Abstract
Free amino acids, dipicolinic acid, and unidentified small molecules were released early in Bacillus spore germination before hydrolysis of the peptidoglycan cortex, but adenine nucleotides and 3-phosphoglycerate were not. These results indicate that early in germination there is a major selective change in the permeability of the spore's inner membrane.
Dormant spores of Bacillus species contain a number of small molecules in their central region or core that become the protoplast of the growing cell. The small molecule present in spores at the highest level (∼600 μmol/g [dry weight]) is pyridine-2,6-dicarboxylic acid (dipicolinic acid [DPA]), which is largely present as a 1:1 chelate with divalent cations, predominantly Ca2+ (Ca-DPA) (17). However, levels of glutamic acid (20 to 25 μmol/g [dry weight]), arginine (∼10 μmol/g [dry weight]), 3-phosphoglyceric acid (3PGA) (5 to 20 μmol/g [dry weight]), AMP (∼1 μmol/g [dry weight]), and ADP (∼0.2 μmol/g [dry weight]) are also significant in spores of Bacillus subtilis and Bacillus megaterium, and there is an even larger amount of sulfolactic acid (∼100 μmol/g [dry weight]) in spores of at least one strain of B. subtilis (1, 7, 8, 17, 19).
DPA plays a significant role in dormant spore resistance to a number of treatments (11, 14). However, when spores are triggered to germinate, DPA and its associated cations are excreted rapidly in the initial stage (stage I) of spore germination (15, 18). This DPA release takes place before the hydrolysis of the spore's peptidoglycan cortex in stage II that completes spore germination and allows progression into outgrowth and ultimately a return to active growth (15, 18). The precise mechanism for DPA release in spore germination is not clear but appears to involve the proteins encoded by the sporulation-specific spoVA operon (23, 25, 26). However, whether these SpoVA proteins comprise all or a part of a gated channel or pore allowing DPA release is not clear.
In addition to DPA, there is a variety of suggestive evidence indicating that other molecules are also released rapidly during spore germination and/or outgrowth. Thus, >80% of the spore's K+ and Na+ are released during germination of B. megaterium spores, and this release appears to precede the release of at least the majority of DPA (21). These monovalent ions may then be reabsorbed, although DPA is not (21). The sulfolactic acid in spores of B. subtilis strain SB133 is also released when these spores germinate (1), although the rate of this release has not been studied. Finally, the great majority of the free amino acids generated within spores at some period late in germination and/or early in outgrowth by the hydrolysis of the spore's large pool of small acid-soluble proteins (SASP) is also released, although these amino acids are also then reabsorbed (20, 22). Since the dormant spore's pools of small molecules are quite stable in spores incubated in water for years, there clearly must be a radical change in the barriers restricting movement of small molecules into and out of the spore when spores germinate and go into outgrowth. Indeed, there is strong evidence that a molecule as small as methylamine crosses the dormant spore's inner membrane and enters the spore core extremely slowly, while moving rapidly across this membrane once spores have completed stage II of germination (5, 21). Even water may enter the dormant spore core slowly (29). The dormant spore's major barrier to movement of small molecules into or out of the spore core appears to be the spore's inner membrane (21), and fluorescent probes in this membrane are largely immobile in the dormant spore, but become freely mobile when spores complete germination (5). The volume encompassed by the spore's inner membrane also increases 2-fold upon completion of spore germination, and thus the inner membrane surface area increases ∼1.5-fold, but without any phospholipid synthesis (5).
While the changes in the inner spore membrane during spore germination noted above require the hydrolysis of the spore's large peptidoglycan cortex, DPA release takes place in stage I of germination prior to cortex hydrolysis. DPA release can be separated from cortex hydrolysis, since B. subtilis spores that lack both redundant cortex-lytic enzymes (CLEs), CwlJ and SleB, or have a cortex that is not recognized by CLEs release DPA relatively normally in response to nutrient germinants (12, 15). As noted above, at least K+ and Na+ release precedes the release of the majority of DPA from B. megaterium spores during germination (21), and a number of other small molecules, including amino acids and sulfolactic acid, are also released at some point after the triggering of spore germination (1, 20, 22), although it is not known if the latter releases take place before or after the inner membrane rearrangements that follow completion of spore germination. In this communication, we report studies on the release of small molecules early in the germination of B. megaterium and B. subtilis spores. This work makes the new finding that in addition to DPA and associated divalent cations, the dormant spore's free amino acid pools as well as some other unidentified small molecules are also released approximately in parallel with DPA during spore germination. However, several other small molecules present at significant levels in dormant spores were released minimally if at all during this period.
