Abstract
The Brucella abortus virB locus contains 12 open reading frames, termed virB1 through virB12, which encode a type IV secretion system. Polar mutations in the virB locus markedly reduce the ability of B. abortus to survive in cultured macrophages or to persist in organs of mice. While a nonpolar deletion of the virB2 gene reduces survival in cultured macrophages and in organs of mice, a nonpolar deletion of virB1 only reduces survival in macrophages, whereas virB12 is dispensable for either virulence trait. Here we investigated the role of the remaining genes in the virB locus during survival in macrophages and virulence in mice. Mutants carrying nonpolar deletions of the virB3, virB4, virB5, virB6, virB7, virB8, virB9, virB10, or virB11 gene were constructed and characterized. All mutations reduced the ability of B. abortus to survive in J774A.1 mouse macrophage-like cells to a degree similar to that caused by a deletion of the entire virB locus. Deletion of virB3, virB4, virB5, virB6, virB8, virB9, virB10, or virB11 markedly reduced the ability of B. abortus to persist in the spleens of mice at 8 weeks after infection. Interestingly, deletion of virB7 did not reduce the ability of B. abortus to persist in spleens of mice. We conclude that virB2, virB3, virB4, virB5, virB6, virB8, virB9, virB10, and virB11 are essential for virulence of B. abortus in mice, while functions encoded by the virB1, virB7, and virB12 genes are not required for persistence in organs with this animal model.
Brucellosis, also known as Malta fever or undulant fever, is the most frequent anthropozoonosis in the world, with over half a million new cases each year (26, 44). The disease is caused by gram-negative bacteria that are members of the genus Brucella. These pathogens cause abortion and sterility in animals, while in humans the disease is characterized by recurrent episodes of fever. In both cases, the pathogens are capable of surviving for extended periods of time in the organs of the reticulo-endothelial system, such as liver, spleen, lymph nodes, and bone marrow. While Brucella species lack many of the classical virulence factors present in other Proteobacteria, such as fimbriae, toxins, or virulence plasmids, their chromosomes carry the virB locus, which encodes a type IV secretion system (T4SS) that is required for infection and modulation of the host immune system (24, 29, 42, 56). T4SSs are ancestrally related to conjugation systems and have been identified in a large number of gram-negative pathogens that cause disease in both mammals and plants (1, 4, 5, 20, 43, 52, 54, 62, 64). Based on sequence comparison of their structural components, T4SSs can be divided into two groups, the virB-like T4SSs (T4A) and the dot/icm-like T4SSs (T4B) (14). Both T4A and T4B secretion systems have been shown to translocate or secrete a range of different substrates from DNA bound to protein to single proteins to multisubunit toxins (4, 8, 11, 12, 16, 36, 37, 39-41, 43, 53, 55, 61, 64). The closest homologues of the virB locus are T4A systems encoded by the conjugative plasmids pSB102, pIPO2T, and pTer331 (38, 51, 60).
The best-studied T4SS is the VirB system of Agrobacterium tumefaciens, which is therefore used as a model for the T4A systems. The current model for assembly of the VirB T4SS postulates a core structure in the periplasmic space composed of the proteins VirB6, VirB7, VirB8, VirB9, and VirB10 (13). Energy for the secretion apparatus is thought to be supplied by three NTPases, VirB4, VirB11, and VirD4, located at the cytoplasmic membrane (2). Only two of these NTPases, VirB4 and VirB11, are present in Brucella. A surface structure composed of VirB2 (major) and VirB5 (minor) subunits is thought to mediate contact with the host cells (13). The Brucella VirB T4SS is composed of 12 genes (virB1 to virB12), which are transcribed from a single promoter located in front of virB1 (6). Previous studies on the role of the Brucella T4SS have shown that this virulence factor is required for persistent infection of mice, for survival in tissue culture cells, and for inhibition of fusion of the Brucella-containing vacuole with lysosomes (10, 15, 18, 19, 24, 29, 32, 42, 45, 46, 56, 59, 63, 67). However, most of these studies used transposon insertion mutants, which likely exerted polar effects on the expression of the downstream genes. Secondly, although the overall structures of the T4A secretion systems are very similar, there are some genetic differences between the different organisms. For example, Brucella is missing a homolog of the coupling protein VirD4 that is present in most T4SSs, but it does have an extra gene in the operon, virB12 (42). The Ptl system of Bordetella pertussis lacks the genes for VirB1 and VirB5 but is still able to secrete the pertussis toxin (64). Similarly, the VirB system of Anaplasma marginale has only the proteins that form the core structure but no VirB1, VirB2, VirB5, or VirB7 homolog (7). This large difference in genetic organization, combined with the use of mostly transposon insertions to study the function of the VirB system of Brucella, led us to ask the question of which of the Brucella virB genes are required for virulence and persistent infection. In two previous articles, we reported that virB2 is required for survival in macrophages and persistent infection of mice, while virB1 and virB12 are dispensable in vivo (19, 59). Here we report on the role of the remaining genes in the operon.
MATERIALS AND METHODS
Bacterial strains, media, and culture conditions.
Bacterial strains used were derivatives of Brucella abortus 2308. Strains were cultured on tryptic soy agar (TSA) (Difco/Becton-Dickinson, Sparks, MD) or in tryptic soy broth (TSB) at 37°C on a rotary shaker. Bacterial inocula for infection of mice were cultured on TSA plus 2%-sheep-blood plates. Antibiotics, when required, were added at the following concentrations: carbenicillin (CAR), 100 mg/liter; kanamycin (Km), 100 mg/liter; chloramphenicol (Cm), 5 to 30 mg/liter. All work with live B. abortus was performed at biosafety level 3.
Recombinant DNA techniques.
Plasmid DNA was isolated using ion exchange columns from Qiagen (Valencia, CA). Standard methods were used for Southern blotting, PCR, restriction endonuclease analyses, ligation, and transformation of plasmid DNA into Escherichia coli (3). PCR products were cloned into pCR2.1-TOPO using a commercial kit (Invitrogen, Carlsbad, CA).
Construction of unmarked in-frame deletions.
Unmarked in-frame deletions of virB3, virB4, virB5, virB6, virB7, virB8, virB9, virB10, and virB11 were constructed in a two-step process. First, 1-kb up- and downstream fragments of each plasmid were amplified with the primers listed in Table 2. The resulting amplicons were cloned into pCR2.1 TOPO (Invitrogen). Next, the up- and downstream fragments of each gene were ligated together in either pCR2.1 (virB3, -4, -7, and -8) or in pBluescript (virB5, -6, -9, and 10), using restriction enzymes incorporated into the primers listed in Table 2. Next, a kanamycin resistance cassette (KSAC or KIXX) was ligated into the plasmid between the up- and downstream fragments, using restriction sites incorporated into the original primers used to amplify the up- and downstream fragments. This plasmid was then introduced into wild-type Brucella by electroporation. After recovery, transformants were selected on potato infusion agar (PIA) plus kanamycin and colonies were checked by southern blotting. To remove the kanamycin resistance cassette, we inserted the sacB gene into the plasmid containing the up- and downstream fragments. Each plasmid was then introduced into its corresponding kanamycin-resistant deletion strain, and transformants containing the sacB plasmid integrated into the genome were selected on PIA plus CAR. Colonies resistant to CAR were inoculated into 3 ml sucrose broth and after 24 h of incubation were plated on sucrose agar plates. Colonies from these plates were screened on PIA, PIA plus kanamycin, and PIA plus CAR to identify those colonies that were kanamycin and CAR sensitive. To confirm deletion of the kanamycin resistance cassette, genomic DNA of the wild type and the isogenic mutants was digested with FspI and electrophoresed on an 0.8% agarose gel. After transfer to a positively charged nylon membrane was carried out, the membrane was probed with DNA fragments corresponding to each of the virB genes.
TABLE 2.
