Abstract
Myxococcus xanthus undergoes a complex starvation-induced developmental program that results in cells forming multicellular fruiting bodies by aggregating into mounds and then differentiating into spores. This developmental program requires at least 72 h and is mediated by a temporal cascade of gene regulators in response to intra- and extracellular signals. espA mutants, encoding an orphan hybrid histidine kinase, alter the timing of this developmental program, greatly accelerating developmental progression. Here, we characterized EspA and demonstrated that it autophosphorylates in vitro on the conserved histidine residue and then transfers the phosphoryl group to the conserved aspartate residue in the associated receiver domain. The conserved histidine and aspartate residues were both required for EspA function in vivo. Analysis of developmental gene expression and protein accumulation in espA mutants indicated that the expression of the A-signal-dependent spi gene was not affected but that the MrpC transcriptional regulator accumulated earlier, resulting in earlier expression of its target, the FruA transcriptional regulator. Early expression of FruA correlated with acceleration of both the aggregation and sporulation branches of the developmental program, as monitored by early methylation of the FrzCD chemosensory receptor and early expression of the sporulation-specific dev and Mxan_3227 (Ω7536) genes. These results show that EspA plays a key role in the timing of expression of genes necessary for progression of cells through the developmental program.
Myxococcus xanthus is a gram-negative, rod-shaped bacterium that is a model system for social behavior in prokaryotes. M. xanthus resides in the soil or on herbivore dung and obtains nutrients by secreting antibiotics and enzymes (such as proteases and lysozyme) that digest macromolecules from prey microorganisms or decaying organic matter (43). Communities of M. xanthus exhibit cooperative growth which is dependent on the amount of secreted enzymes that break down complex macromolecules for subsequent uptake by the individual cells (44). Under starvation conditions, M. xanthus enters an elaborate developmental program in which cells first aggregate into mounds of approximately 100,000 cells and then within these mounds (fruiting bodies), cells differentiate into metabolically quiescent, environmentally resistant spores (reviewed in reference 50). Upon sensing nutrient-rich conditions, spores germinate and reenter the vegetative cycle. It is presumed that formation of spores within fruiting bodies allows M. xanthus to germinate in groups, providing an advantage for cooperative feeding behaviors.
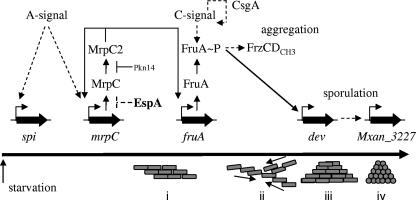
The M. xanthus developmental program is temporally regulated in association with a series of intra- and intercellular signaling events (reviewed in reference 13). A core pathway of molecular events have been identified which are necessary for formation of mature fruiting bodies (summarized in Fig. 7). First, starvation is sensed via the stringent response, resulting in increased guanosine tetra- or pentaphosphate [(p)ppGpp] (10, 51). Rising (p)ppGpp levels trigger A-signaling (51), proposed to be a population (quorum)-sensing mechanism (14, 23) in which individual cells are induced to release amino acids and peptides generated by extracellular proteolysis in a manner dependent on genes of the asg class (20-22, 38). When sufficient A-signal is produced, several genes necessary for the developmental program are induced (14, 23).
FIG. 7.
Molecular events during the M. xanthus developmental program (top) in relation to aggregation and sporulation (bottom). The roles of the developmental gene and protein expression patterns that are assayed in the espA mutants are depicted; see text for details. Pkn14 phosphorylates MrpC (data not shown), which prevents MrpC2 accumulation (35). EspA may repress protein accumulation of MrpC. Solid lines represent direct interactions; dashed lines indicate that mechanisms of action are indirect or unknown. The long horizontal arrow represents time. Groups of M. xanthus cells (gray rectangles) first responding to nutrient limitation and A-signal (i), begin to aggregate (ii) into mounds (iii) and then form spores (gray circles) within the mounds (iv).
One of the genes whose transcriptional upregulation depends upon A-signaling is mrpC, which encodes a transcriptional regulator of the cyclic AMP receptor family (56, 57). The MrpC protein is subject to complex posttranslational regulation, which appears to control its affinity for identified target promoters: phosphorylated MrpC (MrpC-P) affinity for target sequences is reduced, whereas the proteolytically processed MrpC2 protein shows increased affinity (34, 35). In addition, MrpC-P does not appear to be processed into MrpC2. MrpC2 binds to the promoter and is thought to induce transcriptional upregulation of the key developmental transcriptional regulator gene, fruA (60). FruA is an orphan response regulator of the two-component signal transduction family containing a DNA-binding output domain (5, 36). Genetic evidence suggests that FruA is activated by phosphorylation of a conserved aspartic acid in the receiver domain (5).
FruA activation is proposed to occur in response to the C-signal pathway (5). C-signal is encoded by the csgA gene, and csgA mutants are unable to form proper fruiting bodies or to sporulate (8, 9, 16). C-signal arises from proteolytic processing of the cell-surface-associated CsgA protein (28) and has been proposed to be sensed by an unidentified receptor protein on a neighboring cell. As a result of cell-cell contact, CsgA expression is upregulated (15, 17) and C-signaling is amplified (7).
In a current model (reviewed in references 13 and 52), FruA activated in response to C-signaling stimulates development through a branched pathway, with one branch leading to aggregation and a second branch leading to sporulation. FruA is proposed to induce the aggregation branch by, in an unknown mechanism, stimulating methylation of the FrzCD methyl-accepting chemotaxis protein, which directs cells to aggregate into mounds (2, 30, 31, 48, 53). Increased cell-cell contact in mounds is proposed to lead to increased C-signaling and increased activation of FruA. It is then proposed that the higher levels of activated FruA induce the sporulation branch of the developmental pathway. It has been demonstrated that FruA activates transcription of the dev locus (63), which in turn is necessary for expression of Tn5 lacZ Ω7536 (27), which resides in Mxan_3227 (J. Jakobsen, personal communication). Both the dev locus and Mxan_3227 are required for sporulation (27, 58). In this manner, C-signaling and the FruA protein are proposed to coordinate the formation of spores with the completion of fruiting bodies.
Generation of mature fruiting bodies is a relatively slow process; under laboratory conditions, spore-filled fruiting body formation takes at least 72 h. Several mutants in two-component signal transduction genes have been described, including espA (4), todK (41), espC (25), and redCDEF (11), which progress through the developmental program more quickly, forming more disorganized fruiting bodies but otherwise displaying no defect in the ability to form spores. These observations suggest that in wild-type cells, these respective gene products act to repress progression through the developmental program until a specific condition or set of conditions is met. However, it is unclear how these proteins mediate this repression.
In this paper, we focus on EspA, a histidine protein kinase homolog, previously proposed to be necessary for regulating only the timing of sporulation (4). Two particular features of EspA make it difficult to predict how EspA may function to alter the developmental program. First; it is an orphan (i.e., not genetically organized together with a cognate response regulator gene [49]), so that it is not clear as to the nature of the output mechanism. Second, EspA is a hybrid kinase containing an associated receiver domain located at the carboxy terminus of the protein and the role of the receiver in EspA activity is unclear.
Here we show that the histidine kinase activity is required early during the developmental program to inhibit developmental progression, both aggregation and sporulation. Our data suggest that in espA mutants, while A-signaling is unaffected, MrpC accumulates earlier compared to the wild type, resulting in earlier expression of FruA and premature induction of the aggregation and sporulation branches of the developmental program. Analysis of double mutants between espA and key developmental regulators confirms that A-signaling and FruA are required for EspA-mediated modulation of developmental progression, but shows that C-signaling can be partially bypassed.
