Abstract
The pathogenic bacterium Pseudomonas aeruginosa utilizes the 3-oxododecanoyl homoserine lactone (3OC12-HSL) autoinducer as a signaling molecule to coordinate the expression of virulence genes through quorum sensing. 3OC12-HSL also affects responses in host cells, including the upregulation of genes encoding inflammatory cytokines. This proinflammatory response may exacerbate underlying disease during P. aeruginosa infections. The specific mechanism(s) through which 3OC12-HSL influences host responses is unclear, and no mammalian receptors for 3OC12-HSL have been identified to date. Here, we report that 3OC12-HSL increases mRNA levels for a common panel of proinflammatory genes in murine fibroblasts and human lung epithelial cells. To identify putative 3OC12-HSL receptors, we examined the expression patterns of a panel of nuclear hormone receptors in these two cell lines and determined that both peroxisome proliferator-activated receptor beta/delta (PPARβ/δ) and PPARγ were expressed. 3OC12-HSL functioned as an agonist of PPARβ/δ transcriptional activity and an antagonist of PPARγ transcriptional activity and inhibited the DNA binding ability of PPARγ. The proinflammatory effect of 3OC12-HSL in lung epithelial cells was blocked by the PPARγ agonist rosiglitazone, suggesting that 3OC12-HSL and rosiglitazone are mutually antagonistic negative and positive regulators of PPARγ activity, respectively. These data identify PPARβ/δ and PPARγ as putative mammalian 3OC12-HSL receptors and suggest that PPARγ agonists may be employed as anti-inflammatory therapeutics for P. aeruginosa infections.
Inflammation is a complex biological reaction of the innate immune system in response to harmful stimuli, such as pathogens, damaged cells, or irritants (1). This inflammatory response serves to destroy, dilute, or contain the injurious agent and sets into motion events that promote tissue repair. Although fundamentally a protective response, inflammation may contribute to a host of disease processes (1). Chronic inflammation underlies several degenerative diseases, such as rheumatoid arthritis, atherosclerosis, and lung fibrosis, and acute inflammation is responsible for life-threatening hypersensitivity reactions to insect bites, drugs, and toxins (16, 29, 30, 55). In chronic lung infections, tissue repair by fibrosis may lead to remodeling and loss of function (29). For example, cystic fibrosis (CF) patients are colonized by the gram-negative pathogen Pseudomonas aeruginosa, which may become highly resistant to antibiotics (4). Persistent inflammation and infection cause the dilation and destruction of the bronchioles, resulting in airway obstruction and death from respiratory failure (38).
In chronically infected CF patients, P. aeruginosa may adopt a sessile biofilm lifestyle that is resistant to antimicrobial treatment (34). The communities of bacteria coordinate changes in gene expression through a cell-to-cell signaling mechanism termed quorum sensing (QS) (14, 54). QS systems consist of small soluble signaling molecules called autoinducers and receptors that act as transcriptional regulators (20). As the bacterial cell density increases, P. aeruginosa augments the production of virulence factors in response to the increased production of autoinducers, such as the acyl homoserine lactone (AHL) N-(3-oxododecanoyl)-l-homoserine lactone (3OC12-HSL) (22). 3OC12-HSL functions as a ligand for a transcriptional regulator in P. aeruginosa, but recent studies indicate that 3OC12-HSL can also alter mammalian cell responses (56). For example, 3OC12-HSL can promote the upregulation of proinflammatory cytokines and chemokines and the induction of apoptosis (44). These changes may potentiate inflammatory responses in the host tissue and lead to the exacerbation of disease symptoms and permanent organ damage in chronic infections. Although several independent studies have shown that 3OC12-HSL can elicit changes in mammalian gene transcription, little is known about the mechanism(s) through which autoinducers influence mammalian cells.
We propose that 3OC12-HSL alters host cell responses by interacting with endogenous receptor proteins and exploiting mammalian signaling pathways in a process termed interkingdom signaling (41, 43). Several studies have identified proteins, such as the transcriptional regulators nuclear factor kappa B (NF-κB) and activator protein-2 (AP-2), as mammalian components of 3OC12-HSL interkingdom signaling pathways (45); however, no direct targets of 3OC12-HSL in mammalian cells have yet been identified. Based on the observations that 3OC12-HSL elicits effects similar to those of some endogenous mammalian hormones (44, 46, 51) and that autoinducers can enter and retain their functions within mammalian cells (56), we hypothesize that 3OC12-HSL crosses cell membranes and utilizes intracellular mammalian receptors to alter gene transcription. Lipidic signaling compounds commonly elicit responses in mammalian cells by acting as ligands for transcriptional regulators of the nuclear hormone receptor (NHR) superfamily (10). NHRs and LuxR-type proteins (bacterial AHL receptors) function as ligand-regulated transcription factors, and both undergo conformational changes upon ligand binding. Although LuxR proteins and NHRs are not encoded by orthologous genes, their functional similarities have identified NHRs as candidate mammalian AHL receptors. The NHR superfamily is composed of 48 distinct members in humans (26), several of which are associated with the regulation of proinflammatory genes. For example, the peroxisome proliferator-activated receptors (PPARs) are a subset of NHRs with documented anti-inflammatory effects (15, 25, 48), and several 3OC12-HSL-activated genes are also PPARγ targets (24). Here, we demonstrate that 3OC12-HSL modulates the transcriptional and DNA binding activities of PPARβ and/or PPARγ and that, thus, these proteins may mediate some of the actions of 3OC12-HSL within mammalian cells.
MATERIALS AND METHODS
Cell culture and reagents.
The murine fibroblast cell line NIH 3T3 (ATCC, Rockville, MD) was cultured in Dulbecco's modified Eagle's medium (BioWhittaker, Walkersville, MD) supplemented with 10% Cosmic calf serum (HyClone, Logan, UT) and 100 U of penicillin ml−1 and 100 μg of streptomycin ml−1 (BioWhittaker, Walkersville, MD). The human alveolar epithelial cell line A549 (ATCC, Rockville, MD) was cultured in Kaighn's modification of Ham's F-12K medium (Mediatech Inc., Herndon, VA) supplemented with 10% fetal bovine serum, 100 U of penicillin ml−1, and 100 μg of streptomycin ml−1. For reverse transcription (RT)-PCR analysis, cells were seeded onto six-well tissue culture plates (1.5 × 105 cells per well). Cells were stimulated with autoinducer in the appropriate medium lacking fetal bovine serum for 4 h.
Autoinducer preparation.
N-Butyryl-homoserine lactone (C4-HSL) was purchased from Sigma (St. Louis, MO) and dissolved in ethyl acetate containing 0.001% glacial acetic acid for stable storage at −20°C. 3-O-Dodecanoyl homoserine lactone (3OC12-HSL) was synthesized as described previously (11, 42) and was also dissolved in ethyl acetate containing 0.001% glacial acetic acid for stable storage at −20°C. For addition to cell cultures, autoinducers were placed under a gentle stream of nitrogen gas to evaporate the ethyl acetate solvent and were dissolved in the cell culture medium immediately prior to being added to the cell culture.
RT-PCR.