Primers used in this worka
| Primer name | Sequence (restriction enzyme) | Fragment |
|---|---|---|
| VirB3up-F | GTTGTCGTGCCCATGATC | virB3 upstream |
| VirB3up-R | CCCGGGGATCTGTGTCCCTTTACC (SmaI) | virB3 upstream |
| VirB3dn-F | CCCGGGATGGGCGCTCAATCCAAA (SmaI) | virB3 downstream |
| VirB3dn-R | CTGCAGTAATGATATTCACCCATG (PstI) | virB3 downstream |
| VirB4up-F | ATCTGCGATCTGGAGCAT | virB4 upstream |
| VirB4up-R2 | CCCGGGTTATGATTCTCTTTTGCGC (SmaI) | virB4 upstream |
| VirB4dn-F | CCCGGGCACTATGAAGAAGATAAT (SmaI) | virB4 downstream |
| VirB4dn-R | CTGCAGCATATTCATGGTGTTGGA (PstI) | virB4 downstream |
| VirB5up-F3 | TCTAGAGGGACTATTCTTGCCATT (XbaI) | virB5 upstream |
| VirB5up-R2 | CTGCAGAGTGTCACCTTCCTGTTG (PstI) | virB5 upstream |
| VirB5dn-F2 | CTGCAGCTACCCGACTAAGGAGTA (PstI) | virB5 downstream |
| VirB5dn-R2 | GTCGACTGCCACCGTATTCATAGA (SalI) | virB5 downstream |
| VirB6up-F2 | TCTAGAATTGCCGAAGTCGGGGAC (XbaI) | virB6 upstream |
| VirB6up-R2 | CTGCAGCACGAAACTCCTTCACCC (PstI) | virB6 upstream |
| VirB6dn-F2 | CTGCAGGGCCTTACACGCGGCGGC (PstI) | virB6 downstream |
| VirB6dn-R3 | GTCGACGTGACATTGAAACCCAGCG (SalI) | virB6 downstream |
| VirB7up-F2 | TCTAGAAAATTCATGCTGATTGCT (XbaI) | virB7 upstream |
| VirB7up-R2 | CTGCAGTATGATTACCTGCGGTGC (PstI) | virB7 upstream |
| VirB7dn-F2 | CTGCAGTCATGTTTGGACGCAAAC (PstI) | virB7 downstream |
| VirB7dn-R2 | GTCGACGTGGGAGAGCGTCATGGA (SalI) | virB7 downstream |
| VirB8up-F | CGCAGCCATGCTCTTTGA | virB8 upstream |
| VirB8up-R | CCCGGGGATTAGTCCTCGTAAGTG (SmaI) | virB8 upstream |
| VirB8dn-F | CCCGGGGCAATGAAAAGATTCCTG (SmaI) | virB8 downstream |
| VirB8dn-R | CTGCAGCGATTTGATGACGCGCTT (PstI) | virB8 downstream |
| VirB9up-F2 | TCTAGACTGGCACATCATGGGCGA (XbaI) | virB9 upstream |
| VirB9up-R2 | CTGCAGTGCACCACTCCCATTTCT (PstI) | virB9 upstream |
| VirB9dn-F2 | CTGCAGATGACACAGGAAAACATT (PstI) | virB9 downstream |
| VirB9dn-R2 | GTCGACAGGTTGATCTGATTTGAA (SalI) | virB9 downstream |
| VirB10up-F | GCGGCCGCAACAACGCACTGGATCGC (NotI) | virB10 upstream |
| VirB10up-R | GGATCCTGCAGGTTCTCCCCGGGC (BamHI) | virB10 upstream |
| VirB10dn-F | GATATCATGATGTCAAACCGAAGT (EcoRV) | virB10 downstream |
| VirB10dn-R | GTCGACTATGGCGGCCAGTGCCGT (SalI) | virB10 downstream |
| virB8351F | GTCGACGCCCACTCTTGCCTATG (SalI) | virB11 upstream |
| virB9822R | CTGCAGTCATTCACTTCGGTTTGACATCATACAC (PstI) | virB11 upstream |
| virB10850 | CTGCAGAACGGGCACTGTCACTGC (PstI) | virB11 downstream |
| virB12441 | GGATCCAGACGAGCGGATAATGCC (EcoRI) | virB11 downstream |
| KSAC707-F | GGATCCTAACCAATTCTGATTAGA (BamHI) | Kan cassette |
| KSAC1638-R | GATATCCGTTGTGTCTCAAAATCTCTG (EcoRV) | Kan cassette |
| SacB-F-SpeI | ACTAGTGCTGGCTTAACTATGCGGCA (SpeI) | sacB |
| SacB-R-SpeI | ACTAGTTCAAACAGGAGGGCTGGAAG (SpeI) | sacB |
| VirB-P-F2 | CTCGAGATGACAGGCATATTTCAA (XhoI) | virB promoter |
| VirB-P-R | CTGCAGGTCTCCTTCTCAGAGAAT (PstI) | virB promoter |
| VirB3-F2 | CTGCAGATGACAACGGCACCACAGGGA (PstI) | virB3 complementation |
| VirB3-R2 | ACTAGTTTATGATTCTCTTTTGCG (SpeI) | virB3 complementation |
| VirB4-F2 | CTGCAGATGGGCGCTCAATCCAAATAC (PstI) | virB4 complementation |
| VirB4-R2 | ACTAGTTCACCTTCCTGTTGATTT (SpeI) | virB4 complementation |
| VirB5-F2 | CTGCAGATGAAGAAGATAATTCTCAGC (PstI) | virB5 complementation |
| VirB5-R2 | ACTAGTTTAATAGGCGGCTTCCAG (SpeI) | virB5 complementation |
| VirB6-F2 | CTGCAGATGGTTAATCCTGTAATCTTT (PstI) | virB6 complementation |
| VirB6-R2 | ACTAGTCTAATCCCTGTTGAACTG (SpeI) | virB6 complementation |
| VirB7-F2 | CTGCAGATGAAAAAGGTAATCCTTGCT (PstI) | virB7 complementation |
| VirB7-R2 | ACTAGTTTAGTCCTCGTAAGTGTC (SpeI) | virB7 complementation |
| VirB8-F2 | CTGCAGATGTTTGGACGCAAACAATCT (PstI) | virB8 complementation |
| VirB8-R2 | ACTAGTTCATTGCACCACTCCCAT (SpeI) | virB8 complementation |
| VirB9-F2 | CTGCAGATGAAAAGATTCCTGCTTGCG (PstI) | virB9 complementation |
| VirB9-R2 | ACTAGTTCATTGCAGGTTCTCCCC (SpeI) | virB9 complementation |
| VirB10-F2 | CTGCAGATGACACAGGAAAACATTCCG (PstI) | virB10 complementation |
| VirB10-R2 | ACTAGTTCACTTCGGTTTGACATC (SpeI) | virB10 complementation |
| VirB11-F2 | CTGCAGATGTCAAACCGAAGTGAC (PstI) | virB11 complementation |
| VirB11-R2 | ACTAGTTCATTGCGTCTTCTCACT (SpeI) | virB11 complementation |
| RTgyrA-F | TGATGCCCTCGTGCGTATG | RT-PCR |
| RTgyrA-R | TTCCGTGACCTTTTCCAGACG | RT-PCR |
| RTvirB-F | TGCCATTCCTTGTCCTCGC | RT-PCR |
| RTvirB-R | CCCACCAACGACGCCTATTG | RT-PCR |
| RTvirB6-F | TATGATTGCCACGACTGCTGTC | RT-PCR |
| RTvirB6-R | ATGACGAGTTGCGAAAACGG | RT-PCR |
| RTvirB11F | CAGGCAAGACCACACTGATGAAAG | RT-PCR |
| RTvirB11R | CGCTTCGCTCGGATAAAACAG | RT-PCR |
Nucleotides in primers are numbered according to position in the B. abortus virB locus as described under GenBank accession number AF226278 (56).
Cell lines.
The mouse macrophage-like cell line J774A.1 (47) was cultured in Dulbecco's modified Eagle's medium (DMEM) (Gibco, Rockville, MD) supplemented with 10% heat-inactivated fetal bovine serum, 1% nonessential amino acids (DMEMsup). For macrophage killing assays, 24-well microtiter plates were seeded with macrophages at a concentration of 1 × 105 to 2 × 105 cells/well in 0.5 ml of DMEMsup and incubated overnight at 37°C in 5% CO2. For preparation of the inoculum, B. abortus strains were grown for 24 h and then diluted in DMEMsup, and about 5 × 107 bacteria in 0.01 ml of DMEMsup were added to each well of macrophages. Microtiter plates were centrifuged at 210 × g for 5 min at room temperature in order to synchronize infection. Cells were incubated for 20 min at 37°C in 5% CO2, free bacteria were removed by three washes with phosphate-buffered saline (PBS), and the zero-time-point sample was taken as described below. DMEMsup plus 50 mg gentamicin per ml was added to the wells, and the cells were incubated at 37°C in 5% CO2. After 1 h, DMEMsup plus 50 μg/ml gentamicin was replaced with medium containing 25 μg/ml gentamicin. Wells were sampled at 0, 24, and 48 h after infection by aspirating the medium, lysing the macrophages with 0.5 ml of 0.5% Tween 20, and rinsing each well with 0.5 ml of PBS. Viable bacteria were quantified by serial dilution in sterile PBS and plating on TSA. All experiments were performed independently in triplicate at least three times and the standard error for each time point calculated.
Infection of mice.
Female BALB/c ByJ mice were obtained from the Jackson Laboratory (Bar Harbor, ME) and used at an age of 4 to 6 weeks. For infection experiments, groups of four or five mice were inoculated intraperitoneally with 0.2 ml of PBS containing 1 × 105 to 5 × 105 CFU of B. abortus. Infected mice were held in microisolator cages in a biosafety level 3 facility. At the appropriate time points, mice were euthanized by CO2 asphyxiation and the spleens collected aseptically at necropsy. Spleens were homogenized in 3 ml of PBS and serial dilutions of the homogenate plated on TSA containing antibiotics, as appropriate, for enumeration of CFU. All animal experiments were approved by the University of California, Davis, Institutional Laboratory Animal Care and Use Committee and conducted in accordance with institutional guidelines.
