Abstract
We observed a spontaneous amplification of the Streptomyces coelicolor chromosome, including genes encoding biosynthetic enzymes of the antibiotic actinorhodin. A new junction of two tandem segments has, inserted within it, a third copy of a transposable element existing in two places elsewhere in the chromosome, suggesting its involvement in the amplification mechanism.
The linear chromosome of a model actinomycete, Streptomyces coelicolor, whose complete sequence appeared in 2002, extends more than 8 Mb (2). Using DNA microarray analysis, we have characterized 50 S. coelicolor mutants that have gross alterations in their chromosomes caused by genetic instability, showing circular chromosomes and chromosomes with one end replaced by the other after the strains have suffered extremely large deletions and amplifications of DNA (17, 18). The genetic changes in strains that harbored a foreign transposon terminated near native insertion elements, suggesting that the foreign transposon interacted, directly or indirectly, with the native insertion elements (17). Here, we report another gross chromosomal change that involves a native insertion element: the amplification of a 50.5-kb segment that includes the actinorhodin gene cluster. Traditionally, selection for chloramphenicol-sensitive mutants, which detects deletions of large regions from the right end of the chromosome through the loss-of-resistance genes 177 to 321 kb from the terminus, identifies deletion mutants. Since we sought to also identify strains with other genetic changes, we included highly pigmented, chloramphenicol-resistant colonies in our visual screen of over 1,600 colonies of a wild-type S. coelicolor strain (17, 18), as increased antibiotic production is often linked to gross genetic changes (6, 15). A microarray experiment that compared the genomic DNA of one highly pigmented strain, MR3b, and that of the wild-type parent, M145, revealed a 50.5-kb amplified region of the center of the S. coelicolor chromosome (Fig. 1A). Ratios of microarray data across the region were 3:1 higher on average (standard deviation, 1.7) for the mutant and, most likely due to noise, reached up to 10:1. Microarray data of other duplicated chromosomal regions have lower ratios (data not shown), suggesting that MR3b has more than two copies of its amplified region. The amplified DNA extended from approximately SCO5065 through SCO5111, including the entire gene cluster for actinorhodin (SCO5070-SCO5092) (Fig. 1B). While the amplification of biosynthetic gene clusters for antibiotics has been observed for other bacteria, including streptomycetes (14, 20), this work represents the first observation for S. coelicolor.
FIG. 1.
Plot of microarray data for S. coelicolor mutant MR3b and for the wild-type parent strain. (A) All results displayed graphically. The log2 scale shows red/green ratios. A log2(red/green) value of 0 represents the point at which the gene copy numbers of the mutant equal that of the wild type. (B) The upper diagram is a color representation of the microarray data shown in panel A but with the amplification region magnified. In the color scale, green, black, and red represent gene copy numbers that are less than, equal to, or greater than the copy numbers of the wild type, respectively. The lower diagram represents the M145 (wild-type) chromosome arrangement in the amplified region, showing the actinorhodin gene cluster.
The chromosome of strain M145 has two copies of the 1.5-kb transposase gene IS466 (SCO3469 and SCO3490). The microarray data indicate that MR3b has at least threefold more copies of IS466 than the wild-type strain, i.e., six or more copies of the gene. This observation suggests that the amplified region might comprise six or more copies, if IS466 resides at each junction of the amplified region. Thus, increased production of actinorhodin (Fig. 2) might result from the presence of more copies of the entire antibiotic cluster, a mutation observed with improved strains of other streptomycetes (20), or from the presence of more copies of the regulatory gene actII-ORF4, which increases actinorhodin production (7). Note that the amplified region persisted in progeny of MR3b after five generations of growth on solid medium (17; data not shown).
FIG. 2.
Actinorhodin production of the S. coelicolor wild type (M145) and mutant MR3b on solid soy flour mannitol plates after 5 days of growth.
