Abstract
The two-component system CesRK of Listeria monocytogenes responds to cell wall-acting antibiotics. We show here that CesRK controls the transcription of several cell envelope-related genes. The CesRK-dependent induction of these genes may be viewed as an attempt by L. monocytogenes to protect itself against the damaging effects of cell wall-acting antibiotics.
The gram-positive human pathogen Listeria monocytogenes is able to cause serious food-borne infections (16). Individuals suspected of being infected by L. monocytogenes are typically treated with ampicillin, either alone or in combination with an aminoglycoside (15). Despite the use of ampicillin in the treatment of listeriosis, the exact mechanism by which L. monocytogenes senses and responds to β-lactam antibiotics is currently unknown.
The CesRK two-component system contributes to the intrinsic resistance of L. monocytogenes LO28 to antibiotics of the β-lactam family. In addition, mutants lacking cesR or cesK are more tolerant to ethanol (7, 8). The genes encoding the CesRK two-component system are located immediately upstream from a small open reading frame, orf2420. Subinhibitory concentrations of a large range of cell wall-active antimicrobial agents induce the transcription of orf2420 in a CesRK-dependent fashion (7). These findings suggested a role for CesRK in sensing and responding to changes in the cell wall integrity.
Recently, a comparative transcriptome study of L. monocytogenes EGD-e revealed that several genes with putative cell wall-related functions were among those with the highest alcohol-induced differential expression, having up to 40-fold higher expression during growth in the presence of sublethal concentrations of isopropanol (A. Gravesen, H. Jarmer, K. Kutchmina, J. Bresciani, S. Knøchel, T. Chakraborty, and T. Hain, unpublished data). Since the activity of CesRK is strongly induced by ethanol, we found it likely that some of these alcohol-inducible genes may be under the control of CesRK. In order to test this, DNA fragments containing the putative promoter regions of eight genes induced more than threefold by isoproponal (lmo0443. lmo1037, lmo1215, lmo1416, lmo2210, lmo2442, lmo2522, and lmo2812) were amplified by PCR (primers are listed in Table S1 in the supplemental material). The DNA fragments were fused to lacZ in the promoterless lacZ fusion vector pTCV-lac (10) and introduced into L. monocytogenes LO28 wild-type, ΔcesR, and ΔcesK strains. The CesRK-regulated gene orf2420 was included in these experiments as a positive control. Cells containing promoter-lacZ fusions were grown in brain heart infusion (BHI) medium to an optical density at 600 nm (OD600) of 0.2. The cultures were split, and the inducers ethanol, ampicillin, or vancomycin were added at subinhibitory concentrations. Cells were collected 1 h after the addition of inducers and assayed for β-galactosidase activity as described previously (7). As expected, the expression of orf2420-lacZ was induced in a CesRK-dependent manner (Table 1). Interestingly, the expression of lmo0443-lacZ, lmo1416-lacZ, and lmo2812-lacZ was clearly induced in the wild-type strain. Induction was completely abolished in the ΔcesR and ΔcesK strains, indicating that the expression of these three genes is controlled by CesRK (Table 1). lmo2210-lacZ was clearly induced as well, but the induction was not affected by the absence of cesR or cesK. The expression of lmo2522-lacZ was induced by ethanol only, and CesRK is not involved in this regulation (Table 1). Finally, the specific β-galactosidase activities in cells containing lmo1037-lacZ, lmo1215-lacZ, or lmo2442-lacZ were very low under all of the conditions tested, indicating that these three genes are not preceded by inducible promoters that can be detected by this assay.
TABLE 1.
