (UT5600; Table 1). The protease digestion profiles of IcsAWT and 22 IcsAi mutants were compared after SDS-PAGE and Western immunoblotting with polyclonal anti-IcsA antibody. Major alterations to the protein conformation were expected to alter the accessibility of trypsin to sites within IcsA, thereby altering the profile of tryptic fragments. When the IcsAWT protein was subjected to in situ proteolysis with trypsin for either 5 or 10 min (Fig. 3), although band intensities varied between blots, three molecular mass bands of approximately 120 kDa, 100 kDa, and 85 kDa were consistently observed. The mutants IcsAi185, IcsAi219, IcsAi228, IcsAi244, IcsAi271, IcsAi292, IcsAi297, IcsAi330a, IcsAi563, IcsAi633, IcsAi643, IcsAi677, and IcsAi716 had a proteolysis profile different from that of IcsAWT, indicative of an altered sensitivity to trypsin. The latter suggests that these IcsAi mutant proteins have an altered conformation and interpretation of their phenotypes should be considered cautiously. Notably, five of these mutants (IcsAi633, IcsAi643, IcsAi677, and IcsAi716) possessed linker insertions within the putative autochaperone region. The mutants IcsAi226, IcsAi230, IcsAi248, IcsAi268, IcsAi330b, IcsAi342, IcsAi346, IcsAi381, and IcsAi532 had proteolysis profiles comparable to that of IcsAWT, signifying that they were likely to have a native conformation (Fig. 3). Hence, the corresponding phenotypes exhibited by S. flexneri expressing these proteins were unlikely to have resulted from major conformational changes. Interestingly, the mutants IcsAi330a and IcsAi330b had different proteolysis profiles despite harboring linker insertions in the same position. These mutants differed only in the encoded 5-aa insertion, with IcsAi330a harboring the insertion “TAAAI” and IcsAi330b the insertion “GAAAT” (see Table S1 in the supplemental material). However, these mutants did not differ phenotypically in any of the other functional assays.
FIG. 3.
Trypsin accessibility of IcsAi mutants. Log-phase cultures of E. coli UT5600 expressing IcsAWT or IcsAi mutants were treated with 0.1 μg/ml of trypsin at 25°C. Aliquots were taken at 0 min, 5 min, and 10 min and supplemented with 1 mM phenylmethylsulphonylfluoride to inhibit further proteolysis. Whole-cell lysates were electrophoresed on a 7.5% SDS-PAGE gel and subjected to Western blot analysis with anti-IcsA antibody. Samples represent the equivalent of 1 × 109 cells.
Effect of linker insertions on IcsA surface expression and polar distribution.
In order to assess the impact of each linker insertion on IcsA expression and distribution on the surface of bacteria, S. flexneri ΔicsA strains expressing IcsAi were examined by IF microscopy with anti-IcsA antibody. Of the six mutants that demonstrated reduced production by Western immunoblotting, IcsAi633, IcsAi643, IcsAi677, and IcsAi716 could not be detected on the surface of bacteria while IcsAi595 and IcsAi598 could be detected only weakly (Table 2). The remaining 41 IcsAi mutants could all be readily detected on the surface of S. flexneri (Table 2).
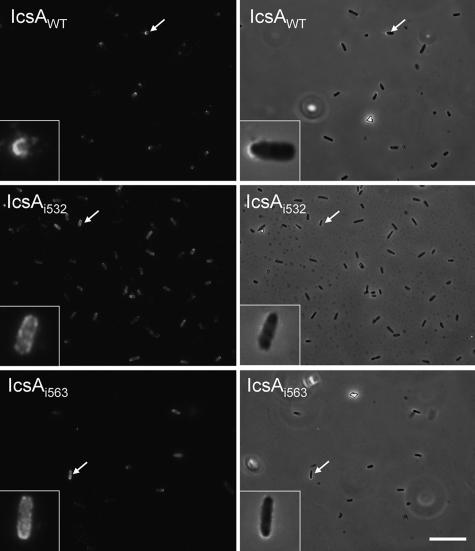
Thirty-nine IcsAi mutants that had WT production levels exhibited a surface distribution that was comparable to that of IcsAWT, with these proteins being predominantly localized to the pole(s) and little or no protein detected on the lateral regions of bacteria. However, two mutants, IcsAi532 and IcsAi563, displayed altered localization (Fig. 4; Table 2). IcsAi532 was distributed polarly on only 5% of bacteria, while for the majority of bacteria this protein was either distributed uniformly on the surface (47.5%) or distributed over the entire surface of the bacteria with some polar reinforcement (47.5%) (see Table S3 in the supplemental material). Similarly, the IcsAi563 mutant was distributed polarly on only 22.5% of the bacteria, while for the majority of bacteria this protein was either distributed uniformly on the surface (35%) or distributed over the entire surface of the bacteria with some polar reinforcement (42.5%) (see Table S3 in the supplemental material). Notably, mutants with linker insertions in polar localization region 1 (aa 1 to 104) (IcsAi56, IcsAi81, and IcsAi87) were all polarly distributed on at least 90% of bacteria (see Table S3 in the supplemental material). IcsAi595 and IcsAi598, which have a linker insertion in polar region 2, appeared to be polarly distributed (data not shown), but the percentage of bacteria with polar distribution could not be determined since the low level of expression of these proteins on the bacterial surface prevented reliable quantitation.
FIG. 4.
Nonpolar distribution of IcsAi532 and IcsAi563 mutants on the surface of S. flexneri. IF microscopy of IcsA surface distribution. Log-phase cultures of S. flexneri strains expressing either IcsAWT or IcsAi were formalin fixed and labeled with anti-IcsA antibodies and Alexa 488-conjugated goat antirabbit secondary antibodies. For clarity, the IF image for each strain is accompanied by an overlay of the IF image with the corresponding phase-contrast image. Bar = 10 μm.