The Bacillus strains used in this work are listed in Table 1. All B. subtilis strains are derived from strain PS832, which is a prototrophic derivative of strain 168. Strain PS533 (wild type) carries plasmid pUB110 encoding resistance to kanamycin (10 μg/ml) (16). Spores of B. subtilis strains were prepared at 37°C on 2× SG medium agar plates, and the spores were harvested, purified, and stored as described previously (9). B. megaterium strain PS1029 is a derivative of strain QM B1551, and B. megaterium spores were made at 30°C in liquid SNB medium and were purified and stored as described previously (20). Spores of B. subtilis strains were germinated at an optical density at 600 nm (OD600) of 2 to 5 in 10 mM KPO4 buffer (pH 7.4)-1 mM l-alanine following a heat shock (30 min at 70°C) of spores in water. After incubation of germinating cultures at 37°C, 1-ml aliquots were centrifuged for 1 min in a microcentrifuge or 20-ml aliquots were centrifuged for 5 min at 14,000 × g, the latter for 3PGA analyses. The pellet fractions were isolated, washed twice with 1 ml water, and suspended in ∼1 ml water, and the samples were either boiled for 30 min (samples for nuclear magnetic resonance [NMR]) or added to 4 ml boiling 1-propanol and boiled for 5 min (6, 19). In some cases, the supernatant fluid was also boiled in 4 ml of 1-propanol. The samples boiled in water were centrifuged, the supernatant fluid was run through a 1-ml Chelex column to remove divalent cations, most importantly Mn2+, the run-through fractions were pooled and lyophilized, and the dry residue was dissolved in D2O for proton NMR spectroscopy as described previously (6, 24). The samples boiled in 1-propanol were flash evaporated, the residue was dissolved in 1 ml water and centrifuged, and the supernatant fluid was analyzed for 3PGA, ATP, ADP, and AMP as described previously (19), but using a luminometer for assays of the adenine nucleotides. B. megaterium spores were germinated following a heat shock for 10 min at 60°C of spores in water. Germination used spores at 1 to 7 mg/ml (dry weight) in 10 mM KPO4 buffer (pH 7.5) plus 50 mM KBr and with or without 10 mM NaF to greatly slow 3PGA catabolism as described previously (19). Samples of 1 or 2 ml were harvested (the latter for ultimate analysis of 3PGA) and then processed and analyzed as described for B. subtilis spores, except that pellet fractions were not washed, and the supernatant fluid from germinating spores was also boiled directly and passed through Chelex, and the run-through fractions were lyophilized and dissolved in D2O for proton NMR spectroscopy.
TABLE 1.
Bacillus strains used in this study
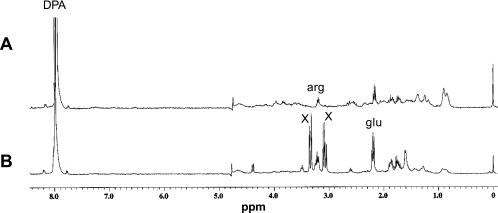
As noted above, DPA, glutamic acid, and arginine are present at significant levels in both B. megaterium and B. subtilis spores, although levels of other amino acids are much lower (7, 8, 17, 19). In addition to the peaks due to these known compounds, NMR spectra of hot water extracts of B. subtilis spores contained a number of large peaks not seen in the spectrum of an extract from B. megaterium spores (Fig. 1A and B; peaks labeled “X” in Fig. 1A). These additional large peaks in the B. subtilis spectrum are due to small molecules, as the compounds giving these peaks eluted after DPA and glutamic acid on a Biogel P2 column (data not shown). At least some of these additional large peaks have provisionally been identified as due to glyceric acid (C. A. Loshon, P. G. Wahome, and P. Setlow, unpublished results). B. megaterium and B. subtilis dormant spores also contained significant levels of AMP and ADP as well as 3PGA (see below), although these molecules were not seen in NMR spectra of processed extracts. The reason these molecules were not seen in NMR spectra is not known, but may be due to small amounts of residual Mn2+ broadening the peaks from these molecules considerably.
FIG. 1.