To test if the amplified DNA had a tandem arrangement, we attempted to detect, by PCR, a new DNA junction with SCO5111 and SCO5065 juxtaposed (Fig. 3B). A PCR with a forward primer within SCO5111 and a reverse primer within SCO5065 produced an ∼6-kb fragment, whose sequence showed the amplified DNA extending from part of SCO5065 to all of SCO5111 (Fig. 1B). The DNA sequence revealed sequences of SCO5111 and SCO5065 joined by a copy of IS466 (Fig. 3C). To test whether IS466 was also present at the external junctions of the amplified region (Fig. 3C, junctions between white and black boxes at each end), we sequenced the left and right junctions and found only the native sequence with no rearrangement or insertions of IS466 (data not shown).
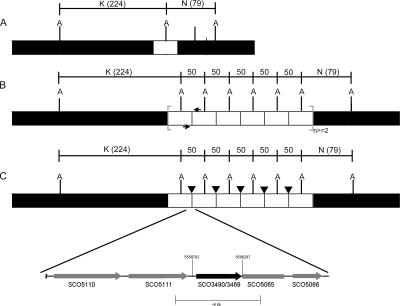
FIG. 3.
Genomic structure of the newly formed junction in the MR3b mutant. (A) Wild-type genome arrangement and AseI-cut sites (indicated by A). (B) Hypothesized arrangement based on microarray data, showing the PCR primer location (black arrows). (C) PCR product sequencing results of the newly formed junction in the genome with the additional insertion element IS466 (black arrowheads), with the exact start and end points of the amplified region noted (numbers above region indicate base pairs). Reactions used genomic DNA and Expand High Fidelity polymerase. K, AseI-K fragment; N, AseI-N fragment.
PCR amplification and sequencing detected the presence of both original IS466 copies (SCO3469 and SCO3490) in their native genome locations in the M145 and MR3b strains (data not shown). Thus, MR3b contains at least three copies of the insertion element in its genome, suggestive of replicative or cut-and-paste transposition.
To obtain further information about the physical location of the amplified DNA, we examined genomic DNA of strain MR3b by pulsed-field gel electrophoresis (PFGE) (10). The 50.5-kb amplified region contains two AseI sites, separated by several base pairs, such that tandem amplification of this sequence generates an additional AseI band that is 50.5 kb, as was observed in AseI-digested genomic DNA of MR3b (Fig. 3B and 4). Southern hybridization showed the presence of telomeres in the MR3b chromosome, indicating a linear chromosome (data not shown). PFGE of undigested DNA showed no extrachromosomal DNA fragments (data not shown). A densitometry scan of the AseI fragments showed a copy number of approximately 7.
FIG. 4.
Results of PFGE of S. coelicolor mutant MR3b, in which a 50.5-kb fragment underwent spontaneous amplification. Genomic DNA was digested with AseI. G through P, AseI-G fragment through AseI-P fragment.
Sequence comparisons showed a lack of synteny between the region amplified in MR3b, including S. coelicolor genes SCO5065 and SCO5098, and the corresponding regions in Streptomyces avermitilis and Streptomyces scabies, two other sequenced streptomycetes. Figure 5A and B show synteny plots for S. coelicolor and S. avermitilis and for S. coelicolor and S. scabies, respectively. The left boundaries of the amplified DNA and the interruption of synteny coincide. Beyond the left end of the amplified DNA, synteny exists between S. coelicolor SCO5035-SCO5062, S. avermitilis SAV3210-SAV3329, and S. scabies SSC3345-SSC3363. An interruption in synteny occurs for 37 genes. Near the right end of the amplified DNA, synteny resumes at S. coelicolor SCO5099, S. avermitilis SAV3190, and S. scabies SSC3171. The right end of the amplified DNA contains 13 genes that possess synteny with the other genomes. In addition, S. coelicolor SCO5064, which resides at the left boundary of the amplified region, has a sequence similar to that of S. avermitilis SAV3192, whose location corresponds to the right boundary. The majority of genes adjacent to the amplified regions in S. coelicolor, S. avermitilis, and S. scabies show synteny. Note that the DNA sequence of the actinorhodin gene cluster lacks similarities to other sequences in the public database, leaving the origin of the actinorhodin gene cluster unknown. We cannot rule out the unlikely possibility that the amplified region is a multicopy circular DNA, which somehow can replicate in S. coelicolor.