Effect of linker insertions on IcsA function in intercellular spreading.
The ability of each IcsAi protein to support ABM was investigated by observing the capacity of S. flexneri ΔicsA strains expressing the mutated proteins to form plaques on HeLa cell monolayers. Plaque formation is an indication of intercellular spreading, a process that requires IcsA-dependent ABM. Eighteen of the S. flexneri strains expressing IcsAi proteins formed WT plaques (Table 2), indicating that linker insertions in these IcsAi mutants were located in functionally permissive sites. Twenty-two of the S. flexneri strains were unable to form plaques in three independent experiments (Table 2). These strains expressed IcsAi mutants with linker insertions either within the “GRR” region (aa 140 to 307) (IcsAi185, IcsAi219, IcsAi226, IcsAi228, IcsAi230, IcsAi244, IcsAi248, IcsAi268, IcsAi271, IcsAi292, and IcsAi297), the IcsB binding region (aa 320 to 433) (IcsAi330a, IcsAi330b, IcsAi342, IcsAi346, and IcsAi381), within polar localization region 2 (aa 507 to 620) (IcsAi532 and IcsAi563), or within or adjacent to the putative autochaperone domain (aa 634 to 735) (IcsAi633, IcsAi643, IcsAi677, and IcsAi716). The remaining strains had intermediate phenotypes, exhibiting a WT frequency of plaque formation but forming plaques that varied in size compared to the control. Strains expressing the mutants IcsAi56, IcsAi595, and IcsAi598 formed plaques that were smaller in diameter than those of bacteria expressing IcsAWT (30%, 51%, and 27% smaller, respectively). Additionally, while strains expressing IcsAi193, IcsAi288, IcsAi312, and IcsAi502 were unable to form plaques, they did form small foci of dead HeLa cells that were not observed for S. flexneri ΔicsA, suggesting that the mutants IcsAi193, IcsAi288, IcsAi312, and IcsAi502 still retained a limited capacity to promote intercellular spread (Table 2).
Effect of linker insertions on IcsA function in N-WASP recruitment and F-actin comet tail formation.
To further investigate the molecular basis for the defects in intercellular spreading exhibited by strains expressing IcsAi proteins, the ability of these strains to recruit host N-WASP and form F-actin comet tails was examined by IF microscopy using anti-N-WASP antibodies and FITC-phalloidin, respectively. Nineteen out of the 29 strains expressing IcsAi mutants were unable to either recruit N-WASP or form F-actin comet tails (Table 2). All of these strains had also been negative for plaque formation. This included strains expressing the mutant proteins IcsAi185, IcsAi219, IcsAi226, IcsAi230, IcsAi244, IcsAi248, IcsAi268, IcsAi271, and IcsAi297, with insertions within the GRR region, strains expressing IcsAi330a, IcsA330b, IcsAi342, IcsAi346, and IcsAi381, with linker insertions within the IcsB binding region, and strains expressing IcsAi532 and IcsAi563 with linker insertions within polar localization region 2.
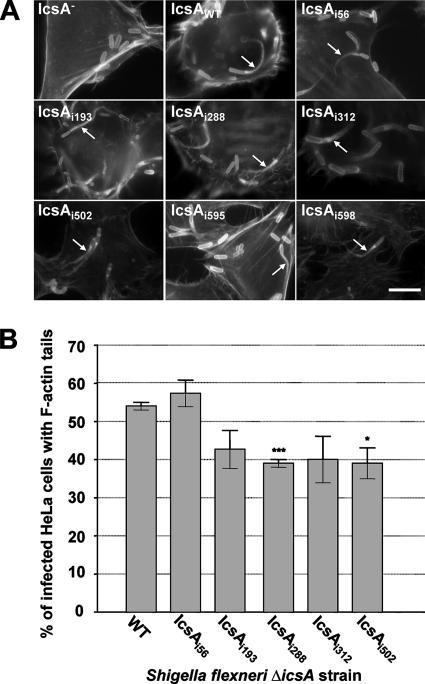
Although a strain producing IcsAi346 was unable to form F-actin comet tails, capping of the bacterial pole with F-actin was observed, despite undetectable N-WASP recruitment (Table 2). Also, while they were unable to form detectable plaques or foci, F-actin tail formation was seen at a very low frequency (in <10% of infected cells) for strains producing IcsAi228, IcsAi292 (which had insertions located in the GRR region), and IcsAi633 (which had an insertion in the putative autochaperone region), and N-WASP recruitment could be detected only with the latter two mutants (Table 2). Strains producing IcsAi595 and IcsAi598 that had reduced levels of protein were readily able to recruit N-WASP (Table 2) and form F-actin comet tails (Fig. 5A).
FIG. 5.
F-actin comet tail formation by intracellular S. flexneri ΔicsA strains expressing IcsA-i mutants. (A) IF microscopy of F-actin tail formation by intracellular S. flexneri ΔicsA strain expressing IcsA-i mutants. HeLa cells infected with S. flexneri were labeled with anti-LPS antibodies and Alexa 594-conjugated donkey antirabbit antibodies, and F-actin was labeled with FITC-phalloidin. Arrows indicate F-actin tail formation. Strains were assessed in three independent experiments. Scale bar = 10 μm. (B) Frequency of F-actin tail formation by S. flexneri ΔicsA strains expressing IcsA-i mutants. HeLa cells were infected with S. flexneri strains and examined by IF microscopy as detailed in Materials and Methods. The frequency of F-actin tail formation was determined by observing the percentage of infected HeLa cells (n = 100) that had at least 1 F-actin tail. Data represent means ± standard errors. *, P < 0.05; ***, P < 0.001 (determined by Student's unpaired two-tailed t test). Data are from three independent experiments.