Proton NMR spectra of small molecules in B. megaterium (A) and B. subtilis (B) spores. Small molecules were extracted from 10 mg (dry weight) of dormant spores of B. megaterium QM B1551 (wild type) (A) and B. subtilis strain PS533 (wild type) (B), extracts were processed, and the proton NMR spectra of the small molecules were obtained as described in the text. Peaks due to known compounds are labeled, although some of the peaks in the arginine region are from glutamate and both arginine and glutamate give additional peaks that are not labeled. The peaks labeled “X” that flank the arginine region are due to an unknown compound or compounds in B. subtilis spores. The spectrum in panel A has been magnified ∼2.5 times more than the spectrum in panel B.
Previous work has shown that large amounts of many different amino acids are released during Bacillus spore germination, with these amino acids coming primarily from SASP hydrolysis (20). The release of large amounts of amino acids generated by SASP hydrolysis would obscure the release of dormant spores' pools of free amino acids. Consequently, we examined amino acid release from B. subtilis spores in which cortex hydrolysis does not take place, since SASP hydrolysis by the SASP-specific germination protease, GPR, requires at least the initiation of cortex hydrolysis (15, 18). The spores used were from strains FB113 (cwlJ sleB) and PS2307 (cwlD) that either lack both CLEs (strain FB113) or have a cortex that does not contain muramic acid-δ-lactam (strain PS2307), a modification essential for CLEs to recognize and act on cortex peptidoglycan (12, 18). While the spores of these two strains do not degrade their cortex in response to nutrient germinants and do not initiate enzyme action in the spore core, they do go through events in stage I of spore germination, most notably DPA release (15). Indeed not only was DPA released upon addition of the nutrient germinant l-alanine to these spores, the spore's pools of free l-arginine and l-glutamic acid also disappeared from the spores and with approximately the same kinetics as for DPA release (Fig. 2A and B). The unknown small molecule or molecules that give rise to the peaks labeled “X” in NMR spectra of small molecules extracted from B. subtilis spores also disappeared from the germinating spores with similar kinetics (Fig. 2A and B).
FIG. 2.
Release of small molecules during germination of B. subtilis spores. Spores of B. subtilis strains FB113 (cwlJ sleB) (A) and PS2307 (cwlD) (B) were germinated, and at various times the small molecules remaining in the germinated spores were extracted, extracts were processed, and small molecules were analyzed by NMR spectroscopy to quantitate small molecules as described in the text. ○, DPA; ▴, glutamic acid; ▵, arginine; and •, X. Note that whatever gives rise to all of the X peaks was released together. The percentage release of the various molecules was calculated by taking the amount in dormant spores as 100% and assuming that there was no change in this total value throughout the experiment.
Mutant strains of B. megaterium lacking CLEs or CwlD are not available. Consequently, to block release of free amino acids generated by SASP hydrolysis during spore germination and outgrowth, we used an isogenic mutant strain (PS1029) in which the gpr gene that encodes the SASP-specific protease has been inactivated. The spores of this strain initiate germination normally, but SASP degradation is greatly slowed (13). These spores were germinated with the salt KBr, which most likely acts by stimulating the spore's nutrient germinant receptors (3, 4), and use of this germinant allowed analysis of amino acids in germination exudates by NMR spectroscopy without interference by the germinant itself. Again, these spore's pools of both glutamic acid and arginine were released with approximately the same kinetics as DPA (Fig. 3) (data not shown). There were also no changes in the level of the total amounts of these compounds present in the germinating spore culture (Fig. 3) (data not shown).
FIG. 3.
Release of small molecules during germination of B. megaterium spores. Spores of B. megaterium strain PS1029 (gpr) were germinated, and at various times aliquots were centrifuged, the pellet fractions were suspended in water, both supernatant and suspended pellet fractions were boiled, and extracts were processed and analyzed by NMR spectroscopy to quantitate small molecules as described in the text. ○, DPA released; ▵, total DPA in the culture; •, glutamic acid released; and ▴, total free glutamic acid in the culture. The average of the total DPA or glutamic acid present at various times was set at 100%, and the percentage released was calculated from values in the supernatant and pellet fractions at each time point. The rate of arginine release was essentially identical to that for glutamic acid release.