Detection of VirB proteins.
B. abortus cultures were inoculated in TSB and incubated at 37°C with shaking at 200 rpm for 18 to 24 h. Bacteria were pelleted and resuspended in equal volumes of 1× modified minimal E-medium (pH 5) (34, 46). The cultures were incubated at 37°C with shaking at 200 rpm for 4 to 5 h. Bacteria were pelleted and resuspended in 2× Laemmli sample buffer at a concentration of 1 × 1010 CFU/ml and heated at 100°C for 5 min, and the total protein from 1 × 108 CFU (0.01 ml) loaded per well for separation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (35). Proteins were transferred to polyvinylidene difluoride membranes by electroblotting and were detected using polyclonal rabbit sera specific for VirB5, VirB8, VirB9, VirB10, VirB11, VirB12. and Bcsp31 and goat anti-rabbit immunoglobulin G conjugated to horseradish peroxidase. Horseradish peroxidase activity was detected with a chemiluminescent substrate (Perkin-Elmer, Waltham, MA).
RNA isolation.
Bacterial RNA was isolated using the Ribopure bacterial RNA kit from Ambion (Austin, TX). One milliliter of culture grown to expression conditions was pelleted and resuspended in 350 μl of RNAwiz. This suspension was transferred to 250 μl of Zirconia beads and shaken for 45 min using a vortex. The beads were pelleted, and the supernatant was transferred to a new tube. Volumes (0.2) of chloroform were added and mixed thoroughly. After a 10-min incubation, the suspension was centrifuged for 5 min at 14,000 rpm. The aqueous-phase material was transferred to a new tube, and 0.5 volumes of 100% ethanol were added. The liquid was transferred to a filter cartridge and centrifuged for 1 min. The filter was washed with 700 μl wash solution 1 and 2 × 500 μl wash solution 2/3. The RNA was eluted with 25 to 50 μl elution solution. The RNA was treated with DNAse I to remove genomic DNA contamination.
RT-PCR.
Reverse transcriptase PCR (RT-PCR) was performed using the Taqman reverse transcription kit from Applied Biosystems (Foster City, CA). Reverse transcription of total RNA (0.5 to 1 μg) in 5.5 mM MgCl2, 500 μM deoxynucleoside triphosphates, 2.5 μM random hexamers, 1 U RNase inhibitor, and 1.25 U/μl MultiScribe reverse transcriptase was performed at 48°C for 30 min. RT-PCR was performed for each cDNA sample (2 μl per reaction) with gene-specific primers.
RESULTS
Construction of nonpolar unmarked virB deletion mutants.
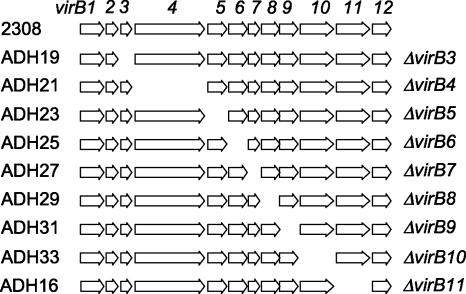
To determine whether each component of the virB T4SS is essential for virulence, we constructed in-frame unmarked deletions of each gene in the operon (deletions and strain designations are shown in Fig. 1). This was done in a two-step process. First, each gene was replaced with a kanamycin resistance cassette by electroporating a plasmid containing 1-kb up- and downstream fragments of the gene to be deleted separated by a kanamycin resistance cassette (plasmids and primers are listed in Tables 1 and 2). In the second step, the kanamycin cassette was removed using the same up- and downstream fragments without the kanamycin cassette but with the counterselectable marker sacB. Each deletion was confirmed by Southern blot analysis using FspI-digested genomic DNA of each of the deletion strains and probes specific for each virB gene (Fig. 2). There are eight FspI sites in the virB operon, which leads to a size difference for several of the signals. For example, virB4 contains two FspI sites, so when probing this mutant with fragments of virB1 to virB8, there was an increase in the size of the hybridizing fragment. These results showed that each deletion strain lacked only the deleted gene and that neighboring genes were intact.
FIG. 1.
Map of virB deletions. Strain names are listed on the left, with the deleted gene indicated on the right. 2308 is wild-type Brucella abortus.
TABLE 1.
Bacterial strains and plasmidsa
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Brucella abortus | ||
| 2308 | Wild type | B. Deyoe |
| ADH4.2 | ΔvirB1-virB12 | 19 |
| ADH6 | ΔvirB1 (nonpolar) | 19 |
| ADH3 | ΔvirB2 (nonpolar) | 19 |
| ADH18 | ΔvirB3::Km (polar) | This work |
| ADH19 | ΔvirB3 (nonpolar) | This work |
| ADH20 | ΔvirB4::Km (polar) | This work |
| ADH21 | ΔvirB4 (nonpolar) | This work |
| ADH22 | ΔvirB5::Km (polar) | This work |
| ADH23 | ΔvirB5 (nonpolar) | This work |
| ADH24 | ΔvirB6::Km (polar) | This work |
| ADH25 | ΔvirB6 (nonpolar) | This work |
| ADH26 | ΔvirB7::Km (polar) | This work |
| ADH27 | ΔvirB7 (nonpolar) | This work |
| ADH28 | ΔvirB8::Km (polar) | This work |
| ADH29 | ΔvirB8 (nonpolar) | This work |
| ADH30 | ΔvirB9::Km (polar) | This work |
| ADH31 | ΔvirB9 (nonpolar) | This work |
| ADH32 | ΔvirB10::Km (polar) | This work |
| ADH33 | ΔvirB10 (nonpolar) | This work |
| ADH15 | ΔvirB11::Km (polar) | This work |
| ADH16 | ΔvirB11 (nonpolar) | This work |
| AK/ORF12 | ΔvirB12::Km | 59 |
| E. coli | ||
| DH5α | endA1 hsdR17(rK− mK−) supE44 thi-1 recA1 gyrA relA1 Δ(lacZYA-argF)U169 deoR φ80dlacZΔ(M15) | 65 |
| BL21(DE3) | F−ompT gal [dcm] [lon] hsdSB(rB− mB−) DE3 | 58 |
| Plasmids | ||
| pBluescript KS | colE1, bla | Stratagene |
| pCR2.1-TOPO | TA cloning vector | Invitrogen |
| pUC4-KSAC | Carries Tn903 kanamycin resistance cassette | Pharmacia |
| pUC4-KIXX | Carries Tn5 kanamycin resistance cassette | Pharmacia |
| pEX18Ap | sacB | 28 |
| pBBRmcs4 | ampR | 33 |
| pADH100 | virB 940-1939 and virB 2290-3290 cloned into pCR2.1-TOPO | This work |
| pADH101 | virB 940-1939 and virB 2290-3290 separated by a kanamycin resistance cassette KIXX cloned into pCR2.1-TOPO | This work |
| pADH102 | SacB cloned into pADH100 | This work |
| pADH103 | virB 1290-2289 and virB 4786-5785 cloned into pCR2.1-TOPO | This work |
| pADH104 | virB 1290-2289 and virB 4786-5785 separated by kanamycin resistance cassette KIXX cloned into pCR2.1-TOPO | This work |
| pADH105 | sacB cloned into pADH103 | This work |
| pADH106 | virB 2772-4707 and virB 5507-6506 cloned into pBluescript | This work |
| pADH107 | virB 2772-4707 and virB 5507-6506 separated by kanamycin resistance cassette KSAC cloned into pBluescript | This work |
| pADH108 | sacB cloned into pADH106 | This work |
| pADH109 | virB 4690-5689 and virB 6734-7751 cloned into pBluescript | This work |
| pADH110 | virB 4690-5689 and virB 6734-7751 separated by kanamycin resistance cassette KSAC cloned into pBluescript | This work |
| pADH111 | sacB cloned into pADH109 | This work |
| pADH112 | virB 5897-6896 and virB 7071-8070 cloned into pBluescript | This work |
| pADH113 | virB 5897-6896 and virB 7071-8070 separated by a kanamycin resistance cassette, KSAC, cloned into pBluescript | This work |
| pADH114 | sacB cloned into pADH112 | This work |
| pADH115 | virB 6073-7072 and virB 7786-8637 cloned into pCR2.1-TOPO | This work |
| pADH116 | virB 6073-7072 and virB 7786-8637 separated by a kanamycin resistance cassette, KIXX, cloned into pCR2.1-TOPO | This work |
| pADH117 | sacB cloned into pADH115 | This work |
| pADH118 | virB 6789-7788 and virB 8655-9658 cloned into pBluescript | This work |
| pADH119 | virB 6789-7788 and virB 8655-9658 separated by a kanamycin resistance cassette, KSAC, cloned into pBluescript | This work |
| pADH120 | sacB cloned into pADH118 | This work |
| pADH121 | virB 7654-8654 and virB 9802-10819 cloned into pBluescript | This work |
| pADH122 | virB 7654-8654 and virB 9802-10819 separated by a kanamycin resistance cassette, KSAC, cloned into pBluescript | This work |
| pADH123 | sacB cloned into pADH121 | This work |
| pADH124 | virB 8351-9822 and virB 10850-12441 cloned into pBluescript | This work |
| pADH125 | virB 8351-9822 and virB 10850-12441 separated by kanamycin resistance cassette KSAC cloned into pBluescript | This work |
| pADH126 | SacB cloned into pADH124 | This work |
| pVP | 450-bp fragment of virB promoter cloned into pBBR-MCS4 | This work |
| pVP3 | virB3 cloned into pVP | This work |
| pVP4 | virB4 cloned into pVP | This work |
| pVP5 | virB5 cloned into pVP | This work |
| pVP6 | virB6 cloned into pVP | This work |
| pVP7 | virB7 cloned into pVP | This work |
| pVP8 | virB8 cloned into pVP | This work |
| pVP9 | virB9 cloned into pVP | This work |
| pVP10 | virB10 cloned into pVP | This work |
| pVP11 | virB11 cloned into pVP | This work |
Cloned fragments of the virB operon are numbered according to the sequence found under GenBank accession number AF226278 (56).
