Abstract
The occurrence of unilateral flagellar phase variation was previously demonstrated in Escherichia coli strains carrying the non-fliC flagellin-specifying locus flk. In this study, we investigated the mechanism involved in this process. By using sequencing and sequence analysis, the flk region between the chromosomal genes yhaC and rnpB was characterized in all described flk-positive E. coli strains, including the H35 strain identified in this study (the other strains used are H3, H36, H47, and H53 strains), and this region was found to contain a putative integrase gene and flanking direct repeats in addition to the flk flagellin-specifying gene flkA and a fliC repressor gene, flkB, indicating that there is a typical genomic islet (GI), which was designated the flk GI. The horizontal transfer potential of the flk GI was indicated by detection of the excised extrachromosomal circular form of the flk GI. By generating fliC-expressing variants of H3 and H47 strains, unilateral flagellar phase variation in flk-positive strains was shown to be mediated by excision of the flk GI. The function of the proposed integrase gene was confirmed by deletion and a complementation test. The potential integration sites of the flk GI were identified. A general model for flagellar phase variation in flk-positive E. coli strains can be expressed as fliCoff + flkAon → fliCon + flkAnone. This is the first time that a molecular mechanism for flagellar phase variation has been reported for E. coli.
Flagella are best known for conferring motility to bacteria, which allows the bacteria to swim toward attractants and away from repellents (5). Flagella also play a variety of other roles in many bacterial lifestyles, including bacterial pathogenesis and biofilm formation (27, 45). Pathogenic bacteria specifically produce flagella to promote colonization and invasion of mucosa (27). Flagellar antigen, also known as H antigen, is one of the major antigens in gram-negative bacteria. The serological variety of flagella is important for intrageneric differentiation of bacteria (12). Flagellin is the protein subunit of the flagellar filament and determines the specificity of the flagellar antigen.
Phase variation of antigenic expression, especially expression of surface structures, such as flagella, fimbria, capsular polysaccharide, and lipopolysaccharide, is a common strategy used by many bacteria for adaptation to particular environments (38). Phase variation of flagellar antigens was first described in Salmonella enterica serovar Typhimurium, in which two different flagellin genes, fljB and fliC, are alternatively expressed, giving rise to two different H phases (18, 50). Flagellar phase variation is thought to be related to bacterial survival in the presence of host defense systems and therefore is linked to bacterial virulence (19). The molecular mechanism for flagellar phase variation in S. enterica has been well studied (1, 20, 22, 40, 47, 51). Alternating expression of fljB and fliC is controlled by site-specific inversion of an approximately 996-bp DNA segment (H segment) containing a promoter for the cotranscription of fljB and fljA (encoding a posttranscriptional repressor of the unlinked gene fliC), and the fljBA promoter is turned on in one orientation but not in the other (51). The hin gene encoding a recombinase (Hin invertase) and a 26-bp inverted repeat sequence (hix) are responsible for the invertible recombination of the H segment (20, 40). Recently, flagellar phase variation caused by deletion of the fljAB-like operon in a z66 antigen-positive strain of S. enterica serovar Typhi was reported (17), but the exact mechanism involved is unclear.
A total of 53 different H type strains of Escherichia coli have been officially registered (26). In 44 of these strains the manifested flagellins are encoded at the fliC locus, while in all of the rest of them except the H35 type strain they are encoded at the flk (H3, H36, H47 and H53), fll (H44 and H55), flm (H54), or fliC// (H17) locus (4, 28-33, 43, 44, 46). H35 has a disrupted fliC gene (46), and its flagellin-specifying locus has not been determined. Although E. coli is generally considered monophasic (24), the occurrence of unilateral flagellar phase variation in flk-positive H3 and H47 strains has been reported (28, 32, 33). The flk region containing the flagellin-specifying gene flkA and the repressor gene flkB was located between chromosomal genes rnpB and yhaC in H3 and H53 strains, and the flkB product repressed the expression of fliC in E. coli and, in some cases, S. enterica; therefore, the flkB gene is functionally similar to fljA of S. enterica (43). The unilateral phase variation in E. coli H3 and H47 was expressed as fliCoff + flkAon → fliCon + flkAoff (33).
Genomic islets or islands (GIs) represent a large group of mobile elements in bacteria (39). GIs are known to encode many different functions and are related to bacterial virulence, antibiotic resistance, symbiosis, fitness, and adaptation (9, 39). The number of described GIs and GI-bearing hosts is constantly increasing (25). Some GIs have features of integrative and conjugative elements, and these elements are excised from the chromosome by site-specific recombination and are transferred to new hosts by conjugation (2, 7, 10). Recombination between two attachment sites, attP on the circular form of a GI and attB on a bacterial chromosome, that leads to the integration of the GI into the chromosome is mediated by an integrase (14, 15, 39). Integration of a GI generates two junctions in the chromosome, which are two direct repeats (DRs) at the left and right ends of the GI. These two DRs are the chimeras of attB and attP. Integrases also mediate the recombination between two flanking DRs of an integrated GI, resulting in excision of the GI from the chromosome via an extrachromosomal circular intermediate (10, 14, 16, 23, 39).
In this study, the mechanism involved in flagellar phase variation in flk-positive E. coli strains was investigated. H35 was identified as a new member of the flk-positive group. The regions between yhaC and rnpB in H35, H36, and H47 strains were sequenced, and the sequences were analyzed together with the corresponding sequences from H3 and H53 strains published previously. In all five flk-positive strains, the flk region was found to contain a putative integrase gene and flanking DRs at both ends in addition to the flagellin gene flkA and the repressor gene flkB, indicating that there is a typical GI, which was designated the flk GI. PCR was carried out to detect the excised extrachromosomal circular form of the flk GI. fliC-expressing variants of H3 and H47 were generated and examined to determine the presence of the flk GI, and the phase variation in the two parental strains was shown to be mediated by excision of the flk GI. The function of Orf486 as an integrase was confirmed by deletion and complementation tests. The frequency of flagellar phase transition was determined. The potential integration sites of the flk GI were identified. A general model for the mechanism mediating unilateral flagellar phase variation in flk-positive E. coli strains is presented below.