FIG. 5.
Dot plot comparison of the S. coelicolor M145 sequence (NC_003888) (x axis) with the corresponding S. avermitilis sequence (NC_003155) (A) or with the corresponding S. scabies sequence (B) (y axis). (Sequence data, produced by the S. scabies sequencing group at the Sanger Institute, were obtained from ftp://ftp.sanger.ac.uk/pub/pathogens/ssc/Ssc.dbs.) The slanted lines indicate significant DNA similarities. SAV and SSC indicate S. avermitilis and S. scabies gene numbers, respectively. The plots were generated using an nucleic acid identity matrix, where the window size was 30, the hash value was 6, the jump was 1, and the minimum percentage score was 75%.
To our knowledge, this work represents the first evidence of a large spontaneous amplification of an antibiotic gene cluster involving a native insertion sequence in a streptomycete. In streptomycetes, amplifications previously associated with transposable elements likely occurred by homologous recombination between insertion sequences that flanked both ends of the amplified DNA (5, 16). Lack of homology between the ends of the 50.5-kb amplified region in strain MR3b precludes amplification by the rolling-circle model of replication, a mechanism that creates tandem amplifications and requires regions of homology at the beginning and end of the amplified unit (20, 21). However, the amplification in MR3b might have arisen after an initial insertion of IS466 by replicative transposition, accompanied by a tandem duplication of the region, and, subsequently, homologous recombination between the duplicated regions. While an exact mechanism for MR3b remains unclear, insertion elements have been implicated in genetic instability for many organisms, including streptomycetes (3).
Possible acquisition of the actinorhodin gene cluster by S. coelicolor through horizontal gene transfer reflects the diverse cargo that mobile genetic elements transfer between bacteria. Gene transfer on autonomous and integrative plasmids, has been well documented for streptomycetes and includes large linear plasmids (12) and the integration of the methylenomycin antibiotic gene cluster, carried by SCP1, into the S. coelicolor chromosome. Phylogeny studies of streptomycetes also provide evidence for the horizontal transfer of antibiotic gene clusters (1, 4, 19). In streptomycetes and other bacteria, such as Streptococcus pneumoniae, which has penicillin resistance genes likely acquired by horizontal gene transfer, the transferred genes often lie adjacent to specific DNA sequences, such as direct repeats or insertion elements, and undergo duplications and amplifications at high frequencies (8, 13).
Acknowledgments
We thank the John Innes Centre for its generous support and, in particular, Mark Buttner, Mervyn Bibb, David Hopwood, Tobias Kieser, and Maureen Bibb for their scientific guidance.
C.W.C. acknowledges the support of a research grant from the National Science Council, Republic of China (NSC95-2321-B-010-002).
Footnotes
Published ahead of print on 25 April 2008.