RNA was isolated from cells using the Versagene RNA cell kit according to the specifications of the manufacturer (Gentra Systems, Minneapolis, MN). cDNA was prepared by combining 2 μg of total RNA, 400 U of SuperScript RT (Invitrogen, Carlsbad, CA), and 500 ng of oligo(dT) and incubating the mixture at 37°C for 1 h. Specific primer sets for genes encoding murine glyceraldehyde-3-phosphate dehydrogenase (GAPDH), cyclooxygenase-2 (COX-2), interleukin-6 (IL-6), keratinocyte-derived cytokine (KC; an orthologue of IL-8), and IL-1α and human L19, COX-2, IL-8, IL-6, and IL-1α (Table 1) were used to amplify DNA templates in a T3 thermocycler (Biometra, Goettingen, Germany) with Taq DNA polymerase (New England Biolabs, Ipswich, MA). Oligonucleotides were synthesized by Integrated DNA Technologies (Coralville, IA). Specific murine and human primer sets for the PPARα, PPARγ, PPARβ/δ, retinoid X receptor α (RXRα), RXRβ, RXRγ, pregnane X receptor (PXR), farnesoid X receptor (FXR), and constitutive androstane receptor (CAR) genes (Table 1) were used to amplify DNA templates in a RapidCycler air thermocycler (Idaho Technology, Idaho Falls). PCR products were run on 1.5% agarose gels containing 10 ng of ethidium bromide (Fisher Biosciences, Lafayette, CO), and the gels were visualized under UV light.
TABLE 1.
Primers used in this study
| Gene product | Sequence (5′ to 3′) of:
|
|
|---|---|---|
| Forward primer | Reverse primer | |
| Murine gene products | ||
| GAPDH | AAGGTCGGAGTCAACGGATT | TTGATGACAAGCTTCCCGTT |
| COX-2 | CCCCCACAGTCAAAGACACT | GAGTCCATGTTCCAGGAGGA |
| KC | GCTGGGATTCACCTCAAGAA | TGGGGACACCTTTTAGCATC |
| IL-6 | ACTTCACAAGTCCGGAGAGG | GTTGAAGATATGAATTAGAG |
| IL-1α | CGTCAGGCAGAAGTTTGTCA | GTGCACCCGACTTTGTTCTT |
| PPARα | TGCAAACTTGGACTTGAACG | AATCCCCTCCTGCAACTTCT |
| PPARβ/δ | GCAGCCTCTTCCTCAATGAC | GTACTGGCTGTCAGGGTGGT |
| PPARγ | CTGGCCTCCCTGATGAATAA | ACGTGCTCTGTGACGATCTG |
| RXRα | CTCCTATCAGCACCCTGAGC | TGTTGTCTCGGCAGGTGTAG |
| RXRβ | CTCCTCATTGCGTCCTTCTC | CCTGCTGCTCAGGGTACTTC |
| RXRγ | AATGCTCTTGGCTCTCCGTA | TGAAGAAGCCTTTGCAACCT |
| FXR | GGCAGAATCTGGATTTGGAA | GTGAGCGCGTTGTAGTGGTA |
| PXR | TTTCAGAAGGGCCATGAAAC | CCTGCAGAAACTCTGGAAGC |
| CAR | GGAGGACCAGATCTCCCTTC | GTTCAGAATCAGCGCCATTT |
| Human gene products | ||
| L19 | GCTGATCAAGGATGGGCTGA | CGGGAATGGACAGTCACAGG |
| COX-2 | TGAAACCCACTCCAAACACA | AACTGATGCGTGAAGTGCTG |
| IL-8 | TCTGCAGCTCTGTGTGAAGG | ATTGCATCTGGCAACCCTAC |
| IL-6 | AAAGAGGCACTGGCAGAAAA | AAAGCTGCGCAGAATGAGAT |
| IL-1α | GTAAGCTATGGCCCACTCCA | AGCAGCCGTGAGGTACTGAT |
| PPARα | CTGGAAGCTTTGGCTTTACG | GTTGTGTGACATCCCGACAG |
| PPARβ/δ | GTGTGGAAGCAGTTGGTGAA | GCGTTGAACTTGACAGCAAA |
| PPARγ | TTCAGAAATGCCTTGCAGTG | CACCTCTTTGCTCTGCTCCT |
| RXRα | TTCTCCACCCAGGTGAACTC | CTTGGTGAAGGAAGCCATGT |
| RXRβ | CCTTCTCACACCGATCCATT | CCTGCTGCTCAGGGTACTTC |
| RXRγ | TGTGGTCAACAGTGTCAGCA | CCTCACTCTCAGCTCGCTCT |
| FXR | TTGCTTTGCTGAAAGGGTCT | GAGGATTTTCAGGCTGGTGA |
| PXR | CTGATGGACGCTCAGATGAA | AGGGAGATCTGGTCCTCGAT |
| CAR | GCAGCTGTGGAAATCTGTCA | AGGCCTAGCAACTTCGCATA |
Transcriptional assays.
A Renilla luciferase vector, pRL-TK (Promega, Madison, WI), was included in all experiments as a transfection efficiency control. Firefly and Renilla luciferase activities were assayed using the dual luciferase reporter assay kit according to the instructions of the manufacturer (Promega, Madison, WI). Luminescence was measured with a Modulus single-tube luminometer (Turner Biosystems, Sunnyvale, CA). Transfections were performed using Polyfect according to the instructions of the manufacturer (Qiagen, Valencia, CA). For luciferase assays, NIH 3T3 cells were transfected with plasmids expressing the NHR of interest (either PPARγ or PPARβ/δ) and the PPAR binding partner RXRα, as well as a receptor-specific luciferase reporter plasmid. The reporter plasmid for PPARγ contained a fragment from the rat phosphoenolpyruvate carboxykinase promoter encompassing nucleotides −1130 to +69 (18, 28) in the pGL3-basic plasmid (Promega). The reporter for PPARβ/δ was the 2X Cyp4A6Z pal thymidine kinase (TK)-luciferase plasmid, which contains a TK basal promoter fragment upstream of two copies of the Cyp4A6Z motif from the reporter plasmid pBL-CAT8+ (28) in the pGL2-basic plasmid (Promega). Twenty-four hours after transfection, cells were treated with increasing concentrations of 3OC12-HSL or C4-HSL with or without rosiglitazone for 4 h and then assayed for luciferase activity. The pSG5-PPARβ/δ, pSPORT-PPARγ, pSPORT-RXRα, and phosphoenolpyruvate carboxykinase-luciferase reporter plasmids were generous gifts from Elmus Beale (Texas Tech University Health Sciences Center).
Nuclear extracts and Western blotting.