MATERIALS AND METHODS
Bacterial strains, media, and antisera.
Strains used in this study are listed in Table 1. Flagellin-negative strain EJ34 was used as the host strain for construction of genomic libraries. Bacteria were grown in Luria-Bertani (LB) broth or agar A (BBI) supplemented with ampicillin (100 μg/ml) when necessary. A semisolid medium (motility agar plates) containing 0.2% agar in LB broth was used to enhance the bacterial motility and to screen for motile clones.
TABLE 1.
E. coli strains and plasmids used in this study
| Strain or plasmida | Derivation, genotype, or phenotype | H antigen expressed | Flagellin genotype | Reference or source |
|---|---|---|---|---|
| Strains | ||||
| G1436 | Bi7327-41, H3 type strain | H3 | fliC16offflkA3on | 30 |
| G1393 | 4370-53, H35 type strain | H35 | flkA35on | This study |
| G1394 | 5017-53, H36 type strain | H36 | flkA36on | 28 |
| G1397 | 1755-58, H47 type strain | H47 | fliC21offflkA47on | 28 |
| G1392 | E480-68, H53 type strain | H53 | flkA53on | 28 |
| H1171 | Phase variant of G1436 | H16 | fliC16onflkA3none | This study |
| H1172 | Phase variant of G1436 | H16 | fliC16onflkA3none | This study |
| H1173 | Phase variant of G1436 | H16 | fliC16onflkA3none | This study |
| H1174 | Phase variant of G1436 | H16 | fliC16onflkA3none | This study |
| H1175 | Phase variant of G1436 | H16 | fliC16onflkA3none | This study |
| H1176 | Phase variant of G1436 | H16 | fliC16onflkA3none | This study |
| H1201 | Phase variant of G1397 | H47 | fliC21onflkA47none | This study |
| H1202 | Phase variant of G1397 | H47 | fliC21onflkA47none | This study |
| G1438b | EJ34 | 11 | ||
| G1370 | DH5α | |||
| G1067 | H3 non-type strain | H3 | fliC16offflkA3on | |
| G1145 | H47 non-type strain | H47 | fliC21offflkA47on | |
| H1692 | orf486-deficient mutant of G1436 | H3 | fliC16offflkA3on | This study |
| H1693 | H1692 carrying pLW1330 | H3 | fliC16offflkA3on | This study |
| H1694 | G1436 carrying pLW1330 | H3 | fliC16offflkA3on | This study |
| Plasmids | ||||
| pUC19 | Cloning vector; Apr | Takara | ||
| pLW1249 | pUC19 with a 8.5-kb insert containing the flagellin gene for H35 | This study | ||
| pLW1041 | pUC19 with a 4.1-kb insert containing the flagellin gene for H36 | This study | ||
| pLW1248 | pUC19 with a 3.1-kb insert containing the flagellin gene for H47 | This study | ||
| pKD4 | Contains a kanamycin resistance gene (kan); Kmr Apr | 8 | ||
| pKD20 | RED recombinase expression plasmid; Apr | 8 | ||
| pUC18 | Cloning vector; Apr | Takara | ||
| pLW1330 | pUC18 containing orf486 | This study |
E. coli H antigen type strains were obtained from the Institute of Medical and Veterinary Science, Adelaide, Australia, and the Mechnikov Research Institute for Vaccines and Sera, Russian Academy of Medical Sciences, Moscow, Russia. Other E. coli standard H type strains used have been described previously (12, 46).
A derivative of E. coli K-12 which is nonflagellated and nonmotile because the expression of its single flagellin gene, fliC, is blocked by a mutation.
Antisera against E. coli H type strains were obtained from the Mechnikov Research Institute for Vaccines and Sera, Russian Academy of Medical Sciences, Moscow, Russia, and were used for H antigen identification by means of slide tests (34).
Construction of a genomic library.
A genomic library was constructed by the shotgun cloning method (37). Genomic DNA partially digested with Sau3AI was ligated into pUC19, which was digested with BamHI and treated with calf intestinal alkaline phosphatase (Takara). The library was introduced into EJ34 by transformation, and the recombinant bacterial cells were screened for restoration of swimming motility on motility agar plates containing ampicillin.
Primers and PCR amplification.
The primers used in this study are listed in Table S1 in the supplemental material. Each PCR was carried out using a 50-μl reaction mixture containing 2 mM MgCl2, each deoxynucleoside triphosphate at a concentration of 0.2 mM, each primer at a concentration of 1 μM, and 2 U of Taq DNA polymerase (5 U of LA-Taq DNA polymerase for long-range PCR).
Sequence analysis.
Sequencing was carried out by Tianjin Biochip Cooperation, China, using an ABI 3730 automated DNA sequencer. Sequence data were assembled using the Staden package (41). Artemis (36) was used for annotation. BLAST was used for searching databases, including the GenBank, COG (Clusters of Orthologous Groups), and Pfam protein motif databases. Sequence alignment and comparison were carried out using ClustalX (42). Phylogenetic analysis was conducted using MEGA, version 2.1 (21).
Selection of spontaneous variants with altered H antigen.
Bacteria were grown on semisolid medium containing antiserum against the expressed H antigen, which provided strict conditions for selecting variants expressing an alternative flagellar antigen phase (30, 32). While the parental strain was immobilized, the variants remained motile.
Deletion of orf486 from the E. coli H3 type strain.
