Abstract
Ludox density gradients were used to enrich for Escherichia coli mutants with conditional growth defects and alterations in membrane composition. A temperature-sensitive mutant named Lud135 was isolated with mutations in two related, nonessential genes: yghB and yqjA. yghB harbors a single missense mutation (G203D) and yqjA contains a nonsense mutation (W92TGA) in Lud135. Both mutations are required for the temperature-sensitive phenotype: targeted deletion of both genes in a wild-type background results in a strain with a similar phenotype and expression of either gene from a plasmid restores growth at elevated temperatures. The mutant has altered membrane phospholipid levels, with elevated levels of acidic phospholipids, when grown under permissive conditions. Growth of Lud135 under nonpermissive conditions is restored by the presence of millimolar concentrations of divalent cations Ca2+, Ba2+, Sr2+, or Mg2+ or 300 to 500 mM NaCl but not 400 mM sucrose. Microscopic analysis of Lud135 demonstrates a dramatic defect at a late stage of cell division when cells are grown under permissive conditions. yghB and yqjA belong to the conserved and widely distributed dedA gene family, for which no function has been reported. The two open reading frames encode predicted polytopic inner membrane proteins with 61% amino acid identity. It is likely that YghB and YqjA play redundant but critical roles in membrane biology that are essential for completion of cell division in E. coli.
The envelope of Escherichia coli is a remarkable structure consisting of two membranes, an inner membrane (IM) and an outer membrane (OM), separated by the periplasmic space. The OM is an asymmetric lipid bilayer containing lipopolysaccharide (LPS) in the outer leaflet and phospholipids in the inner leaflet. The lipids of the OM are synthesized in the cytoplasm by use of cytoplasmically oriented enzymes and substrates and exported across the IM in an MsbA-dependent manner (15, 17). An msbA temperature-sensitive mutant (WD2) with the mutation A270T was isolated and accumulates newly synthesized LPS and phospholipids at the inner leaflet of the IM after a short growth at the nonpermissive temperature (15, 17). Following growth at the nonpermissive temperature, WD2 was found to migrate anomalously on density gradients of Ludox (14), a colloidal silica that spontaneously forms gradients during moderate centrifugation (38). Using such a gradient with a population of chemically mutagenized E. coli, it was possible to enrich for a collection of temperature-sensitive mutants, many of which contained mutations in the msbA gene and were complemented at the nonpermissive temperature by a plasmid copy of the msbA (14).
In this report, we characterize an unexpected mutant isolated in the same enrichment protocol. This mutant, Lud135, is temperature sensitive for growth and is complemented at 42°C with a plasmid copy of yghB or yqjA. Lud135 is a double mutant harboring mutations in yghB and yqjA resulting in a synthetic growth defect at elevated temperatures. An engineered double-deletion strain was constructed and is phenotypically similar to Lud135. YghB and YqjA are predicted IM proteins of 219 and 220 amino acids, respectively, with 61% amino acid identity to the highly conserved and widely distributed dedA family, for which no function has been reported. yghB and yqjA are nonessential genes and both individual null mutant strains grow normally at all temperatures. The double mutant displays a striking phenotype in that it forms chains of cells under permissive growth conditions, indicating a defect in septation or constriction. In this respect, the mutant resembles a number of strains reported to have septation defects due to mutations affecting envelope maintenance such as envA, envC, or twin arginine pathway genes (28, 35, 36, 40, 44). Unlike these mutants, the mutant reported here is not hypersensitive to detergents or antibiotics, indicating the presence of an intact OM.
Genes predicted to encode proteins with significant amino acid identity to E. coli DedA are present in most bacterial genomes so far sequenced (often multiple times). They are generally not found in eukaryotes, with the exception of certain unicellular algae (see Discussion). In all cases, no function has been described for any member of this family. We show here that YqjA and YghB carry out essential but redundant functions required for completion of cell division in E. coli, possibly due to an effect on membrane phospholipid composition. This work establishes for the first time a critical function for the conserved DedA family of IM proteins and opens the door for a genetic and biochemical analysis of their roles in membrane biology.