Nuclear extracts were prepared from A549 and NIH 3T3 cells as described previously (7). All steps in the nuclear extract preparation were carried out at 4°C or on ice. The cells were washed twice with phosphate-buffered saline, harvested in ice-cold lysis buffer (Dignam buffer A: 10 mM HEPES [pH 7.9], 10 mM KCl, 1.5 mM MgCl2, 0.5 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, and 2 μg of a protease inhibitor cocktail [Cal Biochem, San Diego, CA]/ml), and incubated on ice for 20 min. The cells were lysed by passing eight times through a 21-gauge needle, and the lysates were centrifuged at 13,000 × g for 45 s to pellet the nuclei. The nuclear pellet was resuspended in Dignam buffer C (20 mM HEPES [pH 7.9], 25% [vol/vol] glycerol, 0.42 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM phenylmethylsulfonyl fluoride, 0.5 mM dithiothreitol, 2 μg of a protease inhibitor cocktail/ml), and the resulting suspension was rotated at 4°C for 30 min. The suspension was then centrifuged at 13,000 × g to pellet nuclear debris, and the supernatant containing the nuclear extract was collected and stored at −80°C. The protein concentration was determined utilizing a protein assay reagent according to the instructions of the manufacturer (Bio-Rad Laboratories, Hercules, CA). A 10-μg sample of nuclear proteins was separated on sodium dodecyl sulfate-8% polyacrylamide gel, and the proteins were electroblotted onto nitrocellulose membranes. Membranes were preblocked in a mixture of phosphate-buffered saline-Tween 20 and 4% milk and probed using anti-PPARγ (Cell Signaling, Danvers, MA) or anti-PPARβ/δ (Santa Cruz) antisera at 1:1,000 dilutions. Membranes were then stripped with 0.1 M glycine at pH 2.6 and reprobed using an antilamin antibody (Santa Cruz) at a 1:1,000 dilution as a loading control.
EMSA.
Double-stranded oligonucleotides containing a consensus PPARγ response element were end labeled with [-32P]ATP using T4 polynucleotide kinase (Promega), purified in Microspin G-25 columns (Sigma), and used as probes for an electrophoretic mobility shift assay (EMSA). Recombinant PPARγ and RXRα proteins were synthesized using the TNT T7 quick translation-transcription system (Promega), and programmed lysate or unprogrammed lysate was incubated with the radioactively labeled wild-type oligonucleotide carrying the consensus PPARγ response element. All binding reactions were performed at 23°C with a 25-μl mixture containing 2 μl of the programmed or unprogrammed lysate, 6% (vol/vol) glycerol, 4% (wt/vol) Ficoll, 10 mM HEPES (pH 7.9), 10 mM dithiothreitol, 0.25 μg of bovine serum albumin, 0.06% bromophenol blue, 1 μg of poly(dI-dC), and 1.25 ng of the probe. For competition assays, a 10× or 100× molar excess of unlabeled oligonucleotides (both wild-type and mutated forms) was included in the reaction mixture, along with the radiolabeled probe. The effects of AHLs on DNA binding activities were assessed by incubating programmed lysates with 100 μM 3OC12-HSL or C4-HSL during the binding reaction. Gels were dried after electrophoresis and subjected to autoradiography.
RESULTS
3OC12-HSL modulates mRNA levels for inflammatory mediators in mammalian cells.
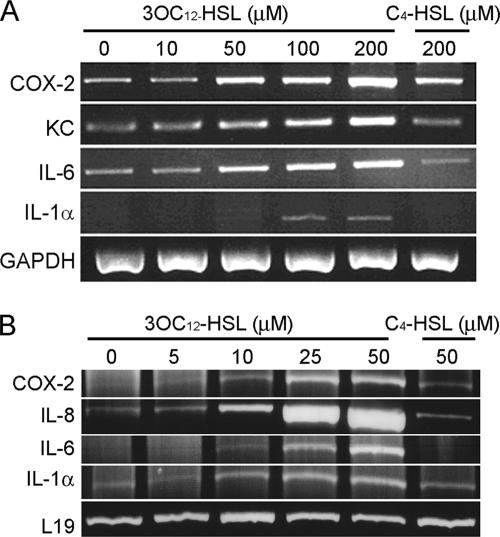
We previously demonstrated that 3OC12-HSL increases mRNA levels for IL-6, COX-2, and KC in mouse fibroblasts, and several other reports have described similar effects in human fibroblasts and epithelial cells (44-46). However, as demonstrated by previous apoptotic studies, 3OC12-HSL may have various cell type-specific effects (50). To determine whether 3OC12-HSL elicits similar effects in murine and human cells, we have examined mRNA levels for an expanded panel of proinflammatory genes in mouse NIH 3T3 fibroblasts and human A549 lung epithelial cells. These cell lines are representative of cell types likely to be exposed to elevated levels of P. aeruginosa autoinducers during lung and wound infections. Cells were exposed to a range of concentrations of 3OC12-HSL for 6 h, RNA was harvested and converted into cDNA, and PCR was performed utilizing primers for several candidate inflammatory mediators (Fig. 1). The range of autoinducer concentrations necessary to elicit changes in cytokine mRNA levels in the A549 human cells was lower than that in the hardier NIH 3T3 mouse fibroblasts. IL-1α, IL-6, IL-8/KC, and COX-2 mRNA levels exhibited dose-dependent increases in both cell lines and were unaffected by the exposure of cells to C4-HSL, the second P. aeruginosa autoinducer, which was used to demonstrate AHL specificity (Fig. 1). These data demonstrate that 3OC12-HSL increases mRNA levels for several inflammatory mediators in both human and murine cells. Thus, we have identified a common response to 3OC12-HSL in cell lines from two distinct mammalian species by using corresponding panels of inflammatory cytokines.
FIG. 1.
3OC12-HSL modulates proinflammatory gene expression in vitro. NIH 3T3 (A) and A549 (B) cells were incubated in the absence or presence of increasing concentrations of 3OC12-HSL or C4-HSL for 6 h, and RNA was extracted and converted into cDNA. mRNA levels for genes encoding the inflammatory mediators COX-2, IL-8, IL-6, and IL-1α were assessed by RT-PCR. GAPDH and L19 mRNAs were used as loading controls.
Mammalian NHRs are expressed in 3OC12-HSL-sensitive cell lines.