FIG. 2.
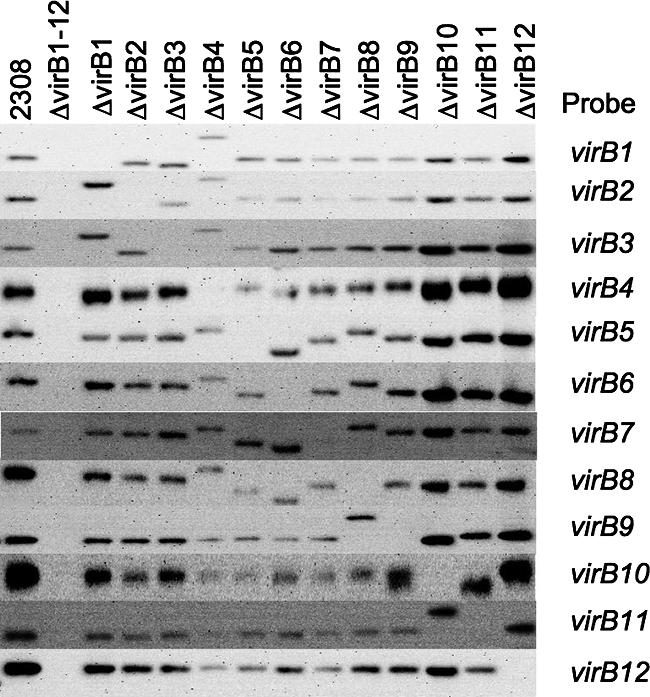
Confirmation of virB gene deletions by Southern blotting. FspI-digested genomic DNA from wild-type B. abortus 2308 and each of its isogenic mutants was electrophoresed and transferred to nylon membranes. Membranes were probed with DNA fragments corresponding to genes listed on the right. Strain genotypes are listed across the top. There are eight FspI sites in the virB operon, including one in the virB4 gene and one in the virB10 gene, whose deletion results in larger hybridizing FspI fragments in the virB4 and virB10 mutants.
Unmarked deletions do not prevent transcription of downstream genes.
For each mutant, we determined whether expression of downstream genes was affected by the unmarked deletion. To this end, we isolated RNA from each mutant grown under conditions for virB expression. Expression of virB1, virB6, and virB11 was assessed in each RNA sample by RT-PCR using specific primers. As a control for DNA contamination, an RNA sample from each mutant was analyzed without adding reverse transcriptase. No PCR product was obtained in this control reaction, suggesting that this method specifically detected mRNA for each gene. As a positive control, mRNA of the gyrA gene was detected by RT-PCR in each sample, confirming the presence of mRNA in each preparation. An additional positive control included RT-PCR using genomic DNA from wild-type B. abortus, which was positive for each primer pair, as expected. A product for virB1 was obtained by RT-PCR in mRNA samples from all strains except the virB1-virB12 deletion mutant (ADH4.2) and the virB1 deletion mutant (ADH6) (Fig. 3). A product was also visible for virB6 and virB11 in all strains except the virB6 mutant (ADH25) and the virB11 mutant (ADH16). These data suggested that unmarked deletions of virB1 (ADH6), virB2 (ADH3), virB3 (ADH19), virB4 (ADH21), virB5 (ADH23), virB6 (ADH25), virB7 (ADH27), virB8 (ADH29), virB9 (ADH31), virB10 (ADH33), and virB11 (ADH16) were nonpolar on the transcription of downstream genes.
FIG. 3.
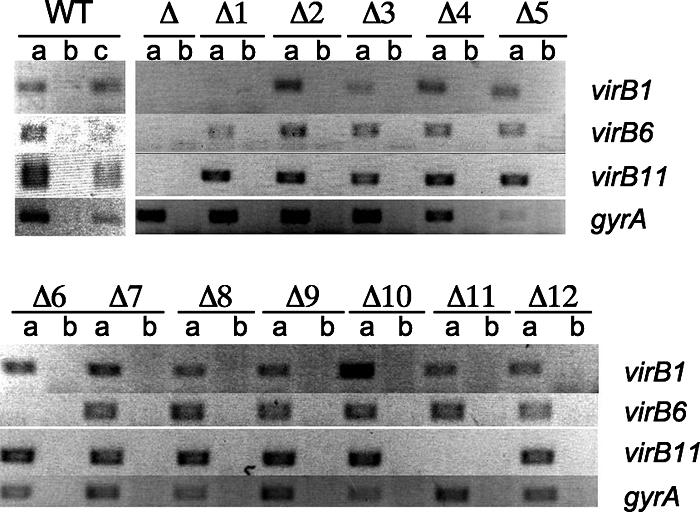
Deletions of virB genes are nonpolar on transcription of downstream genes. PCR amplicons from RNA isolated from wild-type B. abortus and its isogenic mutants with primers for virB1, virB6, virB11, and gyrA are shown. The genotype of each mutant is listed at the top of the figure (Δ1, ΔvirB1; Δ2, ΔvirB2, etc.; WT, wild type). For each strain, a PCR was performed with reverse transcriptase (a) or without reverse transcriptase (b); lane c, wild-type genomic DNA control.
Expression of VirB proteins in strains carrying unmarked deletions.
Although each unmarked deletion appeared to be nonpolar, we considered that these mutations may affect translation of downstream genes. Furthermore, the absence of one component may affect assembly of the secretion apparatus, thereby possibly resulting in misfolding and/or degradation of other components of the T4SS. To determine the effect of each unmarked deletion on abundance of other T4SS components, we performed Western blot analysis with all strains, using polyclonal rabbit sera against VirB5, VirB8, VirB9, VirB10, VirB11, and VirB12 (Fig. 4). A previously described polyclonal antibody against the Brucella outer membrane protein Bcsp31 was used as a loading control (59). Deletion of virB3 (ADH19) led to reduced levels of VirB5, VirB8, VirB9, VirB10, and VirB11, suggesting either an effect of the mutation on translation or a requirement of the VirB3 protein for stability of other T4SS proteins. In the virB2 mutant (ADH3) and the virB4 mutant (ADH21), the abundance of VirB5 was markedly reduced while VirB8, VirB9, VirB10, VirB11, and VirB12 were detected at wild-type levels. These data suggested that reduced expression of VirB5 in the virB2 mutant (ADH3) and the virB4 mutant (ADH21) was not due to polar effects on transcription but likely reflected a requirement of the VirB2 and VirB4 proteins for the assembly/stability of VirB5. Deletion of virB7 (ADH27) reduced protein levels of VirB8, VirB9, VirB10, and VirB11 under in vitro growth conditions, as indicated by our Western blot analysis. With the virB8 mutant (ADH29), we were not able to detect VirB9, VirB10, and VirB11 by Western blotting (Fig. 4) despite the presence of mRNA encoding these proteins (Fig. 3), suggesting that VirB8 is required for the assembly/stability of VirB9, VirB10, and VirB11. Alternatively, deletion of virB8 may reduce the efficiency by which the virB9, virB10, and virB11 open reading frames are translated. Deletion of virB9 (ADH31) resulted in reduced levels of VirB10 and VirB11 (Fig. 4). The virB10 mutant (ADH33) exhibited reduced levels of VirB11. These data suggested either that deletions of virB9 and virB10 may reduce translation of downstream genes or that the encoded proteins are required for the stability of VirB11. VirB12 was expressed in all strains at the same level except in the virB11 mutant (ADH16), in which its abundance was reduced. In summary, while unmarked deletion of each gene within the virB locus was nonpolar on transcription of downstream genes (Fig. 3), several of these mutations affected abundance of other components of the T4SS, suggesting effects on either translation or stability of these proteins.
FIG. 4.