Although S. flexneri strains expressing IcsAi56, IcsAi193, IcsAi288, IcsAi312, and IcsAi502 formed reduced-size plaques, these mutants still displayed efficient N-WASP recruitment (Table 2) and F-actin comet tail formation (Fig. 5A). To reconcile these data, it was hypothesized that the frequency of tail formation of these strains may have been only slightly reduced, resulting in a decrease in intercellular spreading and plaque formation. Accordingly, F-actin comet tail formation was more rigorously quantitated (see Materials and Methods) in order to detect any small changes in F-actin tail formation frequency. The mutants IcsAi598 and IcsAi595 were not included in these studies since the low-level production of these mutant proteins would complicate interpretation of their phenotypes. Interestingly, for all strains tested, F-actin comet tail formation was found to occur at a frequency that was not statistically different from that with IcsAWT, except for strains expressing IcsAi288 (P < 0.001) and IcsAi502 (P < 0.05) (Fig. 5B).
Effect of LPS Oag on F-actin comet tail formation and N-WASP recruitment by S. flexneri expressing IcsA mutants.
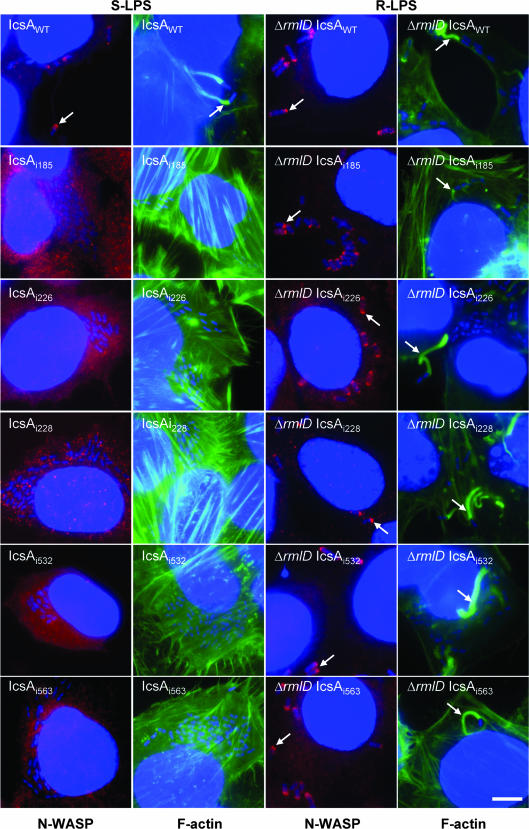
We have previously shown that smooth-LPS (S-LPS) populations, possessing the Oag component, can mask IcsA function (31, 32). Therefore, it was of interest to determine if IcsAi mutants that were unable to recruit N-WASP in an S-LPS background could do so in an S. flexneri strain that has only “rough” LPS (R-LPS) populations that lack the Oag component. Furthermore, it was of interest to determine if expression of IcsAi mutants in this background would restore the ability of these IcsAi mutants to form F-actin comet tails. Sixteen IcsAi mutants (Table 2) that were either unable or had a reduced ability to recruit N-WASP and induce F-actin comet tails were expressed in an R-LPS S. flexneri ΔicsA ΔrmlD strain (RMA2043), and the intracellular behavior of each strain was examined by IF microscopy.
Remarkably, when either the IcsAi185, IcsAi226, IcsAi532, or IcsAi563 mutants were expressed in an R-LPS S. flexneri ΔicsA ΔrmlD strain, F-actin comet tail formation and N-WASP recruitment were observed (Fig. 6; Table 2), albeit at a lower frequency (10 to 20% of infected cells contained F-actin comet tails) than with the S. flexneri ΔicsA ΔrmlD strain expressing IcsAWT (RMA2107; Table 1) (54% of infected cells had F-actin comet tails). For IcsAi228, N-WASP recruitment could not be detected when this mutant was expressed in an S-LPS S. flexneri ΔicsA strain, despite this strain occasionally forming F-actin comet tails (Table 2). However, when IcsAi228 was expressed in the R-LPS S. flexneri ΔicsA ΔrmlD strain, N-WASP recruitment could be detected and F-actin comet tail formation occurred more frequently (albeit still at a lower frequency than with the S. flexneri ΔicsA ΔrmlD strain expressing IcsAWT). Additionally, for the S. flexneri ΔicsA ΔrmlD strain expressing IcsAi271, N-WASP recruitment could be detected, albeit faintly (Table 2). For the remaining R-LPS strains that were investigated, N-WASP recruitment and F-actin comet tail formation could not be detected (Table 2).
FIG. 6.
N-WASP recruitment and F-actin comet tail formation by intracellular S. flexneri strains expressing the IcsAi proteins with either smooth or rough LPS. IF microscopy of F-actin tail formation and N-WASP recruitment by intracellular S. flexneri strains expressing IcsA-i mutants. HeLa cells were infected with S. the flexneri ΔicsA (S-LPS) or S. flexneri ΔicsA ΔrmlD (R-LPS) strain expressing IcsAi proteins and formalin fixed. Bacteria were labeled with DAPI (blue), F-actin was labeled with FITC-phalloidin (green), and N-WASP was labeled with anti-N-WASP and Alex 594-conjugated donkey antirabbit antibodies (red) as detailed in Materials and Methods. Arrows indicate F-actin tail formation and N-WASP recruitment. Strains were assessed in two independent experiments. Bar = 10 μm.