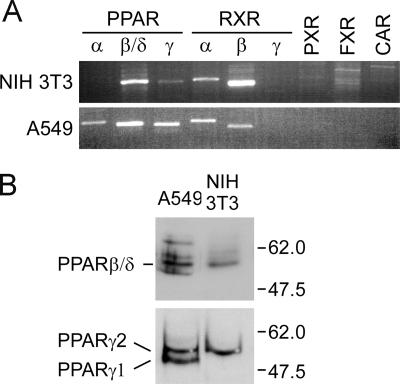
Despite growing evidence that AHLs directly influence mammalian responses, endogenous mammalian receptors have not been identified. Our previous studies suggested that at least two distinct 3OC12-HSL receptors may be present in mammalian cells (41, 44). One receptor that mediates a Ca2+ signaling-based apoptotic response appears to be located at or close to the cell membrane, while a second, uncharacterized receptor may be responsible for the proinflammatory effects of 3OC12-HSL. We previously showed that P. aeruginosa autoinducers can enter and function in mammalian cells, suggesting that the second receptor may be an intracellular protein (56). As many hydrophobic signaling compounds can pass through cell membranes and directly affect transcription by interacting with members of the NHR superfamily, we next sought to identify NHRs that may function as 3OC12-HSL receptors (17). We extracted endogenous mRNA from untreated NIH 3T3 and A549 cells and performed RT-PCR to identify mammalian NHRs common to both cell lines. RT-PCR assays were performed using primers specific for three PPAR (PPARα, PPARβ/δ, and PPARγ) genes (NR1C1, NR1C2, and NR1C3) (36), three RXR (RXRα, RXRβ, and RXRγ) genes (NR2B1, NR2B2, and NR2B3), and the PXR (NR1I1), FXR (NR1H4), and CAR (NR1I3) genes (Fig. 2A). These genes were examined based on the associations between their products and inflammatory or xenobiotic reactions and the fact that several exhibit flexibility in accommodating structurally diverse ligands. Strong signals for PPARβ/δ, PPARγ, RXRα, and RXRβ were detected in RNAs from both NIH 3T3 and A549 cells. PPARα mRNA was detected only in A549 cells, while RXRγ, PXR, FXR, and CAR mRNAs were not detected in either cell line. Nuclear extracts from NIH 3T3 and A549 cells were subjected to immunoblotting analysis to confirm that PPARβ/δ and PPARγ proteins were also expressed in these cell lines. Both PPARγ1 and PPARγ2 isoforms were detected in A549 cells, while only the PPARγ1 isoform was detected in NIH 3T3 cells (Fig. 2B). PPARβ/δ was also detected in NIH 3T3 and A549 cells (Fig. 2B).
FIG. 2.
NHRs are expressed in 3OC12-HSL-responsive cell lines. RNA from cell lines that exhibit 3OC12-HSL-dependent changes in proinflammatory mRNAs was extracted and converted into cDNA. (A) RT-PCR was used to screen for a panel of NHRs in A549 cells. (B) Western blot analysis revealed PPARγ and PPARβ/δ proteins in the nuclear extracts of A549 and NIH 3T3 cells.
3OC12-HSL affects the transcriptional activities of PPARβ and PPARγ.
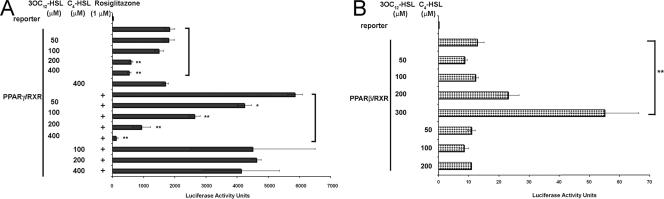
The PPAR family consists of three isoforms with high degrees of sequence and structural similarities, PPARα, PPARγ, and PPARβ. All PPARs form heterodimers with the RXR and bind to the same DNA response element after activation. This sequence, containing multiple AGGTCA repeats, is termed the specific PPAR response element (PPRE) and is found within the promoters of target genes. Recent evidence has credited PPARs with the ability to decrease inflammation through the transrepression of transcription factors involved in the inflammatory response (13). Transcriptional activation assays were performed to test whether 3OC12-HSL affected the activity of PPARβ/δ and PPARγ. NIH 3T3 cells were transfected with plasmids expressing either PPARβ or PPARγ and the PPAR binding partner RXRα, along with a receptor-specific luciferase reporter plasmid. Twenty-four hours after transfection, cells were stimulated with various concentrations of 3OC12-HSL and cultured for a further 24 h, and luciferase activity in the cell extracts was assayed. C4-HSL was again used as an AHL specificity control. The transcriptional activity of PPARγ was specifically inhibited by 3OC12-HSL in a dose-dependent manner both in the presence and in the absence of the potent PPARγ agonist rosiglitazone (Fig. 3A). In contrast, the activity of PPARβ-RXRα was specifically enhanced in a dose-dependent manner (Fig. 3B). Importantly, the activities of both PPARs were not significantly altered in cells exposed to C4-HSL in the absence or presence of rosiglitazone, indicating that these responses are dependent on a specific AHL.
FIG. 3.
3OC12-HSL modulates the transcriptional activities of NHRs. NIH 3T3 cells were transfected with expression vectors for either PPARγ and RXRα (A) or PPARβ/δ and RXRα (B), along with the appropriate PPAR-responsive luciferase reporter plasmid and the TK-Renilla luciferase control plasmid. Twenty-four hours after transfection with the expression vectors, cells were stimulated with 3OC12-HSL or C4-HSL in the presence (+) or absence of rosiglitazone as indicated and incubated for 6 h. Cell lysates were prepared and assayed for luciferase activity. Each bar represents the average of results obtained from three transfections, and each experiment was performed at least three times. Statistical analysis was performed using a one-way analysis of variance with the Tukey-Kramer multiple-comparisons test. The error bars represent standard errors of the means. *, P < 0.01; **, P < 0.001.
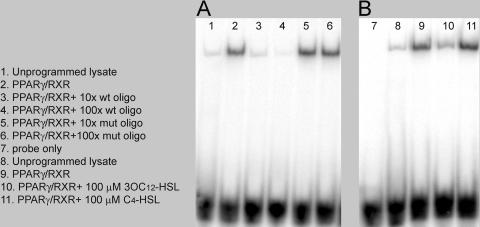
3OC12-HSL inhibits the DNA binding activity of PPARγ.
An EMSA was performed to determine whether 3OC12-HSL affects the ability of PPARγ to bind an oligonucleotide containing a PPRE. Recombinant PPARγ and RXRα were synthesized in reticulocyte lysates and added to binding reaction mixtures. Strong binding was observed in samples containing programmed lysates but was essentially absent in reactions using unprogrammed lysates (Fig. 4A, compare lanes 1 and 2). The specificity of this binding reaction was confirmed by competition with an excess of unlabeled PPRE oligonucleotide and a lack of competition with an excess of a mutated form of the oligonucleotide (Fig. 4A, compare lanes 3 and 4 with lanes 5 and 6). Importantly, the preincubation of the programmed lysate with 100 μM 3OC12-HSL resulted in a decrease in binding activity, while binding activity was essentially unaffected when the lysate was preincubated with 100 μM C4-HSL (Fig. 4B, compare lanes 10 and 11). We interpret these data to indicate that 3OC12-HSL is potentially a ligand for PPARγ which induces a conformational change that diminishes the DNA binding ability of PPARγ.
FIG. 4.
3OC12-HSL affects the DNA binding activity of PPARγ. Recombinant PPARγ and RXRα proteins were synthesized in vitro utilizing the TNT in vitro transcription-translation kit. Equal amounts of programmed or unprogrammed lysates were incubated with a radioactively labeled wild-type oligonucleotide carrying a consensus PPARγ-responsive element. (A) Competition assays were performed using 10× or 100× molar excesses of unlabeled wild-type (wt) or mutated (mut) oligonucleotides (oligo; lanes 3 to 6). (B) The effects of autoinducers on PPARγ DNA binding activity were assessed by adding 100 μM 3OC12-HSL or C4-HSL to the binding reaction mixtures in lanes 10 and 11.
3OC12-HSL antagonizes the transrepression of cytokine expression by a PPARγ agonist.