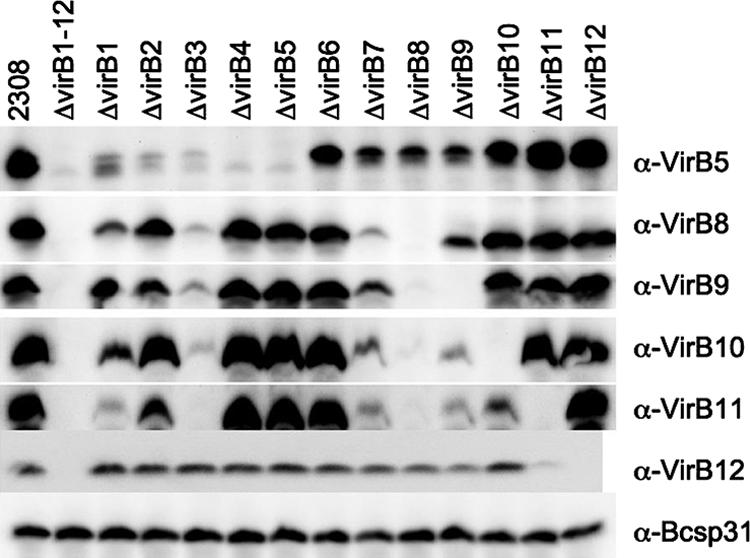
Western blot of wild type and deletion mutant strains. All strains were grown under expression conditions described by Patey et al. (34). Expression was detected with polyclonal antisera raised against VirB5 (α-VirB5), VirB8 (α-VirB8), VirB9 (α-VirB9), VirB10 (α-VirB10), VirB11 (α-VirB11), VirB12 (α-VirB12), and Bcsp31 (α-Bcsp31) (loading control).
Requirement of all virB genes for growth of B. abortus in macrophages.
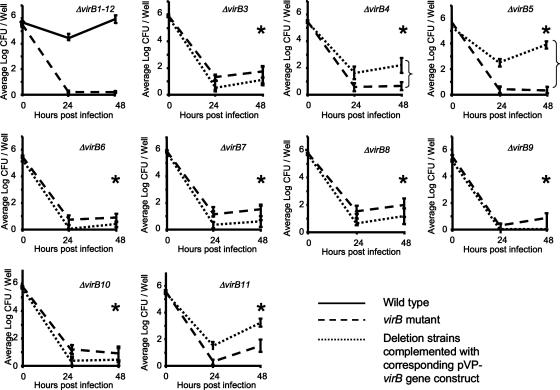
To determine the role of the virB genes in vitro, we infected the J774A.1 macrophage-like cell line at a multiplicity of infection of 100:1. Deletion of virB3 (ADH19), virB4 (ADH21), virB5 (ADH23), virB6 (ADH25), virB7 (ADH27), virB8 (ADH29), virB9 (ADH31), virB10 (ADH33), and virB11 (ADH16) significantly decreased the ability of B. abortus to survive inside macrophages (Fig. 5). Strains carrying mutations in virB4 (ADH21), virB5 (ADH23), or virB11 (ADH16) could be partially complemented by introducing plasmids (pVP derivatives) carrying each virB gene under control of the upstream promoter of the virB operon. Western blot analysis showed that complementation of the virB8 mutant (ADH29), the virB9 mutant (ADH31), or the virB10 mutant (ADH33) resulted in expression of VirB8, VirB9, and VirB10, respectively (Fig. 6). However, introduction of the appropriate plasmids did not complement the phenotype caused by deletion of virB3 (ADH19), virB6 (ADH25), virB8 (ADH29), virB9 (ADH31), or virB10 (ADH33) (Fig. 5). One possible interpretation of these data is that full functionality of the T4SS requires production of each component in the correct stoichiometry, which cannot be reproduced by expressing components from a multicopy plasmid. For example, deletion of virB6 (ADH25) did not result in polar effects on transcription of downstream genes (Fig. 3), nor did it alter expression of VirB8, VirB9, VirB10, VirB11, or VirB12 (Fig. 4). However, the respective mutant could not be complemented for macrophage survival by introducing a plasmid carrying the cloned virB6 gene expressed from the virB promoter (Fig. 5). These data suggested that functionality of the T4SS may require a balanced expression of the VirB6 protein. In conclusion, our results indicated that the virB3, virB4, virB5, virB6, virB7, virB8, virB9, virB10, and virB11 genes are required for survival and replication in macrophages.
FIG. 5.
Survival and replication in J774A.1 macrophages of B. abortus virB deletion mutants. Each deletion mutant was complemented with a pVP derivative carrying the corresponding virB gene under the control of the virB operon promoter. In each graph, the solid line represents CFU of B. abortus 2308; the dashed line represents CFU of the ΔvirB mutant indicated in the upper-right corner of each graph; and the dotted line represents CFU of each deletion mutant complemented with a pVP derivative expressing the corresponding virB gene from the virB promoter. Introduction of the empty pVP plasmid had no effect on intracellular survival of any of the deletion mutants (data not shown). Data presented are averages of results from three experiments with three wells per strain. Error bars represent standard errors of the means. Asterisks denote significant differences (P < 0.05) between numbers of CFU of the mutant and of its complemented strain, determined by Student's t test.
FIG. 6.
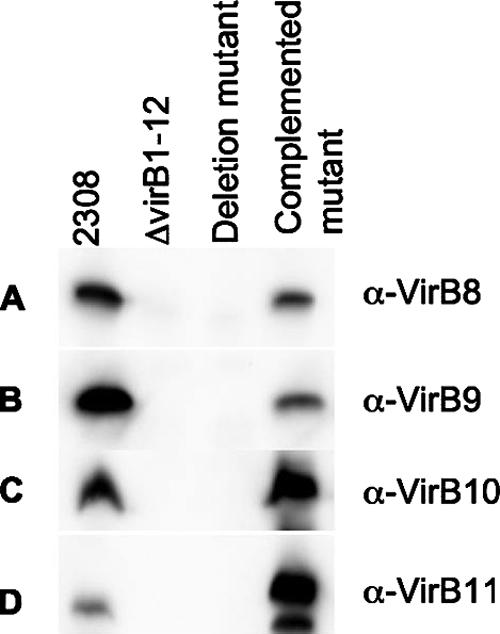
Western blots showing expression of virB genes from plasmids used for complementation of deletion mutants. The antiserum (anti-VirB8, -VirB9, -VirB10, or -VirB11) used to probe each blot is shown on the right. (A) virB8 deletion mutant. (B) virB9 deletion mutant. (C) virB10 deletion mutant. (D) virB11 deletion mutant.
VirB7 is not required for persistent infection of mice by B. abortus.
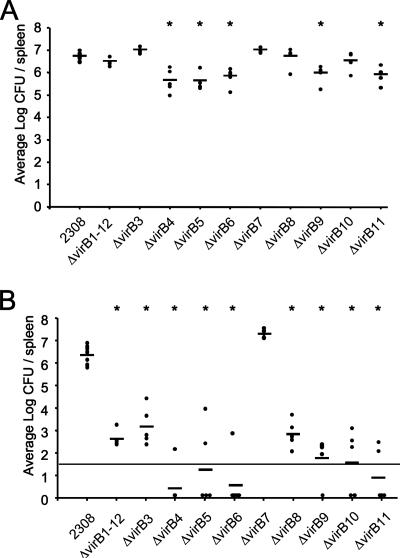
In order to determine which of the virB genes are required for persistence of B. abortus in vivo, we infected mice with the wild type and each of the virB deletion strains. Groups of 10 BALB/c mice were infected with the B. abortus wild type (2308) or one of the isogenic mutant strains. At 1 and 8 weeks after infection, groups of five mice were euthanized and the bacterial load in the spleen determined by plating serial dilutions. Statistical differences were determined by one-way analysis of variance. Figure 7A shows that at 1 week after infection, strains carrying deletions of virB4 (ADH21), virB5 (ADH23), virB6 (ADH25), virB9 (ADH31), and virB11 (ADH16) were significantly attenuated compared to the wild type, while strains with deletions of virB1 to virB12 (ADH4.2), virB3 (ADH19), virB7 (ADH27), virB8 (ADH29), and virB10 (ADH33) were recovered at equal levels compared to the wild type (2308). The fact that at 1 week after infection some of the mutants (ADH21, ADH23, ADH25, ADH31, and ADH16) were attenuated more strongly than a strain carrying a precise deletion of the virB locus (ADH4.2) suggests that the presence of an incompletely assembled secretion apparatus may make B. abortus more vulnerable to host defense mechanisms encountered in the initial days after inoculation. At 8 weeks after infection, all strains except the virB7 mutant (ADH27) were recovered at 3- to 4-log-lower levels than the wild type (2308) (Fig. 7B). This result was unexpected, because the virB7 mutant (ADH27) was attenuated for macrophage survival (Fig. 5). To verify that the group of mice had been infected with the virB7 deletion mutant (ADH27), we isolated genomic DNA from bacteria recovered from mice and confirmed the deletion by PCR using primers flanking the deletion site. Sequence analysis of the PCR fragment confirmed that virB7 was deleted (data not shown). These data suggested that the virB7 gene is not required for mouse virulence of B. abortus. Furthermore, our data showed that the complete absence of the T4SS (ADH4.2), as well as defects in the T4SS caused by mutations in virB3 (ADH19), virB4 (ADH21), virB5 (ADH23), virB6 (ADH25), virB8 (ADH29), virB9 (ADH31), virB10 (ADH33), and virB11 (ADH16), markedly reduced the ability of B. abortus to persist in mice at 8 weeks after infection. Together with previous studies showing that virB1 and virB12 are dispensable during infection (19, 59), these data show that 9 out of the 12 genes present in the virB locus are required for persistence in organs of mice.