MATERIALS AND METHODS
Strains and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. M. xanthus strains were grown vegetatively at 32°C on CYE agar plates [1% Casitone, 0.5% yeast extract, 10 mM 3-(N-morpholino)propanesulfonic acid (MOPS; pH 7.6), 4 mM MgSO4, 1.5% agar] or in CYE broth (CYE lacking agar). Plates were supplemented with 100 μg ml−1 kanamycin or 10 μg ml−1 oxytetracycline where necessary. Escherichia coli cells were grown under standard laboratory conditions in Luria-Bertani broth supplemented with 100 μg ml−1 ampicillin or 50 μg ml−1 kanamycin where necessary (29).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype | Source or reference |
|---|---|---|
| Strains | ||
| M. xanthus | ||
| DZ2 | Wild type | 3 |
| DZ4227 | DZ2 ΔespA573 | 4 |
| PH1008 | DZ2 espA(H407A) | This study |
| PH1009 | DZ2 espA(D696A) | This study |
| PH1007 | DZ2 espAΔREC | This study |
| PH1010 | DZ2 asgA::pPH127 | This study |
| PH1011 | DZ4227 asgA::pPH127 | This study |
| DK9035 | DK1622 csgA::Tn5-132ΩLS205 Δfrz(′CD-F)::Kan′ | 54 |
| PH1014 | DZ2 csgA::Tn5-132ΩLS205 | This study |
| PH1015 | DZ4227 Tn5-132ΩLS205 | This study |
| PH1013 | DZ2 fruA::pPH128 | This study |
| PH1012 | DZ4227 fruA::pPH128 | This study |
| DZ4169 | DZ2 frzCD::Tn5 | 47 |
| PH1016 | DZ4227 frzCD::Tn5 | This study |
| E. coli | ||
| TOP10 | Host for cloning | Invitrogen |
| BL21λ(DE3) | Host for protein expression | Novagen |
| Plasmids | ||
| pBJ114 | Backbone for deletions; galK Kmr | 12 |
| pPH150 | pBJ114 espA(H407A) | This study |
| pPH148 | pBJ114 espA(D696A) | This study |
| pPH124 | pBJ114 espAΔREC | This study |
| pPH127 | pBJ114 asgA | This study |
| pPH128 | pBJ114 fruA | This study |
| pGEX-4T-1 | GST expression plasmid; Apr | Amersham |
| pPH141 | pGEX-4T-1 espA kinase | This study |
| pPH143 | pGEX-4T-1 espA kinase receiver | This study |
| pPH156 | pGEX-4T-1 espA kinase (H407A) | This study |
| pPH157 | pGEX-4T-1 espA kinase receiver (D696A) | This study |
Analysis of M. xanthus developmental phenotypes.
Development was assayed under submerged culture conditions modified from reference 19. Briefly, cells were grown under vegetative conditions overnight in CYE broth and then diluted to an optical density at 550 nm (OD550) of 0.035 in fresh CYE medium. Sixteen milliliters or 0.5 ml of diluted cells was added to 9-cm petri plates or per well to 24-well tissue culture plates, respectively, and incubated at 32°C for 24 h. To initiate the developmental program, CYE medium was replaced by an equivalent volume of MMC starvation medium (10 mM MOPS [pH 7.6], 2 mM CaCl2, 4 mM MgSO4) and plates were incubated at 32°C for the respective times indicated. For analysis of development on clone fruiting (CF) agar plates, cells were grown to mid-log phase in CYE broth, washed, and resuspended to an OD550 of 0.35 in MMC starvation medium and 10 μl of cells was spotted onto CF plates [0.15% Casitone, 0.2% sodium citrate, 0.1% sodium pyruvate, 0.02% (NH4)2SO4, 10 mM MOPS (pH 7.6), 8 mM MgSO4, 1 mM KH2PO4, 1.5% agar] and incubated at 32°C. Developmental phenotypes were recorded at the times indicated with a Leica MZ8 stereomicroscope and attached Leica DFC320 camera.
Construction of mutants.
In-frame deletion of the receiver domain in espA was generated by homologous recombination modified from a previously reported method (61). Briefly, approximately 500-bp PCR fragments corresponding to regions upstream and downstream of the deletion were separately amplified and then fused together by overlap extension PCR. The resulting approximately 1,000-bp PCR fragments were then cloned into the EcoRI and BamHI sites of pBJ114. Clones were sequenced to confirm sequences were error free at the codon level. The plasmids were integrated into the genome by homologous recombination and selected for by resistance to kanamycin. Loss of the integrated plasmid via a second homologous recombination event was then screened for by galK-mediated counterselection on CYE plates containing 2.5% galactose. Resulting kanamycin-sensitive (Kans), galactose-resistant (Galr) colonies were then screened by PCR for those that generated a deletion as opposed to the original wild type. Deletions were confirmed by Southern blot analysis. The espA(H407A) or espA(D696A) substitution mutations were generated by a similar procedure, except the overlap PCR generated a GCG (Ala) codon in replacement of the CAC (His407) or GAT (Asp696) codons, respectively. Furthermore, Kans Galr colonies were screened by PCR using primers containing either the wild-type codon or the mutant GCG codon at the extreme 3′ end. The desired codon substitutions were then confirmed by PCR amplification of the relevant areas of the espA gene using the mutant strain genomic DNA as template, cloning the PCR products into pGEX-4T-1, and sequencing the cloned product.
Insertion mutants in asgA were generated by cloning internal fragments containing bp 257 to 797 of the asgA sequence into the EcoRI/BamHI sites of pBJ114, generating pPH127. pPH127 was introduced by electroporation into strains DZ2 (wild type) and DZ4227 (DZ2 ΔespA), and plasmid integration was selection for by kanamycin resistance, resulting in strains PH1010 and PH1011, respectively. Insertion mutants in fruA were similarly generated, except bp 30 to 451 of fruA were cloned, generating plasmid pPH128 and strains PH1013 and PH1012, respectively.
To generate csgA mutants in the DZ2 background, genomic DNA isolated from strain DK9035 was electroporated into either DZ2 or DZ4227, and the resulting double homologous recombination events were selected by oxytetracycline resistance, generating strains PH1014 and PH1015, respectively. Strain PH1016 (DZ4227 frzCD::Tn5) was similarly generated by electroporation of genomic DNA from strain DZ4169 into DZ4227 with kanamycin selection.
Cloning, overexpression, and purification of GST-EspAkin proteins.
Overexpression plasmids pPH141 and pPH156 encode the glutathione S-transferase (GST) affinity tag fused at the amino terminus to the EspA kinase region (EspAkin), which constitutes the HisKA and HATPase_c domains (Fig. 1A), or to the kinase mutant [EspAkin(H407A)], respectively (Fig. 1B). pPH141 and pPH156 were constructed by PCR amplification of the kinase region of espA (codons 390 to 646) using genomic DNA isolated from strain DZ2 (wild type) and PH1008 (DZ2 espA(H407A), respectively, as a template. The resulting PCR products were cloned into the EcoRI site of pGEX4T-1. Plasmids pPH143 and pPH157 encoding GST fused to the kinase and receiver domains of EspA (EspAkin-rec) (Fig. 1A), or to the kinase and point-mutated receiver [EspAkin-rec(D696A)], respectively, were similarly constructed by amplifying the region of espA encoding HisKA to the protein end (codons 390 to 768) from DZ2 and PH1009 [DZ2 espA(D696A)] genomic DNA, respectively (Fig. 1B). All constructs were sequenced to confirm the absence of PCR-generated errors. E. coli BL21λ(DE3) was transformed with the relevant overexpression plasmids, and expression of the GST-tagged proteins was induced at an OD550 of approximately 0.7 by the addition of 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) followed by growth for 3 h at 25°C. Cells were then harvested, resuspended in 0.05 volume phosphate-buffered saline (PBS) lysis buffer (135 mM NaCl, 3.6 mM KCl, 8 mM Na2HPO4, 2 mM KH2PO4) containing 1 mg ml−1 lysozyme and 1:200 dilution mammalian protease inhibitor cocktail (Sigma), and incubated on ice for 30 min. Cells were lysed by French press three times at approximately 18,000 lb/in2 and then incubated with rotation in the presence of 1% Triton X-100 for 40 min at 4°C. Insoluble protein was removed by centrifugation at 600 × g for 30 min, and the supernatant was incubated for 3 h at 4°C with 1 ml of GST bind resin (Novagen) preequilibrated with PBS. The resin was washed with 30 ml of PBS prior to elution of protein with 3 ml of elution buffer (50 mM Tris [pH 8.0], 10 mM reduced glutathione). The eluted fractions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), peak elution fractions were pooled, and the protein was dialyzed overnight against TGMNKD buffer (50 mM Tris-HCl [pH 8.0], 10% [vol/vol] glycerol, 5 mM MgCl2, 150 mM NaCl, 50 mM KCl, 1 mM dithiothreitol) for further assays.
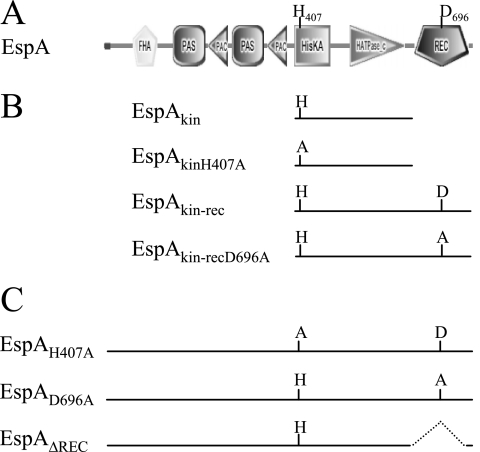
FIG. 1.
(A) Domain organization of EspA protein depicted by SMART analysis (26, 46). PAS/PAC, energy-sensing domains; HisKA histidine kinase dimerization domain; HATPase_c, histidine kinase ATPase domain; REC, receiver domain. (B) Constructs for analysis of EspA kinase activity in vitro. (C) Mutations generated in the espA locus for in vivo analyses.