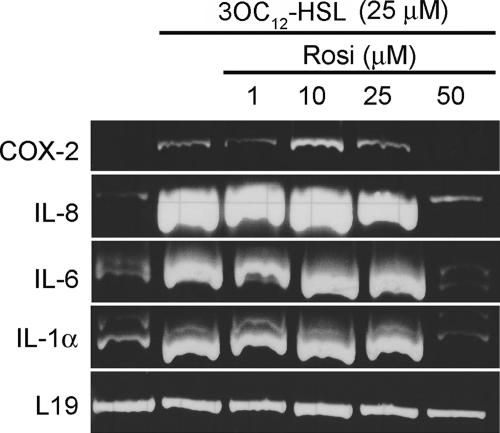
Ligand-bound PPARγ possesses an anti-inflammatory function mediated by the ability of PPARγ to directly inhibit transcription from the promoters of proinflammatory genes. For example, PPARγ has been proposed to inhibit the transcription of NF-κB-dependent genes, such as the inducible nitric oxide synthase gene, by stabilizing corepressor complexes and preventing coactivator recruitment to the target promoter (25, 37, 39). Additionally, ligand-bound PPARγ can inhibit the transcription of AP-1-regulated genes by competing for coactivator complexes (49). Collectively, these inhibitory functions of PPARγ are termed transrepression and presumably represent a mechanism for dampening inflammatory responses. We therefore tested whether the potent PPARγ agonist rosiglitazone could prevent the 3OC12-HSL-dependent increase in proinflammatory mRNA levels in A549 cells. Separate cultures of A549 cells were exposed to a constant concentration of 3OC12-HSL and increasing concentrations of rosiglitazone, and RNA was prepared for RT-PCR analysis. As before, IL-1α, IL-6, IL-8, and COX-2 mRNA levels were elevated in cells exposed to 25 μM 3OC12-HSL (Fig. 5, compare lanes 1 and 2). The levels of each mRNA were essentially unaffected in cells cultured in the presence of up to 25 μM rosiglitazone but were decreased nearly or completely to the unstimulated levels in cells cultured in 50 μM rosiglitazone (Fig. 5, lanes 3 to 6). These data suggest that 3OC12-HSL and rosiglitazone are mutually antagonistic in controlling the activity of PPARγ.
FIG. 5.
Antagonistic effects of 3OC12-HSL and rosiglitazone on mRNA levels for proinflammatory genes. A549 cells were incubated in the absence or presence of 25 μm 3OC12-HSL, either alone or in the presence of various concentrations of rosiglitazone (Rosi) for 6 h. RNA was extracted and converted into cDNA, and mRNA levels for a panel of proinflammatory genes were assessed by RT-PCR. The mRNA encoding the ribosomal protein L19 was analyzed as a loading control.
DISCUSSION
Eukaryotes and prokaryotes have coexisted for approximately 2 billion years, often existing in close apposition in a shared hormonal environment. We postulate that organisms from different kingdoms have acquired mechanisms to sense and respond to one another's signaling molecules. This cross-kingdom communication has been termed interkingdom signaling and has potentially important implications for the relationship between pathogenic organisms and their hosts. One example of interkingdom signaling emerged from the observation that the QS system of enterohemorrhagic Escherichia coli can be activated by epinephrine and norepinephrine produced by mammalian hosts (12). We have focused on a signaling pathway that appears to function in the opposite direction, in which AHLs produced by P. aeruginosa alter gene expression in mammalian cells. This pathway represents a previously uncharacterized aspect of host-pathogen interaction and innate immunity. In this report, we identify two members of the PPAR subfamily of NHRs as candidate mammalian receptors for the AHL 3OC12-HSL.
Several earlier studies have implicated transcriptional regulators and protein kinases as components of AHL signaling pathways in mammalian cells, but no specific receptors have been identified. For example, the NF-κB transcriptional regulator was implicated in the AHL-dependent activation of IL-8 and COX-2 transcription, and the AP-2 transcription factor was identified as a potential regulator of IL-8 (45, 46). Neither of these proteins is likely to interact directly with AHLs, and thus, these proteins are presumably targets of signaling pathways activated by AHLs. In addition, a number of protein kinases, including members of the mitogen-activated protein kinase family and Akt, were implicated previously as intermediates in AHL signaling; however, these kinases are also not likely to be direct targets of AHLs (23, 45). Subsequently, studies with genetically null mice revealed that the canonical Toll-like receptor pathways are not involved in AHL signaling (27). Together, these studies have highlighted the need to identify bona fide AHL receptors in order to clarify the mechanisms of action of these molecules in mammalian cells.
The concept that 3OC12-HSL interacts with multiple mammalian receptors emerged from our earlier studies on two distinct effects of 3OC12-HSL on mammalian cells, namely, apoptotic induction and increased mRNA levels for proinflammatory genes (44). These studies revealed that apoptotic induction is mediated by a calcium-dependent signaling pathway, apparently initiated by the interaction of 3OC12-HSL with an unknown receptor located at or close to the cell membrane, and that the inhibition of this pathway blocks apoptosis but not proinflammatory responses. Thus, we proposed that a second receptor was associated with the inflammatory response to AHLs. The candidacy of members of the NHR superfamily was supported by our previous data showing that autoinducers can enter and retain functionality in mammalian cells (56) and the recent direct demonstration that radiolabeled 3OC12-HSL can be detected in T cells (40). In addition, clear functional similarities exist between LuxR-AHL and NHR-ligand interactions, including the induction of conformational changes in the receptor protein upon ligand binding (3, 19). Although LuxR-type proteins and NHRs are not evolutionarily related (41), their functional similarities led us to propose NHRs as candidate AHL receptors (41, 43).
The PPARs were particularly attractive candidate AHL receptors for several reasons, particularly their responsiveness to ligands of relatively disparate structures and their association with inflammatory gene regulation (45). The PPAR family consists of three closely related gene products, PPARα, PPARγ, and PPARβ. All PPARs bind to DNA as obligate heterodimers with the RXR at specific PPREs within the promoters of target genes. In the absence of a ligand, they most likely bind to DNA and form complexes with corepressor proteins. Under these conditions, the transcription of target genes is repressed. Ligand binding induces a structural change that displaces corepressors, facilitates interaction with coactivators, and promotes the transcription of target genes (21). Of the three isotypes, PPARβ has the broadest expression pattern, and functions in the skin, gut, placenta, skeletal muscle, adipose tissue, and brain have been assigned to this isotype (5, 6, 31). PPARγ is expressed as two isoforms, PPARγ1 and PPARγ2, that differ at their N termini. PPARγ2 is found at high levels in the different adipose tissues (52), whereas PPARγ1 has a broader expression pattern that extends to settings such as the gut, brain, vascular cells, and specific kinds of immune and inflammatory cells (53, 57). Our data show that two members of the PPAR family, PPARβ/δ and PPARγ, as well as several of their heterodimeric partners from the RXR family, are expressed in cell types that are AHL responsive. Furthermore, we have shown that the transcriptional activities of both PPARβ/δ and PPARγ are affected by 3OC12-HSL, although in opposite directions. In theory, 3OC12-HSL may function as a ligand for either partner in these heterodimeric complexes. The RXRs were the first orphan nuclear receptors for which an endogenous ligand (9-cis-retinoic acid) was identified, and RXRs are also receptors for a variety of other dietary lipids, as well as some nonmammalian lipids (10). However, as RXRα was a common partner for PPARβ/δ and PPARγ in these transcriptional assays, we interpret the opposite effects of 3OC12-HSL on the two heterodimeric complexes as evidence that the PPAR partner mediates the effect of the AHL. Several independent assays, including direct binding assays with radiolabeled autoinducers, are currently under way to confirm this conclusion.
