Abstract
Conjugative transfer of the Ti plasmids of Agrobacterium tumefaciens is controlled by a quorum-sensing system composed of TraR and its signal N-(3-oxo-octanoyl)-l-homoserine lactone. This system is, in turn, controlled by the conjugative opines produced by crown gall tumors induced on plants by the bacteria. Using nonpolar traI mutants, we examined the kinetics of induction of conjugative transfer in response to exogenous acyl-homoserine lactone. In the absence of the antiactivator TraM, onset of induction of transfer requires about 30 min, 15 to 20 min of which is needed for expression and construction of the conjugative apparatus. TraM delays the onset of conjugation by 30 min. While the rate of development of conjugative competence was not significantly affected by levels of TraR, maximum efficiencies of transfer were correlated with amounts of the activator in the donors. Donors harboring Ti plasmids lacking TraM were fully induced by the quormone at concentrations as low as 100 pM. TraM raised the concentration of signal required for maximum activity to 1 nM. Donors grown in batch culture retained conjugative competence following signal removal, even when in stationary phase. However, donors kept in balanced growth rapidly lost transfer ability following signal removal. Loss of transfer was mirrored by a decrease in levels of active TraR. Decreases in TraR activity and conjugative competence could be accounted for by dilution associated with cell division, suggesting that while induction of Ti plasmid conjugation is an active process, the cells lack a mechanism for disassembling the conjugative apparatus when signals become limiting.
Conjugative transfer of the Ti plasmids of Agrobacterium tumefaciens is a tightly regulated process involving two signaling systems arranged in a hierarchy. Expression of the three Ti plasmid transfer operons, traAFB, traCDG, and traI-trb, requires first a small molecule signal, the conjugative opine, which is produced by the crown gall tumors induced on susceptible plants by this phytopathogen (7, 8). In addition to acting as a signal, the conjugative opine also can be catabolized by the bacteria by using functions coded for by the Ti plasmid. The conjugative opines regulate transfer by controlling the expression of a Ti plasmid operon of which TraR, the direct regulator of the three transfer operons, is a member. For example, transfer of pTiC58 is induced by the sugar phosphodiester opines agrocinopine A and agrocinopine B. In this plasmid, traR is a member of the five-gene arc operon, the expression of which is regulated by the opine-responsive repressor AccR (1). In the absence of the conjugative opines, the expression of arc is repressed. When the opines are present, as when the bacteria are colonizing an agrocinopine-producing crown gall tumor, repression by AccR is relieved, and the arc operon, including traR, is expressed.
TraR, a member of the LuxR family of quorum-sensing transcription factors, activates expression of the transfer operons in response to the second signal, an acyl-homoserine lactone (acyl-HSL) (27, 34). The cognate quormone N-(3-oxo-octanoyl)-l-homoserine lactone (3-oxo-C8-HSL) is produced by the bacteria themselves via the acyl-HSL synthase TraI, also encoded by the Ti plasmid (16, 24). This signal transits out of and back into the cells in a stochastic fashion, and its concentration in a diffusion-limited environment increases with increasing populations of the producing bacteria. At some critical concentration, nascently expressed TraR binds the quormone, folds correctly, and forms active dimers (28, 35, 36). The activator then binds an 18-base-pair (bp) inverted repeat, the tra box, located in the promoter regions of the three tra operons (11, 19, 35), thereby activating transcription of the regulon.
The activity of TraR is modulated by a small antiactivator, TraM, which also is coded for by the Ti plasmid (12, 14). This protein binds to the carboxy-terminal region of TraR and prevents the activator from binding to its DNA target sites (15, 22, 32). TraM is essential for the quorum-sensing phenomenon; otherwise, wild-type Ti plasmids with mutations in traM transfer constitutively (12, 14). This observation suggested that even in the absence of the conjugative opine, traR is expressed at a basal level that is sufficient to activate transfer. Based on this observation, we proposed that one role of TraM is to prevent premature conjugation attendant to this low level of expression of the activator gene (14, 25). In addition, Ti plasmids with mutations in both traM and accR are hyperconjugative, suggesting that the antiactivator also serves to govern the maximum level of expression of the tra regulon under opine-inducing conditions (14).
Although we have a good understanding of TraR and its modulation by TraM at the molecular levels, we know little about the kinetics of activation of the conjugative transfer system by TraR as the acyl-HSL signal becomes nonlimiting. In previous studies, we showed that responsiveness to the quormone is delayed for several hours following opine induction (25). While we postulated that this delay is due to TraM, we could not test the hypothesis, since traM mutants are constitutive for transfer. To explore the kinetics of signal-mediated induction of conjugation, we constructed nonpolar mutations in traI in three transfer-constitutive derivatives of pTiC58. Studies with these mutants indicate that TraR can be rapidly activated by the acyl-HSL but that TraM significantly delays this process. We also determined the time required to express and construct the conjugative apparatus. Finally, the mutants allowed us to assess how the conjugative competence of Ti plasmids responds when the inducing signals disappear.
MATERIALS AND METHODS
Bacterial strains and media.
The strains of A. tumefaciens and the plasmids used in this study are listed in Table 1. Ti plasmids pCMA1 (traM), pTiC58ΔaccR (accR), and pKMA1 (accR traM) are three transfer-constitutive [Tra(Con)] derivatives of pTiC58 (1, 15). Agrobacterium strains were grown at 28°C on nutrient agar (Difco) and in L broth or AB minimal medium (6) containing 0.2% mannitol (ABM medium). When required, antibiotics were added at the following concentrations, in μg/ml: kanamycin, 50; tetracycline, 2; carbenicillin, 50; streptomycin, 200; rifampin, 50; and gentamicin, 50.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or description | Reference or source |
|---|---|---|
| Strains | ||
| A. tumefaciens | ||
| NTL4 | Derivative of C58 cured of its Ti plasmid; ΔtetAR | 21 |
| C58C1RS | Ti plasmid-cured C58; recipient strain for Ti plasmid conjugation; Rmr Smr | 7 |
| E. coli | ||
| DH5α | F− φ80dlacZΔM15 endA1 recA1 hsdR17(rK− mK−) supE44 thi-1 gyrA96 Δ(lacZYA-argF)U169 | 29 |
| S17-1 | Pro− Res− Mod+recA; integrated RP4-Tet::Mu-Kan::Tn7; Mob+ | 31 |
| Plasmids | ||
| pWM91 | f1(+) ori lacZα of pBluescript II (SK+); oriRR6KγsacB plasmid | 23 |
| pWMΔtraI | Deletion allele of traI from pTiC58 cloned in pWM91 | This study |
| pZLR4 | Broad-host-range plasmid containing traR and a traCDG::lacZ fusion reporter | 4 |
| pH4I41 | TraR-dependent traCDG::lacZ fusion reporter | 14 |
| pTiC58 | Nopaline-type Ti plasmid | Our collection |
| pCMA1 | pTiC58ΔtraM::nptII; transfer constitutive | 14 |
| pTiC58ΔaccR | accR deletion derivative of pTiC58; transfer constitutive | 1 |
| pKMA1 | pTiC58accR; traM::nptII; transfer constitutive | 14 |
| pCMA1ΔtraI | Nonpolar traI deletion mutant of pCMA1 | This study |
| pTiC58ΔaccRΔtraI | Nonpolar traI deletion mutant of pTiC58ΔaccR | This study |
| pKMA1ΔtraI | Nonpolar traI deletion mutant of pKMA1 | This study |
| pTiC58ΔaccRΔtraIKm | pTiC58ΔaccRΔtraI into which an nptII cassette was marker exchanged; transfer constitutive | This study |
Reagents.