Radiolabeled in vitro phosphorylation and ATPase assays.
In vitro phosphorylation of proteins was carried out in TGMNKD buffer (above) containing 0.5 mM [γ-32P]ATP (14.8 GBq mmol−1; Amersham) and 5 μM EspA in a 25-μl total volume for 120 min at room temperature. Aliquots of 10 μl were quenched with 5 μl of 3× SDS-EDTA loading dye (7.5% [wt/vol] SDS, 90 mM EDTA, 37.5 mM Tris-HCl [pH 6.8], 37.5% glycerol, 0.3 M dithiothreitol), loaded without prior heating on a 11% polyacrylamide gel, and separated by SDS-PAGE. Gels were exposed to a PhosphorImager screen overnight, and images were detected on a Typhoon Trio PhosphorImager (Amersham Biosciences) and analyzed using the ImageQuant version 5.0 software (Molecular Dynamics). Gels were subsequently stained by Coomassie dye to detect protein.
ATPase activity was measured under the conditions of the in vitro phosphorylation assay, except that 0.5 mM [α-32P]ATP (110 TBq mmol−1; Amersham) was used as a substrate. The products of [α-32P]ATP hydrolysis were analyzed by thin-layer chromatography (TLC) as per reference 42. Briefly, the reaction products were separated from EspA proteins using Microcon-Y10 ultrafiltration columns (Millipore). Adenine nucleotides were separated by spotting 0.5 μl of the elute to a poly(ethyleneimine)-cellulose F TLC plate (Merck) with 2.4 M formic acid as the solvent system for 20 min at room temperature. The labeled nucleotides on the TLC plates were visualized on a PhosphorImager and analyzed using ImageQuant software as described above.
Real-time PCR analyses.
RNA for real-time PCR analyses was harvested from cells developing under submerged culture. RNA was isolated by the hot-phenol method as per references 29 and 37. Briefly, cells were harvested at the indicated time points, pelleted, and immediately stored at −80°C. Pellets were resuspended in 1 ml ice-cold solution I (0.3 M sucrose, 0.01 M sodium acetate [pH 4.5]) and added to 1 ml solution II (2% SDS, 0.01 M sodium acetate [pH 4.5]) at 65°C. Two milliliters of 65°C phenol was added, followed by incubation at 65°C for 5 min. Reaction mixtures were flash cooled, and the aqueous layer was again extracted with 2 ml 65°C phenol followed by 2 ml phenol-chloroform-isoamyl alcohol as described above. RNA was precipitated from the final aqueous layer with 0.1 volume sodium acetate (pH 4.5) and 2 volumes ethanol followed by centrifugation. Pellets were washed with 75% ethanol and resuspended in RNase-free water. RNA (10 μg) was treated with DNase I (Fermentas) at 37°C for 60 min and then purified with RNeasy RNA purification columns (Qiagen) according to the manufacturer's instructions. One microgram of DNA-free RNA was reverse transcribed into cDNA using random hexamer primers (Amersham) and Superscript III reverse transcriptase (Invitrogen) in a 20-μl reaction according to the manufacturer's instructions. Real-time PCR was performed on 2 μl of a 1:50 dilution of the cDNA reaction using Sybr green PCR master mix (Applied Biosystems) and primers specific to the indicated gene in a 7300 real-time PCR system (Applied Biosystems). For each sample, a control reaction was performed on RNA to be certain no contaminating DNA was present. All samples were normalized to the wild-type strain at zero hours of development. Representative real-time PCR patterns are shown, but similar patterns were obtained from at least two biological replicate experiments.
Immunoblot analysis.
Protein lysates for Western blot analyses were obtained from cells developing under submerged culture for the indicated time points. Lysates were prepared from cells pelleted and frozen at −20°C. Pellets were resuspended in 0.4 ml MMC starvation buffer containing a 1:200 dilution of mammalian protease inhibitor cocktail (Sigma) and disrupted by sonication with cooling using a Branson 250 sonifier equipped with a microtip. Cell lysates were quantitated using a Bradford assay (Bio-Rad Laboratories), resuspended in 2× or 3× Laemmli sample buffer (LSB) (24) to 0.5 μg μl−1, heated at 99°C for 5 to 7 min, and stored at −20°C. Protein lysates (10 μg) were resolved by denaturing SDS-PAGE using the following acrylamide concentrations: 8% for EspA, 12% for MrpC and FrzCD, and 13% for FruA. CsgA was resolved on Tris-Tricine gels as per reference 45. Proteins were transferred to polyvinylidene difluoride membrane using a semidry transfer apparatus (Hoeffer). Western blot analyses were performed using the following antibody dilutions: anti-EspA polyclonal antibodies (pAb) at 1:1,500; anti-FruA pAb, anti-MrpC pAb (35), and anti-FrzCD (32) at 1:5,000; anti-CsgA C-terminal pAb (28) at 1:2,500; and anti-PilC pAb (V. Jakovljevic and L. Sogaard-Andersen, unpublished data) at 1:10,000. Secondary anti-rabbit immunoglobulin G (IgG)-horseradish peroxidase antibody (Pierce) was used at 1:20,000, and signals were detected with enhanced chemiluminescence substrate (Pierce) followed by exposure to autoradiography film. Representative immunoblot patterns are shown, but similar patterns were obtained from at least two biological replicates.
RESULTS
Analysis of EspA kinase activity.
EspA (768 amino acids) is a two-component signal transduction hybrid histidine protein kinase homologue (4) (Fig. 1A). The sensor module in EspA consists of a Forkhead-associated (FHA) domain and two adjacent PAS/PAC domains. In this paper, we focused on the signal transmitter/output domains: (i) residues 395 to 468 encode a HisKA dimerization domain containing a conserved histidine at position 407 (H407) predicted to be the site of autophosphorylation, (ii) residues 518 to 624 encode a HATPase_c domain predicted to be necessary for ATP binding and catalysis, and (iii) residues 650 to 760 encode a receiver domain containing a conserved aspartic acid at position 696 (D696) predicted to be a phosphoacceptor residue.
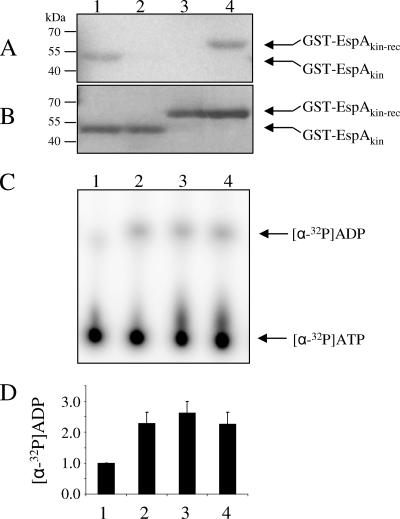
To determine whether EspA has histidine kinase activity, we purified a GST-tagged recombinant protein containing EspA's kinase region (HisKA and HATPase_c domains) as well as a version bearing an H407-to-alanine substitution (GST-EspAkin and GST-EspAkin(H407A), respectively) (Fig. 1A and B). These two proteins were assayed for autophosphorylation in the presence of [γ-32P]ATP. While a radioactive band corresponding to GST-EspAkin could be readily detected, the corresponding band for GST-EspAkin(H407A) was not detected, indicating that the kinase domain of EspA is capable of autophosphorylation on His407 (Fig. 2A and B, lanes 1 and 2). To determine what effect the receiver domain has on this reaction, we purified and analyzed a protein containing both the kinase and receiver domains (GST-EspAkin-rec) and the same protein bearing a substitution of the conserved aspartic acid in the receiver domain (GST-EspAkin-rec(D696A) (Fig. 1B). When these proteins were analyzed under the same conditions as those employed for the kinase, no radiolabeled GST-EspAkin-rec product was detected, whereas the GST-EspAkin-rec(D696A) band was readily detected (Fig. 2A and B, lanes 3 and 4). The GST-EspAkin-rec protein was active because we could detect turnover of ATP to ADP when the proteins were incubated in the presence of [α-32P]ATP and the reaction supernatant was subsequently analyzed by TLC for [α-32P]ADP accumulation (Fig. 2C and D). These results suggest that EspA becomes autophosphorylated on His407 followed by two possibilities: (i) the phosphoryl group is transferred to D696 of the receiver domain but is subsequently released as inorganic phosphate such that the kinase signal is depleted, either due to intrinsic instability or because EspA's kinase domain also displays phosphatase activity; or (ii) the intact receiver domain prevents autophosphorylation but not ATP hydrolysis.
FIG. 2.