MATERIALS AND METHODS
Materials.
Tryptone and yeast extract were from Difco. Radioisotopes were purchased from Perkin Elmer. Restriction enzymes were purchased from New England Biolabs. Polyvinylpyrrolidone and Ludox were purchased from Sigma-Aldrich. All other chemicals were reagent grade and purchased from either Sigma-Aldrich or VWR. The antibiotics tetracycline (Tet; 12.5 μg/ml), ampicillin (100 μg/ml), kanamycin (30 μg/ml), and chloramphenicol (30 μg/ml) were added to LB growth medium as necessary.
Ludox density gradients.
Ludox HS-40 is colloidal silica that forms a density gradient spontaneously upon exposure to moderate ultracentrifugation. Ludox density gradient solutions were made as described previously (14), and gradient formation was carried out by centrifugation at 50,000 × g for 1 h at 4°C. Nitrosoguanidine mutagenesis was carried out as described previously (17). W3110A, WD2, and mutagenized W3110A were grown to an optical density (OD) of approximately 0.5 to 1.0 at 30°C in a volume of 25 ml of LB, and the entire culture was then diluted into 75 ml of LB prewarmed to 44°C. Growth was continued for 30 min at this temperature and cultures were harvested. Cell pellets (∼3 × 1010 cells) were washed two times in ice-cold phosphate-buffered saline (PBS) and then resuspended in 0.2 ml of PBS and layered on top of the preformed Ludox gradients. Gradients were centrifuged at 50,000 × g for 1 hour at 4°C in an SW41 rotor to separate cells by density. Twelve fractions of 0.6 ml were collected by inserting an 18-gauge needle into the side of the ultracentrifuge tube approximately 2 cm from the bottom. LB (0.6 ml) was added immediately to each fraction and an OD at 600 nm (OD600) was measured for each fraction. Fractions containing cells that migrated to a similar density as WD2 from each gradient were pooled, diluted to 25 ml with LB-20% glycerol, and stored at −80°C (14).
Isolation and analysis of temperature-sensitive mutants.
Ludox-enriched dense mutants were plated on LB containing Tet and 0.1% sodium dodecyl sulfate (SDS). The SDS was included to remove mutants containing truncated LPS (“deep rough”), which were isolated at high frequency using this method (14). Surviving colonies were replica plated at 30°C and 42°C and temperature-sensitive colonies were isolated and purified. Competent cells were made by the method of Inoue et al. (21), and colonies were transformed with an E. coli genomic library made in pACYC184 (16). Transformants were selected for chloramphenicol resistance and growth at 42°C. Plasmid DNA was isolated from surviving clones and subjected to DNA sequencing using primers flanking the pACYC184 BamHI site (16). Several plasmids isolated in this manner from Lud135 contained inserts that included the gene yghB. The yghB gene was amplified from Lud135, cloned into plasmid pSC-B by use of the StrataClone blunt PCR cloning kit (Stratagene) according to the manufacturer's instructions, and sequenced. Since E. coli has several homologues of yghB (4), we also sequenced yabI, dedA, yohD, and yqjA from Lud135 in a similar manner.
Construction of plasmid DNA.