Antibiotics, 3-oxo-C8-HSL, 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal), ο-nitrophenyl β-galactoside, and nopaline were purchased from Sigma.
Genetic manipulations.
Plasmid DNA was introduced into Escherichia coli by CaCl2-mediated transformation (29) and into A. tumefaciens strains by electroporation (3) or by biparental mating using E. coli strain S17-1 (9).
Construction of nonpolar traI mutants.
A 545-bp EcoRI-BamHI fragment containing 323 bp of 5′ upstream noncoding sequence, along with the first 332 bp of the traI coding region and a 1,499-bp XbaI-EcoRI fragment containing 219 bp from the 3′ end of traI and the entire trbB gene, were amplified using Pfu DNA polymerase (Stratagene), ligated together, and cloned between the BamHI and SpeI sites of the suicide plasmid pWM91 (23). This vector also contains a sacB gene, which can be used for selecting for loss of the plasmid. The resulting plasmid, pWMΔtraI, carrying an in-frame deletion of a 204-bp internal region of traI, was introduced into strains NTL4(pCMA1), NTL4(pTiC58ΔaccR), and NTL4(pKMA1), respectively, by conjugation with selection for resistance to carbenicillin. Recombinants in which wild-type traI had been replaced by the ΔtraI allele were identified by screening carbenicillin-resistant colonies for resistance to sucrose. The sucrose-resistant derivatives were screened for the inability to produce 3-oxo-C8-HSL (30).
Construction of a kanamycin-marked derivative of pTiC58ΔaccRΔtraI.
An nptII cassette was introduced into pTiC58ΔaccRΔtraI between atu6160 and atu6161 as follows. A 1,465-bp PCR amplicon containing the two open reading frames was cloned into pBluescript II KS(+), and an nptII cassette from pMKm was ligated into the unique HincII site between atu6160 and atu6161. The clone, which cannot replicate in Agrobacterium, was electroporated into NTL4(pTiC58ΔaccRΔtraI) with selection for resistance to kanamycin. Isolates resistant to kanamycin but sensitive to carbenicillin conferred by the vector were screened by PCR for double crossovers in which the cassette was marker exchanged into pTiC58ΔaccRΔtraI. One such strain, NTL4(pTiC58ΔaccRΔtraIKm), was retained for this study.
Culture manipulations.
For batch growth, overnight cultures of traI mutants were diluted 1:100 into 50 ml of ABM medium containing appropriate antibiotics and incubated at 28°C with shaking. For induction experiments, 3-oxo-C8-HSL was added to give a final concentration of 25 nM. For experiments involving removal of the signal, when the optical densities at 600 nm (OD600) of the cultures reached 0.25, cells were harvested by centrifugation and washed up to six times with fresh ABM medium by centrifugation. After being washed, the cells were resuspended in 50 ml of fresh ABM medium containing appropriate antibiotics and the cultures were further incubated as described in Results. For balanced growth, cultures were grown with 25 nM 3-oxo-C8-HSL to an OD600 of 0.25, and the cells were harvested by centrifugation and washed extensively with fresh ABM medium. The cells were then suspended in the same volume of fresh ABM medium with appropriate antibiotics, and incubation was continued. When the culture reached an OD600 of 0.5, corresponding to one generation of cell growth, samples were collected and assayed for conjugation, acyl-HSL retention, and β-galactosidase levels. The master cultures were diluted back to OD600 of 0.25 with fresh ABM medium lacking the quormone, and incubation was continued. The cycle of growth to an OD600 of 0.5, sampling, and dilution to an OD600 of 0.25 was continued for a total of 12 generations.
Conjugation assays.
Frequencies of conjugative transfer were measured by the spot mating method and expressed as numbers of transconjugants recovered per input donor, as described previously (2). Briefly, the recipient strain C58C1RS was spread as a confluent lawn over the surfaces of the selection plates, containing rifampin, streptomycin, and either kanamycin or 1 mM nopaline as the sole carbon source. Five-microliter volumes of serially diluted donor cells were spotted in triplicate onto the surface of the recipient lawn, and the cultures were incubated at 28°C for 72 h. Transconjugants appearing within the donor inoculum spots were enumerated with the aid of a dissecting microscope. Titers of donor cells were determined by dilution plating in triplicate with ABM plates at the time of mating.
β-Galactosidase assay.
β-Galactosidase activity, expressed as units per 109 viable cells, was quantified as described previously (19).
Detection of intracellular acyl-HSLs.
Cells were harvested by centrifugation from 1.5 ml of culture and washed up to six times with 0.9% NaCl. Cells collected after the final wash were extracted two times with 1.5-ml volumes of ethyl acetate, the organic fractions were pooled and evaporated to dryness, and residues were dissolved in 20 μl of ethyl acetate. A series of twofold dilutions of the extracts and similar dilutions of authentic 3-oxo-C8-HSL at known concentrations in ethyl acetate were prepared, and 2-μl volumes of each diluted sample were spotted onto an analytical C18 reversed-phase thin-layer chromatography plate. The samples were allowed to dry, and the plate was overlaid with 150 ml of ABM medium with 1.5% agar, 40 μg/ml of X-Gal, and 50 ml of early-stationary-phase culture of the acyl-HSL bioreporter strain NTL4(pZLR4) (30). The assay plates were incubated overnight at 28°C and examined for blue spots. The amount of intracellular quormone was quantified by comparison to the authentic 3-oxo-C8-HSL standards spotted on the same plate (10).
Detection of TraR by Far Western analysis.
Cultures of A. tumefaciens were grown in the absence or presence of 25 nM 3-oxo-C8-HSL in ABM at 28°C for 12 h. Cells were collected by centrifugation, washed once with modified Agrowash (20 mM Tris-HCl, pH 8.0, 0.5 M NaCl, 1% sodium Sarkosyl), and lysed as described previously (20). Lysates were centrifuged at 100,000 × g for 30 min, proteins in the soluble fractions were resolved by electrophoresis in 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels, and the separated proteins were transferred to a nitrocellulose membrane. The membranes were incubated with purified TraM, washed, and reincubated with 1,000-fold-diluted polyclonal murine antiserum directed against TraM from pTiC58 (22). The TraR-TraM complexes were visualized by chemiluminescence with horseradish peroxidase-conjugated monkey anti-mouse antiserum, using an enhanced chemiluminescence kit (Amersham Biosciences), and the developed X-ray films were archived by digital scanning.
RESULTS
Construction and characterization of Ti plasmids with a nonpolar mutation in traI.