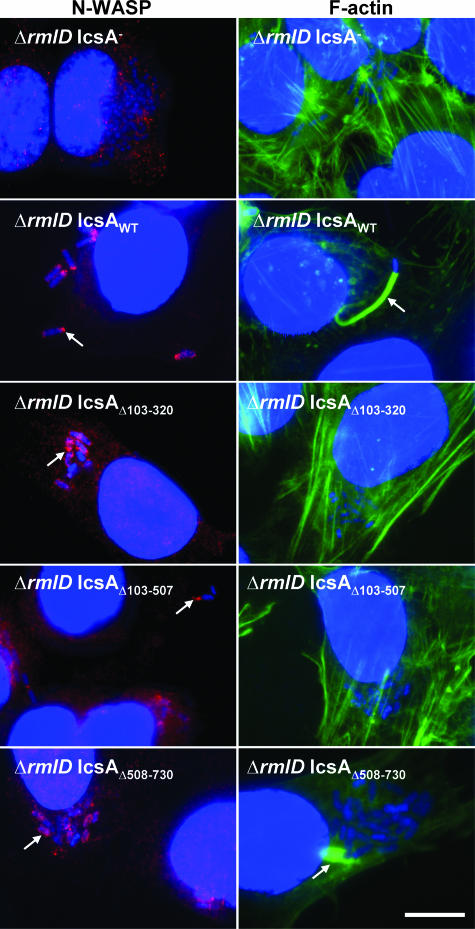
The identification of IcsAi proteins that were able to recruit N-WASP and induce F-actin comet tails in an R-LPS background but not in the presence of S-LPS was interesting. Based on this finding, it was hypothesized that previously reported IcsA deletion mutants (49-51) would also be subject to masking by LPS Oag, perhaps to even a greater extent since these IcsA constructs possessed large deletions ranging in size from 188 aa to 404 aa. Suzuki et al. (51) reported that IcsAΔ103-320, IcsAΔ508-730, and IcsAΔ103-507 were unable to induce F-actin comet tail formation when expressed by an S-LPS S. flexneri ΔicsA strain, although IcsAΔ508-730 did induce F-actin accumulation. Furthermore, the latter mutant was reported to recruit N-WASP, while the others did not (49, 50). Hence, plasmids encoding IcsAΔ103-320 (pD10-virG1), IcsAΔ508-730 (pD10-virG3), and IcsAΔ103-507 (pD10-virG4) were expressed in an R-LPS S. flexneri ΔicsA ΔrmlD strain (RMA2043), and N-WASP recruitment and F-actin comet tail formation were examined. As reported previously for S-LPS strains, when these proteins were expressed in an R-LPS background, none of the IcsA deletion mutant proteins were able to induce F-actin tail formation (Fig. 7; Table 3). The N-WASP recruitment and circumferential accumulation of F-actin observed when IcsAΔ508-730 was expressed in an S-LPS S. flexneri background were also observed when this construct was expressed in an R-LPS background (Fig. 7; Table 3). Surprisingly, though, the other two mutants, IcsAΔ103-320 and IcsAΔ103-507, also exhibited a low level of N-WASP recruitment when expressed in an R-LPS S. flexneri strain (Fig. 7; Table 3). These results indicate that these deletion mutations retain some residual N-WASP recruitment activity that is masked in an S-LPS background.
FIG. 7.
N-WASP recruitment and F-actin comet tail formation by intracellular S. flexneri strains expressing IcsA deletion mutant proteins with either smooth or rough LPS. IF microscopy of F-actin tail formation and N-WASP recruitment by intracellular S. flexneri strains expressing IcsAΔ mutants. HeLa cells were infected with S. flexneri ΔicsA (S-LPS) or S. flexneri ΔicsA ΔrmlD (R-LPS) strains expressing IcsA deletion mutant proteins and formalin fixed. Bacteria were labeled with DAPI (blue), F-actin was labeled with FITC-phalloidin (green), and N-WASP was labeled with anti-N-WASP and Alex 594-conjugated donkey antirabbit antibodies (red) as detailed in Materials and Methods. Arrows indicate F-actin tail formation or N-WASP recruitment. Strains were assessed in two independent experiments. Bar = 10 μm.
TABLE 3.
F-actin comet tail formation and N-WASP recruitment by R-LPS S. flexneri ΔicsA ΔrmlD strains expressing IcsAi mutants
| Protein expressed | Phenotype of S. flexneri ΔicsA ΔrmlD straina
|
|
|---|---|---|
| F-actin tail formation | N-WASP recruitment | |
| IcsAWT | +++ | +++ |
| IcsAi185 | ++ | ++ |
| IcsAi219 | − | − |
| IcsAi226 | ++ | ++ |
| IcsAi228 | ++ | ++ |
| IcsAi244 | − | − |
| IcsAi248 | − | − |
| IcsAi268 | − | − |
| IcsAi271 | − | + |
| IcsAi297 | − | − |
| IcsAi330a | − | − |
| IcsAi330b | − | − |
| IcsAi342 | − | − |
| IcsAi346 | − | +/− |
| IcsAi381 | − | − |
| IcsAi532 | ++ | ++ |
| IcsAi563 | ++ | ++ |
| IcsAΔ103-320 | − | + |
| IcsAΔ508-730 | +/− | + |
| IcsAΔ103-507 | − | + |
+++, WT N-WASP recruitment/F-actin comet tail formation; ++, 50 to 90% reduction in N-WASP recruitment/F-actin tail formation relative to IcsAWT; +, >90% reduction in N-WASP recruitment/F-actin tail formation; +/−, F-actin capping; −, N-WASP/F-actin tail formation not detected. Two independent experiments (n = 20) were carried out.