The orf486 gene was replaced by a kanamycin resistance gene (kan) using the RED recombination system of phage lambda (8, 48). The kan gene was PCR amplified from plasmid pKD4 by using primers wl-11719 and wl-11720 binding to the 5′ and 3′ ends of the gene, and each primer contained 40 bp based on the H3 DNA, which flanks orf486. The PCR product was transformed into the H3 type strain (G1436) carrying pKD20, and kanamycin-resistant transformants were selected after induction of the RED genes by the protocol described by Datsenko and Wanner (8). PCR with primers specific for the DNA of the kan gene and the H3 flk region was carried out to confirm the replacement. To complement the orf486-deficient H3 mutant, the orf486 gene was amplified from the H3 type strain using primers wl-10352 and wl-10353. The resulting PCR products were cloned into pUC18 to obtain plasmid pLW1330.
Determination of flagellar phase transition frequency in H3 strains.
Bacteria were grown on LB agar for 24 h. A block of agar bearing a single colony was cut out and transferred to 15 ml of LB broth. After 6 h of incubation at 37°C, the culture was transferred into two centrifuge tubes. Fifty microliters of antiserum against the expressed H antigen (H3 antiserum in this study) was added to the first tube, and 50 μl of some other antiserum (H47 antiserum in this study) which did not contain antibodies to any surface antigens of the strain used (H3 in this study) was added to the second tube. The contents of each tube were gently mixed. After incubation at 37°C for 30 min, a small sample was taken from the second tube to make dilutions for plating to determine the cell number. The total number of bacterial cells was deduced.
The two tubes were centrifuged simultaneously (40 min, 40 × g). Sediment was produced in the first tube, and the second tube served as a control in which no sediment should have been produced. Immediately after centrifugation, 0.5 ml supernatant was taken from the first tube and used to prepare serial 10-fold dilutions in saline. For plating, 0.5 ml of each dilution was grown on semisolid medium containing antiserum against the expressed H antigen. The number of spreading colonies was determined, and the total number of flagellar phase variants was calculated.
Flagellar phase transition frequency was calculated by using the equation described previously (13, 49), as follows: flagellar phase transition frequency 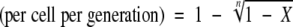 , where n is the number of generations and X is (total number of flagellar phase variants)/(total number of bacterial cells). The number of generations (n) was determined by using the following equation: n = (log N − log N0)/log 2, where N is the total number of bacterial cells and N0 is number of bacterial cells in the inoculum.
, where n is the number of generations and X is (total number of flagellar phase variants)/(total number of bacterial cells). The number of generations (n) was determined by using the following equation: n = (log N − log N0)/log 2, where N is the total number of bacterial cells and N0 is number of bacterial cells in the inoculum.
Nucleotide sequence accession numbers.
The DNA sequences of flk regions between rnpB and yhaC of the E. coli H35, H36, and H47 type strains and E. coli strains H1171, H1173, H1174, and H1201 have been deposited in the GenBank database under accession numbers EF392692 to EF392698.
RESULTS
Characterization of the flagellin-specifying locus in H35, H36, and H47 strains.
To localize the flagellin-specifying locus in the H35 strain and to examine the flk region in the H36 and H47 strains, a genomic library was constructed for each strain. From each library, a single recombinant plasmid (pLW1249, pLW1041, and pLW1248 carrying 8.5-, 4.1-, and 3.1-kb inserts, respectively) conferring motility to strain EJ34, which is a derivative of E. coli K-12 lacking flagellin, was isolated and confirmed to carry the respective flagellin gene by means of serotyping. Sequencing analysis revealed the presence of flkA and flkB in all three inserts. To test whether the flk regions in H35, H36, and H47 are located between yhaC and rnpB as they are in H3 and H53, PCRs were carried out to amplify the region between yhaC and flkB using primers wl-3622 and wl-5272, wl-5276, or wl-5274 and PCRs were carried out to amplify the region between flkA and rnpB using primers wl-3623 and wl-5273, wl-5277, or wl-5275. Sequencing analysis of the PCR products revealed the presence of the flk region between rnpB and yhaC in all three strains. The results indicated that H35 is a new member of the flk-positive group, and the flk region containing the flkAB operon is generally present between yhaC and rnpB in flk-positive E. coli.
Sequence analysis of the yhaC-rnpB region in H3, H35, H36, H47, and H53 strains.
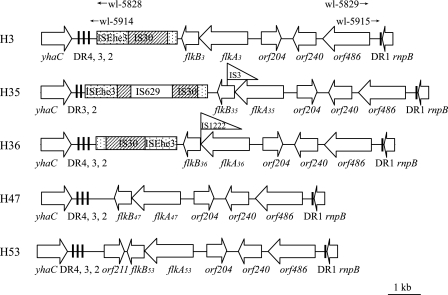
Sequences that were 13,317, 12,193, and 8,737 bp long were obtained for the region between yhaC and rnpB in the H35, H36, and H47 type strains, respectively, and these sizes are similar to the sizes obtained for the H3 (10,867 bp) and H53 (9,310 bp) type strains retrieved from the GenBank database (AB128916 and AB128917). flkB, flkA, orf204, orf240, and orf486 in the same order were found in all five strains (Fig. 1). orf486 encodes a putative integrase belonging to the tyrosine recombinase family (also known as the λ integrase family), orf240 encodes a putative invertase belonging to the serine recombinase family, and orf204 encodes a protein with an unknown function. An IS1222 element was found between flkA and flkB in H36, suggesting that the cotranscription of these two genes may be disrupted, resulting in expression of only flkA. This explains the absence of repressor activity for fliC in H36 reported previously (28). The flkB gene in H35 was found to be disrupted by an IS3 element, and therefore, the absence of repressor activity in H35 is also expected. Several insertion elements are present between yhaC and flkB in H3, H35, and H36 strains (Fig. 1).
FIG. 1.