Analysis of EspA kinase activity. (A) Autoradiograph of GST-EspAkin (lane 1), GST-EspAkin(H407A) (lane 2), GST-EspAkin-rec (lane 3), and GST-EspAkin-rec(D696A) (lane 4) incubated in the presence of [γ-32P]ATP. (B) Coomassie-stained gel corresponding to panel A. (C) TLC analysis of [α-32P]ADP produced by [α-32P]ATP (lane 1), GST-EspAkin (lane 2), GST-EspAkin-rec (lane 3), and GST-EspAkin-rec(D696A) (lane 4). (D) Quantification of [α-32P]ADP signal in panel C as an average from triplicate experiments.
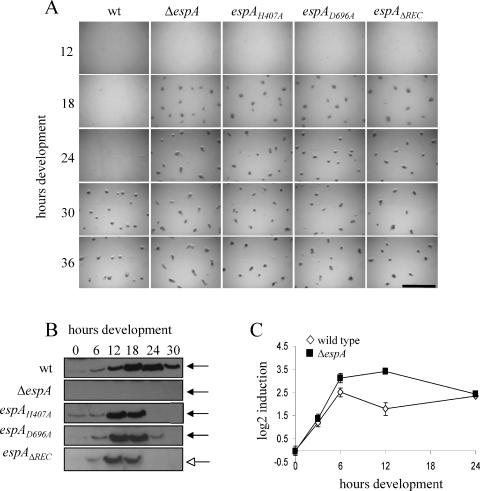
To determine whether kinase activity is necessary for EspA activity in vivo, we generated a strain (PH1008) bearing a substitution of H407 to alanine [espA(H407A)] at the native espA locus (Fig. 1C). Analysis of the developmental phenotypes of the espA(H407A) mutant in parallel with the wild type (strain DZ2) and the espA-null mutant (ΔespA; strain DZ4227) under submerged culture conditions demonstrated that the espA(H407A) mutant behaved like the ΔespA mutant: the cells began to aggregate between 12 and 18 h, approximately 12 h earlier than the wild type (Fig. 3A). Like the ΔespA mutant, the espA(H407A) mutant also produced spores earlier than the wild type and produced spores outside of fruiting bodies (data not shown). The espA(H407A) and ΔespA mutants also exhibited similar phenotypes when development was induced on CF agar plates (data not shown). In addition, immunoblot analysis indicated that EspA(H407A) is produced as a stable protein (Fig. 3B) (data described below). Together, these data indicate that the conserved H407 residue is absolutely required for EspA function and imply that kinase activity is necessary for EspA-mediated control over developmental progression.
FIG. 3.
(A) Developmental phenotype of espA mutants compared to the wild type. Cultures of DZ2 (wild type [wt]), DZ4227 (ΔespA), PH1008 [espA(H407A)], PH1009 [espA(D696A)], and PH1007 (espAΔREC) cells were induced to develop under submerged culture conditions at 32°C. Pictures were recorded at the indicated hours. Bar, 1 mm. (B) Western blot analysis of EspA expression. Protein lysates (10 μg) prepared from cells in panel A harvested at the indicated hours of development were subjected to immunoblotting with anti-EspA polyclonal antisera. Black arrows, 83 kDa; white arrow, 71 kDa. (C) Quantitative real-time PCR analysis of espA mRNA expression. Total RNA isolated from DZ2 wild-type (white diamonds) or PH1008 mutant [espA(H407A)] (black squares) cells at the indicated hours of development was transcribed into cDNA. espA transcripts were detected by real-time PCR.
To analyze the role of the associated receiver domain in EspA, we similarly generated mutants bearing either an alanine substitution of the conserved D696 in the receiver domain [espA(D696A); strain PH1009] or a deletion of the entire receiver domain (espAΔREC; strain PH1007) (Fig. 1C). When subject to the same analyses described above, these mutants both phenocopied the espA(H407A) and ΔespA mutants (Fig. 3A), indicating that the receiver domain and in particular the D696 residue are absolutely required for EspA function.
Expression of EspA during development.
Analysis of espA-lacZ fusions demonstrated that espA is transcribed at the onset of starvation and peaks at approximately 24 h of development in the DZ2 strain (4). To determine the protein expression pattern of EspA, we analyzed cell lysates harvested from a developmental time course by immunoblot analysis using affinity-purified antibodies generated against the full-length EspA protein. An EspA-specific band was detected which migrated at 83 kDa, near the predicted molecular mass for EspA (Fig. 3B). In a developmental time course, EspA was barely detectable under vegetative conditions, but increased after the onset of starvation, with peak expression at 18 to 24 h, after which expression decreased (Fig. 3B). When we examined EspA expression in the espA(H407A), espA(D696A), and espAΔREC strains, we detected EspA proteins at the predicted molecular masses of 83, 83, and 71 kDa, respectively (Fig. 3B). Interestingly however, we observed that mutations rendering EspA inactive changed the expression pattern of the mutant EspA. While both wild-type EspA and mutant EspA showed similar levels from 0 to 6 h, during the 6- to 12-h period mutant EspA showed maximal expression between 12 and 18 h rather than at 18 to 24 h as observed in the wild type. Furthermore, the mutant EspA proteins were not detected after 18 h, while expression continued in the wild type, albeit at decreased levels after 24 h. It should be noted that the relative amounts of EspA protein detected were the same between EspA(H407A), EspA(D696A), and wild-type EspA. A less intense signal for EspAΔREC likely arose from fewer epitopes in this deletion mutant. The early decrease in EspA signal in the espA mutants is likely a secondary effect resulting from early sporulation in these mutants: either EspA is not released from spores under the lysis conditions employed or is not present in spores. However, our data suggest that wild-type EspA activity may be necessary for delaying espA transcription and/or protein accumulation from 6 to 12 h and that EspA is involved in regulating its own expression.
We were therefore interested in determining whether the transcription of the espA gene was upregulated earlier in an EspA-inactive strain compared to a strain in which EspA was active. Quantitative real-time PCR analysis of espA expression from the wild type versus the espA(H407A) mutant suggested that espA was indeed upregulated in the espA(H407A) mutant between 6 and 12 h compared to the wild type (Fig. 3C). These observations suggest that EspA likely is involved in a phosphorylation-dependent signaling pathway that ultimately downregulates its own transcription between 6 and 12 h of development.
Developmental marker gene expression analysis in espA mutants.
ΔespA mutants aggregate and sporulate earlier than the wild type, suggesting that in wild-type cells EspA transiently represses development progression. However, it is unclear how and when in the developmental pathway EspA mediates this developmental repression. The M. xanthus developmental program is characterized by temporal cascades of gene expression. To gain insight into the nature of EspA control, we examined the expression of several characterized developmental genes (see Fig. 7) in wild-type versus ΔespA strains under submerged culture conditions to determine how defects in EspA affect other genes involved in the developmental pathway.
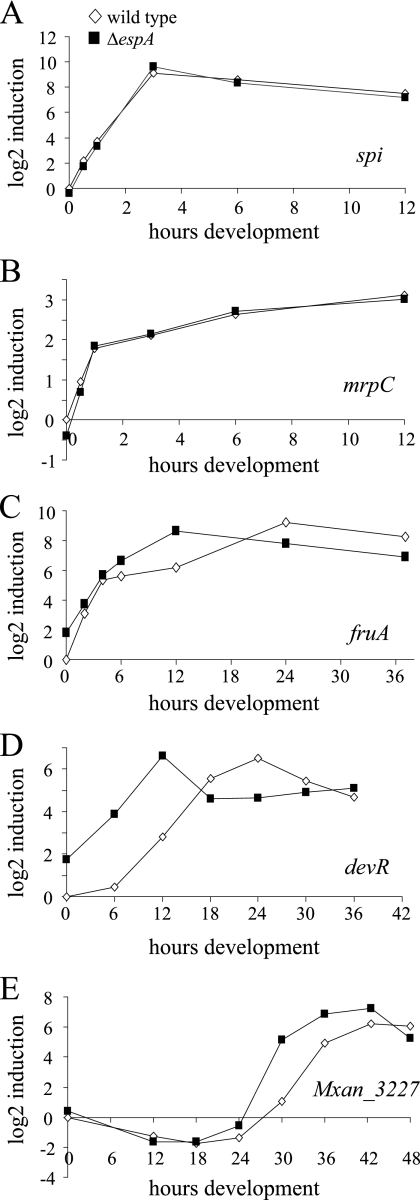
To address whether ΔespA mutants were altered in production or reception of the A-signal, we analyzed the expression of the A-signal-responsive gene, spi (21) (originally identified as Tn5 lac Ω4521) (18), by real-time PCR as the cells progressed through the developmental program. Our analyses indicated that in wild-type DZ2 cells, spi was upregulated by 30 min after induction of starvation, with peak expression by 2 to 4 h (Fig. 4A). We observed no significant difference in transcription of spi between the wild type and the ΔespA mutant, suggesting that espA mutants are not impaired in production or reception of the A-signal.
FIG. 4.