Expression of promoter-lacZ fusions in response to the addition of ethanol, ampicillin, or vancomycin as determined by β-galactosidase assays
| Gene | Expressiona
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt
|
ΔcesR mutant
|
ΔcesK mutant
|
||||||||||
| None | EtOH | Amp | Van | None | EtOH | Amp | Van | None | EtOH | Amp | Van | |
| Inducible and CesRK-dependent genes | ||||||||||||
| orf2420 | 22 | 1,210 | 1,430 | 1,320 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| lmo0443 | 54 | 1,150 | 1,300 | 740 | 42 | 57 | 55 | 54 | 46 | 62 | 63 | 60 |
| lmo1416 | 4 | 90 | 72 | 92 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| lmo2812 | 4 | 80 | 108 | 50 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Inducible but mainly CesRK-independent genes | ||||||||||||
| lmo2210 | 3 | 18 | 30 | 13 | 3 | 9 | 36 | 12 | 3 | 8 | 27 | 12 |
| lmo2522 | 3 | 10 | 3 | ND | 3 | 9 | 3 | ND | 3 | 9 | 3 | ND |
| Noninducible genes | ||||||||||||
| lmo1037 | 4 | 4 | 3 | ND | 4 | 4 | 3 | ND | 3 | 4 | 4 | ND |
| lmo1215 | 5 | 5 | 4 | ND | 5 | 5 | 4 | ND | 5 | 4 | 4 | ND |
| lmo2442 | 4 | 6 | 5 | ND | 4 | 5 | 3 | ND | 4 | 5 | 3 | ND |
The expression of promoter-lacZ fusions in response to the addition of 2% ethanol (EtOH), 0.1 μg of ampicillin per ml (Amp), or 0.3 μg of vancomycin per ml (Van) was determined by β-galactosidase assays. The specific β-galactosidase activity was measured for wild-type (wt) or ΔcesR or ΔcesK mutant cells containing promoter-lacZ fusions, grown for 1 h in the presence or absence (None) of inducer. ND, not determined. The data represent the mean of three experiments, in which the observed variation did not exceed 10%.
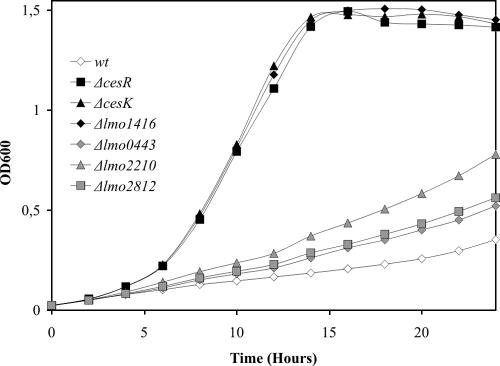
Curiously, the majority of the highly inducible genes encode proteins with putative cell wall-related functions. Lmo0443 is a 309-amino-acid protein belonging to the LytR/CpsA/Psr family of envelope-related regulatory proteins (9, 12). Lmo1416 belongs to the VanZ family of proteins. The vanZ gene is located within Tn1546 from Enterococcus faecium, together with genes required for glycopeptide resistance (1). VanZ is an accessory protein that confers low-level resistance to teicoplanin by an unknown mechanism. Lmo2812 is a putative d-alanyl-d-alanine carboxypeptidase that may catalyze the removal of the C-terminal d-alanine residue from peptidoglycan pentapeptides (2), whereas Lmo2210 shows no homology to other known proteins. For further characterization of lmo0443, lmo1416, lmo2812, and lmo2210, mutants with in-frame deletions were constructed in L. monocytogenes LO28 (7) (primers for the construction of in-frame deletion mutants are listed in Table S1 in the supplemental material). The growth rate of the mutant strains in BHI medium was comparable to the growth rate of the wild-type strain (data not shown). As observed previously, the ΔcesR and ΔcesK strains were able to grow in the presence of ethanol, whereas growth of the wild-type strain was restricted (Fig. 1). The Δlmo1416 mutant was clearly tolerant to ethanol as well, whereas small but significant effects on growth were observed for the Δlmo0443, Δlmo2210, and Δlmo2812 mutants relative to the wild-type strain (Fig. 1).
FIG. 1.
Ethanol tolerance of wild-type and mutant strains. L. monocytogenes LO28 wild-type (wt) and mutant strains carrying in-frame deletions of the cesR, cesK, lmo0443, lmo1416, lmo2210, and lmo2812 genes were grown in BHI medium containing 5% ethanol. The data represent the means of three experiments, in which the observed variation did not exceed 10%.