DISCUSSION
The IcsA (VirG) protein of S. flexneri is essential for virulence, and through its interaction with host N-WASP, it is able to initiate F-actin comet tail formation, leading to bacterial ABM and intercellular spreading throughout the colonic epithelium (2, 17, 18, 24, 52). The aim of our study was to refine our understanding of the IcsA structure-function relationship using pentapeptide mutagenesis, since previous studies have for the most part utilized either large deletion mutations or expression of small fragments of IcsA to identify functional domains. Linker insertion mutagenesis is an effective tool for examining structure-function relationships and has been used successfully for other AT proteins (7, 12, 22). There is the potential for such insertions to impact on the overall structure of the target protein, and the effects may not be strictly localized to the sight of the insertion. Therefore, we have attempted to address this issue by assessing the protease accessibility of mutants as a probe for major alterations of protein conformation. Nonetheless, our data obtained using the linker insertion mutagenesis approach are consistent with those of previous studies and have additionally allowed us to identify novel functional regions within the passenger domain.
Of the 47 IcsAi mutants that were created in this study, 6 proteins (IcsAi595, IcsAi598, IcsAi633, IcsAi643, IcsAi677, and IcsAi716) demonstrated reduced production of both cell-associated and secreted forms in S. flexneri. However, production of IcsAi595, IcsAi598, and IcsAi633 was restored to WT levels when they were expressed in E. coli UT5600 (ΔompP ΔompT), consistent with the hypothesis that these proteins may be sensitive to degradation by endogenous OM proteases. Production of IcsAi643, IcsAi677, and IcsAi716 was still greatly reduced in E. coli UT5600, suggesting sensitivity to degradation by periplasmic proteases, such as DegP. Indeed, DegP has been implicated in IcsA export (20, 43, 44). Additionally, all six mutants demonstrated increased sensitivity to trypsin. A change in protease accessibility of these mutants implies a conformational alteration compared to IcsAWT. Oliver et al. (39) have previously described a putative intramolecular chaperone region of the passenger domain conserved between several AT proteins, including IcsA (aa 634 to 735). In the B. pertussis AT protein, BrkA, deletion of this autochaperone region (aa 601 to 692) made the protein susceptible to degradation by OM proteases and trypsin. Similarly, this has also been shown for homologous regions within other autotransporter proteins, SSP, Pet, and AIDA-1 (3, 12, 38). The role of this region in IcsA biogenesis had not been previously investigated. Our study provides the first experimental evidence implicating this region of IcsA as a putative autochaperone domain. Owing to the associated production defect, it was not possible to determine the contribution of this region to N-WASP recruitment and F-actin comet tail formation.
Two regions of the passenger domain (aa 1 to 104 and aa 507 to 620) have previously been attributed to polar localization of IcsA (9, 51). We have identified two mutants, IcsAi532 and IcsAi563, with altered IcsA localization on the surface of S. flexneri. Both of these mutants possessed linker insertions within polar localization region 2 (aa 507 to 620); three mutants (IcsAi56, IcsAi81, and IcsAi87) that possessed linker insertions within polar localization region 1 (aa 1 to 104) were all polarly distributed. Our data suggest that polar targeting region 1 is not able to compensate for mutational alterations in region 2. These results refute the significance of polar targeting region 1, which has previously been reported to be targeted to the pole independently of polar targeting region 2 (9).
Another significant finding from our study was that the interaction between N-WASP and some IcsAi and IcsAΔ proteins inside host cells was influenced by LPS Oag. By assessing protein function in an R-LPS background, we were able to account for masking of mutant proteins by LPS Oag when interpreting the resulting phenotypes. The mutants IcsAi185, IcsAi226, IcsAi532, and IcsAi563 were unable to recruit N-WASP and induce F-actin comet tails when expressed in S-LPS S. flexneri ΔicsA strains. However, these mutants were able to recruit N-WASP and form F-actin tails in an R-LPS S. flexneri background (albeit at a lower frequency than R-LPS S. flexneri expressing IcsAWT). Additionally, the IcsAΔ103-320 and IcsAΔ103-507 deletion mutants examined in our study, although unable to recruit N-WASP when expressed by S-LPS S. flexneri, were able to recruit N-WASP when expressed by R-LPS S. flexneri strains. Hence, mutations in IcsA that abolish N-WASP recruitment do not necessarily indicate mutational alteration of N-WASP binding regions of IcsA but may be due to LPS-Oag-dependent interference in IcsA-N-WASP interactions, possibly due to conformational alterations or truncation of mutant proteins.
In our study, the ability of mutants to facilitate F-actin comet tail formation correlated with N-WASP recruitment. However, we identified icsAi mutants that had proficient F-actin comet tail formation but were defective in intercellular spread. The molecular basis of this defect is currently under investigation, but these findings highlight the need for intercellular spreading to be directly assessed when determining the relative contributions of various host and bacterial factors to the process of ABM.
The region spanning aa 103 to 433 of IcsA has previously been shown to be adequate for N-WASP binding, as established by in vitro pull-down assays (49, 50), and IcsA aa 53 to 508 are sufficient to induce actin polymerization during in vitro assays (17). Therefore, it was not surprising that most of the linker insertions within this region disrupted IcsA function in N-WASP recruitment and ABM. Fifteen IcsAi mutants had linker insertions within the GRR region (aa 140 to 307), and nine of these mutant proteins (IcsAi185, IcsAi219, IcsAi226, IcsAi230, IcsAi244, IcsAi248, IcsAi268, IcsAi271, and IcsAi297) were to unable induce N-WASP recruitment and F-actin comet tail formation when expressed by S. flexneri in infected cells. Although deletion of aa 104 to 226 has been reported to abolish F-actin comet tail formation (51), this had not been correlated with N-WASP recruitment. Data from our study confirm the involvement of this region in N-WASP recruitment. Five mutants (IcsAi330a, IcsA330b, IcsAi342, IcsAi346, and IcsAi381) with linker insertions within the IcsB binding region (aa 320 to 433) were also unable either to form plaques or F-actin comet tails or to recruit N-WASP when expressed by S. flexneri. These icsAi mutants differ phenotypically from the icsB mutants from previous studies (36, 37), which were unable to form plaques in tissue culture monolayers but were able to form F-actin tails and exhibit actin-based motility at a frequency similar to that of the WT up to 3 h postinfection (37).