Comparative map of the flk regions in E. coli H3, H53, H47, H36, and H35 strains. The orientation corresponds to that of the E. coli K-12 chromosome. Open arrows indicate the locations and orientations of open reading frames of putative genes. Insertion elements are indicated by rectangles or burgees. The imperfect 23-bp DRs are indicated by bars. orf204 in H53 strain was designated orf210 in a previous study (43). The positions of the wl-5828/wl-5829 and wl-5914/wl-5915 primer pairs used for detection of the circular form of flk GIH3 are indicated by arrows.
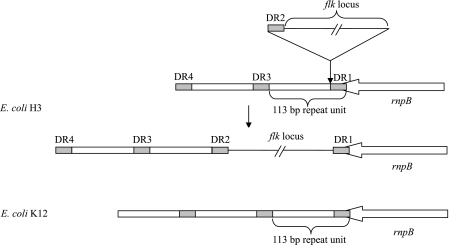
In all five strains, three or four copies of an imperfect 23-bp DR, corresponding to the last 23 bp of the rnpB gene (5′-CGGCTTATCGGTCAGTTTCACCT-3′), were found at the right (DR1) and left (DR2, DR3, and DR4) ends of the flk region, and DR1 overlapped the 23 bp of rnpB (Fig. 1). One or two single-nucleotide polymorphic sites were found in some of the DRs (data not shown). In E. coli K-12, a 113-bp repeated DNA sequence beginning with the last 23 bp of the rnpB gene is present in the flanking region downstream of rnpB and is repeated several times (6, 35). Each 113-bp unit consists of the 23-bp sequence (DR in flk-positive strains) and the region for intrinsic transcription termination. In flk-positive E. coli strains, the first 23-bp sequence (DR1) and the transcription termination region were found to be separated by the flk locus and the second 23-bp sequence (DR2) (Fig. 2).
FIG. 2.
Schematic representation of the 113-bp repeats downstream of the rnpB gene in E. coli strain K-12 and insertion of the flk locus in the same region in the H3 strain. The 113-bp repeats are indicated by open rectangles. The 23-bp sequences corresponding to the last 23 bp of the rnpB gene are indicated by shaded rectangles. The positions of DRs in H3 are indicated.
In addition to the presence of the flanking DRs and a putative integrase gene (orf486), several other features of typical GIs were also found in the region, including the large size (6,951 to 11,651 bp) and lower G+C content of the region (42.2 to 46.2% versus 50.5% for the E. coli genome) and the absence of the region in closely related strains, such as E. coli K-12 (accession no. NC_000913) and O157:H7 (AE005174) (39). All these findings indicate that the flk regions between yhaC and rnpB in the five flk-positive strains are integrated GIs (referred to as flk GIH3, flk GIH35, flk GIH36, flk GIH47, and flk GIH53) (Fig. 2). While DR1 and DR2 are the two flanking DRs of the flk GI, DR3 and DR4 appear to be intrinsic sequences in the host chromosome.
We also sequenced the yhaC-rnpB region in two nontype strains, E. coli O53:H3 strain G1067 and E. coli O156:H47 strain G1145, and each of the sequences obtained showed 99% DNA identity to the corresponding sequence in the corresponding type strain. This indicates that the flk GI is conserved in E. coli strains carrying the same H antigen.
Detection of the excised extrachromosomal circular form of the flk GI.
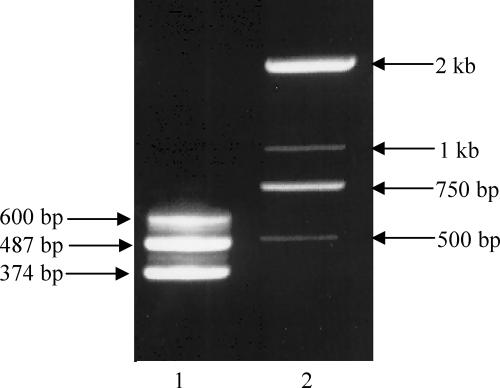
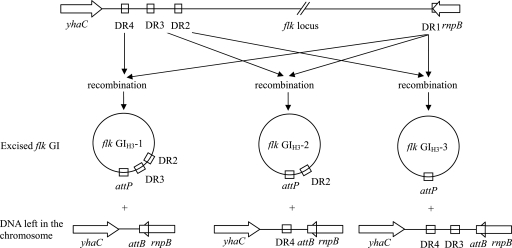
A two-step PCR was carried out with the H3 type strain to detect the extrachromosomal circular form of flk GIH3. The first-round PCR was performed using primers wl-5828 and wl-5829 oriented toward the left and right ends of flk GIH3, respectively. The second-round PCR was carried out using an aliquot of the products from the first round PCR as the template and primers wl-5914 and wl-5915 designed based on the sequence of the expected product from the first-round PCR. Three PCR products that were 600, 487, and 374 bp long were detected (Fig. 3), indicating that there was formation of three types of extrachromosomal circular forms. Sequence analysis of the three PCR products indicated that the recombination events occurred between DR1 and DR2, between DR1 and DR3, and between DR1 and DR4, respectively, and the three types of circular forms were designated flk GIH3-1, flk GIH3-2, and flk GIH3-3 (Fig. 4). Using the same procedure but different primer pairs, three types of extrachromosomal circular forms of the flk GI were also detected in the H36, H47, and H53 type strains. However, only two types were detected in the H35 strain, consistent with the presence of one and two DRs at the two ends of flk GIH35 (data not shown).
FIG. 3.
Detection of the circular forms of flk GIH3 by two-step PCR. Lane 1, three PCR products obtained from the H3 type strain (374, 487, and 600 bp); lane 2, size markers. The wl-5828/wl-5829 and wl-5914/wl-5915 primer pairs were used.
FIG. 4.
Model for the excision of flk GIH3. Recombination events for the generation of flk GIH3-1, flk GIH3-2, and flk GIH3-3 are shown.