E. coli yghB, yqjA, dedA, yabI, and yohD were amplified from genomic DNA by use of primers (Table 1) in a PCR (0.1 ml) containing 2 U Vent polymerase (New England Biolabs), 200 μM deoxynucleoside triphosphates, 200 ng genomic DNA, and 1× ThermoPol reaction buffer. The reaction conditions were as follows: 94°C (denaturing) for 2 min followed by 25 cycles of 94°C for 45 seconds, 55°C (annealing) for 45 seconds, and 72°C (extension) for 1 minute. PCR products were gel purified and digested with NdeI-HindIII or NheI-HindIII and cloned into similarly digested and dephosphorylated vector pET23a. Vectors p-yghB, p-yqjA, p-yabI, and p-yohD were generated by cloning the respective XbaI-HindIII fragments from pET23 into similarly digested and dephosphorylated pACYC184 (Table 2). dedA was cloned in frame with its native ribosomal binding site directly into pACYC184, generating vector p-dedA. Ligation reactions were used to transform competent NovaBlue cells (EMD Biosciences) that were then selected for appropriate antibiotic resistance. The Qiagen Miniprep kit was used to prepare plasmid DNA. Genomic DNA was prepared using the Easy-DNA kit from Invitrogen. DNA sequencing was carried out at the LSU College of Basic Science Genomics Facility.
TABLE 1.
PCR primers used in this study
| E. coli gene | Primer sequence (forward; reverse)a |
|---|---|
| yghB | 5′-CGCGCATATGGCTGTTATTCAAGATATCATCGCTG-3′ (NdeI); 5′-CGCGAAGCTTGGGGAAAATCGTCAGGCGTTACAG-3′ (HindIII) |
| yqjA | 5′-GCGCCATATGGAACTTTTGACCCAATTGCTGCAAGCC-3′ (NdeI); 5′-GCGCAAGCTTGCGAAGCGACATGCGAGGTATTTGCATC-3′ (HindIII) |
| dedA | 5′-GCGCTCTAGAGTAGTTACAGCCTGAAAGATGACGAGTAC-3′ (XbaI); 5′-GCGCAAGCTTGAACCGCGGAGTTACTTTTTATTTTGCG-3′ (HindIII) |
| yabI | 5′-GCGCCATATGGGCACATTATGCAAGCATTGCTGGAAC-3′ (NdeI); 5′-GCGCAAGCTTTCCTAAACCCCAACCACTTTACGC-3′ (HindIII) |
| yohD | 5′-GCGCCATATGGACTGGCCTATGGATCTCAATACACTTATC-3′ (NdeI); 5′-GCGCAAGCTTCAATGGTTTTACGCCTGATGATCCGG-3′ (HindIII) |
Underlining for each primer sequence indicates the site for the restriction endonuclease (given in parentheses) for that sequence.
TABLE 2.
Bacterial strains and plasmids
| Strain or plasmid | Relevant genotype and descriptiona | Source or reference |
|---|---|---|
| E. coli strains | ||
| W3110 | Wild type, F−, λ− | E. coli genetic stock center, Yale University |
| W3110A | aroA::Tn10 (Tetr P1vir transductant of W3110; LCB273 donor) | 15 |
| Lud135 | W3110A (temp-sensitive mutant), yghB1(G203D), yqjA1(W92TGA) | This work |
| JW3066 | Δ(araD-araB)567 ΔlacZ4787(::rrnB-3) λ− ΔyqjA785::Kanrrph-1 Δ(rhaD-rhaB)568 hsdR514 | 1 |
| JW2975 | Δ(araD-araB)567 ΔlacZ4787(::rrnB-3) λ− ΔmetC790::Kanrrph-1 Δ(rhaD-rhaB)568 hsdR514 | 1 |
| JW2976 | Δ(araD-araB)567 ΔlacZ4787(::rrnB-3) λ− ΔyghB781::Kanrrph-1 Δ(rhaD-rhaB)568 hsdR514 | 1 |
| JW3065 | Δ(araD-araB)567 ΔlacZ4787(::rrnB-3) λ− ΔexuR784::Kanrrph-1 Δ(rhaD-rhaB)568 hsdR514 | 1 |
| BC201 | JW2976, ΔyqjA::Tetr | This work |
| BC202 | W3110, ΔyqjA::Tetr ΔyghB781::Kanr | This work |
| BC203 | W3110, ΔyqjA::Tetr | This work |
| BC204 | W3110, ΔyghB781::Kanr | This work |
| Plasmids | ||
| pET23a | Expression vector; T7 promoter; Ampr | Novagen |
| pET23-yghB | pET23 containing E. coli yghB (NdeI/HindIII sites) | This work |
| pET23-yqjA | pET23 containing E. coli yqjA (NdeI/HindIII sites) | This work |
| pET23-yabI | pET23 containing E. coli yabI (NdeI/HindIII sites) | This work |
| pET23-yohD | pET23 containing yohD (NdeI/HindIII sites) | This work |