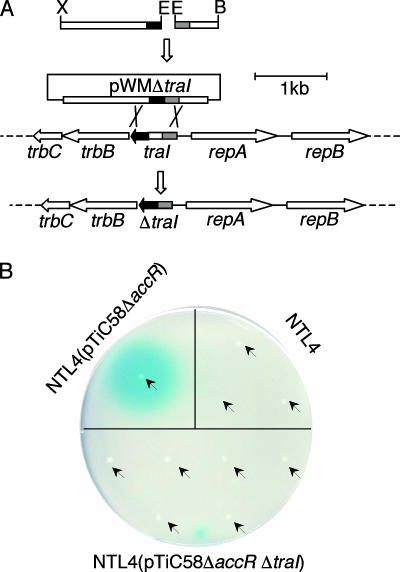
TraR-mediated transcriptional activation is dependent on the activator binding its acyl-HSL quormone, 3-oxo-C8-HSL. The signal, in turn, is synthesized by TraI, coded for by the first gene of the Ti plasmid trb operon, which codes for the essential type IV conjugative secretion system. Insertion mutations in traI are strongly polar on the downstream trb operon; such mutants fail to conjugate at detectable frequencies, even when cultures are supplied with exogenous quormone (18). We constructed a nonpolar mutation in the traI gene of three transfer-constitutive derivatives of pTiC58, pTiC58ΔaccR, pCMA1 (traM), and pKMA1 (accR traM) (Table 1 and Fig. 1A), as described in Materials and Methods.
FIG. 1.
Construction and characterization of A. tumefaciens traI mutants. (A) Strategy for construction of ΔtraI mutants. An EcoRI (E)-BamHI (B) fragment containing the 5′ end of traI (dashed region) and upstream sequences and an XbaI (X)-EcoRI (E) fragment containing the 3′ end of traI (filled region) and downstream sequences were ligated together and cloned into the suicide vector pWM91 (Table 1). The resultant plasmid, pWMΔtraI, was used to introduce the mutant allele into the respective Ti plasmids via double crossover recombination as described in Materials and Methods. The organizations of the wild-type and the truncated traI regions from the Ti plasmids are shown, with arrows indicating the directions of transcription. (B) The traI deletion mutants do not produce Agrobacterium autoinducer. Colonies of NTL4, NTL4(pTiC58ΔaccR), and NTL4(pTiC58ΔaccRΔtraI) were patched onto an indicator plate seeded with the acyl-HSL bioreporter strain NTL4(pZLR4) (Table 1). The plate was incubated at 28°C for 15 h, and the results were recorded. Arrows indicate the locations at which colonies were patched on the plate.
Strains harboring the parent Ti plasmid of each mutant produced large amounts of quormone, yielding easily detectable blue zones on plates seeded with the acyl-HSL bioreporter strain (Fig. 1B and data not shown). In contrast, a subset of sucrose-resistant isolates of each mutant tested failed to produce blue zones on the indicator plate (Fig. 1B and data not shown). Representative quormone-negative isolates of each Ti plasmid type were selected and assessed for the expected deletion in traI by PCR analysis. All such tested isolates yielded a 432-bp PCR fragment corresponding to the deleted form of traI (data not shown). One such traI deletion derivative of each Ti plasmid was retained for the study (Table 1).
The three parent Ti plasmids were transferred by conjugation to A. tumefaciens C58C1RS at high frequencies (Table 2). When tested as donors, strains harboring all three traI deletion mutants failed to transfer their Ti plasmids at detectable frequencies (Table 2). However, transfer of each traI mutant was restored to near-wild-type levels when cultures of the three donors were incubated with 25 nM 3-oxo-C8-HSL for 10 to 12 h prior to the matings (Table 2). Clearly, traI is the only functional acyl-HSL synthetase in A. tumefaciens C58 and the requirement for the acyl-HSL to induce conjugation can be satisfied by incubating the cells with exogenous quormone.
TABLE 2.
traI deletion mutants of pTiC58 require exogenous quormone for conjugative transfer
| Ti plasmid | traI allele | Transfer frequencya
|
|
|---|---|---|---|
| With quormone | Without quormoneb | ||
| pTiC58accR | Wild type | (3.75 ± 1.75) × 10−3 | NA |
| ΔtraI | <10−8 | (2.05 ± 0.25) × 10−3 | |
| pCMA1 | Wild type | (5.25 ± 2.15) × 10−4 | NA |
| ΔtraI | <10−8 | (3.05 ± 1.25) × 10−4 | |
| pKMA1 | Wild type | (2.65 ± 0.75) × 10−2 | NA |
| ΔtraI | <10−8 | (2.00 ± 0.7) × 10−2 | |
Expressed as numbers of transconjugants recovered per input donor in matings with strain C58C1RS as a recipient. Values are the averages for two independent experiments.
N-(3-Oxo-octanoyl)-l-homoserine lactone was added at a final concentration of 25 nM as indicated. NA, not applicable (strains with the wild-type allele of traI synthesize their own quormone).
Kinetics of initiation of Ti plasmid conjugative transfer.
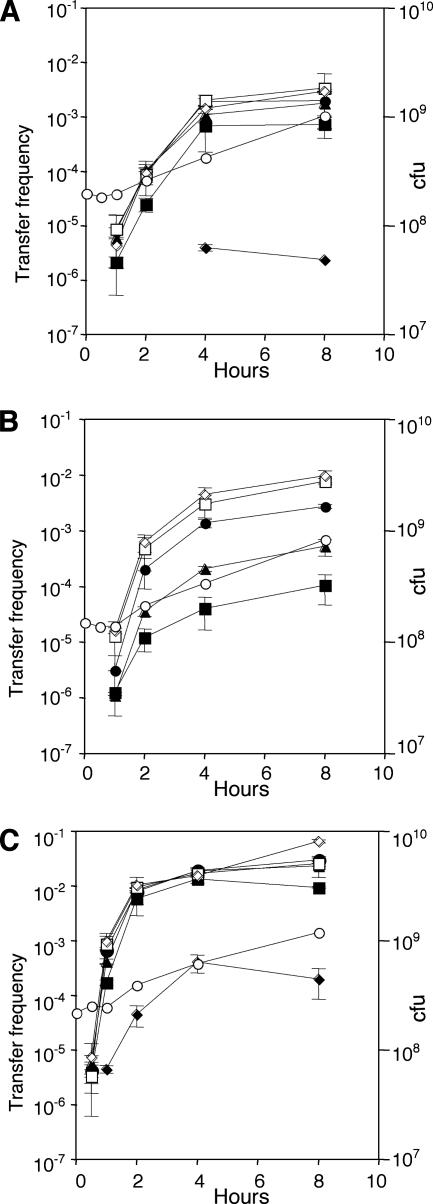
We used this dependency of the traI mutants on an exogenous source of the quorum-sensing signal to determine the rate at which the Ti plasmid initiates conjugative transfer when cultures transition from signal-deprived to signal-nonlimiting conditions. Simultaneously, we determined the relationship between induction of conjugation and quormone concentration. Donor cultures harboring the three mutant Ti plasmids were grown to exponential phase, and 3-oxo-C8-HSL was added to subcultures of each at concentrations ranging from 10 pM to 25 nM. Growth was continued, samples were taken at intervals, and the donors were tested for conjugative competence. Transfer of pKMA1, which produces TraR constitutively and lacks TraM (15), was maximally induced by signal at a concentration as low as 100 pM, although conjugative competence was detectable in donors exposed to as little as 10 pM signal (Fig. 2C). At saturating levels of quormone, transfer of pKMA1 was first detectable 30 min after addition of signal, rose rapidly, and peaked by 4 hours (Fig. 2C). Donors harboring pCMA1, which expresses repressed levels of TraR but lacks TraM (15), responded maximally to concentrations of signal as low as 250 pM but showed a somewhat reduced level of response to signal at 100 pM (Fig. 2A). While transfer frequencies were very low, donors harboring pCMA1 did respond to signal at a concentration of 10 pM. In comparison to donors harboring pKMA1, donors harboring pCMA1 did not initiate transfer until about 1 hour after signal addition, conjugative competence developed more slowly, and transfer frequencies peaked at levels about 50-fold lower than that of pKMA1 (Fig. 2A). Donors harboring pTiC58ΔaccR, in which expression of TraR is constitutive and TraM is present and active, required signal at a concentration of at least 1 nM for maximal transfer frequencies (Fig. 2B). Lower concentrations of signal resulted in an ordered decrease in maximal conjugation rates. Moreover, while donors harboring pTiC58ΔaccR responded to signal at 100 pM, no transconjugants were detected in the culture grown with quormone at 10 pM (Fig. 2B). Rates of increase in development of conjugative competence were similar to those for donors harboring pCMA1.