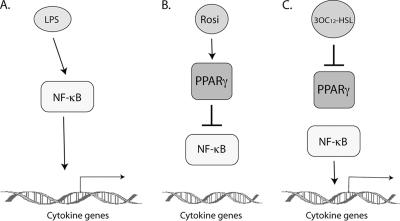
The observation that 3OC12-HSL inhibited DNA binding by recombinant PPARγ-RXRα heterodimers at a consensus PPRE is consistent with a model whereby 3OC12-HSL is directly recognized by PPARγ and induces a conformational alteration that negatively affects the DNA binding activity of PPARγ. However, as mentioned above, certain ligand-bound NHRs can also inhibit the expression of promoters that do not contain PPREs, via a phenomenon known as transrepression (8). In the case of PPARγ-dependent transrepression of NF-κB-regulated genes, ligand-bound PPARγ stabilizes repressor complexes associated with target genes and prevents the recruitment of coactivators in response to proinflammatory stimuli such as lipopolysaccharide (37) (Fig. 6A and B). We propose that 3OC12-HSL, as an antagonist of PPARγ, can compete with PPARγ agonists such as rosiglitazone, thereby inhibiting transrepression and potentiating the expression of proinflammatory genes (Fig. 6C). This exaggerated inflammation can lead to tissue destruction, which often precedes bacteremia. A similar scenario of mutual antagonism between 3OC12-HSL as a PPARγ antagonist and PPARγ agonists would also be consistent with other models of PPAR-dependent transrepression, such as the sequestration of coactivators of AP-1 on the COX-2 promoter (49).
FIG. 6.
Hypothetical model of mutual antagonism between 3OC12-HSL and rosiglitazone in the regulation of NF-κB-dependent genes. (A) Proinflammatory stimuli such as lipopolysaccharide (LPS) activate NF-κB and stimulate the transcription of several inflammatory mediators. (B) Ligand-bound PPARγ transrepresses NF-κB by stabilizing corepressor complexes bound at promoters of NF-κB-dependent genes. (C) 3OC12-HSL inhibits PPARγ activity, possibly by competing with rosiglitazone for binding within the PPARγ ligand binding domain, and relieves the transrepression of NF-κB activity.
Finally, it is important that PPARγ agonists are currently being employed as anti-inflammatory treatments for multiple diseases, such as inflammatory bowel disease (2), atherosclerosis (35), and asthma (47). The PPARγ agonists rosiglitazone and pioglitazone have protective effects against a variety of inflammation-related kidney injuries, such as diabetic nephropathy, hypertensive nephropathy, and ischemia-reperfusion injuries. PPARγ agonists have also been shown to decrease inflammation in injuries related to the digestive tract, lung, and heart. For example, the treatment of lung ischemia-reperfusion injuries in mouse models with the PPARγ agonist pioglitazone resulted in the inhibition of proinflammatory cytokines (tumor necrosis factor alpha and cytokine-induced neutrophil chemoattractant 1), the infiltration of neutrophils into the lung interstitium, and reduced pulmonary edema (32). Therefore, PPARγ agonists may be effective as anti-inflammatory agents in individuals with P. aeruginosa infections, including diabetic and CF patients.
Lastly, it is important that autoinducer concentrations ranging from 0 to 400 μM were utilized for the experiments presented in this paper. Currently, in vivo measurements of autoinducers in CF patient sputum have been in the nanomolar range (33), while experimental concentrations of synthetic autoinducers required to elicit effects in immortalized mammalian cell lines are in the micromolar range. However, these in vivo measurements most likely underestimate the local concentrations of autoinducers within the vicinity of a P. aeruginosa biofilm. AHLs are diluted in the sputum and lung fluid, and the local AHL concentration during an active P. aeruginosa lung infection remains unknown. Local and systemic concentrations may differ in vivo, where cells in close proximity to a high concentration of bacteria (e.g., a biofilm) are exposed to high concentrations of AHLs. In fact, AHL concentrations of up to 600 μM have been measured in the supernatants of biofilms grown in vitro (9). Also, researchers in our lab have shown that nonimmortalized cells are more sensitive to AHLs than immortalized cell lines (44). This work suggests that cells in vivo may be more sensitive to AHL concentrations than those previously studied in culture. Thus, the autoinducer concentrations utilized in these experiments may reflect the relative AHL concentrations to which primary cells are exposed in vivo. Unfortunately, this issue will remain uncertain until more accurate measurements of AHL concentrations in vivo are available.
Acknowledgments
We thank Liliane Michalik for her critical review of the manuscript and helpful comments.
This study was supported by the American Lung Association (K.P.R.), the American Heart Association (S.C.W.), and the Welch Foundation (G.L.).
Footnotes
Published ahead of print on 4 January 2008.
REFERENCES
- 1.Abbas, A. K. 2003. Cellular and molecular immunology, 5th ed. Saunders, Philadelphia, PA.