In order to determine the role of Lmo0443, Lmo1416, Lmo2210, and Lmo2812 in the resistance of L. monocytogenes to cell wall-active antibiotics, disk diffusion assays were performed as described previously (7) (Table 2). As expected, the ΔcesR mutant was more sensitive to cell-active antibiotics of the β-lactam family, in particular the cephalosporins (7). Interestingly, deletion of lmo1416 resulted in an increased sensitivity toward cefuroxime and ampicillin as well, suggesting that Lmo1416 contributes to the resistance of L. monocytogenes to β-lactam antibiotics.
TABLE 2.
β-Lactam and glycopeptide resistance of L. monocytogenes LO28 wild-type and ΔcesR, ΔcesK, Δlmo0443, Δlmo1416, Δlmo2210, and Δlmo2812 mutant strains
| Substance (amt [μg]) | Avg zone of inhibition (mm) ± SDa
|
||||||
|---|---|---|---|---|---|---|---|
| wt | ΔcesR mutant | ΔcesK mutant | Δlmo0443 mutant | Δlmo1416 mutant | Δlmo2210 mutant | Δlmo2812 mutant | |
| Cefuroxime (30) | 19.2 ± 0.8 | 31.0 ± 0.5* | 28.7 ± 0.3* | 18.2 ± 0.8 | 30.2 ± 0.8* | 19.3 ± 1.3 | 17.5 ± 0.5 |
| Ampicillin (10) | 33.5 ± 0.5 | 36.2 ± 0.3* | 36.3 ± 0.8* | 30.8 ± 0.8 | 37.0 ± 0.5* | 33.3 ± 0.3 | 33.8 ± 0.3 |
| Vancomycin (30) | 22.8 ± 0.3 | 23.8 ± 0.3 | 24.3 ± 0.8 | 23.0 ± 1.0 | 22.8 ± 0.3 | 20.8 ± 0.3* | 19.5 ± 0.5* |
| Teicoplanin (30) | 22.5 ± 0.5 | 22.2 ± 0.3 | 21.8 ± 0.3 | 21.7 ± 0.8 | 20.8 ± 0.3 | 19.5 ± 0,0* | 18.5 ± 0.9* |
The results are averages of triplicate experiments. *, significant difference (P < 0.01) between the mutant strain and the wild type.
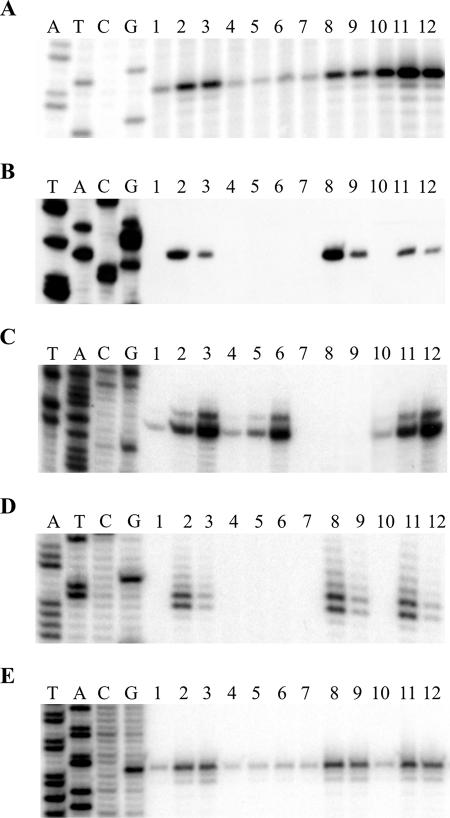
In order to map potential CesRK-dependent transcription start sites upstream from lmo0443, lmo1416, lmo2210, and lmo2812, we performed primer extension analysis on total RNA purified from wild-type and ΔcesR mutant strains treated with subinhibitory concentrations of ethanol or cefuroxime as described previously (7). In the presence of inducers, putative transcription start sites were observed upstream from all four genes in the wild-type strain (Fig. 2A to D). Importantly, CesR is required for induction of the transcription of lmo0443, lmo1416, and lmo2812 but not for the induction of lmo2210.
FIG. 2.