FIG. 7.
Persistence of nonpolar mutants in spleens of BALB/c mice. Each dot represents CFU recovered from a single mouse; the mean number of CFU in each group is indicated by a horizontal line. Asterisks indicate statistical significance of each group compared to wild-type B. abortus, as determined by one-way analysis of variance followed by a Tukey-Kramer multiple comparison. (A) One week postinoculation. (B) Eight weeks postinoculation. The horizontal line across each graph indicates the limit of detection.
DISCUSSION
T4SSs have been identified in many pathogenic bacteria. They are found in both extracellular and intracellular pathogens, where they allow the bacteria to adapt to changes in the environment and by doing so enable them to survive. The B. abortus T4SS has been shown to be essential for persistent infection in both in vitro and in vivo models (15, 19, 29, 42, 56, 67). More recently it was shown that the T4SS allows B. abortus to set up its replicative niche by modulating the immune response of the host (48, 49). In our current study, we focused on determining the role that each of the virB genes has on the ability of B. abortus to establish persistent infection. To this end, we constructed nonpolar deletions and were able to demonstrate that all of them, except a deletion of virB7, impaired the ability of B. abortus to establish a replicative niche in the spleens of mice. Our data are in agreement with the reports of Watarai et al., Patey et al., and Comerci et al., who used nonpolar deletions to show that virB4, virB8, and virB10 are required for persistent infection of Brucella (15, 46, 63). The finding that not all components of the B. abortus virB locus are required for infection of mice is reminiscent of data obtained with the cag pathogenicity island of Helicobacter pylori, which contains 27 genes, only 17 of which are required for translocation of CagA (23). However, in the case of the ptl genes of Bordetella pertussis, all the genes of the secretion apparatus are required for secretion of pertussis toxin (17). Our data are also in agreement with what has been reported for the Bartonella VirB system. The Bartonella VirB T4SS is required for establishing intraerythrocytic infection, and a strain lacking virB4 was shown to be avirulent (52).
Interestingly, our data on the B. abortus T4SS differ from those reported for the closely related T4SS of Agrobacterium tumefaciens, in which the homologous virB7 gene is required for the formation of a functional secretion apparatus. This finding was not the result of an error in the construction of the virB7 mutant, since we did confirm that our virB7 deletion strain is correct by sequencing genomic DNA isolated from bacteria recovered from the mice. Although our protein data are in agreement with data in previously reported studies on A. tumefaciens VirB protein levels, in which deletion of virB7 led to the absence of the VirB8 and VirB9 proteins and to reduced levels of VirB4, VirB10, and VirB11 (5, 21), we did not see that the virB7 mutant strain is avirulent. A. tumefaciens VirB7 has a cysteine residue at position 24 that is required for the formation and stabilization of VirB7 homodimers and VirB7-VirB9 heterodimers, which are thought to link the pilus (VirB2 and VirB5) to the periplasmic core structure of the T4SS (57). Interestingly, this critical cysteine residue is not conserved within the B. abortus VirB7 primary structure. However, despite the missing cysteine residue, yeast two-hybrid analysis showed that VirB7 and VirB9 of Brucella spp. can still interact (E. Botella and D. O'Callaghan, personal communication). The overall similarity between the B. abortus and A. tumefaciens VirB proteins is quite low (20 to 30% similarity [42]) compared to the similarity between the biosynthetic proteins AroC and PurE, which share 83% similarity between the two species (not shown). This likely reflects the acquisition of the T4SS genes by A. tumefaciens and B. abortus by independent horizontal gene transfer events, as suggested by Frank et al. (25). In support of this idea, a phylogenetic analysis of T4SS components by Cao and Saier showed that the Brucella VirB proteins always clustered more closely with B. pertussis Ptl proteins than with VirB proteins from more closely related species, such as A. tumefaciens or Bartonella henselae (9). It is thus possible either that the function of VirB7 in the B. abortus T4SS differs from that of its counterpart in the T4SS of A. tumefaciens or that the molecular interactions between VirB7 and other components of the T4SS are not the same in the two species.
Effects of B. abortus virB gene deletion on abundance of other T4SS components mirrored in some cases what has been reported for A. tumefaciens. For example, deletion of B. abortus virB6 did not affect levels of VirB3, VirB5, VirB9, and VirB10, similar to what has been reported by Judd et al. for a virB6 deletion mutant (31). However, it should be noted that other studies have found that virB6 deletion in A. tumefaciens led to a reduction in levels of these proteins (27, 30, 50). Further, deletion of B. abortus virB4 led to an absence of VirB5, as was reported by Finberg et al. for an A. tumefaciens virB4 mutant (22). Furthermore, we did not note a stabilizing effect of VirB4 on VirB8, which has been reported for A. tumefaciens (66). We also observed that deletion of certain core proteins led to reduced levels of other core proteins (Fig. 4), as has been observed in A. tumefaciens (21). These results suggest that there are both similarities and differences in the interactions between T4SS components that stabilize the secretion apparatus in A. tumefaciens and B. abortus.
Although we were able to demonstrate restoration of protein production by complementation for some mutants, this did not lead to wild-type replication levels in our in vitro macrophage survival assays. One possible explanation for these observations may be that the VirB proteins need to be expressed at a fixed ratio to one another for the secretion complex to form properly. The plasmid we used to complement virB deletion mutants was a medium-copy-number derivative of pBBR1MCS4, which may have resulted in an inappropriate expression level of the respective virB gene, thereby impairing assembly of the secretion system. This would be in agreement with a previous study in which we found that virB2 from the lac promoter on pBBR1MCS impaired intracellular survival of B. abortus (19). The plasmids used for complementation of each mutant expressed each virB gene from the virB promoter, and therefore their expression should be coordinated with that of the chromosomal virB genes. Recent studies did show complementation of a virB8 deletion strain with a plasmid-borne virB8 gene driven by the virB promoter (45, 46). The difference between the plasmid used in the previous studies and the plasmid used in our study is that Paschos et al. used a 1.1-kb region upstream of virB1 to drive expression of virB8, while in our construct, 450 bp were used. This difference, or a difference in the strain background used (B. suis versus B. abortus 2308), may account for the disparate results with complementation in these studies. However, we were able to show that VirB8 is expressed in the complemented virB8 deletion mutant (pVP8) (Fig. 6), so lack of complementation in our study was not due to a lack of virB8 expression from this construct.
Our data show that deletion of virB7 reduces survival in J774A.1 cells, while the ability to colonize organs of mice was not affected by this mutation. Similarly, a nonpolar deletion of virB1 reduces the ability of B. abortus to survive in J774A.1 cells, while mouse virulence is not altered (19). Differences in the outcome of in vitro and in vivo models of infection suggest that tissue culture systems may not always accurately reflect all aspects of host-pathogen interaction in vivo. Since the signals controlling the expression of the virB genes and stability of the T4SS apparatus in vivo are poorly understood, it is possible that the other core components may have increased expression or stability in vivo in the absence of VirB7 (compared to results in vitro), thereby allowing for assembly of a more functional T4SS. Alternatively, a protein expressed by B. abortus in tissues of the mouse but not in the in vitro tissue culture assays may compensate for the lack of VirB7 to mediate stability and function of the T4SS apparatus. Finally, VirB7 may be required for efficient secretion of a subset of effector proteins that are more crucial for replication in tissue culture cells than for persistence in vivo. Identification of the secreted effectors of the B. abortus T4SS will aid in distinguishing between these different possibilities and help us to understand the differences between the minimal sets of VirB proteins required for T4SS functionality in vitro and in vivo.
Acknowledgments
We thank Christian Baron for the generous gift of antiserum against VirB8, VirB9, VirB10, and VirB11 and Andreas Bäumler for critical comments on the manuscript.
This work was supported by PHS grant AI050553 to R.M.T.
Footnotes
Published ahead of print on 9 May 2008.
REFERENCES
- 1.Andrews, H. L., J. P. Vogel, and R. R. Isberg. 1998. Identification of linked Legionella pneumophila genes essential for intracellular growth and evasion of the endocytic pathway. Infect. Immun. 66950-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atmakuri, K., E. Cascales, and P. J. Christie. 2004. Energetic components VirD4, VirB11 and VirB4 mediate early DNA transfer reactions required for bacterial type IV secretion. Mol. Microbiol. 541199-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1994. Current protocols in molecular biology. J. Wiley & Sons, Hoboken, NJ.