FIG. 2.
Induction of Ti plasmid conjugative transfer and its sensitivity to the acyl-HSL quorum-sensing signal. Donors harboring pCMA1ΔtraI (traM) (A), pTiC58ΔaccRΔtraIKm (accR) (B), or pKMA1ΔtraI (accR traM) (C) were grown to exponential phase and split into five subcultures, and acyl-HSL was added at concentrations of 10 pM (♦), 100 pM (▪), 250 pM (▴), 500 pM (•), 1 nM (□), and 25 nM (⋄). Growth was continued, samples were removed at the indicated times, and the donors were tested for conjugative proficiency. Growth of a representative culture (○) is expressed as numbers of CFU per ml. Transfer frequencies are expressed as numbers of transconjugants obtained per input donor.
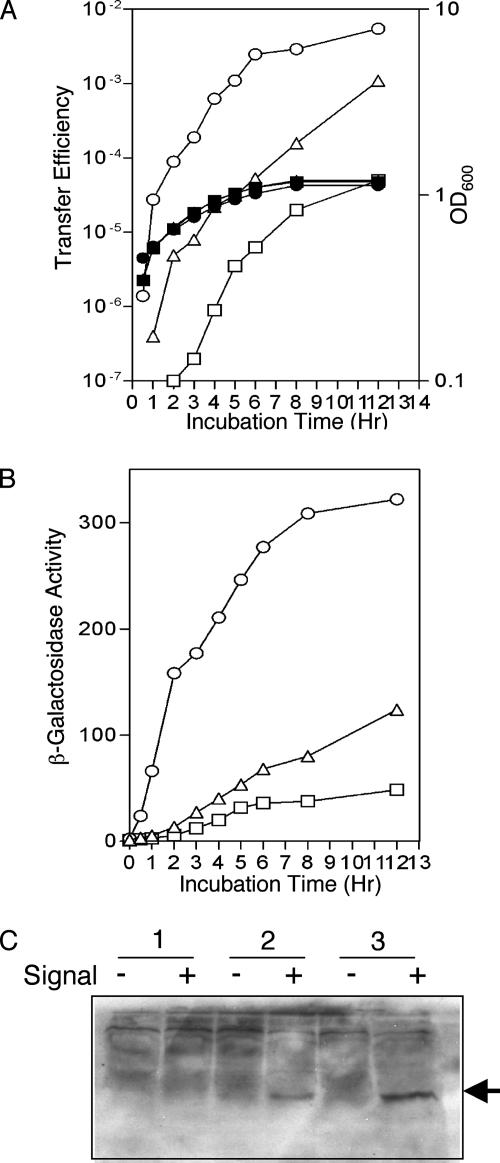
We then examined the kinetics of induction and maintenance of conjugative competence of donors harboring each of the three Ti plasmids over the culture cycle. The donors were grown with quormone at a concentration of 25 nM to ensure saturating levels of signal over the course of the experiment. Each donor also harbored pH4I41, a recombinant clone containing a TraR-dependent traCDG::lacZ reporter fusion (Table 1), to monitor levels of TraR activity. Each culture was sampled at intervals after signal addition, and the cells were tested for conjugative transfer efficiencies and for levels of β-galactosidase activity.
Conjugative competence in the three donors developed in a manner similar to that described above, with donors harboring pKMA1 (accR traM) rapidly responding to signal while donors harboring pTiC58ΔaccR and pCMA1 (traM) showed delays in induction of 30 to 60 min (Fig. 3A). Remarkably, all three donors retained high levels of conjugative competence at 8 to 12 h after signal addition, when these cultures were well into stationary phase.
FIG. 3.
Kinetics of initiation of Ti plasmid conjugative transfer. Donors harboring pCMA1ΔtraI (traM) (□, ▪), pTiC58ΔaccRΔtraI (▵, ▴), or pKMA1ΔtraI (accR traM) (○, •) were grown to early exponential phase; at zero time, 3-oxo-C8-HSL was added to give a concentration of 25 nM, and growth was continued. Samples were collected at intervals and assayed for conjugative transfer (A) and TraR activity as assessed by activation of the traCDG::lacZ reporter on pH4I41 (B). Open symbols show conjugative transfer efficiency, expressed as numbers of transconjugants obtained per input donor, or β-galactosidase activity, expressed as numbers of units per 109 cells. Closed symbols show growth of the cultures as measured by OD600. Samples of cells harboring pCMA1ΔtraI (1), pTiC58ΔaccRΔtraI (2), or pKMA1ΔtraI (3) grown with (+) or without (−) 25 nM 3-oxo-C8-HSL for 12 h were assayed for TraR levels by immunoblot analysis (C). Proteins in the soluble fractions were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and TraR was detected by immunoblotting as described in Materials and Methods. The arrow indicates the position of the TraR protein. Matings shown in panels A and B were repeated once, with similar patterns of results, and data from one experiment are shown.
TraR activity, as assessed by activation of the traCDG::lacZ reporter on pH4I41, was detectable in NTL4(pKMA1) as early as 30 min after addition of the quormone, and levels of the reporter increased rapidly, peaking at about 8 hours after addition of the signal (Fig. 3B). Donors harboring pTiC58ΔaccR or pCMA1 exhibited detectable activity only after 2 hours of exposure to the signal. While levels of β-galactosidase activity increased in these two donor strains, the rates of increase were markedly lower than that observed in the donor harboring pKMA1. Moreover, in neither strain did β-galactosidase activity increase to the levels observed in the donor harboring pKMA1 (Fig. 3B). In all three donors, β-galactosidase activity remained at peak levels even after the cells transitioned into stationary phase.
We also assessed the levels of TraR protein in the soluble fraction from each donor type by Far Western analysis as described in Materials and Methods. TraR was clearly detectable in lysates of NTL4(pKMA1ΔtraI) and barely detectable in lysates of NTL4(pTiC58ΔaccRΔtraI) (Fig. 3C). However, we could not detect the activator in lysates of NTL4(pCMA1ΔtraI) under the conditions tested (Fig. 3C). Moreover, TraR was detectable only in lysates from cells grown with the quorum-sensing signal (Fig. 3C). The antibody did not visualize a protein of the correct size in lysates of strain NTL4 lacking a Ti plasmid (data not shown).