Primer extension analysis of genes induced by cell wall-acting antimicrobial agents. (A to E) Expression of lmo0443 (A), lmo1416 (B), lmo2210 (C), lmo2812 (D), and lmo0441 (E) genes in response to subinhibitory concentrations of ethanol and cefuroxime. The analysis was performed with RNA purified from the wild-type (lanes 1 to 3), ΔcesR (lanes 4 to 6), ΔlisR (lanes 7 to 9), or Δlmo0443 (lanes 10 to 12) strain. Cells were grown in BHI medium to an OD600 of 0.3. The cell cultures were split and treated with 2% ethanol (lanes 2, 5, 8, and 11) or 4 μg of cefuroxime per ml (lanes 3, 6, 9, and 12) for 20 min. Controls without treatment were included (lanes 1, 4, 7, and 10). Lanes G, A, T, and C are sequencing ladders. The primers used for the primer extension analysis are shown in Table S1 in the supplemental material.
In addition to CesRK, other transcriptional regulatory systems may be involved in the control of alcohol- and antibiotic-inducible genes in L. monocytogenes. Like CesRK, the LisRK two-component system mediates ethanol sensitivity and β-lactam resistance in L. monocytogenes (3, 4). In addition, lmo0443 encodes a putative regulatory protein, suggesting that the CesRK-dependent induction of gene expression could be an indirect effect mediated though Lmo0443. To test whether LisR or Lmo0443 affects the expression of lmo0443, lmo1416, lmo2210, or lmo2812, we performed primer extension analyses of these four genes in mutant strains with lisR or lmo0443 deleted (Fig. 2A to D). In comparison to the wild-type strain, the expression of lmo0443 was clearly higher in the Δlmo0443 strain, indicating that lmo0443 is subject to negative autoregulation. Interestingly, no induction of lmo2210 was observed in the ΔlisR strain, indicating that antimicrobial agents affecting the bacterial cell wall induce the expression of lmo2210 in a LisR-dependent manner.
β-Lactam antibiotics bind to and inhibit the activity of penicillin-binding proteins (PBPs), which carry out the assembly of the peptidoglycan of the bacterial cell envelope. The L. monocytogenes EGD-e genome contains ten genes encoding proteins with similarities to PBPs (2). A recent study of seven genes encoding PBP-like proteins showed that interruption of lmo0441 and, to a lesser extent, lmo2229 resulted in an increased sensitivity of L. monocytogenes EGD-e to β-lactam antibiotics (6). We tested whether the addition of subinhibitory concentrations of ethanol or cefuroxime alters the expression of lmo0441 and lmo2229 in L. monocytogenes LO28. We found that in the wild-type, ΔcesR, and ΔlisR strains the expression of a lmo2229-lacZ fusion (5) was not affected by the presence of inducers (data not shown). In contrast, a primer extension analysis of lmo0441 revealed the presence of an ethanol- and cefuroxime-inducible transcription start site within the lmo0441 promoter region (Fig. 2E). Transcription is not affected by the absence of Lmo0443 or LisR; however, CesR is clearly needed in order to induce the expression of lmo0441.
Transcriptional regulatory proteins are known to activate transcription by interacting with single or multiple DNA binding sites located within or upstream of the promoter. By inspection of the promoter regions of the CesR-regulated genes lmo0441, lmo0443, lmo1416, orf2420, and lmo2812, a conserved nine-nucleotide sequence element (aatCTTTAA) was found to be present in all five promoter regions located from bp −40 to bp −65 upstream from the transcription start sites (Fig. 3). We speculate that this sequence element may correspond to a DNA-binding site recognized by a transcriptional activator, such as CesR, under inducing conditions. To analyze whether this conserved element is required for the CesRK-dependent expression of orf2420, a site-directed mutagenesis experiment was performed. DNA fragments extending from positions −130 to +42 with respect to the transcription start site, containing various substitutions within the conserved sequence element, were generated by PCR using the primers shown in Table S1 in the supplemental material and fused to lacZ in pTCV-lac. The resulting plasmids were introduced into wild-type and ΔcesR strains, and cells were assayed for β-galactosidase activity before (t = 0 h) and 1 h after the addition of the inducer cefuroxime. As observed previously (7), the expression of orf2420 is highly dependent on the presence of CesR, even in the absence of a cell wall-acting antimicrobial agent (i.e., at t = 0 h; Table 3). As expected, the expression of the wild-type promoter was strongly induced by cefuroxime in a CesR-dependent manner. Substitutions of all nine nucleotides (from −57 to −65) completely abolished induction by cefuroxime. The CesR-dependent induction by cefuroxime was lost, either partly or completely, by substitution of only three of the nine nucleotides (Table 3). These results indicate that the conserved nine-nucleotide sequence element extending from positions −57 to −65 in the orf2420 promoter region is part of a CesRK-responsive, antibiotic-inducible element. We note that the consequence of substitutions at positions −60 to −62 is equally as dramatic as that which occurs when all nine nucleotides are altered, indicating that one or more nucleotides at positions −60 to −62 are highly significant for the CesRK-dependent induction of orf2420.