- 4.Backert, S., E. Ziska, V. Brinkmann, U. Zimny-Arndt, A. Fauconnier, P. R. Jungblut, M. Naumann, and T. F. Meyer. 2000. Translocation of the Helicobacter pylori CagA protein in gastric epithelial cells by a type IV secretion apparatus. Cell Microbiol. 2155-164. [DOI] [PubMed] [Google Scholar]
- 5.Berger, B. R., and P. J. Christie. 1994. Genetic complementation analysis of the Agrobacterium tumefaciens virB operon: virB2 through virB11 are essential virulence genes. J. Bacteriol. 1763646-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boschiroli, M. L., S. Ouahrani-Bettache, V. Foulongne, S. Michaux-Charachon, G. Bourg, A. Allardet-Servent, C. Cazevieille, J. P. Liautard, M. Ramuz, and D. O'Callaghan. 2002. The Brucella suis virB operon is induced intracellularly in macrophages. Proc. Natl. Acad. Sci. USA 991544-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brayton, K. A., L. S. Kappmeyer, D. R. Herndon, M. J. Dark, D. L. Tibbals, G. H. Palmer, T. C. McGuire, and D. P. Knowles, Jr. 2005. Complete genome sequencing of Anaplasma marginale reveals that the surface is skewed to two superfamilies of outer membrane proteins. Proc. Natl. Acad. Sci. USA 102844-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campodonico, E. M., L. Chesnel, and C. R. Roy. 2005. A yeast genetic system for the identification and characterization of substrate proteins transferred into host cells by the Legionella pneumophila Dot/Icm system. Mol. Microbiol. 56918-933. [DOI] [PubMed] [Google Scholar]
- 9.Cao, T. B., and M. H. Saier, Jr. 2001. Conjugal type IV macromolecular transfer systems of Gram-negative bacteria: organismal distribution, structural constraints and evolutionary conclusions. Microbiology 1473201-3214. [DOI] [PubMed] [Google Scholar]
- 10.Celli, J., C. de Chastellier, D. M. Franchini, J. Pizarro-Cerda, E. Moreno, and J. P. Gorvel. 2003. Brucella evades macrophage killing via VirB-dependent sustained interactions with the endoplasmic reticulum. J. Exp. Med. 198545-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, J., K. S. de Felipe, M. Clarke, H. Lu, O. R. Anderson, G. Segal, and H. A. Shuman. 2004. Legionella effectors that promote nonlytic release from protozoa. Science 3031358-1361. [DOI] [PubMed] [Google Scholar]
- 12.Chen, J., M. Reyes, M. Clarke, and H. A. Shuman. 2007. Host cell-dependent secretion and translocation of the LepA and LepB effectors of Legionella pneumophila. Cell Microbiol. 91660-1671. [DOI] [PubMed] [Google Scholar]
- 13.Christie, P. J., K. Atmakuri, V. Krishnamoorthy, S. Jakubowski, and E. Cascales. 2005. Biogenesis, architecture, and function of bacterial type IV secretion systems. Annu. Rev. Microbiol. 59451-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christie, P. J., and J. P. Vogel. 2000. Bacterial type IV secretion: conjugation systems adapted to deliver effector molecules to host cells. Trends Microbiol. 8354-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Comerci, D. J., M. J. Martinez-Lorenzo, R. Sieira, J. P. Gorvel, and R. A. Ugalde. 2001. Essential role of the VirB machinery in the maturation of the Brucella abortus-containing vacuole. Cell Microbiol. 3159-168. [DOI] [PubMed] [Google Scholar]
- 16.Conover, G. M., I. I. Derre, J. P. Vogel, and R. R. Isberg. 2003. The Legionella pneumophila LidA protein: a translocated substrate of the Dot/Icm system associated with maintenance of bacterial integrity. Mol. Microbiol. 48305-321. [DOI] [PubMed] [Google Scholar]
- 17.Craig-Mylius, K. A., and A. A. Weiss. 1999. Mutants in the ptlA-H genes of Bordetella pertussis are deficient for pertussis toxin secretion. FEMS Microbiol. Lett. 179479-484. [DOI] [PubMed] [Google Scholar]
- 18.Delrue, R. M., M. Martinez-Lorenzo, P. Lestrate, I. Danese, V. Bielarz, P. Mertens, X. De Bolle, A. Tibor, J. P. Gorvel, and J. J. Letesson. 2001. Identification of Brucella spp. genes involved in intracellular trafficking. Cell Microbiol. 3487-497. [DOI] [PubMed] [Google Scholar]
- 19.den Hartigh, A. B., Y. H. Sun, D. Sondervan, N. Heuvelmans, M. O. Reinders, T. A. Ficht, and R. M. Tsolis. 2004. Differential requirements for VirB1 and VirB2 during Brucella abortus infection. Infect. Immun. 725143-5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dillard, J. P., and H. S. Seifert. 2001. A variable genetic island specific for Neisseria gonorrhoeae is involved in providing DNA for natural transformation and is found more often in disseminated infection isolates. Mol. Microbiol. 41263-277. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez, D., G. M. Spudich, X. R. Zhou, and P. J. Christie. 1996. The Agrobacterium tumefaciens VirB7 lipoprotein is required for stabilization of VirB proteins during assembly of the T-complex transport apparatus. J. Bacteriol. 1783168-3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finberg, K. E., T. R. Muth, S. P. Young, J. B. Maken, S. M. Heitritter, A. N. Binns, and L. M. Banta. 1995. Interactions of VirB9, -10, and -11 with the membrane fraction of Agrobacterium tumefaciens: solubility studies provide evidence for tight associations. J. Bacteriol. 1774881-4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer, W., J. Puls, R. Buhrdorf, B. Gebert, S. Odenbreit, and R. Haas. 2001. Systematic mutagenesis of the Helicobacter pylori cag pathogenicity island: essential genes for CagA translocation in host cells and induction of interleukin-8. Mol. Microbiol. 421337-1348. [DOI] [PubMed] [Google Scholar]
- 24.Foulongne, V., G. Bourg, C. Cazevieille, S. Michaux-Charachon, and D. O'Callaghan. 2000. Identification of Brucella suis genes affecting intracellular survival in an in vitro human macrophage infection model by signature-tagged transposon mutagenesis. Infect. Immun. 681297-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frank, A. C., C. M. Alsmark, M. Thollesson, and S. G. Andersson. 2005. Functional divergence and horizontal transfer of type IV secretion systems. Mol. Biol. Evol. 221325-1336. [DOI] [PubMed] [Google Scholar]
- 26.Godfroid, J., A. Cloeckaert, J. P. Liautard, S. Kohler, D. Fretin, K. Walravens, B. Garin-Bastuji, and J. J. Letesson. 2005. From the discovery of the Malta fever's agent to the discovery of a marine mammal reservoir, brucellosis has continuously been a re-emerging zoonosis. Vet. Res. 36313-326. [DOI] [PubMed] [Google Scholar]
- 27.Hapfelmeier, S., N. Domke, P. C. Zambryski, and C. Baron. 2000. VirB6 is required for stabilization of VirB5 and VirB3 and formation of VirB7 homodimers in Agrobacterium tumefaciens. J. Bacteriol. 1824505-4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoang, T. T., R. R. Karkhoff-Schweizer, A. J. Kutchma, and H. P. Schweizer. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 21277-86. [DOI] [PubMed] [Google Scholar]
- 29.Hong, P. C., R. M. Tsolis, and T. A. Ficht. 2000. Identification of genes required for chronic persistence of Brucella abortus in mice. Infect. Immun. 684102-4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jakubowski, S. J., V. Krishnamoorthy, and P. J. Christie. 2003. Agrobacterium tumefaciens VirB6 protein participates in formation of VirB7 and VirB9 complexes required for type IV secretion. J. Bacteriol. 1852867-2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Judd, P. K., D. Mahli, and A. Das. 2005. Molecular characterization of the Agrobacterium tumefaciens DNA transfer protein VirB6. Microbiology 1513483-3492. [DOI] [PubMed] [Google Scholar]
- 32.Kim, S., M. Watarai, Y. Kondo, J. Erdenebaatar, S. Makino, and T. Shirahata. 2003. Isolation and characterization of mini-Tn5Km2 insertion mutants of Brucella abortus deficient in internalization and intracellular Growth In HeLa cells. Infect. Immun. 713020-3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kovach, M. E., P. H. Elzer, D. S. Hill, G. T. Robertson, M. A. Farris, R. M. Roop II, and K. M. Peterson. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166175-176. [DOI] [PubMed] [Google Scholar]
- 34.Kulakov, Y. K., P. G. Guigue-Talet, M. R. Ramuz, and D. O'Callaghan. 1997. Response of Brucella suis 1330 and B. canis RM6/66 to growth at acid pH and induction of an adaptive acid tolerance response. Res. Microbiol. 148145-151. [DOI] [PubMed] [Google Scholar]
- 35.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227680-685. [DOI] [PubMed] [Google Scholar]