While some small molecules are clearly released approximately in parallel with DPA early in spore germination, it seems extremely unlikely that this should be the fate of all small molecules. Indeed, ATP generation from catabolism of endogenous 3PGA as well as exogenous compounds, including amino acids released after SASP hydrolysis, also begins either late in stage II of spore germination or early in outgrowth (15, 18-20). To examine release of other spore small molecules, levels of ATP in germinating PS533 (wild type) spores were determined in both the spore pellet fraction and in the germination exudate. As expected, <5% of total ATP was found in the germination exudate (Table 2). Similarly, <5% of the pools of AMP and ADP present in dormant cwlJ sleB B. subtilis spores was released into the supernatant fluid upon germination of these spores with l-alanine (Table 2). These cwlJ sleB spores that are blocked in germination after completion of stage I do not synthesize any appreciable ATP (15). Thus, at least free adenine nucleotides are not released during spore germination.
TABLE 2.
Levels of adenine nucleotides and 3PGA retained by Bacillus spores during spore germinationa
| Strain | Adenine nucleotide or 3PGA | Level of nucleotide or 3GPA in spores (μmol/g spores [dry wt])
|
|
|---|---|---|---|
| Dormant | Germinatedb | ||
| B. subtilis | |||
| PS533 (wild type) | ATP | ≤0.002 | 0.4-0.6 (≤5)c |
| FB113 (cwlJ sleB) | ADP | 0.12 | 0.13 (<8)d |
| AMP | 0.6 | 0.6 (<2)d | |
| B. megaterium | |||
| QM B1551 | 3PGA | 18 | 14 (14)e |
| 18 | 11 (19)f | ||
| 18 | 1.5g | ||
| B. subtilis | |||
| FB113 (cwlJ sleB) | 3PGA | 5 | 4h |
Spores of various strains were germinated and extracted, extracts were processed, and adenine nucleotides and 3PGA were quantitated as described in the text. All values are the result of duplicate determinations.
Values not in parentheses are the levels found in the germinated spore pellet fraction; values in parentheses are the percentage of the total released in the germination exudate.
Values are for spores germinated at an OD600 of 2 for 15, 30, and 45 min; DPA release was >85% complete in 30 min.
Values are for spores germinated at an OD600 of 2 for 90 min; at this time, DPA release was >90% complete.
Spores were germinated for 15 min at an OD600 of 50 with NaF present; at this time, DPA release was 85% complete.
Spores were germinated for 30 min at an OD600 of 50 with NaF present; at this time, DPA release was >95% complete.
Spores were germinated for 30 min at an OD600 of 50 without NaF; at this time, DPA release was >95% complete.
Spores were germinated at an OD600 of 5 for 60 min; at this time, DPA release was >90% complete.
As noted above, 3PGA is normally catabolized either late in spore germination or early in outgrowth to generate ATP (15, 18, 19), and ≥90% of the 3PGA in dormant B. megaterium spores had disappeared after 30 min of germination, as expected (19) (Table 2). However, when B. megaterium spores were germinated in the presence of NaF to block 3PGA catabolism by inhibition of enolase (19), the great majority of the 3PGA was retained in these spores, even after DPA release was >80% complete (Table 2). Analysis of 3PGA retained in germinating B. subtilis cwlJ sleB spores in which 3PGA metabolism, and indeed all metabolism, was blocked due to the lack of cortex hydrolysis (15) also showed that the great majority of 3PGA was not released from these spores when >90% of DPA was released (Table 2).
The results in this communication lead to one major new conclusion: that in stage I of spore germination not only are DPA and its associated divalent cations released rapidly, but so are a number of other small molecules, including glutamic acid, arginine, and one or more compounds that have not yet been definitively identified. This clearly is the case during B. subtilis spore germination, since release of additional small molecules took place with spores in which progression to stage II of germination was blocked. While no such spores of B. megaterium are currently available, the fact that release of dormant B. megaterium spores' pools of glutamic acid and arginine largely paralleled DPA release during germination strongly suggests that release of these amino acids from these spores also does not require cortex hydrolysis. While a number of small molecules were released rapidly during spore germination, clearly this release is at least somewhat selective, as AMP, ADP, ATP, and 3PGA were released minimally if at all during B. megaterium and B. subtilis spore germination.