- 2.Adachi, M., R. Kurotani, K. Morimura, Y. Shah, M. Sanford, B. B. Madison, D. L. Gumucio, H. E. Marin, J. M. Peters, H. A. Young, and F. J. Gonzalez. 2006. Peroxisome proliferator activated receptor gamma in colonic epithelial cells protects against experimental inflammatory bowel disease. Gut 551104-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aranda, A., and A. Pascual. 2001. Nuclear hormone receptors and gene expression. Physiol. Rev. 811269-304. [DOI] [PubMed] [Google Scholar]
- 4.Bals, R., and B. Jany. 2001. Identification of disease genes by expression profiling. Eur. Respir. J. 18882-9. [DOI] [PubMed] [Google Scholar]
- 5.Bastie, C., D. Holst, D. Gaillard, C. Jehl-Pietri, and P. A. Grimaldi. 1999. Expression of peroxisome proliferator-activated receptor PPARδ promotes induction of PPARγ and adipocyte differentiation in 3T3C2 fibroblasts. J. Biol. Chem. 27421920-5. [DOI] [PubMed] [Google Scholar]
- 6.Braissant, O., F. Foufelle, C. Scotto, M. Dauca, and W. Wahli. 1996. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology 137354-66. [DOI] [PubMed] [Google Scholar]
- 7.Bretz, J. D., S. C. Williams, M. Baer, P. F. Johnson, and R. C. Schwartz. 1994. C/EBP-related protein 2 confers lipopolysaccharide-inducible expression of interleukin 6 and monocyte chemoattractant protein 1 to a lymphoblastic cell line. Proc. Natl. Acad. Sci. USA 917306-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castrillo, A., M. J. Diaz-Guerra, S. Hortelano, P. Martin-Sanz, and L. Bosca. 2000. Inhibition of IκB kinase and IκB phosphorylation by 15-deoxy-Δ12,14-prostaglandin J2 in activated murine macrophages. Mol. Cell. Biol. 201692-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charlton, T. S., R. de Nys, A. Netting, N. Kumar, M. Hentzer, M. Givskov, and S. Kjelleberg. 2000. A novel and sensitive method for the quantification of N-3-oxoacyl homoserine lactones using gas chromatography-mass spectrometry: application to a model bacterial biofilm. Environ. Microbiol. 2530-41. [DOI] [PubMed] [Google Scholar]
- 10.Chawla, A., J. J. Repa, R. M. Evans, and D. J. Mangelsdorf. 2001. Nuclear receptors and lipid physiology: opening the X-files. Science 2941866-70. [DOI] [PubMed] [Google Scholar]
- 11.Chhabra, S. R., C. Harty, D. S. Hooi, M. Daykin, P. Williams, G. Telford, D. I. Pritchard, and B. W. Bycroft. 2003. Synthetic analogues of the bacterial signal (quorum sensing) molecule N-(3-oxododecanoyl)-L-homoserine lactone as immune modulators. J. Med. Chem. 4697-104. [DOI] [PubMed] [Google Scholar]
- 12.Clarke, M. B., and V. Sperandio. 2005. Events at the host-microbial interface of the gastrointestinal tract. III. Cell-to-cell signaling among microbial flora, host, and pathogens: there is a whole lot of talking going on. Am. J. Physiol. Gastrointest. Liver Physiol. 288G1105-G1109. [DOI] [PubMed] [Google Scholar]
- 13.Daynes, R. A., and D. C. Jones. 2002. Emerging roles of PPARs in inflammation and immunity. Nat. Rev. Immunol. 2748-59. [DOI] [PubMed] [Google Scholar]
- 14.De Kievit, T. R., and B. H. Iglewski. 1999. Quorum sensing, gene expression, and Pseudomonas biofilms. Methods Enzymol. 310117-28. [DOI] [PubMed] [Google Scholar]
- 15.Devchand, P. R., H. Keller, J. M. Peters, M. Vazquez, F. J. Gonzalez, and W. Wahli. 1996. The PPARα-leukotriene B4 pathway to inflammation control. Nature 38439-43. [DOI] [PubMed] [Google Scholar]
- 16.Doggrell, S. A. 2005. Inflammation, the key to much pathology. Drug News Perspect. 18531-9. [PubMed] [Google Scholar]
- 17.Downward, J. 2001. The ins and outs of signalling. Nature 411759-62. [DOI] [PubMed] [Google Scholar]
- 18.Eubank, D. W., E. Duplus, S. C. Williams, C. Forest, and E. G. Beale. 2001. Peroxisome proliferator-activated receptor gamma and chicken ovalbumin upstream promoter transcription factor II negatively regulate the phosphoenolpyruvate carboxykinase promoter via a common element. J. Biol. Chem. 27630561-9. [DOI] [PubMed] [Google Scholar]
- 19.Fuqua, C., and E. P. Greenberg. 2002. Listening in on bacteria: acyl-homoserine lactone signalling. Nat. Rev. Mol. Cell Biol. 3685-95. [DOI] [PubMed] [Google Scholar]
- 20.Fuqua, C., S. C. Winans, and E. P. Greenberg. 1996. Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum-sensing transcriptional regulators. Annu. Rev. Microbiol. 50727-51. [DOI] [PubMed] [Google Scholar]
- 21.Glass, C. K., and M. G. Rosenfeld. 2000. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 14121-41. [PubMed] [Google Scholar]
- 22.Hardman, A. M., G. S. Stewart, and P. Williams. 1998. Quorum sensing and the cell-cell communication dependent regulation of gene expression in pathogenic and non-pathogenic bacteria. Antonie van Leeuwenhoek 74199-210. [DOI] [PubMed] [Google Scholar]
- 23.Imamura, Y., K. Yanagihara, Y. Mizuta, M. Seki, H. Ohno, Y. Higashiyama, Y. Miyazaki, K. Tsukamoto, Y. Hirakata, K. Tomono, J. Kadota, and S. Kohno. 2004. Azithromycin inhibits MUC5AC production induced by the Pseudomonas aeruginosa autoinducer N-(3-oxododecanoyl) homoserine lactone in NCI-H292 cells. Antimicrob. Agents Chemother. 483457-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaul, D., P. K. Anand, and A. Khanna. 2006. Functional genomics of PPAR-gamma in human immunomodulatory cells. Mol. Cell. Biochem. 290211-5. [DOI] [PubMed] [Google Scholar]
- 25.Kostadinova, R., W. Wahli, and L. Michalik. 2005. PPARs in diseases: control mechanisms of inflammation. Curr. Med. Chem. 122995-3009. [DOI] [PubMed] [Google Scholar]
- 26.Krasowski, M. D., K. Yasuda, L. R. Hagey, and E. G. Schuetz. 2005. Evolutionary selection across the nuclear hormone receptor superfamily with a focus on the NR1I subfamily (vitamin D, pregnane X, and constitutive androstane receptors). Nucl. Recept. 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kravchenko, V. V., G. F. Kaufmann, J. C. Mathison, D. A. Scott, A. Z. Katz, M. R. Wood, A. P. Brogan, M. Lehmann, J. M. Mee, K. Iwata, Q. Pan, C. Fearns, U. G. Knaus, M. M. Meijler, K. D. Janda, and R. J. Ulevitch. 2006. N-(3-Oxo-acyl)homoserine lactones signal cell activation through a mechanism distinct from the canonical pathogen-associated molecular pattern recognition receptor pathways. J. Biol. Chem. 28128822-30. [DOI] [PubMed] [Google Scholar]
- 28.Krey, G., H. Keller, A. Mahfoudi, J. Medin, K. Ozato, C. Dreyer, and W. Wahli. 1993. Xenopus peroxisome proliferator activated receptors: genomic organization, response element recognition, heterodimer formation with retinoid X receptor and activation by fatty acids. J. Steroid Biochem. Mol. Biol. 4765-73. [DOI] [PubMed] [Google Scholar]
- 29.Kumar, V., A. K. Abbas, and N. Fausto. 2005. Robbins and Cotran pathologic basis of disease, 7th ed. Elsevier Saunders, Philadelphia, PA.