Significantly, we observed that an R-LPS S. flexneri strain expressing IcsAΔ103-507 exhibited a low level of N-WASP recruitment. Since this deletion mutation removes both the GRR region and the IcsB regions, it suggests that an additional region(s) was involved in N-WASP recruitment. Deletion of aa 508 to 730 of IcsA has previously been shown to affect F-actin comet tail formation (51). However, the IcsAΔ508-730 mutant also displayed aberrant localization on the surface of bacteria, complicating the interpretation of this mutant's phenotype (51). Indeed, the authors of that paper suggested that the defect in F-actin comet tail formation resulted from nonpolar localization of IcsA. Our study has identified two mutants, IcsAi532 and IcsAi563, which are also nonpolarly expressed on the surface of S. flexneri and are unable to induce F-actin comet tail formation in S-LPS strains (like IcsAΔ508-730). However, F-actin comet tail formation was restored, albeit at a low frequency, when these mutants were expressed in an R-LPS strain. The same was not observed for IcsAΔ508-730, which was unable to induce F-actin comet tail formation in R-LPS strains. The latter indicates that an additional defect occurs as a result of deleting this region that is independent of the effect on IcsA polarity.
The results of our study suggest a previously unidentified region of IcsA (aa 508 to 730) is important for N-WASP recruitment and F-actin comet tail formation, and this warrants further investigation. We propose a model to reconcile this data, in which multiple regions of IcsA are required for N-WASP recruitment. Our model is supported by the demonstration that WASP homology domain 1 and the Cdc42/Rac interactive binding domain of N-WASP are independently recruited to S. flexneri inside N-WASP-deficient cell lines or HeLa cells (25, 30). The determination of the structure of IcsA and IcsA in complex with N-WASP (with and without LPS Oag) would clearly assist our understanding of the binding process.
Supplementary Material
Acknowledgments
We thank Hiroaki Miki for the kind gifts of N-WASP antisera and His-tagged and glutathione S-transferase-tagged N-WASP constructs and Chihiro Sasakawa for virG deletion constructs. We gratefully acknowledge Luisa Van Den Bosch and Lisa Clark for technical assistance and Marcin Grabowicz for critically reading the manuscript.
This work was supported by a project grant from the National Health and Medical Research Council of Australia awarded to R.M. K.L.M. was the recipient of a Faculty of Science Postgraduate Scholarship from the University of Adelaide.
Footnotes
Published ahead of print on 2 May 2008.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Baker, S., J. Gunn, and R. Morona. 1999. The Salmonella typhi melittin resistance gene pqaB affects intracellular growth in PMA-differentiated U937 cells, polymyxin B resistance and lipopolysaccharide. Microbiology 145367-378. [DOI] [PubMed] [Google Scholar]
- 2.Bernardini, M. L., J. Mounier, H. d'Hauteville, M. Coquis-Rondon, and P. J. Sansonetti. 1989. Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc. Natl. Acad. Sci. USA 863867-3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berthiaume, F., N. Rutherford, and M. Mourez. 2007. Mutations affecting the biogenesis of the AIDA-I autotransporter. Res. Microbiol. 158348-354. [DOI] [PubMed] [Google Scholar]
- 4.Brahmbhatt, H. N., A. A. Lindberg, and K. N. Timmis. 1992. Shigella lipopolysaccharide: structure, genetics, and vaccine development. Curr. Top. Microbiol. Immunol. 18045-64. [DOI] [PubMed] [Google Scholar]
- 5.Brandon, L. D., N. Goehring, A. Janakiraman, A. W. Yan, T. Wu, J. Beckwith, and M. B. Goldberg. 2003. IcsA, a polarly localized autotransporter with an atypical signal peptide, uses the Sec apparatus for secretion, although the Sec apparatus is circumferentially distributed. Mol. Microbiol. 5045-60. [DOI] [PubMed] [Google Scholar]
- 6.Brandon, L. D., and M. B. Goldberg. 2001. Periplasmic transit and disulfide bond formation of the autotransported Shigella protein IcsA. J. Bacteriol. 183951-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charbonneau, M.-E., and M. Mourez. 2007. Functional organization of the autotransporter adhesin involved in diffuse adherence. J. Bacteriol. 1899020-9029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charles, M., J. Magdalena, J. A. Theriot, and M. B. Goldberg. 1999. Functional analysis of a rickettsial OmpA homology domain of Shigella flexneri IcsA. J. Bacteriol. 181869-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charles, M., M. Perez, J. H. Kobil, and M. B. Goldberg. 2001. Polar targeting of Shigella virulence factor IcsA in Enterobacteriacae and Vibrio. Proc. Natl. Acad. Sci. USA 989871-9876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cossart, P. 2000. Actin-based motility of pathogens: the Arp2/3 complex is a central player. Cell. Microbiol. 2195-205. [DOI] [PubMed] [Google Scholar]