Involvement of the flk GI in flagellar phase variation.
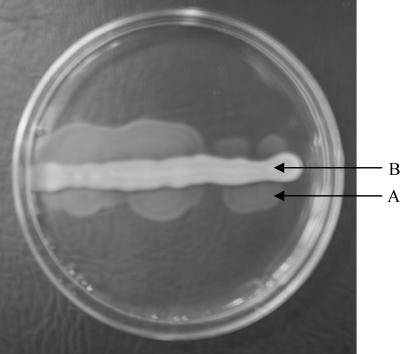
By growing the H3 and H47 type strains on motility agar containing antiserum against H3 and H47, respectively, and screening for clones which exhibited spreading growth (Fig. 5), six and two spontaneous phase variants were obtained. All six H3 variants (strains H1171 to H1176) produced agglutination with H16 antiserum but not with H3 antiserum and were strongly immobilized when they were grown on semisolid motility medium containing H16 antiserum. Both H47 variants (strains H1201 and H1202) agglutinated with H21 antiserum but not with H47 antiserum and were strongly immobilized in the presence of H21 antiserum. The results are in agreement with the previous findings that the fliC genes in H3 and H47 strains encode H16 and H21 antigens, respectively (28, 29, 46). The strong immobilization of the variants by H16 or H21 antiserum indicated that there was unilateral phase change, as reported for the two strains previously.
FIG. 5.
Selection of spontaneous flagellar phase variants from E. coli H3 on motility agar containing antiserum against H3 antigen. Arrow A indicates spreading growth produced by the phase variants. Arrow B indicates immobilized growth of the H3 parental strain in the presence of H3 antiserum.
To find out whether the flk GI is still present in the variants, the yhaC-rnpB regions in all eight variant strains were sequenced using primers wl-3622 and wl-3623. In the H3 variants, the region is 500 bp long and contains two complete DRs (DR3 and DR4) in H1171, H1172, and H1175, is 387 bp long and contains one DR (DR4) in H1173 and H1176, and is 274 bp long and contains no DRs in H1174 (Fig. 4). The region is 507 bp long and contains two DRs (DR3 and DR4) in both H47 variant strains (H1201 and H1202). These results indicate that the phase variation of flagellar antigens in the H3 and H47 strains was caused by the excision of the flk GI and that recombination could occur between DR1 and DR2, between DR1 and DR3, and between DR1 and DR4.
No PCR products were obtained from any of the eight variant strains using the flkA-specific primer pairs (wl-5141/wl-5142 and wl-3820/wl-3821), indicating the absence of the flkAB operon and therefore the absence of the excised flk GI in the variants (data not shown). The excision and irrevocable loss of the flk GI provide an explanation for the unilateral phase variation observed in flk-positive strains.
Characterization of Orf486 as a functional integrase.
To confirm the function of Orf486 as an integrase for the excision of the flk GI, an orf486-deficient mutant of the H3 type strain (H1692) and the corresponding transcomplementary strain (H1693) were generated. PCR was carried out to detect extrachromosomal circular forms of flk GIH3 in the two strains generated, and the expected PCR products indicative of the circular forms of the GI were detected only in transcomplementary strain H1693 and not in mutant strain H1692. By growing the two strains on motility agar containing antiserum against H3, spontaneous flagellar antigen phase variants with the H16 phenotype were detected for transcomplementary strain H1693 but not for the mutant. These results confirmed that orf486 is a functional integrase gene required for excision of the flk GI from the chromosome.
The frequency of flagellar phase transition was also determined in H1692 (H3 type strain), H1693 (transcomplementary strain of orf486 mutant), and H1694 (H3 type strain carrying pLW1330 containing orf486). The transition rates were determined to be 1.98 × 10−8 per bacterium per generation for H1692, 1.93 × 10−2 per bacterium per generation for H1693, and 2.83 × 10−2 per bacterium per generation for H1694. The increased transition frequencies in H1693 and H1694 could apparently be attributed to the overexpression of Orf486. The results further indicated that orf486 is a functional integrase gene.
Identification of the potential integration sites.
In all phase variant strains generated (H1171 to H1176 for H3 and H1201 and H1202 for H53), the last 23 bp of rnpB (corresponding to DR1 in the integrated flk GI) is retained (Fig. 4), indicating that the 3′-terminal 23 bp of the rnpB gene (5′-CGGCTTATCGGTCAGTTTCACCT-3′) is the potential chromosome attachment site (attB) of the flk GI. The sequence of the potential attB site is conserved in all variants except H1174, which has a single-nucleotide polymorphic site at position 19. The potential attB site was also found in many E. coli strains of other types either by sequencing (H2, H8, H11, H16, H23, H27, and H55) or through genome searching (accession no. AE014075, BA000007, AE005174, NC_000913, AE005541, AP009048, CP000468, CP000247, CP000243, and AE014075), as well as in strains belonging to other species of the family Enterobacteriaceae, including S. enterica, Shigella, Klebsiella pneumoniae, and Pantoea agglomerans (data not shown). Therefore, this site is conserved not only in E. coli but also in other closely related species.
The potential attP site (5′-CGGCTTATCGGTCAGCTTCAACT-3′) in the extrachromosomal circular form of flk GIH3 was identified at the junction of the two ends of flk GIH3 (Fig. 4), which is identical to the sequence of DR1. Two single-nucleotide polymorphic sites between the attP and attB sites were found to be at positions 16 and 21. The attP site was formed by recombination between DR1 and one of the other DRs, but the exact mechanism involved is not clear. One or two single-nucleotide polymorphic sites were also detected in some of the DRs, including DR1, which had different residues at positions 16 and 21 than flk GIH3. Presumably, the site polymorphism between the attP and attB genes might arise from site polymorphisms in DRs. To prove this, further studies are needed. Although not investigated in this study, polymorphism sites may also be present in the attP site of the flk GI in other flk-positive strains for the same reason.