| pACYC184 | Low-copy-number vector; Tetr Camr | New England Biolabs |
| p-yghB | XbaI/HindIII fragment of pET23-yghB cloned into pACYC184 | This work |
| p-yqjA | XbaI/HindIII fragment of pET23-yqjA cloned into pACYC184 | This work |
| p-dedA | pACYC184 containing E. coli dedA (XbaI/HindIII sites) | This work |
| p-yabI | XbaI/HindIII fragment of pET23-yabI cloned into pACYC184 | This work |
| p-yohD | XbaI/HindIII fragment of pET23-yohD cloned into pACYC184 | This work |
Ampr, ampicillin resistance; Camr, chloramphenicol resistance.
Targeted deletion of yqjA and isolation of strain BC201/BC202.
Replacement of YqjA with the Tet resistance (Tetr) gene from TN10 was carried out using λ-RED-mediated recombination. The Tetr gene was amplified from W3110A genomic DNA by use of forward primer 5′-ATGGAACTTTTGACCCAATTGCTGCAAGCCCTGTGGGCGCAGGATTTTGACAAGAGGGTCATTATATTTCG-3′ and reverse primer 5′-CTTACCCCCGATTTCCATATTTCTTTTTCCATAACACGACCAGAGAACCTACTCGACATCTTGGTTACCG-3′. The gel-purified DNA was used to transform strain DY330 by electroporation (42, 47). Following a 2-hour outgrowth in LB medium at 30°C, Tetr colonies were isolated following an overnight incubation at 30°C. Disruption of the yqjA gene was confirmed for one of these colonies by using PCR amplification with primers designed to flank the yqjA gene. This strategy results in the insertion of the Tetr gene and the deletion of all but the first and last 50 bp of the yqjA gene. A P1vir lysate was prepared from DY330 (ΔyqjA::Tetr) and used to transduce JW2976 (Table 2) to Tetr. This strain, containing the ΔyqjA::Tetr and ΔyghB::Kanr mutations, is referred to here as BC201. BC202 contains the same mutations in the W3110 genetic background (Table 2). Both strains were used in these experiments and were essentially interchangeable. BC203 and BC204 contain the individual ΔyqjA::Tetr and ΔyghB::Kanr mutations, respectively, in the W3110 background (Table 2).
Phospholipid analysis.
W3110A, Lud135, and BC202 were grown at 30°C to OD600s of ∼1.0. Cells were diluted 1:3 into fresh LB medium prewarmed to either 30 or 44°C and grown in a shaking water bath/incubator for 30 min. 32Pi was added to a concentration of 10 μCi/ml and growth was continued for 10 min. The cells were placed in an ice-water bath to cool and final cell density was measured; cell density varied between 0.4 and 0.6. Out of concerns that the mutant cells may be prone to lyse during routine washing and centrifugation, phospholipids were extracted directly from the cell culture without washing. Volumes utilized were corrected for final cell density. Chloroform and methanol were added to cells to a final concentration for chloroform:methanol:water of 1:2:0.8. For acidic extraction, methanol containing 0.1 N HCl was used instead of methanol (34). The extraction mixture was allowed to incubate for 1 h at room temperature with occasional mixing. Insoluble material including LPS was removed by centrifugation for 10 min at 20,000 × g. The supernatant was transferred to a new tube and chloroform and water were added to adjust the ratio of chloroform:methanol:water to 1:1:0.8, resulting in a two-phase mixture. The aqueous upper phase was discarded and the lower phase was washed twice with fresh preequilibrated upper phase. Lipid species were resolved by thin-layer chromatography (TLC) on silica gel 60 plates (Merck) by use of the solvent chloroform:methanol:acetic acid (65:25:10) and analyzed using a Phosphorimager equipped with IQMac software.