- 11.d'Hauteville, H., R. Dufourcq Lagelouse, F. Nato, and P. Sansonetti. 1996. Lack of cleavage of IcsA in Shigella flexneri causes aberrant movement and allows demonstration of a cross-reactive eukaryotic protein. Infect. Immun. 64511-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dutta, P. R., B. Q. Sui, and J. P. Nataro. 2003. Structure-function analysis of the enteroaggregative Escherichia coli plasmid-encoded toxin autotransporter using scanning linker mutagenesis. J. Biol. Chem. 27839912-39920. [DOI] [PubMed] [Google Scholar]
- 13.Egile, C., H. d'Hauteville, C. Parsot, and P. J. Sansonetti. 1997. SopA, the outer membrane protease responsible for polar localization of IcsA in Shigella flexneri. Mol. Microbiol. 231063-1073. [DOI] [PubMed] [Google Scholar]
- 14.Frischknecht, F., S. Cudmore, V. Moreau, I. Reckmann, S. Rottger, and M. Way. 1999. Tyrosine phosphorylation is required for actin-based motility of Vaccinia but not Listeria or Shigella. Curr. Biol. 989-92. [DOI] [PubMed] [Google Scholar]
- 15.Fukuda, I., T. Suzuki, H. Munakata, N. Hayashi, E. Katayama, M. Yoshikawa, and C. Sasakawa. 1995. Cleavage of Shigella surface protein VirG occurs at a specific site, but the secretion is not essential for intracellular spreading. J. Bacteriol. 1771719-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukuoka, M., H. Miki, and T. Takenawa. 1997. Identification of N-WASP homologs in human and rat brain. Gene 19643-48. [DOI] [PubMed] [Google Scholar]
- 17.Goldberg, M. B. 2001. Actin-based motility of intracellular microbial pathogens. Microbiol. Mol. Biol. Rev. 65595-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldberg, M. B., O. Barzu, C. Parsot, and P. J. Sansonetti. 1993. Unipolar localization and ATPase activity of IcsA, a Shigella flexneri protein involved in intracellular movement. J. Bacteriol. 1752189-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henderson, I. R., F. Navarro-Garcia, M. Desvaux, R. C. Fernandez, and D. Ala'Aldeen. 2004. Type V protein secretion pathway: the autotransporter story. Microbiol. Mol. Biol. Rev. 68692-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jain, S., and M. B. Goldberg. 2007. Requirement for YaeT in the outer membrane assembly of autotransporter proteins. J. Bacteriol. 1895393-5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jann, K., and B. Jann. 1987. Polysaccharide antigens of Escherichia coli. Rev. Infect. Dis. 9S517-S526. [DOI] [PubMed] [Google Scholar]
- 22.Klemm, P., L. Hjerrild, M. Gjermansen, and M. A. Schembri. 2004. Structure-function analysis of the self-recognizing antigen 43 autotransporter protein from Escherichia coli. Mol. Microbiol. 51283-296. [DOI] [PubMed] [Google Scholar]
- 23.Kotloff, K. L., F. Noriega, G. A. Losonsky, M. B. Sztein, S. S. Wasserman, J. P. Nataro, and M. M. Levine. 1996. Safety, immunogenicity, and transmissibility in humans of CVD 1203, a live oral Shigella flexneri 2a vaccine candidate attenuated by deletions in aroA and virG. Infect. Immun. 644542-4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lett, M. C., C. Sasakawa, N. Okada, T. Sakai, S. Makino, M. Yamada, K. Komatsu, and M. Yoshikawa. 1989. virG, a plasmid-coded virulence gene of Shigella flexneri: identification of the VirG protein and determination of the complete coding sequence. J. Bacteriol. 171353-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lommel, S., S. Benesch, K. Rottner, T. Franz, J. Wehland, and R. Kuhn. 2001. Actin pedestal formation by enteropathogenic Escherichia coli and intracellular motility of Shigella flexneri are abolished in N-WASP-defective cells. EMBO Rep. 2850-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lugtenberg, B., J. Meijers, R. Peters, P. van der Hoek, and L. van Alphen. 1975. Electrophoretic resolution of the “major outer membrane protein” of Escherichia coli K12 into four bands. FEBS Lett. 58254-258. [DOI] [PubMed] [Google Scholar]
- 27.Makino, S., C. Sasakawa, K. Kamata, T. Kurata, and M. Yoshikawa. 1986. A genetic determinant required for continuous reinfection of adjacent cells on large plasmid in S. flexneri 2a. Cell 46551-555. [DOI] [PubMed] [Google Scholar]
- 28.Miki, H., and T. Takenawa. 2003. Regulation of actin dynamics by WASP family proteins. J. Biochem. 134309-313. [DOI] [PubMed] [Google Scholar]
- 29.Monack, D. M., and J. A. Theriot. 2001. Actin-based motility is sufficient for bacterial membrane protrusion formation and host cell uptake. Cell. Microbiol. 3633-647. [DOI] [PubMed] [Google Scholar]
- 30.Moreau, V., F. Frischknecht, I. Reckmann, R. Vincentelli, G. Rabut, D. Stewart, and M. Way. 2000. A complex of N-WASP and WIP integrates signalling cascades that lead to actin polymerization. Nat. Cell Biol. 2441-448. [DOI] [PubMed] [Google Scholar]
- 31.Morona, R., C. Daniels, and L. Van Den Bosch. 2003. Genetic modulation of Shigella flexneri 2a lipopolysaccharide O antigen modal chain length reveals that it has been optimized for virulence. Microbiology 149925-939. [DOI] [PubMed] [Google Scholar]
- 32.Morona, R., and L. Van Den Bosch. 2003. Lipopolysaccharide O antigen chains mask IcsA (VirG) in Shigella flexneri. FEMS Microbiol. Lett. 221173-180. [DOI] [PubMed] [Google Scholar]
- 33.Morona, R., and L. Van Den Bosch. 2003. Multicopy icsA is able to suppress the virulence defect caused by the wzzSF mutation in Shigella flexneri. FEMS Microbiol. Lett. 221213-219. [DOI] [PubMed] [Google Scholar]