In all five flk-positive strains, only one DR (DR1) was found to be at the right end of the flk GI, and the rest of the DRs were found to be at the left end, indicating that any flk GIs could integrate only into the attB site and not into other 23-bp DR sequences in the 3′ flanking region of rnpB.
Absence of the flk region in other H type strains.
The region between yhaC and rnpB was amplified from 53 E. coli H type strains by PCR using primers wl-3622 and wl-3623. In E. coli K-12, which does not contain any non-fliC flagellin genes, this region contains a 615-bp intergenic DNA region (6). In comparison, the region is 8,737 to 13,317 bp long in the five flk-positive strains. Therefore, the presence of the flk region can be indicated by the size of the PCR product. The results showed that except for the five flk-positive strains described above, none of the strains gave PCR products larger than 1.5 kb (data not shown). PCRs were also performed using the primers targeting flkAB (wl-5136 and wl-5033) and orf486 (wl-5830 and wl-5831), and the expected PCR products were detected for the five flk-positive strains but not for other strains (data not shown). Therefore, none of the type strains except those expressing H3, H35, H36, H47, and H53 antigens carries the flk region.
DISCUSSION
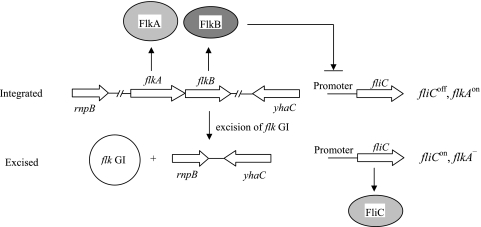
In this study, we examined the involvement of a GI, the flk GI, in flagellar phase variation in E. coli strains. A general model for the phase variation in the FlkA and FliC flagellins in E. coli is shown in Fig. 6. The principle is that when the flk GI is present in the chromosome, the flkAB operon is expressed to allow coproduction of the FlkA flagellin and the repressor protein FlkB, and the expression of fliC is repressed. When the flk GI is excised from the chromosome, flkAB is irreversibly deleted, and therefore the repression of the fliC is released and the FliC flagellin is produced. Therefore, the formula for the unilateral phase variation (fliCoff + flkAon → fliCon + flkAoff) (33) should be revised to fliCoff + flkAon → fliCon + flkAnone.
FIG. 6.
Model for the phase variation between FliC and FlkA in E. coli. When the flk GI is present in the chromosome, the flkAB operon is expressed. FlkA flagellin is produced, and the fliC gene is repressed by FlkB. After the flk GI is excised from the chromosome, the fliC gene is expressed.
In the five flk-positive E. coli strains, the fliC genes in the H35, H36, and H53 strains are disrupted or defective (33, 43, 46). Therefore, any flk-negative variants of these strains would be nonmotile. Whether this can occur was not investigated here due to a lack of screening methods for nonmotile variants. However, it is more likely that such strains would become monophasic under physiological conditions, as the survival of the bacteria may be affected in the absence of a flagellin, and the flagellin encoded by flkA may offer a selective advantage to these strains in their particular environments. On the other hand, repression of fliC in these strains is not needed, and this is also reflected by the disruption of the repressor gene flkB by insertion elements in H35 and H36. The insertions must have happened after the bacteria gained the flk GI, so that the survival of bacteria would not be affected. Although the flkB gene is intact in H53, disruption of this gene is also expected sooner or later from an evolutionary point of view.
Although the principles for flagellar phase variation in E. coli flk-positive strains and S. enterica strains are rather similar and both organisms require cotranscription of a flagellin gene and a fliC repressor gene, different mechanisms are utilized. While the flk GI described here mediates only unilateral phase change owing to the irreversible loss of the flk GI, flagellar phase variation mediated by the site-specific inversion of the H segment in S. enterica is bilateral (1, 20, 22, 40, 47, 51). Although the flagellar phase variation in a z66-positive strain of S. enterica serovar Typhi is also caused by deletion of the fljAB-like operon (17), a mechanism different from that described here seems to be involved, as indicated by a recent report showing that the fljB-like gene in this strain is located on a novel liner plasmid (3). Still, mechanisms involved in flagellar phase variation in other E. coli strains carrying different non-fliC flagellin loci and showing flagellar phase variation, such as H17 carrying the fliC// locus (28, 32, 33), remain to be investigated.
In addition to the integrase gene (orf486) characterized, orf204 encoding a putative invertase/resolvase is also present in the region. Site-specific recombination mediated by the invertase requires the presence of inverted repeats (14), such as the hix sequence in S. enterica, which are absent in the flk region. Therefore, the orf204 gene is likely to be nonfunctional or not related to the flk region.
At this stage, we could not obtain any indication of the possible origin of the flk GI. The flk GI could have evolved from a single ancestor and been integrated into other hosts through recombination at the attB site. The presence of the 23-bp attB site in many bacterial species belonging to the family Enterobacteriaceae brings up the possibility of the presence of flk-like GIs in other bacterial species. The other possibility is that all five flk loci may have evolved from a common ancestor in which the flk GI had already been integrated at the attB site. The integration potential of the flk GI remains to be studied further. In future studies, it should also be worthwhile to investigate the factors or environmental conditions which can induce the flagellar phase variation and mechanisms for the maintenance of flagellar variants in the population.
Supplementary Material
Acknowledgments
This work was supported by the National 863 program of China (grants 2006AA020703 and 2006AA06Z409), by the National Natural Science Foundation of China (NSFC) Key Programs (grants 30530010 and 20536040), by the Chinese National Science Fund for Distinguished Young Scholars (grant 30788001), by the NSFC General Program (grant 30670038), and by the Tianjin Municipal Fund for Science and Technology Innovation (grant 05FZZDSH00800).