- 30.Libby, P., P. M. Ridker, and A. Maseri. 2002. Inflammation and atherosclerosis. Circulation 1051135-43. [DOI] [PubMed] [Google Scholar]
- 31.Michalik, L., B. Desvergne, N. S. Tan, S. Basu-Modak, P. Escher, J. Rieusset, J. M. Peters, G. Kaya, F. J. Gonzalez, J. Zakany, D. Metzger, P. Chambon, D. Duboule, and W. Wahli. 2001. Impaired skin wound healing in peroxisome proliferator-activated receptor (PPAR)α and PPARβ mutant mice. J. Cell Biol. 154799-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michalik, L., and W. Wahli. 2006. Involvement of PPAR nuclear receptors in tissue injury and wound repair. J. Clin. Investig. 116598-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Middleton, B., H. C. Rodgers, M. Camara, A. J. Knox, P. Williams, and A. Hardman. 2002. Direct detection of N-acylhomoserine lactones in cystic fibrosis sputum. FEMS Microbiol. Lett. 2071-7. [DOI] [PubMed] [Google Scholar]
- 34.Murray, T. S., M. Egan, and B. I. Kazmierczak. 2007. Pseudomonas aeruginosa chronic colonization in cystic fibrosis patients. Curr. Opin. Pediatr. 1983-8. [DOI] [PubMed] [Google Scholar]
- 35.Neve, B. P., J. C. Fruchart, and B. Staels. 2000. Role of the peroxisome proliferator-activated receptors (PPAR) in atherosclerosis. Biochem. Pharmacol. 601245-50. [DOI] [PubMed] [Google Scholar]
- 36.Nuclear Receptors Committee. 1999. A unified nomenclature system for the nuclear receptor subfamily. Cell 971-20. [DOI] [PubMed] [Google Scholar]
- 37.Pascual, G., A. L. Fong, S. Ogawa, A. Gamliel, A. C. Li, V. Perissi, D. W. Rose, T. M. Willson, M. G. Rosenfeld, and C. K. Glass. 2005. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature 437759-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramsey, M. L., and J. M. Fenlin, Jr. 1996. Use of an antibiotic-impregnated bone cement block in the revision of an infected shoulder arthroplasty. J. Shoulder Elbow Surg. 5479-82. [DOI] [PubMed] [Google Scholar]
- 39.Ricote, M., A. C. Li, T. M. Willson, C. J. Kelly, and C. K. Glass. 1998. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature 39179-82. [DOI] [PubMed] [Google Scholar]
- 40.Ritchie, A. J., C. Whittall, J. J. Lazenby, S. R. Chhabra, D. I. Pritchard, and M. A. Cooley. 2007. The immunomodulatory Pseudomonas aeruginosa signalling molecule N-(3-oxododecanoyl)-L-homoserine lactone enters mammalian cells in an unregulated fashion. Immunol. Cell Biol. 85596-602. [DOI] [PubMed] [Google Scholar]
- 41.Rumbaugh, K. P. 2007. Convergence of hormones and autoinducers at the host/pathogen interface. Anal. Bioanal. Chem. 387425-35. [DOI] [PubMed] [Google Scholar]
- 42.Shiner, E. K., S. Reddy, C. Timmons, G. Li, S. C. Williams, and K. P. Rumbaugh. 2004. Construction of a bacterial autoinducer detection system in mammalian cells. Biol. Proced. Online 6268-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shiner, E. K., K. P. Rumbaugh, and S. C. Williams. 2005. Interkingdom signaling: deciphering the language of acyl homoserine lactones. FEMS Microbiol. Rev. 29935-47. [DOI] [PubMed] [Google Scholar]
- 44.Shiner, E. K., D. Terentyev, A. Bryan, S. Sennoune, R. Martinez-Zaguilan, G. Li, S. Gyorke, S. C. Williams, and K. P. Rumbaugh. 2006. Pseudomonas aeruginosa autoinducer modulates host cell responses through calcium signalling. Cell. Microbiol. 81601-10. [DOI] [PubMed] [Google Scholar]
- 45.Smith, R. S., E. R. Fedyk, T. A. Springer, N. Mukaida, B. H. Iglewski, and R. P. Phipps. 2001. IL-8 production in human lung fibroblasts and epithelial cells activated by the Pseudomonas autoinducer N-3-oxododecanoyl homoserine lactone is transcriptionally regulated by NF-kappa B and activator protein-2. J. Immunol. 167366-74. [DOI] [PubMed] [Google Scholar]
- 46.Smith, R. S., R. Kelly, B. H. Iglewski, and R. P. Phipps. 2002. The Pseudomonas autoinducer N-(3-oxododecanoyl) homoserine lactone induces cyclooxygenase-2 and prostaglandin E2 production in human lung fibroblasts: implications for inflammation. J. Immunol. 1692636-42. [DOI] [PubMed] [Google Scholar]
- 47.Spears, M., C. McSharry, and N. C. Thomson. 2006. Peroxisome proliferator-activated receptor-gamma agonists as potential anti-inflammatory agents in asthma and chronic obstructive pulmonary disease. Clin. Exp. Allergy 361494-504. [DOI] [PubMed] [Google Scholar]
- 48.Standiford, T. J., V. G. Keshamouni, and R. C. Reddy. 2005. Peroxisome proliferator-activated receptor-γ as a regulator of lung inflammation and repair. Proc. Am. Thorac. Soc. 2226-31. [DOI] [PubMed] [Google Scholar]
- 49.Subbaramaiah, K., D. T. Lin, J. C. Hart, and A. J. Dannenberg. 2001. Peroxisome proliferator-activated receptor gamma ligands suppress the transcriptional activation of cyclooxygenase-2. Evidence for involvement of activator protein-1 and CREB-binding protein/p300. J. Biol. Chem. 27612440-8. [DOI] [PubMed] [Google Scholar]
- 50.Tateda, K., Y. Ishii, M. Horikawa, T. Matsumoto, S. Miyairi, J. C. Pechere, T. J. Standiford, M. Ishiguro, and K. Yamaguchi. 2003. The Pseudomonas aeruginosa autoinducer N-3-oxododecanoyl homoserine lactone accelerates apoptosis in macrophages and neutrophils. Infect. Immun. 715785-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Telford, G., D. Wheeler, P. Williams, P. T. Tomkins, P. Appleby, H. Sewell, G. S. Stewart, B. W. Bycroft, and D. I. Pritchard. 1998. The Pseudomonas aeruginosa quorum-sensing signal molecule N-(3-oxododecanoyl)-l-homoserine lactone has immunomodulatory activity. Infect. Immun. 6636-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tontonoz, P., E. Hu, R. A. Graves, A. I. Budavari, and B. M. Spiegelman. 1994. mPPARγ2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 81224-34. [DOI] [PubMed] [Google Scholar]
- 53.Tontonoz, P., E. Hu, and B. M. Spiegelman. 1994. Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell 791147-56. [DOI] [PubMed] [Google Scholar]
- 54.Van Delden, C., and B. H. Iglewski. 1998. Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerg. Infect. Dis. 4551-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wallace, W. A., P. M. Fitch, A. J. Simpson, and S. E. Howie. 2007. Inflammation-associated remodelling and fibrosis in the lung: a process and an end point. Int. J. Exp. Pathol. 88103-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williams, S. C., E. K. Patterson, N. L. Carty, J. A. Griswold, A. N. Hamood, and K. P. Rumbaugh. 2004. Pseudomonas aeruginosa autoinducer enters and functions in mammalian cells. J. Bacteriol. 1862281-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu, Y., C. Qi, J. R. Korenberg, X. N. Chen, D. Noya, M. S. Rao, and J. K. Reddy. 1995. Structural organization of mouse peroxisome proliferator-activated receptor gamma (mPPAR gamma) gene: alternative promoter use and different splicing yield two mPPAR gamma isoforms. Proc. Natl. Acad. Sci. USA 927921-5. [DOI] [PMC free article] [PubMed] [Google Scholar]