FIG. 3.
Sequence alignment of the promoter regions of the CesR-regulated genes lmo0441, lmo0443, lmo1416, orf2420, and lmo2812. Transcription start sites (+1) are indicated in boldface. The putative −10 boxes are underlined, whereas the 9-bp putative CesR-responsive elements, located between positions −40 and −65 relative to the transcription start sites, are shown in boldface.
TABLE 3.
Induction of orf2420 by cefuroxime requires an intact CesR-responsive elementa
| orf2420-lacZ derivative | CesR-responsive element | Sp act (t = 0)
|
Sp act (t = 1 h after induction)
|
||||
|---|---|---|---|---|---|---|---|
| wt
|
ΔcesR mutantb
|
||||||
| wt | ΔcesR mutant | - | + | - | + | ||
| Wild type | AATCTTTAA | 17 | 4 | 19 | 201 | 3 | 3 |
| Mut-57-65 | TTAGAAATT | 4 | 4 | 4 | 3 | 3 | 3 |
| Mut-57-59 | TTACTTTAA | 5 | 4 | 4 | 13 | 4 | 3 |
| Mut-60-62 | AATGAATAA | 4 | 4 | 3 | 3 | 3 | 3 |
| Mut-63-65 | AATCTTATT | 4 | 4 | 4 | 9 | 3 | 3 |
The wild-type (wt) or ΔcesR mutant strains contain a wild-type orf2420-lacZ fusion or mutant (Mut) orf2420-lacZ derivatives carrying various substitutions within the CesR-responsive element situated from positions −57 to −65 with respect to the orf2420 transcription start site. Cells were grown in BHI medium to an OD600 of 0.2. Samples were harvested and subjected to β-galactosidase assays (t = 0; no cefuroxime treatment). The residual of the cell cultures was split, and half of the culture was treated with 4 μg of cefuroxime/ml for 1 h. Samples were harvested from both untreated (−) and treated (+) samples and subjected to β-galactosidase assays (t = 1 h after induction).
Substitutions are indicated in boldface.
Multiresistant strains of L. monocytogenes have been identified in a number of studies, including strains with resistance to the antibiotics commonly used to treat human listeriosis (11, 13, 14). These findings clearly emphasize the need for improving our understanding of how L. monocytogenes senses and responds to antimicrobial agents. Our results suggest that CesRK is part of a complex regulatory network in L. monocytogenes that controls the expression of genes involved in cell wall maintenance in response to the presence of cell wall-acting antimicrobials, including antibiotics used in the treatment of bacterial infections. In addition to CesRK, this regulatory network includes at least one other two-component system, LisRK, and the LytR-like regulatory protein Lmo0443. The signal recognized by CesK appears to be generated when the cell wall is damaged. In an attempt to defend itself against these antimicrobial agents, L. monocytogenes will induce the expression of genes involved in cell wall synthesis, such as lmo0441 and lmo2812 encoding PBP-like proteins. Whether CesRK controls the expression of additional genes, such as stress resistance genes that function to protect L. monocytogenes from the stress imposed by cell wall-acting antimicrobial agents, is an obvious possibility that should be addressed in future studies.
Supplementary Material
Acknowledgments
This study was supported by a grant from the Danish Natural Science Research Council.
We thank Christina Kirkegaard for expert technical assistance.