Footnotes
Published ahead of print on 25 April 2008.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Aldridge, P. D., C. Wu, J. Gnerer, J. E. Karlinsey, K. T. Hughes, and M. S. Sachs. 2006. Regulatory protein that inhibits both synthesis and use of the target protein controls flagellar phase variation in Salmonella enterica. Proc. Natl. Acad. Sci. USA 10311340-11345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antonenka, U., C. Nolting, J. Heesemann, and A. Rakin. 2005. Horizontal transfer of Yersinia high-pathogenicity island by the conjugative RP4 attB target-presenting shuttle plasmid. Mol. Microbiol. 57727-734. [DOI] [PubMed] [Google Scholar]
- 3.Baker, S., J. Hardy, K. E. Sanderson, M. Quail, I. Goodhead, R. A. Kingsley, J. Parkhill, B. Stocker, and G. Dougan. 2007. A novel linear plasmid mediates flagellar variation in Salmonella typhi. PLoS Pathog. 3e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beutin, L., and E. Strauch. 2007. Identification of sequence diversity in the Escherichia coli fliC genes encoding flagellar types H8 and H40 and its use in typing of Shiga toxin-producing E. coli O8, O22, O111, O174, and O179 strains. J. Clin. Microbiol. 45333-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blair, D. F. 1995. How bacteria sense and swim. Annu. Rev. Microbiol. 49489-522. [DOI] [PubMed] [Google Scholar]
- 6.Blattner, F. R., G. I. Plunkett, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, et al. 1997. The complete genome sequence of Escherichia coli K-12. Science 2771453-1474. [DOI] [PubMed] [Google Scholar]
- 7.Burrus, V., G. Pavlovic, B. Decaris, and G. Guedon. 2002. Conjugative transposons: the tip of the iceberg. Mol. Microbiol. 46601-610. [DOI] [PubMed] [Google Scholar]
- 8.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 976640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dobrindt, U., B. Hochhut, U. Hentschel, and J. Hacker. 2004. Genomic islands in pathogenic and environmental microorganisms. Nat. Rev. Microbiol. 2414-424. [DOI] [PubMed] [Google Scholar]
- 10.Doublet, B., D. Boyd, M. R. Mulvey, and A. Cloeckaert. 2005. The Salmonella genomic island 1 is an integrative mobilizable element. Mol. Microbiol. 551911-1924. [DOI] [PubMed] [Google Scholar]
- 11.Enomoto, M., and B. A. Stocker. 1975. Integration, at hag or elsewhere, of H2 (phase-2 flagellin) genes transduced from Salmonella to Escherichia coli. Genetics 81595-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ewing, W. H. 1986. Edwards and Ewing's identification of Enterobacteriaceae, 4th ed. Elsevier Science Publishing, Inc., New York, NY.
- 13.Gally, D. L., J. A. Bogan, B. I. Eisenstein, and I. C. Blomfield. 1993. Environmental regulation of the fim switch controlling type 1 fimbrial phase variation in Escherichia coli K-12: effects of temperature and media. J. Bacteriol. 1756186-6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grindley, N. D., K. L. Whiteson, and P. A. Rice. 2006. Mechanisms of site-specific recombination. Annu. Rev. Biochem. 75567-605. [DOI] [PubMed] [Google Scholar]
- 15.Hallet, B., and D. J. Sherratt. 1997. Transposition and site-specific recombination: adapting DNA cut-and-paste mechanisms to a variety of genetic rearrangements. FEMS Microbiol. Rev. 21157-178. [DOI] [PubMed] [Google Scholar]
- 16.Hochhut, B., and M. K. Waldor. 1999. Site-specific integration of the conjugal Vibrio cholerae SXT element into prfC. Mol. Microbiol. 3299-110. [DOI] [PubMed] [Google Scholar]
- 17.Huang, X., V. Phung le, S. Dejsirilert, P. Tishyadhigama, Y. Li, H. Liu, K. Hirose, Y. Kawamura, and T. Ezaki. 2004. Cloning and characterization of the gene encoding the z66 antigen of Salmonella enterica serovar Typhi. FEMS Microbiol. Lett. 234239-246. [DOI] [PubMed] [Google Scholar]
- 18.Iino, T. 1969. Genetics and chemistry of bacterial flagella. Bacteriol. Rev. 33454-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikeda, J. S., C. K. Schmitt, S. C. Darnell, P. R. Watson, J. Bispham, T. S. Wallis, D. L. Weinstein, E. S. Metcalf, P. Adams, C. D. O'Connor, and A. D. O'Brien. 2001. Flagellar phase variation of Salmonella enterica serovar Typhimurium contributes to virulence in the murine typhoid infection model but does not influence Salmonella-induced enteropathogenesis. Infect. Immun. 693021-3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson, R. C., and M. I. Simon. 1985. Hin-mediated site-specific recombination requires two 26 bp recombination sites and a 60 bp recombinational enhancer. Cell 41781-791. [DOI] [PubMed] [Google Scholar]
- 21.Kumar, S., K. Tamura, I. B. Jakobsen, and M. Nei. 2001. MEGA2: molecular evolutionary genetics analysis software. Bioinformatics 171244-1245. [DOI] [PubMed] [Google Scholar]
- 22.Kutsukake, K., and T. Iino. 1980. A trans-acting factor mediates inversion of a specific DNA segment in flagellar phase variation of Salmonella. Nature 284479-481. [DOI] [PubMed] [Google Scholar]
- 23.Lesic, B., S. Bach, J. M. Ghigo, U. Dobrindt, J. Hacker, and E. Carniel. 2004. Excision of the high-pathogenicity island of Yersinia pseudotuberculosis requires the combined actions of its cognate integrase and Hef, a new recombination directionality factor. Mol. Microbiol. 521337-1348. [DOI] [PubMed] [Google Scholar]
- 24.Macnab, R. M. 1992. Genetics and biogenesis of bacterial flagella. Annu. Rev. Genet. 26131-158. [DOI] [PubMed] [Google Scholar]
- 25.Mantri, Y., and K. P. Williams. 2004. Islander: a database of integrative islands in prokaryotic genomes, the associated integrases and their DNA site specificities. Nucleic Acids Res. 32D55-D58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orskov, F., and I. Orskov. 1984. Serotyping of Escherichia coli. Academic Press, London, United Kingdom.