It is notable that the release of the additional small molecules during spore germination takes place before hydrolysis of the spore's cortex and the reorganization of the spore's inner membrane that takes place in fully germinated spores. Somehow a membrane that has not allowed passage of DPA and several other small molecules, perhaps for years, allows the rapid passage of the same molecules once spore germination is triggered. This triggering can be by nutrients, as shown in the present work, or by elevated pressures or the cationic surfactant dodecylamine, as shown previously (24, 26). The major question then is how this might be accomplished. One formal possibility is that this is via some type of gated mechanosensitive channel (2). However, deletion of all known mechanosensitive channels from B. subtilis has no effect on at least DPA release during spore germination (27, 28), and known mechanosensitive channels also allow release of molecules up to the size of ATP and even larger (2). Whatever the mechanisms allowing the rapid release of DPA and other small molecules in the first minute of spore germination, this large increase in the inner membrane's permeability to small molecules appears to be selective, as a number of small molecules are not released. Some of this selectivity may be based on molecular size, as the molecules released almost completely (DPA, glutamate, and arginine) have a lower molecular weight than those released poorly if at all (3PGA and adenine nucleotides), although this selectivity may depend primarily on the hydrated dimensions of these molecules. In addition, a number of these molecules are chelated to and likely released with divalent ions, which further complicates analysis of selectivity based solely on molecular weight. The large increase in membrane permeability early in spore germination, most likely in stage I, is also almost certainly transient, since a number of these small molecules are subsequently taken back up. Indeed, the continued high permeability of a plasma membrane to hydrophilic small molecules such as amino acids would seem to be incompatible with life. It seems likely that establishing the mechanism for and regulation of this transient change in the permeability properties of the spore's inner membrane during spore germination will greatly increase our understanding not only of spore germination, but also features of dormant spores as well.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (GM-19697) to P.S.
Sonali Ghosh assisted in the size exclusion chromatography of the small molecules in B. subtilis spores.
Footnotes
Published ahead of print on 9 May 2008.
REFERENCES
- 1.Bonsen, P. P. M., J. A. Spudich, D. L. Nelson, and A. Kornberg. 1969. Biochemical studies of bacterial sporulation and germination. XII. A sulfonic acid as a major sulfur compound of Bacillus subtilis spores. J. Bacteriol. 9862-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Booth, I. R., M. D. Edwards, S. Black, V. Schumann, and S. Miller. 2007. Mechanosensitive channels in bacteria: signs of closure. Nat. Rev. Microbiol. 5431-440. [DOI] [PubMed] [Google Scholar]
- 3.Christie, G., and C. R. Lowe. 2007. Role of chromosomal and plasmid-borne receptor homologues in the response of Bacillus megaterium QM B1551 spores to germinants. J. Bacteriol. 1894375-4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cortezzo, D. E., B. Setlow, and P. Setlow. 2004. Analysis of the action of compounds that inhibit the germination of spores of Bacillus species. J. Appl. Microbiol. 96725-741. [DOI] [PubMed] [Google Scholar]
- 5.Cowan, A. E., E. M. Olivastro, D. E. Koppel, C. A. Loshon, B. Setlow, and P. Setlow. 2004. Lipids in the inner membrane of dormant spores of Bacillus species are immobile. Proc. Natl. Acad. Sci. USA 1017733-7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loshon, C. A., P. G. Wahome, M. W. Maciejewski, and P. Setlow. 2006. Levels of glycine betaine in growing cells and spores of Bacillus species and lack of effect of glycine betaine on dormant spore resistance. J. Bacteriol. 1883153-3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson, D. L., and A. Kornberg. 1970. Biochemical studies of bacterial sporulation and germination. XVIII. Free amino acids in spores. J. Biol. Chem. 2451128-1136. [PubMed] [Google Scholar]
- 8.Nelson, D. L., and A. Kornberg. 1970. Biochemical studies of bacterial sporulation and germination. XIX. Phosphate metabolism during sporulation. J. Biol. Chem. 2451137-1145. [PubMed] [Google Scholar]
- 9.Nicholson, W. L., and P. Setlow. 1990. Sporulation, germination and outgrowth, p. 391-450. In C.R. Harwood and S.M. Cutting (ed.), Molecular biological methods for bacillus. John Wiley and Sons, Chichester, United Kingdom.