Quantitative real-time PCR analysis of developmental marker gene transcription in the wild type (strain DZ2; white diamonds) and ΔespA mutant (strain DZ4227; black squares). Cells were developed in submerged culture and harvested at the indicated time points. RNA was isolated and reverse transcribed into cDNA. Primers specific for spi (A), mrpC (B), fruA (C), devR (D), or Mxan_3227 (E) were used for real-time PCR analysis.
We next analyzed initial mrpC transcription, which is also indirectly responsive to the A-signal (56). In DZ2, mrpC expression was upregulated after onset of starvation and continued to rise over the course of the 12 h of development for which it was analyzed (Fig. 4B). In the espA strain, mrpC expression was similar to that of the wild type (Fig. 4B).
Analysis of fruA gene transcription in DZ2 showed that fruA was upregulated after onset of starvation in DZ2, with peak expression between 12 and 24 h (Fig. 4C). In the espA mutant, fruA expression was essentially similar to that of the wild type from 0 to 6 h. However, by 12 h, fruA transcript was 5.6-fold higher in the ΔespA cells, after which it appeared to decrease (Fig. 4C). This early upregulation of fruA was consistently observed in several biological replicates (data not shown).
Expression of the dev locus depends at least in part on regulation by FruA (62) and expression of Mxan_3227 (Tn5 lacZ Ω7536) depends upon the dev locus (27). In DZ2, devR was upregulated between 6 and 12 h of development and began to decrease between 24 and 30 h (Fig. 4D). In the espA strain, consistent with the premature expression of fruA, devR was expressed approximately 16 times higher from 6 to 12 h in the espA mutant and began to decrease between 12 and 18 h (Fig. 4D). In a similar manner, expression of Mxan_3227 was markedly upregulated in the espA mutant between 24 and 30 h compared to the wild type, which peaks between 30 and 36 h (Fig. 4E). Together, these data suggest that in espA mutants the developmental program begins to be perturbed after A-signaling but before transcription of fruA.
Developmental marker protein expression analysis in espA mutants.
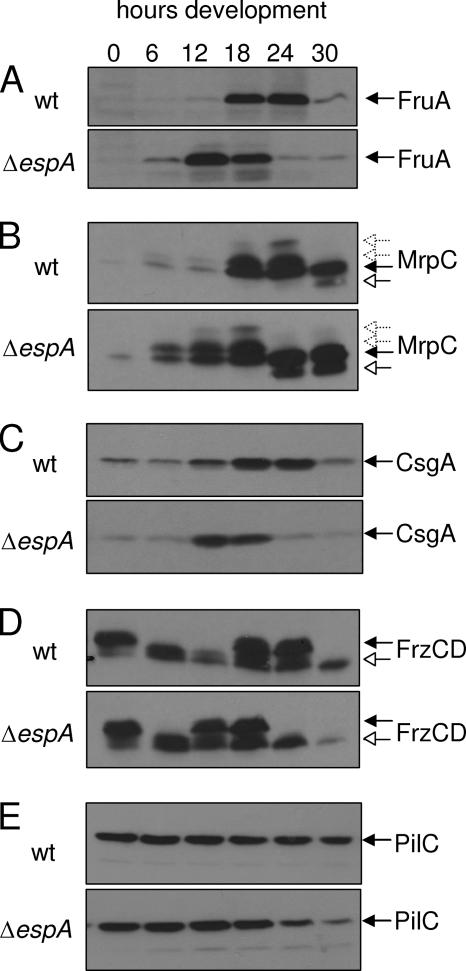
To further examine how the early expression of fruA might be controlled in the ΔespA mutant, we examined the protein expression patterns of several key developmental regulators (Fig. 7). First, we determined that the expression of FruA was upregulated earlier in the ΔespA mutant (between 0 and 6 h) compared to the wild type (12 to 18 h) (Fig. 5A), consistent with the early expression of fruA mRNA as detected by real-time PCR (Fig. 4C).
FIG. 5.
Immunoblot analysis of developmental marker protein expression in the wild type (strain DZ2 [wt]) and ΔespA mutant (strain DZ4227). Cells were grown in submerged culture and harvested at the indicated time points. Samples containing 10 μg protein were subject to immunoblot analysis and probed with anti-FruA (A), anti-MrpC (B), anti-CsgA (C), anti-FrzCD (D), or anti-PilC (E) polyclonal antisera. (B) Black arrows, MrpC; white arrows, MrpC2. (C) CsgA p25. (D) Black arrows, FrzCD unmethylated species; white arrows, FrzCD methylated species.
MrpC protein is required for induction of fruA transcription (34, 35, 60); we therefore examined the accumulation of MrpC. It had previously been determined that in wild-type M. xanthus strain DZF1 (FB), MrpC can be detected in two forms: full-length MrpC present in vegetative and developing cells and proteolytically processed MrpC2 present in only developing cells (35). Immunoblot analysis of MrpC expression in wild-type DZ2 cells revealed four MrpC bands (Fig. 5B). We attribute the full-length form (Fig. 5B) to the species detected in vegetative cells. The smaller MrpC2 species (Fig. 5B) was detected between 24 and 30 h in DZ2. We attribute the additional more slowly migrating bands (Fig. 5B) to phosphorylated forms of MrpC which were inferred, but not detected, in the earlier study (35). It is not clear whether the detection of these putative phosphorylated forms is due to differences in immunoblot techniques or due to the difference of development in strain DZ2 versus DZF1. Strain DZ2 develops significantly more slowly than strain DZF1, perhaps allowing detection of more transient isoforms of MrpC. In the ΔespA mutant, all four forms of MrpC were detected, except that the respective MrpC isoforms began to accumulate 6 to 12 h earlier than in the wild type (Fig. 5B). In vitro data suggest that fruA is regulated by MrpC2 (35, 60). However, in both the wild type and espA mutant, the upregulation of fruA expression correlated with accumulation of the full-length MrpC isoforms (at 18 and 6 h of development, respectively), although low levels of MrpC2 can be detected at earlier time points on extended exposures (data not shown). The observation that MrpC protein accumulation is higher in the espA mutants, whereas mrpC transcription is the same as that of the wild type during the first 12 h of development (Fig. 4B), suggests that EspA may play a role in transiently repressing MrpC accumulation early during the developmental program.
FruA activity is proposed to be modulated by the C-signal pathway (5). We next examined whether we could detect differential expression of CsgA. A sharp increase of CsgA was observed between 6 and 12 h in the espA mutant compared to a more gradual accumulation between 6 and 18 h in the wild-type strain (Fig. 5C). These results suggest that the C-signal amplification pathway is also functioning earlier in the espA mutant.
It has been suggested that activated FruA directly or indirectly influences the activity of the Frz chemotaxis pathway (53). Development-related signaling of the Frz chemosensory pathway can be followed by the increased methylation of FrzCD receptor, which is a methyl-accepting chemotaxis protein that becomes highly methylated during development (31). We therefore next examined the developmental expression pattern of FrzCD in the ΔespA and wild-type strains. FrzCD with different methylation, demethylation, or deamidation levels can be detected as multiple bands on immunoblots using anti-FrzCD serum (30). Under the culture conditions used here, FrzCD harvested from cells incubated in submerged culture with vegetative medium for 24 h was primarily present as unmethylated species (Fig. 5D). After onset of development (vegetative media replaced with starvation media) between 0 and 12 h, FrzCD was detected primarily as a methylated species (Fig. 5D). Between 12 and 24 h, FrzCD became partially less methylated and then, finally after 24 h, commensurate with formation of mounds (Fig. 3), FrzCD was detected as a fully methylated species. This pattern of FrzCD methylation over the course of the developmental program in which FrzCD undergoes partial transient demethylation just before mound formation differs from previously published methylation patterns using wild-type strains DZF1 (FB) (31) and DK1622 (53). As discussed above for MrpC, the more slowly developing wild-type DZ2 strain may allow for detection of more transient FrzCD methylation states. In the espA strain, the same overall pattern of FrzCD methylation was detected, except the pattern was shifted 6 h earlier (between 6 and 12 h of development) than in the wild type. In the ΔespA strain, mound formation was observed between 12 and 18 h, indicating that the FrzCD pool remained as partially demethylated after mounds began to form (Fig. 3). These results suggest that in the ΔespA mutant, the FrzCD methylation status is also modulated earlier than in the wild type, consistent with the early aggregation phenotype displayed by the ΔespA mutant.
As a control for loading, we also examined the expression of PilC, a pilus-associated protein (64, 65) which is not predicted to be developmentally regulated. From 0 to 18 h, PilC protein was detected at the same levels in the ΔespA strain compared to the wild type (Fig. 5E), indicating that the early expression of MrpC, FruA, or CsgA in the espA mutants was not simply due to overloaded protein samples. Consistent with the premature sporulation observed in the espA mutant, PilC was detected at reduced levels during the later developmental time points.
Analysis of espA double mutants with key developmental genes.