- 36.Laguna, R. K., E. A. Creasey, Z. Li, N. Valtz, and R. R. Isberg. 2006. A Legionella pneumophila-translocated substrate that is required for growth within macrophages and protection from host cell death. Proc. Natl. Acad. Sci. USA 10318745-18750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luo, Z. Q., and R. R. Isberg. 2004. Multiple substrates of the Legionella pneumophila Dot/Icm system identified by interbacterial protein transfer. Proc. Natl. Acad. Sci. USA 101841-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mela, F., K. Fritsche, H. Boersma, J. D. van Elsas, D. Bartels, F. Meyer, W. de Boer, J. A. van Veen, and J. H. J. Leveau. 19 March 2008. Comparative genomics of the pIPO2/pSB102 family of environmental plasmids: sequence, evolution, and ecology or pTer331 isolated from Collimonas fungivorans Ter331. FEMS Microbiol. Ecol. doi: 10.1111/j.1574-6941.2008.00472.x. [DOI] [PubMed]
- 39.Murata, T., A. Delprato, A. Ingmundson, D. K. Toomre, D. G. Lambright, and C. R. Roy. 2006. The Legionella pneumophila effector protein DrrA is a Rab1 guanine nucleotide-exchange factor. Nat. Cell Biol. 8971-977. [DOI] [PubMed] [Google Scholar]
- 40.Nagai, H., J. C. Kagan, X. Zhu, R. A. Kahn, and C. R. Roy. 2002. A bacterial guanine nucleotide exchange factor activates ARF on Legionella phagosomes. Science 295679-682. [DOI] [PubMed] [Google Scholar]
- 41.Nagai, H., and C. R. Roy. 2001. The DotA protein from Legionella pneumophila is secreted by a novel process that requires the Dot/Icm transporter. EMBO J. 205962-5970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Callaghan, D., C. Cazevieille, A. Allardet-Servent, M. L. Boschiroli, G. Bourg, V. Foulongne, P. Frutos, Y. Kulakov, and M. Ramuz. 1999. A homologue of the Agrobacterium tumefaciens VirB and Bordetella pertussis Ptl type IV secretion systems is essential for intracellular survival of Brucella suis. Mol. Microbiol. 331210-1220. [DOI] [PubMed] [Google Scholar]
- 43.Odenbreit, S., J. Puls, B. Sedlmaier, E. Gerland, W. Fischer, and R. Haas. 2000. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science 2871497-1500. [DOI] [PubMed] [Google Scholar]
- 44.Pappas, G., P. Papadimitriou, N. Akritidis, L. Christou, and E. V. Tsianos. 2006. The new global map of human brucellosis. Lancet Infect. Dis. 691-99. [DOI] [PubMed] [Google Scholar]
- 45.Paschos, A., G. Patey, D. Sivanesan, C. Gao, R. Bayliss, G. Waksman, D. O'Callaghan, and C. Baron. 2006. Dimerization and interactions of Brucella suis VirB8 with VirB4 and VirB10 are required for its biological activity. Proc. Natl. Acad. Sci. USA 1037252-7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patey, G., Z. Qi, G. Bourg, C. Baron, and D. O'Callaghan. 2006. Swapping of periplasmic domains between Brucella suis VirB8 and a pSB102 VirB8 homologue allows heterologous complementation. Infect. Immun. 744945-4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ralph, P., and I. Nakoinz. 1975. Phagocytosis and cytolysis by a macrophage tumour and its cloned cell line. Nature 257393-394. [DOI] [PubMed] [Google Scholar]
- 48.Rolan, H. G., and R. M. Tsolis. 2007. Mice lacking components of adaptive immunity show increased Brucella abortus virB mutant colonization. Infect. Immun. 752965-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roux, C. M., H. G. Rolan, R. L. Santos, P. D. Beremand, T. L. Thomas, L. G. Adams, and R. M. Tsolis. 2007. Brucella requires a functional type IV secretion system to elicit innate immune responses in mice. Cell Microbiol. 91851-1869. [DOI] [PubMed] [Google Scholar]
- 50.Schmidt-Eisenlohr, H., N. Domke, C. Angerer, G. Wanner, P. C. Zambryski, and C. Baron. 1999. Vir proteins stabilize VirB5 and mediate its association with the T pilus of Agrobacterium tumefaciens. J. Bacteriol. 1817485-7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schneiker, S., M. Keller, M. Droge, E. Lanka, A. Puhler, and W. Selbitschka. 2001. The genetic organization and evolution of the broad host range mercury resistance plasmid pSB102 isolated from a microbial population residing in the rhizosphere of alfalfa. Nucleic Acids Res. 295169-5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schulein, R., and C. Dehio. 2002. The VirB/VirD4 type IV secretion system of Bartonella is essential for establishing intraerythrocytic infection. Mol. Microbiol. 461053-1067. [DOI] [PubMed] [Google Scholar]
- 53.Schulein, R., P. Guye, T. A. Rhomberg, M. C. Schmid, G. Schroder, A. C. Vergunst, I. Carena, and C. Dehio. 2005. A bipartite signal mediates the transfer of type IV secretion substrates of Bartonella henselae into human cells. Proc. Natl. Acad. Sci. USA 102856-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Segal, G., M. Purcell, and H. A. Shuman. 1998. Host cell killing and bacterial conjugation require overlapping sets of genes within a 22-kb region of the Legionella pneumophila genome. Proc. Natl. Acad. Sci. USA 951669-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shohdy, N., J. A. Efe, S. D. Emr, and H. A. Shuman. 2005. Pathogen effector protein screening in yeast identifies Legionella factors that interfere with membrane trafficking. Proc. Natl. Acad. Sci. USA 1024866-4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sieira, R., D. J. Comerci, D. O. Sanchez, and R. A. Ugalde. 2000. A homologue of an operon required for DNA transfer in Agrobacterium is required in Brucella abortus for virulence and intracellular multiplication. J. Bacteriol. 1824849-4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spudich, G. M., D. Fernandez, X. R. Zhou, and P. J. Christie. 1996. Intermolecular disulfide bonds stabilize VirB7 homodimers and VirB7/VirB9 heterodimers during biogenesis of the Agrobacterium tumefaciens T-complex transport apparatus. Proc. Natl. Acad. Sci. USA 937512-7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Studier, F. W., A. H. Rosenberg, J. J. Dunn, and J. W. Dubendorff. 1990. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 18560-89. [DOI] [PubMed] [Google Scholar]
- 59.Sun, Y. H., H. G. Rolan, A. B. den Hartigh, D. Sondervan, and R. M. Tsolis. 2005. Brucella abortus virB12 is expressed during infection but is not an essential component of the type IV secretion system. Infect. Immun. 736048-6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tauch, A., S. Schneiker, W. Selbitschka, A. Puhler, L. S. van Overbeek, K. Smalla, C. M. Thomas, M. J. Bailey, L. J. Forney, A. Weightman, P. Ceglowski, T. Pembroke, E. Tietze, G. Schroder, E. Lanka, and J. D. van Elsas. 2002. The complete nucleotide sequence and environmental distribution of the cryptic, conjugative, broad-host-range plasmid pIPO2 isolated from bacteria of the wheat rhizosphere. Microbiology 1481637-1653. [DOI] [PubMed] [Google Scholar]
- 61.Vergunst, A. C., B. Schrammeijer, A. den Dulk-Ras, C. M. de Vlaam, T. J. Regensburg-Tuink, and P. J. Hooykaas. 2000. VirB/D4-dependent protein translocation from Agrobacterium into plant cells. Science 290979-982. [DOI] [PubMed] [Google Scholar]
- 62.Ward, J. E., D. E. Akiyoshi, D. Regier, A. Datta, M. P. Gordon, and E. W. Nester. 1988. Characterization of the virB operon from an Agrobacterium tumefaciens Ti plasmid. J. Biol. Chem. 2635804-5814. [PubMed] [Google Scholar]
- 63.Watarai, M., S. Makino, and T. Shirahata. 2002. An essential virulence protein of Brucella abortus, VirB4, requires an intact nucleoside-triphosphate-binding domain. Microbiology 1481439-1446. [DOI] [PubMed] [Google Scholar]
- 64.Weiss, A. A., F. D. Johnson, and D. L. Burns. 1993. Molecular characterization of an operon required for pertussis toxin secretion. Proc. Natl. Acad. Sci. USA 902970-2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Woodcock, D. M., P. J. Crowther, J. Doherty, S. Jefferson, E. DeCruz, M. Noyer-Weidner, S. S. Smith, M. Z. Michael, and M. W. Graham. 1989. Quantitative evaluation of Escherichia coli host strains for tolerance to cytosine methylation in plasmid and phage recombinants. Nucleic Acids Res. 173469-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yuan, Q., A. Carle, C. Gao, D. Sivanesan, K. A. Aly, C. Hoppner, L. Krall, N. Domke, and C. Baron. 2005. Identification of the VirB4-VirB8-VirB5-VirB2 pilus assembly sequence of type IV secretion systems. J. Biol. Chem. 28026349-26359. [DOI] [PubMed] [Google Scholar]
- 67.Zygmunt, M. S., S. D. Hagius, J. V. Walker, and P. H. Elzer. 2006. Identification of Brucella melitensis 16M genes required for bacterial survival in the caprine host. Microbes Infect. 82849-2854. [DOI] [PubMed] [Google Scholar]