- 10.Paidhungat, M., K. Ragkousi, and P. Setlow. 2001. Genetic requirements for induction of germination of spores of Bacillus subtilis by Ca2+-dipicolinate. J. Bacteriol. 1834886-4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paidhungat, M., B. Setlow, A. Driks, and P. Setlow. 2000. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J. Bacteriol. 1825505-5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Popham, D. L., J. Helin, C. E. Costello, and P. Setlow. 1996. Muramic lactam in peptidoglycan of Bacillus subtilis spores is required for spore outgrowth but not for spore dehydration or heat resistance. Proc. Natl. Acad. Sci. USA 9315405-15410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanchez-Salas, J.-L., M. L. Santiago-Lara, B. Setlow, M. D. Sussman, and P. Setlow. 1992. Properties of Bacillus megaterium and Bacillus subtilis mutants which lack the protease that degrades small, acid-soluble proteins during spore germination. J. Bacteriol. 174807-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Setlow, B., S. Atluri, R. Kitchel, K. Koziol-Dube, and P. Setlow. 2006. Role of dipicolinic acid in resistance and stability of spores of Bacillus subtilis with or without DNA-protective α/β-type small, acid-soluble proteins. J. Bacteriol. 1883740-3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Setlow, B., E. Melly, and P. Setlow. 2001. Properties of spores of Bacillus subtilis blocked at an intermediate stage of spore germination. J. Bacteriol. 1834894-4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Setlow, B., and P. Setlow. 1996. Role of DNA repair in Bacillus subtilis spore resistance. J. Bacteriol. 1783486-3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Setlow, P. 1994. Mechanisms that contribute to the long-term survival of spores of Bacillus species. J. Appl. Bacteriol. 7649S-60S. [DOI] [PubMed] [Google Scholar]
- 18.Setlow, P. 2003. Spore germination. Curr. Opin. Microbiol. 6550-556. [DOI] [PubMed] [Google Scholar]
- 19.Setlow, P., and A. Kornberg. 1970. Biochemical studies of bacterial sporulation and germination. XXII. Energy metabolism in early stages of germination of Bacillus megaterium spores. J. Biol. Chem. 2453637-3644. [PubMed] [Google Scholar]
- 20.Setlow, P., and G. Primus. 1975. Protein metabolism during germination of Bacillus megaterium spores. I. Protein synthesis and amino acid metabolism. J. Biol. Chem. 250623-630. [PubMed] [Google Scholar]
- 21.Swerdlow, B. M., B. Setlow, and P. Setlow. 1981. Levels of H+ and other monovalent cations in dormant and germinated spores of Bacillus megaterium. J. Bacteriol. 14820-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tovar-Rojo, F., R.-M. Cabrera-Martinez, B. Setlow, and P. Setlow. 2003. Studies on the mechanism of the osmoresistance of spores of Bacillus subtilis. J. Appl. Microbiol. 95167-179. [DOI] [PubMed] [Google Scholar]
- 23.Tovar-Rojo, F., M. Chander, B. Setlow, and P. Setlow. 2002. The products of the spoVA operon are involved in dipicolinic acid uptake into developing spores of Bacillus subtilis. J. Bacteriol. 184584-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vepachedu, V. R., K. Hirneisen, D. G. Hoover, and P. Setlow. 2007. Studies of the release of small molecules during pressure germination of spores of Bacillus subtilis. Lett. Appl. Microbiol. 45342-348. [DOI] [PubMed] [Google Scholar]
- 25.Vepachedu, V. R., and P. Setlow. 2004. Analysis of the germination of spores of Bacillus subtilis with temperature sensitive spo mutations in the spoVA operon. FEMS Microbiol. Lett. 23971-77. [DOI] [PubMed] [Google Scholar]
- 26.Vepachedu, V. R., and P. Setlow. 2007. Role of SpoVA proteins in release of dipicolinic acid during germination of Bacillus subtilis spores triggered by dodecylamine or lysozyme. J. Bacteriol. 1891565-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wahome, P. G., and P. Setlow. 2006. The synthesis and role of the mechanosensitive channel of large conductance (MscL) in growth and differentiation of Bacillus subtilis. Arch. Microbiol. 186377-383. [DOI] [PubMed] [Google Scholar]
- 28.Wahome, P. G., and P. Setlow. 2006. Growth, osmotic downshock resistance and differentiation of Bacillus subtilis strains lacking mechanosensitive channels. Arch. Microbiol. 18949-58. [DOI] [PubMed] [Google Scholar]
- 29.Westphal, A. J., B. P. Price, T. J. Leighton, and K. E. Wheeler. 2003. Kinetics of size changes of individual Bacillus thuringiensis spores in response to changes in relative humidity. Proc. Natl. Acad. Sci. USA 1003461-3466. [DOI] [PMC free article] [PubMed] [Google Scholar]