- 27.Ramos, H. C., M. Rumbo, and J. C. Sirard. 2004. Bacterial flagellins: mediators of pathogenicity and host immune responses in mucosa. Trends Microbiol. 12509-517. [DOI] [PubMed] [Google Scholar]
- 28.Ratiner, Y. A. 1987. Different alleles of the flagellin gene hagB in Escherichia coli standard H test strains. FEMS Microbiol. Lett. 4897-104. [Google Scholar]
- 29.Ratiner, Y. A. 1967. Mutation of E. coli with regard to the H-antigen. I. Isolation of H-antigen mutants from test H-strains of Escherichia culture. Zh. Mikrobiol. Epidemiol. Immunobiol. 4423-29. [PubMed] [Google Scholar]
- 30.Ratiner, Y. A. 1982. Phase variation of the H antigen in Escherichia coli strain Bi7327-41, the standard strain for Escherichia coli flagellar antigen H3. FEMS Microbiol. Lett. 1533-36. [Google Scholar]
- 31.Ratiner, Y. A. 1983. Presence of two structural genes determining antigenically different phase-specific flagellins in Escherichia coli strains. FEMS Microbiol. Lett. 1937-41. [Google Scholar]
- 32.Ratiner, Y. A. 1985. Two genetic arrangement determining flagellar antigen specificities in two diphasic Escherichia coli strains. FEMS Microbiol. Lett. 29317-323. [Google Scholar]
- 33.Ratiner, Y. A. 1998. New flagellin-specifying genes in some Escherichia coli strains. J. Bacteriol. 180979-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ratiner, Y. A. 1991. Serotyping of Escherichia coli flagellar antigens, p. 47-51. In G. Stein and R. Fünfstück (ed.), Harnwegsinfektion: Aktuale Gesichtspunkte zur pathogenese Diagnostik und Therapie. Wissenschaftliches Symposium, Frankfurt am Main, Germany.
- 35.Reed, R. E., and S. Altman. 1983. Repeated sequences and open reading frames in the 3′ flanking region of the gene for the RNA subunit of Escherichia coli ribonuclease P. Proc. Natl. Acad. USA 805359-5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rutherford, K., J. Parkhill, J. Crook, T. Horsnell, P. Rice, M. A. Rajandream, and B. Barrell. 2000. Artemis: sequence visualisation and annotation. Bioinformatics 16944-945. [DOI] [PubMed] [Google Scholar]
- 37.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 38.Saunders, J. R. 1994. Population genetics of phase variable antigens, p. 247-268. In S. Baumberg, J. P. W. Young, E. M. H. Wellington, and J. R. Saunders (ed.), Population genetics of bacteria. Cambridge University Press, London, United Kingdom.
- 39.Schmidt, H., and M. Hensel. 2004. Pathogenicity islands in bacterial pathogenesis. Clin. Microbiol. Rev. 1714-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silverman, M., and M. Simon. 1980. Phase variation: genetic analysis of switching mutants. Cell 19845-854. [DOI] [PubMed] [Google Scholar]
- 41.Staden, R. 1996. The Staden sequence analysis package. Mol. Biotechnol. 5233-241. [DOI] [PubMed] [Google Scholar]
- 42.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 224673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tominaga, A. 2004. Characterization of six flagellin genes in the H3, H53 and H54 standard strains of Escherichia coli. Genes Genet. Syst. 791-8. [DOI] [PubMed] [Google Scholar]
- 44.Tominaga, A., and K. Kutsukake. 2007. Expressed and cryptic flagellin genes in the H44 and H55 type strains of Escherichia coli. Genes Genet. Syst. 821-8. [DOI] [PubMed] [Google Scholar]
- 45.Van Houdt, R., and C. W. Michiels. 2005. Role of bacterial cell surface structures in Escherichia coli biofilm formation. Res. Microbiol. 156626-633. [DOI] [PubMed] [Google Scholar]
- 46.Wang, L., D. Rothemund, H. Curd, and P. R. Reeves. 2003. Species-wide variation in the Escherichia coli flagellin (H-antigen) gene. J. Bacteriol. 1852936-2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamamoto, S., and K. Kutsukake. 2006. FljA-mediated posttranscriptional control of phase 1 flagellin expression in flagellar phase variation of Salmonella enterica serovar Typhimurium. J. Bacteriol. 188958-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu, D., H. M. Ellis, E. C. Lee, N. A. Jenkins, N. G. Copeland, and D. L. Court. 2000. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. USA 975978-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ziebuhr, W., V. Krimmer, S. Rachid, I. Lossner, F. Gotz, and J. Hacker. 1999. A novel mechanism of phase variation of virulence in Staphylococcus epidermidis: evidence for control of the polysaccharide intercellular adhesin synthesis by alternating insertion and excision of the insertion sequence element IS256. Mol. Microbiol. 32345-356. [DOI] [PubMed] [Google Scholar]
- 50.Zieg, J., M. Silverman, M. Hilmen, and M. Simon. 1977. Recombinational switch for gene expression. Science 196170-172. [DOI] [PubMed] [Google Scholar]
- 51.Zieg, J., and M. Simon. 1980. Analysis of the nucleotide sequence of an invertible controlling element. Proc. Natl. Acad. Sci. USA 774196-4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.