Analysis of developmental gene and protein expression markers in the espA mutant indicated that EspA acts on the developmental program after A-signaling but before the action of FruA. We therefore generated asgA, fruA, and csgA mutants in the DZ2 wild-type strain and asgA, fruA, csgA, and frzCD mutants in the ΔespA (DZ4227) strain and analyzed the developmental phenotypes. Mutations in asgA, fruA, and csgA in the DZ2 background yielded the expected phenotypes (Fig. 6) that have been previously observed in the DK1622 or FB (DZF1) backgrounds (5, 9, 36, 39).
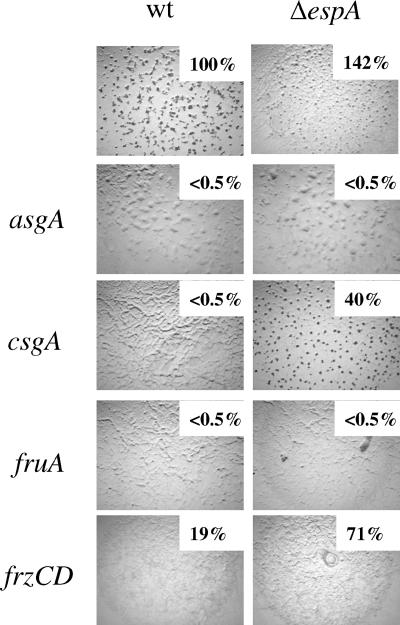
FIG. 6.
Developmental phenotype of key developmental genes in the wild-type (DZ2 [wt]) or ΔespA (DZ4227) background. asgA (PH1010), espA asgA (PH1011), fruA (PH1013), espA fruA (PH1012), csgA (PH1014), espA csgA (PH1015), frzCD (DZ4169), and espA frzCD (PH1016) strains were induced to develop for 72 h on CF plates. Heat- and sonication-resistant spores were enumerated at 72 h of development. Values are displayed as percentages of wild-type spores.
The analysis of spi expression in the ΔespA mutant suggested that A-signaling is not affected in the espA mutant. Mutations in asgA do not produce A-signal and the mutants do not develop. ΔespA asgA double mutants phenocopied the asgA single mutant, indicating that completion of A-signaling is a prerequisite of EspA-mediated developmental repression (Fig. 6). Our model suggests that the early aggregation and sporulation observed in the espA mutant are due to premature expression of FruA. ΔespA fruA double mutants displayed a fruA phenotype (Fig. 6), indicating that EspA's effect on aggregation and sporulation requires FruA. Current models for the molecular mechanisms controlling the developmental program in M. xanthus suggest that FruA activity is modulated in response to the C-signal amplification pathway (5). Interestingly, the double ΔespA csgA mutant was able to aggregate and form spores, albeit at a delayed and reduced level compared to the wild type or ΔespA single mutant (Fig. 6) (data not shown). This phenotype suggests that in espA mutants, C-signaling can be partially bypassed. Finally, analysis of ΔespA frzCD double mutants yielded a mixed phenotype: like the frzCD parent mutant, cells formed frizzy filaments rather than aggregation centers but produced elevated levels of spores consistent with the espA parent (Fig. 6).
DISCUSSION
EspA was previously characterized as a putative histidine kinase that is necessary for appropriate timing of sporulation during development (4). To gain further insight into how EspA affects the developmental program, we first set out to analyze whether EspA was indeed functioning as a histidine protein kinase. Our biochemical analyses indicated that the kinase domain of EspA is capable of in vitro autophosphorylation on the conserved histidine residue at amino acid position 407, and analyses of an espA(H407A) point mutation in vivo indicate that kinase activity is required for EspA-mediated control over developmental progression. The observation that espA(D696A) and espAΔREC mutants phenocopy the kinase-inactive espA(H407A) mutant indicates that phosphotransfer to the receiver domain is a required step in EspA-mediated control over the developmental program. These results clarify our in vitro phosphorylation results and suggest that the presence of the receiver domain disrupts the accumulation of signal on the kinase domain because the phosphoryl group is transferred from the histidine residue to the conserved aspartate at position 696, but the phosphoryl group is rapidly released as inorganic phosphate. A similar result was observed with the hybrid histidine kinase LuxN, which controls luminescence production in Vibrio species (6, 59).
If transfer of the phosphoryl group to the receiver domain is a required step of signal transduction in EspA function, what is the signal output from EspA? One possibility is that EspA participates in a four-step phosphorelay, first to an unidentified histidine phosphotransferase (Hpt) protein and then to a response regulator protein again in a manner analogous to that of the LuxN(Q)/LuxU/LuxO system. Hpt proteins involved in four-step phosphorelay signal transmission are difficult to identify based on sequence alone (1), and current annotation of the M. xanthus genome does not identify convincing candidates that could serve as a partner for EspA. In addition, the M. xanthus genome encodes 64 orphan two-component response regulators (49), indicating that identification of a cognate output protein(s) is challenging. Another intriguing possibility is that EspA may mediate its output directly, perhaps through direct protein interaction with other developmental regulators.
To attempt to identify how EspA might affect the developmental program and thus to pinpoint possible output mechanisms, we next sought to analyze when the developmental program began to differ from the wild type in the espA mutant. We used quantitative real-time PCR to analyze the expression patterns of several established developmental marker genes known to be expressed in a specific order during the developmental pathway (outlined in Fig. 7). These analyses determined that in the espA mutant, expression of the A-signal-dependent spi gene and initial upregulation of mrpC expression (which is also partially dependent on A-signaling) were not altered compared to those of the wild type, whereas expression of fruA, dev, and Mxan_4227 mRNAs is upregulated earlier in the espA mutant than in the wild type (Fig. 4). These data suggest that EspA normally begins to delay the developmental program after the completion of A-signaling but before the upregulation of the major developmental regulator, fruA.
Examination of the protein expression profile of MrpC, a key developmental transcriptional regulator which is necessary for transcription of fruA, clarified why fruA is upregulated earlier in the espA mutants. A key observation here is that MrpC protein accumulates between 0 and 6 h of development in the espA mutant (approximately 12 h earlier than in the wild type) (Fig. 5B), although mrpC expression is not different from that of the wild type during this time period (Fig. 4B). Our data suggest that EspA is involved in regulating MrpC protein accumulation, perhaps at the level of translation or degradation. It should be noted that while the early MrpC accumulation in the espA mutant correlated with early fruA transcription, we did not observe an effect of MprC on mrpC transcription over that of the wild type in the first 12 h of development (Fig. 4B). While a possible explanation is that the MrpC2 isoform displays higher affinity for the fruA promoter than the mrpC promoter, based on in vitro studies (35), it is also likely that in vivo the situation is more complicated, involving factors not yet identified.
MrpC appears to be a key point of developmental control. First, it has previously been determined that early development via premature fruA expression due to modulation of MrpC is observed in pkn8 or pkn14 mutants; in these mutants MrpC cannot be phosphorylated and MrpC2, which is normally only detected during development, is detected under vegetative conditions (34). Second, it has recently been demonstrated that unphosphorylated MrpC acts as an antitoxin for MazF, an mRNA interferase that is necessary for development because it induces a percentage of cells to undergo autolysis (33). MrpC was also shown to be a mazF transcriptional activator, and the binding of MrpC to the mazF promoter was prevented by the MrpC-MazF complex. Interestingly, however, MrpC expression is apparently not altered in a mazF mutant, suggesting the interplay of the various forms of MrpC (MrpC, MprC2, MrpC-P, and MrpC-MazF) may affect MrpC's activity on different target promoters (mrpC, fruA, and mazF) differently (33). It is worth noting that in the espA mutant, the relative proportions of each MrpC isoform were not altered compared to the wild type, confirming that the premature MrpC accumulation is not specifically due to differential processing of MrpC and suggesting that MrpC's interaction with MazF is not perturbed. Differential protein accumulation of MrpC in the espA mutant may then represent a third level of control of MrpC activity.
We suggest that the premature accumulation of MrpC in the espA mutant is the cause of early development in the mutant and can explain most of our results in this paper (Fig. 7). First, A-signaling as monitored by spi gene expression was unaffected in the espA mutant. It has previously been demonstrated that mrpC is developmentally upregulated by the MrpAB two-component signal transduction system (57) in a manner that is facilitated by A-signaling (56). The observation that the initial upregulation of mrpC is not altered in the espA mutant is consistent with the observation that A-signaling is not altered (Fig. 4A). In addition, espA asgA double mutants display an asgA phenotype (Fig. 6), which would be expected since MrpC expression should be reduced (56). Second, premature expression of MrpC induces premature transcription of fruA and consequent production of FruA (Fig. 4C and 5A). Premature FruA expression in turn leads to premature alteration of FrzCD methylation patterns (Fig. 5D), which suggests that signaling is occurring through the Frz chemosensory system promoting aggregation. FruA also acts as a transcription factor for the dev locus (63), and devR is transcribed earlier in the espA strain (Fig. 4D). In addition, Mxan_3227 expression is dependent upon dev expression (27), and the premature expression of Mxan_3227 in espA mutants (Fig. 4E) is likely a consequence of premature expression of dev. dev and Mxan_3227 are both required for sporulation inside of fruiting bodies (27, 58), and their premature expression is consistent with the early sporulation phenotype in espA mutants (4; data not shown). Furthermore, espA fruA double mutants display a fruA phenotype (Fig. 6) consistent with the fact that FruA is an essential component of the pathway affected in espA mutants. However, espA frzCD double mutants display a mixed phenotype: the observed frizzy aggregation is frzCD-like, but the elevated sporulation is espA-like. This result is to be expected if premature FruA expression requires FrzCD to promote normal aggregation, but can still promote sporulation through the dev and Mxan_3227 loci.