Signal removal does not strongly affect conjugative competence of induced donors in batch culture.
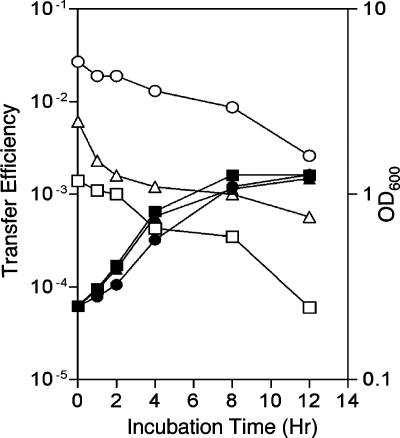
While the quorum-sensing signal is required to induce expression of the Ti plasmid conjugative transfer system, it is not clear what happens to the activated system when the signal is no longer available. We used our traI mutants to assess this condition. Donors harboring the three traI deletion derivatives were grown to exponential phase in medium supplemented with 3-oxo-C8-HSL, harvested, and washed extensively to remove all unbound quormone. The washed cells were resuspended to a population density of about 2.5 × 107 CFU per ml in fresh medium lacking the signal, and the cultures were incubated as before. Samples were taken at timed intervals, and the donors were tested for conjugative efficiency. Donors harboring pKMA1ΔtraI (accR traM) continued to transfer the Ti plasmid at high frequency, with rates of transfer dropping only about twofold over the first 8 hours and 1 order of magnitude over the 12-hour course of the experiment (Fig. 4). The transfer efficiencies of donors harboring pTiC58ΔaccRΔtraI dropped about sevenfold over the first 2 hours and then remained relatively stable over the remaining 10 h of the experiment (Fig. 4). Donors harboring pCMA1ΔtraI (traM) showed an approximately 3-fold drop in transfer frequencies over the first 8 hours and a 10-fold drop over the final 4 hours of the experiment (Fig. 4). However, in all three cases, overall transfer frequencies remained high, not dropping below levels of 10−5 to 10−3 transconjugants per input donor even after 12 h of culturing without signal.
FIG. 4.
Signal removal does not strongly affect conjugative competence of induced donors. Donors harboring pCMA1ΔtraI (traM) (□, ▪), pTiC58ΔaccRΔtraI (accR) (▵), or pKMA1ΔtraI (accR traM) (○, •) were grown in ABM with 25 nM 3-oxo-C8-HSL to exponential phase. Cells were harvested by centrifugation and washed six times. After the final wash, the cells were resuspended in an equal volume of fresh medium without signal, and incubation was continued. Samples were taken at intervals and assayed for conjugative competence. Open symbols represent transfer efficiency, while closed symbols indicate cell growth, both determined as described in the legend to Fig. 2. The experiment was repeated twice, with similar patterns of results, and data from a representative experiment are shown.
Donors in balanced growth lose conjugative competence following signal removal.
We considered it possible that the slow decline in conjugative transfer efficiency following signal removal resulted from the entry of the donors into stationary phase, with attendant alterations in cell growth and division. To determine whether transfer frequencies would decay during extended growth in the absence of signal, we cultured donors for up to 12 generations under balanced growth conditions as described in Materials and Methods. In these experiments, donors harboring pKMA1ΔtraI (accR traM), pTiC58ΔaccRΔtraI, or pCMA1ΔtraI (traM), each also with the traCDG::lacZ reporter pH4I41, were grown in ABM minimal medium containing 25 nM 3-oxo-C8-HSL to a population density corresponding to an OD600 of 0.25. The cells were harvested, washed extensively, and resuspended to an OD600 of 0.25 in the same medium lacking the acyl-HSL signal, and incubation was continued. Growth was followed turbidometrically, and when the OD600 of each culture reached 0.5, a sample was removed and the culture was readjusted to an OD600 of 0.25 by dilution with fresh, unsupplemented medium. The process was continued for an additional 11 generations. Samples taken at each generation were assessed for viable cell number, conjugative efficiency, levels of β-galactosidase activity, and the amounts of the acyl-HSL retained by the cells.
Donors harboring pKMA1ΔtraI retained a very high level of transfer for the first two generations. Conjugative efficiency then declined steadily, dropping 3 orders of magnitude over the next 8 generations and to levels below the limits of detection by the 12th generation (Fig. 5A). Transfer of pTiC58ΔaccRΔtraI, although beginning at a lower efficiency, showed similar kinetics, holding constant for 3 generations, followed by a decay of some 2 orders of magnitude before dropping below the level of detection by the 10th generation (Fig. 5A). Transfer frequencies from donors harboring pCMA1ΔtraI began dropping immediately after removal of the signal and decreased steadily before dropping below detectable levels by the seventh generation (Fig. 5A).
FIG. 5.
Donors in balanced growth lose conjugative competence following signal removal. Donors harboring pCMA1ΔtraI (traM) (□), pTiC58ΔaccRΔtraI (accR) (▵), or pKMA1ΔtraI (accR traM) (○), each also with the traCDG::lacZ reporter pH4I41, were grown in ABM containing 25 nM 3-oxo-C8-HSL to a population density corresponding to an OD600 of 0.25. The cells were harvested, washed extensively, and resuspended to an OD600 of 0.25 in the same medium lacking the acyl-HSL signal, and incubation was continued. Growth was followed turbidometrically, and when the OD600 of the culture reached 0.5, a sample was removed and the culture was readjusted to an OD600 of 0.25 by addition of fresh, unsupplemented medium. The process was continued for a total of 12 generations. Samples taken at each generation were assessed for conjugative efficiency (A) and levels of β-galactosidase activity (B). The matings were repeated once, with similar patterns of results, and data from one experiment are shown.
Unlike transfer frequencies, levels of TraR activity, as measured by β-galactosidase activity from the traCDG::lacZ reporter, declined rapidly and steadily from the first generation in all three donors (Fig. 5B). However, initial levels of β-galactosidase activity were substantially higher in donors harboring pKMA1ΔtraI and, following the removal of signal, did not decay to levels below detection until the 11th generation (Fig. 5B). Donors harboring pTiC58ΔaccRΔtraI and pCMA1ΔtraI showed similar declines, dropping below the level of detection by the sixth generation (Fig. 5B).
We also assessed the amount of quormone retained by the cells as a measure of the amount of intracellular dimer TraR (5, 20). Overall, intracellular quormone levels decreased in parallel to the decay of β-galactosidase activity in the three donors (Fig. 6A and B). However, donors harboring pKMA1ΔtraI initially accumulate levels of signal 2- and 10-fold higher than the levels of signal detected in donors harboring pTiC58ΔaccRΔtraI and pCMA1ΔtraI, respectively (Fig. 6B).
FIG. 6.