REFERENCES
- 1.Alexander, D. C., and S. E. Jensen. 1998. Investigation of the Streptomyces clavuligerus cephamycin C gene cluster and its regulation by the CcaR protein. J. Bacteriol. 1804068-4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bentley, S. D., K. F. Chater, A.-M. CerdnÞo-Tárraga, G. L. Challis, N. R. Thomson, et al. 2002. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417141-147. [DOI] [PubMed] [Google Scholar]
- 3.Chen, C. W., C. H. Huang, H. H. Lee, H. H. Tsai, and R. Kirby. 2002. Once the circle has been broken: dynamics and evolution of Streptomyces chromosomes. Trends Genet. 18522-529. [DOI] [PubMed] [Google Scholar]
- 4.Egan, S., P. Wiener, D. Kalifidas, and E. M. H. Wellington. 2001. Phylogeny of Streptomyces species and evidence for horizontal transfer of antibiotic gene clusters. Antonie van Leeuwenhoek 29127-133. [DOI] [PubMed] [Google Scholar]
- 5.Eichenseer, C., and J. Altenbuchner. 1994. The very large amplifiable element AUD2 from Streptomyces lividans 66 has insertion sequence-like repeats at its ends. J. Bacteriol. 1767107-7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flett, F., and J. Cullum. 1987. DNA deletions in spontaneous chloramphenicol-sensitive mutants of Streptomyces coelicolor A3(2) and Streptomyces lividans 66. Mol. Gen. Genet. 207499-502. [DOI] [PubMed] [Google Scholar]
- 7.Gramajo, H. C., E. Takano, and M. J. Bibb. 1993. Stationary-phase production of the antibiotic actinorhodin in Streptomyces coelicolor A3(2) is transcriptionally regulated. Mol. Microbiol. 7837-845. [DOI] [PubMed] [Google Scholar]
- 8.Hacker, J., G. Blum-Oehler, I. Mühldorfer, and H. Tschäpe. 1997. Pathogenicity islands of virulent bacteria: structure, function and impact on microbial evolution. Mol. Microbiol. 231089-1097. [DOI] [PubMed] [Google Scholar]
- 9.Reference deleted.
- 10.Kieser, H. M., T. Kieser, and D. A. Hopwood. 1992. A combined genetic and physical map of the Streptomyces coelicolor A3(2) chromosome. J. Bacteriol. 1745496-5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kinashi, H., M. Shimaji, and A. Sakai. 1987. Giant linear plasmids in Streptomyces which code for antibiotic biosynthesis genes. Nature 328454-456. [DOI] [PubMed] [Google Scholar]
- 12.Kinashi, H. 1994. Linear plasmids from actinomycetes. Actinomycetologica 887-96. [Google Scholar]
- 13.Maynard Smith, J., C. G. Dowson, and B. G Spratt. 1991. Localized sex in bacteria. Nature 34929-31. [DOI] [PubMed] [Google Scholar]
- 14.Penschke, U., H. Schmidt, H. Zhang, and W. Piepersberg. 1995. Molecular characterization of the lincomycin-production gene cluster of Streptomyces lincolnensis 78-11. Mol. Microbiol. 161137-1156. [DOI] [PubMed] [Google Scholar]
- 15.Sermonti, G., A. Petris, M. Micheli, and L. Lanfolni. 1978. Chloramphenicol resistance in Streptomyces coelicolor A3(2): possible involvement of a transposable element. Mol. Gen. Genet. 16499-103. [DOI] [PubMed] [Google Scholar]
- 16.Volff, J. N., and J. Altenbuchner. 1997. High-frequency transposition of IS1373, the insertion sequence delimiting the amplifiable element AUD2 of Streptomyces lividans. J. Bacteriol. 1795639-5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Widenbrant, E., and C. M. Kao. 2007. Introduction of the foreign transposon Tn4560 in Streptomyces coelicolor leads to genetic instability near the native insertion sequence IS1649. J. Bacteriol. 1899108-9116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Widenbrant, E., H.-H. Tsai, C. W. Chen, and C. M. Kao. 2007. Streptomyces coelicolor undergoes spontaneous end replacement. J. Bacteriol. 1899117-9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiener, P., S. Egan, and E. M. H. Wellington. 1998. Evidence for transfer of antibiotic-resistance genes in soil populations of streptomycetes. Mol. Ecol. 71205-1216. [DOI] [PubMed] [Google Scholar]
- 20.Yanai, K., T. Murakami, and M. Bibb. 2006. Amplification of the entire kanamycin biosynthetic gene cluster during empirical strain improvement of Streptomyces kanamyceticus. Proc. Natl. Acad. Sci. USA 1039661-9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young, M., and J. Cullum. 1987. A plausible mechanism for large-scale chromosomal DNA amplification in streptomycetes. FEBS Lett. 21210-14. [DOI] [PubMed] [Google Scholar]