We observed that expression of CsgA is slightly early in espA mutants (Fig. 5C), which presumably arises because early FruA accumulation stimulates aggregation through the FrzCD pathway. As the cells begin to stream into aggregates, end-to-end contact stimulates C-signaling and CsgA expression is amplified. Interestingly, however, espA csgA mutants are able to aggregate and sporulate, albeit at delayed and reduced levels compared to those of the wild type (Fig. 6), suggesting that espA mutants can partially bypass the requirement for C-signaling. This result suggests that EspA may play an additional unidentified role during development downstream of the C-signal. It is interesting that a similar partial bypass of C-signaling (but not of FruA) has been previously observed with mutants in the unusual histidine protein kinase rodK (40).
But what advantage does EspA's repressive function provide for the developmental program? We speculate that EspA aids in coordinating development by delaying developmental progression until a threshold of an unidentified signal(s) is sensed such that the multiple events (starvation sensing, population sensing, etc.) that must be integrated to generate compact fruiting bodies are coordinated. In the espA strains, premature aggregation and sporulation are associated with slightly disorganized fruiting bodies and formation of spores among the peripheral rods. It is likely that well-organized compact fruiting bodies offer a selective advantage in dissemination of groups of spores, since we presume germination in groups allows for more rapid growth based on the social aspect of M. xanthus feeding. Our results suggest a model in which phosphorylated EspA acts after the completion of A-signaling to inhibit, via an unknown mechanism, accumulation of MrpC (Fig. 7). Upon sensing a change in an unidentified signal, EspA then relieves the inhibition, MrpC protein is allowed to accumulate, and the developmental program progresses.
The nature of the signal(s) sensed by EspA is currently unknown. Genetic epistasis experiments suggest that EspA acts downstream from a signaling module consisting of PktA5 and/or PktB8, two serine/threonine protein kinases, and EspB a membrane protein (4, 55). Interestingly, EspA contains multiple sensing domains: a Forkhead-associated domain which is likely necessary for interaction with PktA5 and/or PktB8 (55), and two adjacent PAS/PAC domains which are likely involved in sensing the redox potential of the cell. We are currently investigating the exact role(s) of the sensing domains in modulation of EspA activity.
The observation that mutants in several other histidine protein kinase homologs (TodK [41], EspC [25], and RedCDEF [11]) display similar mutant phenotypes to espA suggests that regulation of developmental progression is subject to a sophisticated signal transduction network; we are currently exploring how these other signaling systems control this complex developmental process.
Acknowledgments
We gratefully acknowledge S. Inouye for anti-MrpC antibody, L. Søgaard-Andersen for anti-FruA and anti-PilC antibodies, and Namita Kothari for contributions to EspA phosphorylation analyses. We are also grateful to L. Søgaard-Andersen, K. Thorman, and S. Huntley for critical review of the manuscript.
This work was funded by the Max Planck Society (P.I.H.) and National Institutes of Health grants GM64463 and GM20509 (D.Z.).
Footnotes
Published ahead of print on 4 April 2008.
REFERENCES
- 1.Biondi, E. G., S. J. Reisinger, J. M. Skerker, M. Arif, B. S. Perchuk, K. R. Ryan, and M. T. Laub. 2006. Regulation of the bacterial cell cycle by an integrated genetic circuit. Nature 444899-904. [DOI] [PubMed] [Google Scholar]
- 2.Blackhart, B. D., and D. R. Zusman. 1985. “Frizzy” genes of Myxococcus xanthus are involved in control of frequency of reversal of gliding motility. Proc. Natl. Acad. Sci. USA 828767-8770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campos, J. M., and D. R. Zusman. 1975. Regulation of development in Myxococcus xanthus: effect of 3′:5′-cyclic AMP, ADP, and nutrition. Proc. Natl. Acad. Sci. USA 72518-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho, K., and D. R. Zusman. 1999. Sporulation timing in Myxococcus xanthus is controlled by the espAB locus. Mol. Microbiol. 34714-725. [DOI] [PubMed] [Google Scholar]
- 5.Ellehauge, E., M. Norregaard-Madsen, and L. Sogaard-Andersen. 1998. The FruA signal transduction protein provides a checkpoint for the temporal co-ordination of intercellular signals in Myxococcus xanthus development. Mol. Microbiol. 30807-817. [DOI] [PubMed] [Google Scholar]
- 6.Freeman, J. A., and B. L. Bassler. 1999. Sequence and function of LuxU: a two-component phosphorelay protein that regulates quorum sensing in Vibrio harveyi. J. Bacteriol. 181899-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gronewold, T. M., and D. Kaiser. 2001. The act operon controls the level and time of C-signal production for Myxococcus xanthus development. Mol. Microbiol. 40744-756. [DOI] [PubMed] [Google Scholar]
- 8.Hagen, D. C., A. P. Bretscher, and D. Kaiser. 1978. Synergism between morphogenetic mutants of Myxococcus xanthus. Dev. Biol. 64284-296. [DOI] [PubMed] [Google Scholar]
- 9.Hagen, T. J., and L. J. Shimkets. 1990. Nucleotide sequence and transcriptional products of the csg locus of Myxococcus xanthus. J. Bacteriol. 17215-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris, B. Z., D. Kaiser, and M. Singer. 1998. The guanosine nucleotide (p)ppGpp initiates development and A-factor production in Myxococcus xanthus. Genes Dev. 121022-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgs, P. I., K. Cho, D. E. Whitworth, L. S. Evans, and D. R. Zusman. 2005. Four unusual two-component signal transduction homologs, RedC to RedF, are necessary for timely development in Myxococcus xanthus. J. Bacteriol. 1878191-8195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Julien, B., A. D. Kaiser, and A. Garza. 2000. Spatial control of cell differentiation in Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 979098-9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaiser, D. 2004. Signaling in myxobacteria. Annu. Rev. Microbiol. 5875-98. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan, H. B., and L. Plamann. 1996. A Myxococcus xanthus cell density-sensing system required for multicellular development. FEMS Microbiol. Lett. 13989-95. [DOI] [PubMed] [Google Scholar]
- 15.Kim, S. K., and D. Kaiser. 1991. C-factor has distinct aggregation and sporulation thresholds during Myxococcus development. J. Bacteriol. 1731722-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim, S. K., and D. Kaiser. 1990. C-factor: a cell-cell signaling protein required for fruiting body morphogenesis of M. xanthus. Cell 6119-26. [DOI] [PubMed] [Google Scholar]
- 17.Kim, S. K., and D. Kaiser. 1990. Cell alignment required in differentiation of Myxococcus xanthus. Science 249926-928. [DOI] [PubMed] [Google Scholar]
- 18.Kroos, L., A. Kuspa, and D. Kaiser. 1986. A global analysis of developmentally regulated genes in Myxococcus xanthus. Dev. Biol. 117252-266. [DOI] [PubMed] [Google Scholar]
- 19.Kuner, J. M., and D. Kaiser. 1982. Fruiting body morphogenesis in submerged cultures of Myxococcus xanthus. J. Bacteriol. 151458-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuspa, A., and D. Kaiser. 1989. Genes required for developmental signalling in Myxococcus xanthus: three asg loci. J. Bacteriol. 1712762-2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuspa, A., L. Kroos, and D. Kaiser. 1986. Intercellular signaling is required for developmental gene expression in Myxococcus xanthus. Dev. Biol. 117267-276. [DOI] [PubMed] [Google Scholar]
- 22.Kuspa, A., L. Plamann, and D. Kaiser. 1992. Identification of heat-stable A-factor from Myxococcus xanthus. J. Bacteriol. 1743319-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuspa, A., L. Plamann, and D. Kaiser. 1992. A-signalling and the cell density requirement for Myxococcus xanthus development. J. Bacteriol. 1747360-7369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227680-685. [DOI] [PubMed] [Google Scholar]