TraR levels, as measured by amounts of intracellular quormone retained by the cells in balanced growth, decrease following signal removal. Donors harboring pCMA1ΔtraI (traM) (III), pTiC58ΔaccRΔtraI (accR) (II), or pKMA1ΔtraI (accR traM) (I) were cultured for up to 12 generations under balanced growth conditions as described in the legend to Fig. 5. Samples taken at each generation were assessed for retention by the cells of the acyl-HSL signal as described in Materials and Methods. (A) Quantitative assay for retained signal. The extracted acyl-HSLs and a solution of authentic 3-oxo-C8-HSL (Std) at a known concentration were diluted serially twofold in ethyl acetate, and 2-μl volumes of the dilutions were spotted onto C18 thin-layer chromatography plates. The plates were overlaid with the bioreporter strain NTL4(pZLR4) and incubated overnight at 28°C. (B) The amount of signal detected from each strain was quantified by comparison of dot intensity from the biological samples, with a standard curve relating spot intensity to amount of authentic 3-oxo-C8-HSL derived from the dilution series of the standard.
DISCUSSION
Induction of Ti plasmid conjugative transfer is initiated by opines produced by crown gall tumors but ultimately is controlled by TraR through a quorum-sensing system. The level of control by the latter requires that the quorum-sensing signal, an acyl-HSL, accumulate to a concentration high enough to activate the transcriptional factor. Once activated, TraR must transcribe the three tra operons, the trb type IV mating bridge must be constructed, and the Ti plasmid must be processed for transfer.
Our results show that, in the absence of any additional factors, activation of TraR and subsequent development of the conjugative apparatus occur rapidly; transfer of pKMA1ΔtraI, which constitutively expresses TraR and lacks TraM, is detectable about 30 min after addition of the quormone (Fig. 2C and 3A). Since, in the absence of other factors, full activation of TraR by the acyl-HSL signal takes between 10 and 15 min (20), expression and construction of the conjugative transfer apparatus itself require an additional 15 to 20 min.
However, other factors modulate the timing and kinetics of the induction of conjugative transfer (25). In this regard, clearly TraM serves to delay development of conjugative competence; transfer of pTiC58ΔaccRΔtraIKm, which differs from pKMA1ΔtraI only by expressing the antiactivator, does not initiate transfer until 60 min after exposure to the quormone (Fig. 2B and 3A). We suggest that this delay in activation of the tra regulon represents the time required for TraR to accumulate to levels that titrate out the available TraM. This conclusion is consistent with our observation that active TraR, as measured by levels of β-galactosidase expressed from a TraR-dependent promoter::lacZ reporter fusion, was detectable in donors harboring pKMA1ΔtraI within 30 min after addition of the quormone but not until after 2 hours in donors harboring the otherwise isogenic traM+ plasmid pTiC58ΔaccRΔtraI (Fig. 3B).
Our studies suggest that TraM plays a more crucial role in the control of induction of conjugative transfer than was previously appreciated. Donors harboring pKMA1ΔtraI (accR traM), in which traR is expressed constitutively, are fully inducible by 3-oxo-C8-HSL at a concentration as low as 100 pM, while the near-isogenic traM+ Ti plasmid pTiC58ΔaccRΔtraIKm requires the signal at a concentration fully 10-fold higher for maximum induction (compare Fig. 2B and C). Moreover, while donors harboring pKMA1ΔtraI are fully induced by signal at concentrations of 250 and 500 pM (Fig. 2C), these concentrations of quormone induced intermediate levels of transfer of pTiC58ΔaccRΔtraIKm (Fig. 2B). It is important to recognize that these intermediate levels of transfer most probably do not represent a homogeneous population in which each cell is expressing the phenotype at a lower level. Rather, it is likely that under these conditions the donor population is heterogeneous, with some donors being fully induced for transfer, while others remain conjugatively inactive. This observation illustrates the stochastic nature of signal perception attendant to quorum-sensing systems, especially at limiting signal concentrations. Finally, these results strongly suggest that TraM in its interaction with TraR establishes the minimum concentration of the acyl-HSL required to fully induce the system. That donors harboring pCMA1ΔtraI (accR+ traM), which expresses only basal levels of TraR (25), are essentially as sensitive to quormone as those harboring pKMA1ΔtraI (accR traM), which constitutively expresses traR (compare Fig. 2A and C), suggests that sensitivity to signal is not a function of the amount of TraR produced but rather a function of the amount of available TraR, that is, the amount of activator not complexed with TraM. This conclusion is consistent with our hypothesis on the role of TraM in controlling the quorum-sensing system.
Donors harboring pKMA1ΔtraI are exquisitely sensitive to the acyl-HSL signal, with the maximum number of donors being fully induced at a concentration of 100 pM and a few members of the population being induced at a concentration as low as 10 pM (Fig. 2C). If we assume that the signal is fully diffusible between the intracellular and extracellular compartments of the culture and that the individual cell volume is 1.5 pl, a concentration of 10 pM represents about one molecule of signal per 100 cells and, at 100 pM, about one molecule per cell. Since at least two molecules of signal are required to activate a dimer of TraR, donors harboring pKMA1 are about as sensitive to the signal as theoretically possible. This conclusion is consistent with our conclusion that low transfer frequencies at limiting signal concentrations reflect a heterogeneous state within the donor population.
TraM also influences the timing of initial quormone-dependent activation of TraR. Donors harboring pCMA1, in which traR remains repressed but TraM is absent, are constitutive for transfer, demonstrating that in the absence of the antiactivator, basal-level expression of traR is sufficient to activate transcription of the tra regulon at all population sizes tested (25). However, the ΔtraI mutation allowed us to examine the efficiency by which low levels of TraR can activate the tra regulon. Induction of transfer was not detectable until 1 to 2 hours after exposure to signal (Fig. 2A and 3A). Moreover, the initial rate of increase in the transfer frequency, as well as the maximum observed rates of transfer of pCMA1ΔtraI, is substantially lower than that observed for either of the other two Ti plasmids. However, donors harboring pCMA1ΔtraI responded to levels of the acyl-HSL fully 10-fold lower than the minimum level required for induction of pTiC58ΔaccRΔtraIKm, which constitutively expresses traR but also produces TraM. These results again emphasize the importance of TraM in modulating TraR activity but also suggest that TraR is limiting in donors harboring pCMA1. This conclusion is consistent with levels of TraR detectable in cell lysates of the three strains by Far Western analysis (Fig. 3C). Taken as a whole, these results indicate that transfer of the Ti plasmid at maximum frequencies requires full, derepressed expression of TraR, a condition that occurs only on or near crown gall tumors where concentrations of the conjugative opine are expected to be high.
Although the inducibility of Ti plasmid transfer was first demonstrated 30 years ago (13, 17), virtually nothing is known about how conjugative competence, once activated, responds to the loss of inducing signals. In batch culture, removing the quormone had only a modest effect on donors harboring any of the three mutant Ti plasmids (Fig. 4). In matings with all three donors, transfer frequencies remained virtually unchanged for the first 2 to 4 hours and then declined by no more than 10- to 15-fold over the 12-hour course of the experiment. Each of the cultures had entered stationary phase between 6 and 8 hours, representing about three generations, following removal of the signal. Given the growth kinetics, the slow decay in conjugative transfer of these three plasmids over the 12 h of the experiment suggests that stationary-phase cells do not actively degrade the conjugative machinery. Moreover, the cells remain energetically competent to carry out conjugative transfer, even following entry into stationary phase. This conclusion differs from that of Tempé et al. (33), who reported that donors in stationary phase transferred their plasmids at frequencies more than 5 orders of magnitude lower than exponential-phase cells. However, there was no description of how long the cells had been in stationary phase. Moreover, since conjugation was induced by growth with the conjugative opine, it is possible that this signal became limiting, leading to repression of traR and subsequent loss of transfer functions even before the cells had entered stationary phase.