Footnotes
Published ahead of print on 2 May 2008.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Arthur, M., F. Depardieu, C. Molinas, P. Reynolds, and P. Courvalin. 1995. The vanZ gene of Tn1546 from Enterococcus faecium BM4147 confers resistance to teicoplanin. Gene 15987-92. [DOI] [PubMed] [Google Scholar]
- 2.Bierne, H., and P. Cossart. 2007. Listeria monocytogenes surface proteins: from genome predictions to function. Microbiol. Mol. Biol. Rev. 71377-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cotter, P. D., C. M. Guinane, and C. Hill. 2002. The LisRK signal transduction system determines the sensitivity of Listeria monocytogenes to nisin and cephalosporins. Antimicrob. Agents Chemother. 462784-2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cotter, P. D., N. Emerson, C. G. M. Gahan, and C. Hill. 1999. Identification and disruption of lisRK, a genetic locus encoding a two-component signal transduction system involved in stress tolerance and virulence of Listeria monocytogenes. J. Bacteriol. 1816840-6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gravesen, A., B. Kallipolitis, K. Holmstrøm, P. D. Høiby, M. Ramnath, and S. Knøchel. 2004. pbp2229-mediated nisin resistance mechanism in Listeria monocytogenes confers cross-protection to class IIa bacteriocins and affects virulence gene expression. Appl. Environ. Microbiol. 701669-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guinane, C. M., P. D. Cotter, R. P. Ross, and C. Hill. 2006. Contribution of penicillin-binding protein homologs to antibiotic resistance, cell morphology, and virulence of Listeria monocytogenes EGDe. Antimicrob. Agents Chemother. 502824-2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kallipolitis, B. H., H. Ingmer, C. G. Gahan, C. Hill, and L. Søgaard-Andersen. 2003. CesRK, a two-component signal transduction system in Listeria monocytogenes, responds to the presence of cell wall-acting antibiotics and affects β-lactam resistance. Antimicrob. Agents Chemother. 473421-3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kallipolitis, B. H., and H. Ingmer. 2001. Listeria monocytogenes response regulators important for stress tolerance and pathogenesis. FEMS Microbiol. Lett. 204111-115. [DOI] [PubMed] [Google Scholar]
- 9.Lazarevic, V., P. Margot, B. Soldo, and D. Karamta. 1992. Sequencing and analysis of the Bacillus subtilis lytRABC divergon: a regulatory unit encompassing the structural genes of the N-acetylmuramoyl-l-alanine amidase and its modifier. J. Gen. Microbiol. 1381949-1961. [DOI] [PubMed] [Google Scholar]
- 10.Poyart, C., and P. Trieu-Cuot. 1997. A broad-host-range mobilizable shuttle vector for the construction of transcriptional fusions to β-galactosidase in gram-positive bacteria. FEMS Microbiol. Lett. 156193-198. [DOI] [PubMed] [Google Scholar]
- 11.Prazak, M. A., E. A. Murano, I. Mercado, and G. R. Acuff. 2002. Antimicrobial resistance of Listeria monocytogenes isolated from various cabbage farms and packing sheds in Texas. J. Food Prot. 651796-1799. [DOI] [PubMed] [Google Scholar]
- 12.Rossi, J., M. Bischoff, A. Wada, and B. Berger-Bächi. 2003. MsrR, a putative cell envelope-associated element involved in Staphylococcus aureus sarA attenuation. Antimicrob. Agents Chemother. 472558-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Safdar, A., and D. Armstrong. 2003. Antimicrobial activities against 84 Listeria monocytogenes isolates from patients with systemic listeriosis at a comprehensive cancer center (1955-1997). J. Clin. Microbiol. 41483-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srinivasan, V., H. M. Nam, L. T. Nguyen, B. Tamilselvam, S. E. Murinda, and S. P. Oliver. 2005. Prevalence of antimicrobial resistance genes in Listeria monocytogenes isolated from dairy farms. Foodborne Pathog. Dis. 2201-211. [DOI] [PubMed] [Google Scholar]
- 15.Temple, M. E., and M. C. Nahata. 2000. Treatment of listeriosis. Ann. Pharmacother. 34656-661. [DOI] [PubMed] [Google Scholar]
- 16.Vazquez-Boland, J. A., M. Kuhn, P. Berche, T. Chakraborty, G. Dominguez-Bernal, W. Goebel, B. Gonzalez-Zorn, J. Wehland, and J. Kreft. 2001. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 141-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.