- 25.Lee, B., P. I. Higgs, D. R. Zusman, and K. Cho. 2005. EspC is involved in controlling the timing of development in Myxococcus xanthus. J. Bacteriol. 1875029-5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Letunic, I., R. R. Copley, B. Pils, S. Pinkert, J. Schultz, and P. Bork. 2006. SMART 5: domains in the context of genomes and networks. Nucleic Acids Res. 34D257-D260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Licking, E., L. Gorski, and D. Kaiser. 2000. A common step for changing cell shape in fruiting body and starvation-independent sporulation of Myxococcus xanthus. J. Bacteriol. 1823553-3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lobedanz, S., and L. Sogaard-Andersen. 2003. Identification of the C-signal, a contact-dependent morphogen coordinating multiple developmental responses in Myxococcus xanthus. Genes Dev. 172151-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 30.McBride, M. J., T. Köhler, and D. R. Zusman. 1992. Methylation of FrzCD, a methyl-accepting taxis protein of Myxococcus xanthus, is correlated with factors affecting cell behavior. J. Bacteriol. 1744246-4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McBride, M. J., and D. R. Zusman. 1993. FrzCD, a methyl-accepting taxis protein from Myxococcus xanthus, shows modulated methylation during fruiting body formation. J. Bacteriol. 1754936-4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCleary, W. R., M. J. McBride, and D. R. Zusman. 1990. Developmental sensory transduction in Myxococcus xanthus involves methylation and demethylation of FrzCD. J. Bacteriol. 1724877-4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nariya, H., and M. Inouye. 2008. MazF, an mRNA interferase, mediates programmed cell death during multicellular Myxococcus development. Cell 13255-66. [DOI] [PubMed] [Google Scholar]
- 34.Nariya, H., and S. Inouye. 2005. Identification of a protein Ser/Thr kinase cascade that regulates essential transcriptional activators in Myxococcus xanthus development. Mol. Microbiol. 58367-379. [DOI] [PubMed] [Google Scholar]
- 35.Nariya, H., and S. Inouye. 2006. A protein Ser/Thr kinase cascade negatively regulates the DNA-binding activity of MrpC, a smaller form of which may be necessary for the Myxococcus xanthus development. Mol. Microbiol. 601205-1217. [DOI] [PubMed] [Google Scholar]
- 36.Ogawa, M., S. Fujitani, X. Mao, S. Inouye, and T. Komano. 1996. FruA, a putative transcription factor essential for the development of Myxococcus xanthus. Mol. Microbiol. 22757-767. [DOI] [PubMed] [Google Scholar]
- 37.Overgaard, M., S. Wegener-Feldbrügge, and L. Søgaard-Andersen. 2006. The orphan response regulator DigR is required for synthesis of extracellular matrix fibrils in Myxococcus xanthus. J. Bacteriol. 1884384-4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plamann, L., A. Kuspa, and D. Kaiser. 1992. Proteins that rescue A-signal-defective mutants of Myxococcus xanthus. J. Bacteriol. 1743311-3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plamann, L., Y. Li, B. Cantwell, and J. Mayor. 1995. The Myxococcus xanthus asgA gene encodes a novel signal transduction protein required for multicellular development. J. Bacteriol. 1772014-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rasmussen, A. A., S. L. Porter, J. P. Armitage, and L. Sogaard-Andersen. 2005. Coupling of multicellular morphogenesis and cellular differentiation by an unusual hybrid histidine protein kinase in Myxococcus xanthus. Mol. Microbiol. 561358-1372. [DOI] [PubMed] [Google Scholar]
- 41.Rasmussen, A. A., and L. Søgaard-Andersen. 2003. TodK, a putative histidine protein kinase, regulates timing of fruiting body morphogenesis in Myxococcus xanthus. J. Bacteriol. 1855452-5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rasmussen, A. A., S. Wegener-Feldbrugge, S. L. Porter, J. P. Armitage, and L. Sogaard-Andersen. 2006. Four signalling domains in the hybrid histidine protein kinase RodK of Myxococcus xanthus are required for activity. Mol. Microbiol. 60525-534. [DOI] [PubMed] [Google Scholar]
- 43.Reichenbach, H. 1999. The ecology of the myxobacteria. Environ. Microbiol. 115-21. [DOI] [PubMed] [Google Scholar]
- 44.Rosenberg, E., K. H. Keller, and M. Dworkin. 1977. Cell density-dependent growth of Myxococcus xanthus on casein. J. Bacteriol. 129770-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schagger, H., and G. von Jagow. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166368-379. [DOI] [PubMed] [Google Scholar]
- 46.Schultz, J., F. Milpetz, P. Bork, and C. P. Ponting. 1998. SMART, a simple modular architecture research tool: identification of signaling domains. Proc. Natl. Acad. Sci. USA 955857-5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi, W., T. Kohler, and D. R. Zusman. 1993. Chemotaxis plays a role in the social behaviour of Myxococcus xanthus. Mol. Microbiol. 9601-611. [DOI] [PubMed] [Google Scholar]
- 48.Shi, W., F. K. Ngok, and D. R. Zusman. 1996. Cell density regulates cellular reversal frequency in Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 934142-4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi, X., S. Wegener-Feldbrügge, S. Huntley, N. Hamann, R. Hedderich, and L. Søgaard-Andersen. 2008. Bioinformatics and experimental analysis of proteins of two-component systems in Myxococcus xanthus. J. Bacteriol. 190613-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shimkets, L. J. 1999. Intercellular signaling during fruiting-body development of Myxococcus xanthus. Annu. Rev. Microbiol. 53525-549. [DOI] [PubMed] [Google Scholar]
- 51.Singer, M., and D. Kaiser. 1995. Ectopic production of guanosine penta- and tetraphosphate can initiate early developmental gene expression in Myxococcus xanthus. Genes Dev. 91633-1644. [DOI] [PubMed] [Google Scholar]
- 52.Sogaard-Andersen, L. 2004. Cell polarity, intercellular signalling and morphogenetic cell movements in Myxococcus xanthus. Curr. Opin. Microbiol. 7587-593. [DOI] [PubMed] [Google Scholar]
- 53.Sogaard-Andersen, L., and D. Kaiser. 1996. C factor, a cell-surface-associated intercellular signaling protein, stimulates the cytoplasmic Frz signal transduction system in Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 932675-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sogaard-Andersen, L., F. J. Slack, H. Kimsey, and D. Kaiser. 1996. Intercellular C-signaling in Myxococcus xanthus involves a branched signal transduction pathway. Genes Dev. 10740-754. [DOI] [PubMed] [Google Scholar]
- 55.Stein, E. A., K. Cho, P. I. Higgs, and D. R. Zusman. 2006. Two Ser/Thr protein kinases essential for efficient aggregation and spore morphogenesis in Myxococcus xanthus. Mol. Microbiol. 601414-1431. [DOI] [PubMed] [Google Scholar]
- 56.Sun, H., and W. Shi. 2001. Analyses of mrp genes during Myxococcus xanthus development. J. Bacteriol. 1836733-6739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun, H., and W. Shi. 2001. Genetic studies of mrp, a locus essential for cellular aggregation and sporulation of Myxococcus xanthus. J. Bacteriol. 1834786-4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thony-Meyer, L., and D. Kaiser. 1993. devRS, an autoregulated and essential genetic locus for fruiting body development in Myxococcus xanthus. J. Bacteriol. 1757450-7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Timmen, M., B. L. Bassler, and K. Jung. 2006. AI-1 influences the kinase activity but not the phosphatase activity of LuxN of Vibrio harveyi. J. Biol. Chem. 28124398-24404. [DOI] [PubMed] [Google Scholar]
- 60.Ueki, T., and S. Inouye. 2003. Identification of an activator protein required for the induction of fruA, a gene essential for fruiting body development in Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 1008782-8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ueki, T., S. Inouye, and M. Inouye. 1996. Positive-negative KG cassettes for construction of multi-gene deletions using a single drug marker. Gene 183153-157. [DOI] [PubMed] [Google Scholar]
- 62.Viswanathan, P., K. Murphy, B. Julien, A. G. Garza, and L. Kroos. 2007. Regulation of dev, an operon that includes genes essential for Myxococcus xanthus development and CRISPR-associated genes and repeats. J. Bacteriol. 1893738-3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Viswanathan, P., T. Ueki, S. Inouye, and L. Kroos. 2007. Combinatorial regulation of genes essential for Myxococcus xanthus development involves a response regulator and a LysR-type regulator. Proc. Natl. Acad. Sci. USA 1047969-7974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu, S. S., and D. Kaiser. 1995. Genetic and functional evidence that type IV pili are required for social gliding motility in Myxococcus xanthus. Mol. Microbiol. 18547-558. [DOI] [PubMed] [Google Scholar]
- 65.Wu, S. S., J. Wu, and D. Kaiser. 1997. The Myxococcus xanthus pilT locus is required for social gliding motility although pili are still produced. Mol. Microbiol. 23109-121. [DOI] [PubMed] [Google Scholar]