Given the inherent stability of activated TraR (20, 35, 36), retention of conjugative proficiency following signal removal from batch cultures may be due to continued transcription of the tra regulon. However, our results from matings involving donors held in balanced growth argue against this interpretation of the data. When the donor cells were allowed to continue to divide after signal removal, conjugative competence decreased over a range of 3 to 4 orders of magnitude, depending on the mutant plasmid, to frequencies below the level of detection (Fig. 5A). Moreover, the levels of active TraR, as determined by β-galactosidase activity expressed from a TraR-dependent lacZ reporter fusion, also declined in dividing populations of cells (Fig. 5B). Although an indirect measure of TraR levels, results from the β-galactosidase assays are consistent with a decrease in amounts of the acyl-HSL signal retained by the cells, a more direct measure of the amount of active TraR in the cells (Fig. 6B) (5, 20). These observations suggest that, following removal of the quormone, new activated TraR is not produced in significant amounts.
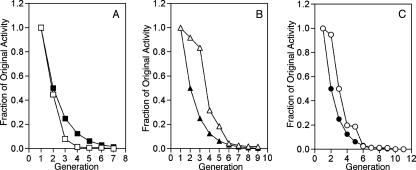
The stability of plasmid transfer following removal of the quorum-sensing signal in donors in batch culture contrasts sharply with the rapid loss of conjugative competence in donor populations maintained in balanced growth. However, an analysis of the decay of conjugative transfer in donors harboring any of the three mutant Ti plasmids indicates that transfer frequencies decline solely as a function of cell division. The loss of conjugative competence of pCMA1ΔtraI (traM) superimposes on a curve relating the theoretical loss of activity as a function of dilution associated with cell division (Fig. 7A). The rates of loss of transfer from donors harboring pTiC58ΔaccRΔtraI and pKMA1ΔtraI (accR traM) are somewhat more complex, deviating from simple dilution in the first few generations (Fig. 7B and C). However, from then on, donors harboring both pTiC58ΔaccRΔtraI and pKMA1ΔtraI lost conjugative competence as a function of cell division. TraR is constitutively expressed from both of these Ti plasmids, and they transfer at frequencies 10- to 100-fold higher than pCMA1ΔtraI, in which TraR is expressed at its repressed level (Fig. 2A and 3A) (14). We suggest that donors harboring pTiC58ΔaccRΔtraI and pKMA1ΔtraI express significantly larger numbers of mating bridges than donors harboring pCMA1ΔtraI. This excess would account for the slower-than-anticipated decay in conjugative competence at early time points. However, once the number of bridges per cell becomes limiting, decay of transfer occurs at a rate compatible with that of simple dilution associated with cell division.
FIG. 7.
Decay of conjugative transfer efficiency compared with theoretical decay by twofold dilution due to cell division. Conjugative transfer frequencies for donors harboring pCMA1ΔtraI (traM) (A), pTiC58ΔaccRΔtraI (accR) (B), or pKMA1ΔtraI (accR traM) (C) from the experiment described for Fig. 5A were recalculated and plotted as fractions of original activity versus generation (open symbols). Closed symbols represent a theoretical decay curve in which the amount of activity decreases by half at each generation.
An analysis of the decline in TraR activity following removal of the quormone in cells in balanced growth yielded similar results. In donors harboring all three Ti plasmids, the level of active TraR, as measured by retention of the acyl-HSL, decreased strictly as a function of dilution associated with cell division (Fig. 8). The rapid decrease in β-galactosidase activity expressed from the TraR-dependent traCDG promoter (Fig. 6B) is entirely consistent with our conclusion that active TraR decreases in growing populations of cells following loss of the quorum-sensing signal.
FIG. 8.
Decay of active TraR compared with theoretical decay by twofold dilution due to cell division. Levels of acyl-HSL retained by donors harboring pCMA1ΔtraI (traM) (A), pTiC58ΔaccRΔtraI (B), or pKMA1ΔtraI (accR traM) (C) from the experiment described for Fig. 6 were recalculated and plotted as fractions of original activity versus generation (open symbols). Closed symbols represent a theoretical decay curve in which the amount of activity decreases by half at each generation.
In summary, expression of the Ti plasmid conjugative transfer system requires active induction involving two signals: the conjugative opine, which controls expression of traR, and the acyl-HSL quormone, produced by the bacteria themselves, which is required to activate TraR. Expression of the tra regulon is modulated by TraM. Following induction of traR, the activator must accumulate to levels high enough to overcome the inhibitory effect of the antiactivator. In donor populations harboring wild-type Ti plasmids, this process can take from 6 to 8 hours following exposure to the conjugative opine (25). While TraR can be activated by the acyl-HSL in as little as 15 min (20), TraM can significantly extend this time (Fig. 2 and 3). Expression of the tra regulon, construction of the mating apparatus, and processing and transfer of the plasmid can occur in as little as 15 to 20 min once active TraR has accumulated to effective levels.
In contrast to induction, there apparently is no active mechanism for disassembling the conjugative system when the quorum-sensing signal is limiting or lost. That donors lose conjugative competence concomitantly with cell division (Fig. 5A and 7) indicates that the mating apparatus is lost solely by partitioning that occurs in dividing populations of cells. Levels of active TraR decrease in a similar fashion (Fig. 6 and 8), suggesting that the cells also lack a mechanism for removing active TraR.
Acknowledgments
We thank the members of the laboratory, especially Yinping Qin, for helpful discussions during the course of this study.
This work was supported in part by grant no. R01-GM52465 from the National Institutes of Health to S.K.F.
Footnotes
Published ahead of print on 18 January 2008.