- 34.Morona, R., L. Van Den Bosch, and P. Manning. 1995. Molecular, genetic, and topological characterization of O-antigen chain length regulation in Shigella flexneri. J. Bacteriol. 1771059-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oaks, E. V., M. E. Wingfield, and S. B. Formal. 1985. Plaque formation by virulent Shigella flexneri. Infect. Immun. 48124-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogawa, M., T. Suzuki, I. Tatsuno, H. Abe, and C. Sasakawa. 2003. IcsB, secreted via the type III secretion system, is chaperoned by IpgA and required at the post-invasion stage of Shigella pathogenicity. Mol. Microbiol. 48913-931. [DOI] [PubMed] [Google Scholar]
- 37.Ogawa, M., T. Yoshimori, T. Suzuki, H. Sagara, N. Mizushima, and C. Sasakawa. 2005. Escape of Intracellular Shigella from Autophagy. Science 307727-731. [DOI] [PubMed] [Google Scholar]
- 38.Ohnishi, Y., M. Nishiyama, S. Horinouchi, and T. Beppu. 1994. Involvement of the COOH-terminal pro-sequence of Serratia marcescens serine protease in the folding of the mature enzyme. J. Biol. Chem. 26932800-32806. [PubMed] [Google Scholar]
- 39.Oliver, D. C., G. Huang, E. Nodel, S. Pleasance, and R. C. Fernandez. 2003. A conserved region within the Bordetella pertussis autotransporter BrkA is necessary for folding of its passenger domain. Mol. Microbiol. 471367-1383. [DOI] [PubMed] [Google Scholar]
- 40.Pallen, M. J., R. R. Chaudhuri, and I. R. Henderson. 2003. Genomic analysis of secretion systems. Curr. Opin. Microbiol. 6519-527. [DOI] [PubMed] [Google Scholar]
- 41.Pantaloni, D., C. L. Clainche, and M.-F. Carlier. 2001. Mechanism of actin-based motility. Science 2921502-1506. [DOI] [PubMed] [Google Scholar]
- 42.Philpott, D. J., J. D. Edgeworth, and P. J. Sansonetti. 2000. The pathogenesis of Shigella flexneri infection: lessons from in vitro and in vivo studies. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355575-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Purdy, G. E., C. R. Fisher, and S. M. Payne. 2007. IcsA surface presentation in Shigella flexneri requires the periplasmic chaperones DegP, Skp, and SurA. J. Bacteriol. 1895566-5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Purdy, G. E., M. Hong, and S. M. Payne. 2002. Shigella flexneri DegP facilitates IcsA surface expression and is required for efficient intercellular spread. Infect. Immun. 706355-6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sansonetti, P. J., J. Arondel, A. Fontaine, H. d'Hauteville, and M. L. Bernardini. 1991. OmpB (osmo-regulation) and icsA (cell-to-cell spread) mutants of Shigella flexneri: vaccine candidates and probes to study the pathogenesis of shigellosis. Vaccine 9416-422. [DOI] [PubMed] [Google Scholar]
- 46.Shere, K. D., S. Sallustio, A. Manessis, T. G. D'Aversa, and M. B. Goldberg. 1997. Disruption of IcsP, the major Shigella protease that cleaves IcsA, accelerates actin-based motility. Mol. Microbiol. 25451-462. [DOI] [PubMed] [Google Scholar]
- 47.Steinhauer, J., R. Agha, T. Pham, A. W. Varga, and M. B. Goldberg. 1999. The unipolar Shigella surface protein IcsA is targeted directly to the bacterial old pole: IcsP cleavage of IcsA occurs over the entire bacterial surface. Mol. Microbiol. 32367-377. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki, T., M.-C. Lett, and C. Sasakawa. 1995. Extracellular transport of VirG protein in Shigella. J. Biol. Chem. 27030874-30880. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki, T., H. Miki, T. Takenawa, and C. Sasakawa. 1998. Neural Wiskott-Aldrich syndrome protein is implicated in the actin-based motility of Shigella flexneri. EMBO J. 172767-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suzuki, T., H. Mimuro, S. Suetsugu, H. Miki, T. Takenawa, and C. Sasakawa. 2002. Neural Wiskott-Aldrich syndrome protein (N-WASP) is the specific ligand for Shigella VirG among the WASP family and determines the host cell type allowing actin-based spreading. Cell. Microbiol. 4223-233. [DOI] [PubMed] [Google Scholar]
- 51.Suzuki, T., S. Saga, and C. Sasakawa. 1996. Functional analysis of Shigella VirG domains essential for interaction with vinculin and actin-based motility. J. Biol. Chem. 27121878-21885. [DOI] [PubMed] [Google Scholar]
- 52.Suzuki, T., and C. Sasakawa. 2001. Molecular basis of the intracellular spreading of Shigella. Infect. Immun. 695959-5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van den Bosch, L., P. A. Manning, and R. Morona. 1997. Regulation of O-antigen chain length is required for Shigella flexneri virulence. Mol. Microbiol. 23765-775. [DOI] [PubMed] [Google Scholar]
- 54.Van den Bosch, L., and R. Morona. 2003. The actin-based motility defect of a Shigella flexneri rmlD rough LPS mutant is not due to loss of IcsA polarity. Microb. Pathog. 3511-18. [DOI] [PubMed] [Google Scholar]
- 55.Yarar, D., W. To, A. Abo, and M. D. Welch. 1999. The Wiskott-Aldrich syndrome protein directs actin-based motility by stimulating actin nucleation with the Arp2/3 complex. Curr. Biol. 9555-559. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.