REFERENCES
- 1.Beck von Bodman, S., G. T. Hayman, and S. K. Farrand. 1992. Opine catabolism and the conjugal transfer of the nopaline Ti plasmid pTiC58 are coordinately regulated by a single repressor. Proc. Natl. Acad. Sci. USA 89643-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck von Bodman, S., J. E. McCutchan, and S. K. Farrand. 1989. Characterization of conjugal transfer functions of Agrobacterium tumefaciens Ti plasmid pTiC58. J. Bacteriol. 1715281-5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cangelosi, G. A., E. A. Best, G. Marinetti, and E. W. Nester. 1991. Genetic analysis of Agrobacterium. Methods Enzymol. 204384-397. [DOI] [PubMed] [Google Scholar]
- 4.Cha, C., P. Gao, Y. C. Chen, P. D. Shaw, and S. K. Farrand. 1998. Production of acyl-homoserine lactone quorum-sensing signals by Gram-negative plant-associated bacteria. Mol. Plant-Microbe Interact. 111119-1129. [DOI] [PubMed] [Google Scholar]
- 5.Chai, Y., and S. C. Winans. 2004. Site-directed mutagenesis of a LuxR-type quorum-sensing transcription factor: alteration of autoinducer specificity. Mol. Microbiol. 51765-776. [DOI] [PubMed] [Google Scholar]
- 6.Chilton, M.-D., T. C. Currier, S. K. Farrand, A. J. Bendich, M. P. Gordon, and E. W. Nester. 1974. Agrobacterium tumefaciens DNA and PS8 bacteriophage DNA not detected in crown gall tumors. Proc. Natl. Acad. Sci. USA 713672-3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellis, J. G., A. Kerr, A. Petit, and J. Tempé. 1982. Conjugal transfer of nopaline and agropine Ti-plasmids—the role of agrocinopines. Mol. Gen. Genet. 186269-273. [Google Scholar]
- 8.Farrand, S. K. 1998. Conjugal plasmids and their transfer, p. 199-233. In H. P. Spaink, A. Kondorosi, and P. J. J. Hooykaas (ed.), The Rhizobiaceae: molecular biology of model plant-associated bacteria. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 9.Farrand, S. K., I. Hwang, and D. M. Cook. 1996. The tra region of the nopaline-type Ti plasmid is a chimera with elements related to the transfer systems of RSF1010, RP4, and F. J. Bacteriol. 1784233-4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farrand, S. K., Y. Qin, and P. Oger. 2002. Quorum-sensing system of Agrobacterium plasmids: analysis and utility. Methods Enzymol. 358452-484. [DOI] [PubMed] [Google Scholar]
- 11.Fuqua, C., and S. C. Winans. 1996. Conserved cis-acting promoter elements are required for density-dependent transcription of Agrobacterium tumefaciens conjugal transfer genes. J. Bacteriol. 178435-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuqua, C., M. Burbea, and S. C. Winans. 1995. Activity of the Agrobacterium Ti plasmid conjugal transfer regulator TraR is inhibited by the product of the traM gene. J. Bacteriol. 1771367-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Genetello, C., N. Van Larebeke, M. Holsters, A. De Picker, M. Van Montagu, and J. Schell. 1977. Ti plasmids of Agrobacterium as conjugative plasmids. Nature 265561-563. [DOI] [PubMed] [Google Scholar]
- 14.Hwang, I., D. M. Cook, and S. K. Farrand. 1995. A new regulatory element modulates homoserine lactone-mediated autoinduction of Ti plasmid conjugal transfer. J. Bacteriol. 177449-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang, I., A. J. Smyth, Z.-Q. Luo, and S. K. Farrand. 1999. Modulating quorum sensing by antiactivation: TraM interacts with TraR to inhibit activation of Ti plasmid conjugal transfer genes. Mol. Microbiol. 34282-294. [DOI] [PubMed] [Google Scholar]
- 16.Hwang, I., P. L. Li, L. H. Zhang, K. R. Piper, D. M. Cook, M. E. Tate, and S. K. Farrand. 1994. TraI, a LuxI homologue, is responsible for production of conjugation factor, the Ti plasmid N-acylhomoserine lactone autoinducer. Proc. Natl. Acad. Sci. USA 914639-4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kerr, A., P. Manigault, and J. Tempé. 1977. Transfer of virulence in vivo and in vitro in Agrobacterium. Nature 265560-561. [DOI] [PubMed] [Google Scholar]
- 18.Li, P., I. Hwang, H. Miyagi, H. True, and S. K. Farrand. 1999. Essential components of the Ti plasmid trb system, a type IV macromolecular transporter. J. Bacteriol. 1815033-5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo, Z.-Q., and S. K. Farrand. 1999. Signal-dependent DNA binding and functional domains of the quorum-sensing activator TraR as identified by repressor activity. Proc. Natl. Acad. Sci. USA 969009-9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo, Z.-Q., S. Su, and S. K. Farrand. 2003. In situ activation of the quorum-sensing transcription factor TraR by cognate and noncognate acyl-homoserine lactone ligands: kinetics and consequences. J. Bacteriol. 1855665-5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo, Z.-Q., T. E. Clemente, and S. K. Farrand. 2001. Construction of a derivative of Agrobacterium tumefaciens C58 that does not mutate to tetracycline resistance. Mol. Plant-Microbe Interact. 1498-103. [DOI] [PubMed] [Google Scholar]
- 22.Luo, Z.-Q., Y. Qin, and S. K. Farrand. 2000. The antiactivator TraM interferes with the autoinducer-dependent binding of TraR to DNA by interacting with the C-terminal region of the quorum-sensing activator. J. Biol. Chem. 2757713-7722. [DOI] [PubMed] [Google Scholar]
- 23.Metcalf, W. M., W. Jiang, L. L. Daniels, S.-K. Kim, A. Haldimann, and B. L. Wanner. 1996. Conditionally replicative and conjugative plasmids carrying lacZ for cloning, mutagenesis, and allele replacement in bacteria. Plasmid 351-13. [DOI] [PubMed] [Google Scholar]
- 24.Moré, M. I., L. D. Finger, J. L. Stryker, C. Fuqua, A. Eberhard, and S. C. Winans. 1996. Enzymatic synthesis of a quorum-sensing autoinducer through use of defined substrates. Science 2721655-1658. [DOI] [PubMed] [Google Scholar]
- 25.Piper, K. R., and S. K. Farrand. 2000. Quorum sensing but not autoinduction of Ti plasmid conjugal transfer requires control by the opine regulon and the antiactivator TraM. J. Bacteriol. 1821080-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reference deleted.
- 27.Piper, K. R., S. Beck von Bodman, and S. K. Farrand. 1993. Conjugation factor of Agrobacterium tumefaciens regulates Ti plasmid transfer by autoinduction. Nature 362448-450. [DOI] [PubMed] [Google Scholar]
- 28.Qin, Y., Z. Luo, A. J. Smyth, P. Gao, S. Beck von Bodman, and S. K. Farrand. 2000. Quorum-sensing signal binding results in dimerization of TraR and its release from membranes into the cytoplasm. EMBO J. 195212-5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 30.Shaw, P. D., G. Ping, S. L. Daly, C. Cha, J. E. Cronan, Jr., K. L. Rinehart, and S. K. Farrand. 1997. Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc. Natl. Acad. Sci. USA 946036-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Bio/Technology 1784-791. [Google Scholar]
- 32.Swiderska, A., A. K. Berndtson, M.-R. Cha, L. Li, G. M. J. Beaudoin III, J. Zhu, and C. Fuqua. 2001. Inhibition of the Agrobacterium tumefaciens TraR quorum-sensing regulator—interactions with the TraM antiactivator. J. Biol. Chem. 27649449-49458. [DOI] [PubMed] [Google Scholar]
- 33.Tempé, J., C. Estrada, and A. Petit. 1978. The biological significance of opines. II. The conjugative activity of the Ti-plasmids of Agrobacterium tumefaciens, p. 153-160. In M. Ridé (ed.), Proceedings of the 4th International Conference on Plant Pathogenic Bacteria. INRA, Angers, France.
- 34.Zhang, L. H., P. J. Murphy, A. Kerr, and M. E. Tate. 1993. Agrobacterium conjugation and gene regulation by N-acyl-l-homoserine lactones. Nature 362446-448. [DOI] [PubMed] [Google Scholar]
- 35.Zhu, J., and S. C. Winans. 1999. Autoinducer binding by the quorum-sensing regulator TraR increases affinity for target promoters in vitro and decreases TraR turnover rates in whole cells. Proc. Natl. Acad. Sci. USA 964832-4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu, J., and S. C. Winans. 2001. The quorum-sensing transcriptional regulator TraR requires its cognate signaling ligand for protein folding, protease resistance, and dimerization. Proc. Natl. Acad. Sci. USA 981507-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]