Lipid A analysis.
To analyze lipid A, the insoluble material from the phospholipid extraction was dissolved in 0.4 ml 12.5 mM Na acetate, pH 4.5, 1% SDS, heated for 30 min at 95°C to release lipid A from the LPS core sugars, and then extracted by the addition of 1 ml chloroform:methanol (1:1) to yield a two-phase solution. The aqueous upper phase was discarded and the lower phase was washed with fresh preequilibrated upper phase. Lipid species were resolved by TLC on silica gel 60 plates (Merck) by use of the solvent chloroform:pyridine:formic acid:water (50:50:16:5) and analyzed using a Phosphorimager equipped with IQMac software.
Determination of PE topology.
The topology of newly synthesized phosphatidylethanolamine (PE) was examined in Lud135 by use of 2,4,6-trinitrobenzene sulfonic acid (TNBS), an amine-reactive dye that is impermeable to the plasma membrane (15). Bacterial strains were grown 44°C for 30 minutes and then labeled for 10 minutes with 32Pi. Cells were harvested and washed twice with ice-cold PBS, and the cell pellet was resuspended in 50 mM NaHCO3, pH 8.5, containing 100 mM NaCl. TNBS was added to a final concentration of 3 mM and incubation was continued on ice for up to 90 min. The reaction was stopped by the addition of bovine serum albumin (final concentration, 0.25%) and trichloroacetic acid (final concentration, 5%). Phospholipids were extracted using chloroform:methanol:H2O (1:2:0.8) and insoluble material was removed by centrifugation for 10 min at 20,000 × g in a microcentrifuge. The supernatant was converted to a two-phase solution by the addition of chloroform and water to give a chloroform:methanol:H2O ratio of 2:2:1.8. The lower phase was washed with fresh preequilibrated upper phase and dried. Lipid species were resolved by TLC with the solvent chloroform:methanol:H2O (65:25:4) and analyzed using a Phosphorimager equipped with IQMac software.
β-Galactosidase activity of culture supernatants.
Strains were grown with shaking at 30°C until late log phase in LB-Tet-1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) and then diluted 1:25 in LB-Tet-1 mM IPTG prewarmed to 44°C, at which time incubation was continued for 180 min. Cells were removed by twice centrifuging the culture medium at 4,000 × g in a centrifuge for 10 minutes, removing the supernatant after each centrifugation, and filtering the supernatant through a 0.45-μm syringe filter. β-Galactosidase assays were performed on 0.025 ml (media from mutants grown at 44°C) or 0.1 ml of cell-free medium according to the method of Miller (33). Cultures grown at 30°C and the parent strain grown at 44°C had low but reproducibly detectable levels of β-galactosidase present in the culture supernatant.
Differential interference contrast microscopy.
Cells were grown in LB medium to mid-log growth phase and visualized using a Nikon Microphot-FXA microscope. Digital images were captured with a SPOT RT slider charge-coupled-device camera and manipulated with Adobe Photoshop CS software.
Electron microscopy.
Cells were fixed in 2% glutaraldehyde and 1% formaldehyde in half-strength growth medium for 10 min and collected on a 0.22-μm-pore polycarbonate filter. Filtered sample was fixed in 2% glutaraldehyde and 1% formaldehyde in 0.1 M cacodylate buffer at pH 7.0 for 50 min, rinsed four times in 0.1 M buffer plus 0.004 M glycine during a 1-h period, postfixed in 2% aqueous osmium tetroxide for 1 h, rinsed briefly in water, en bloc stained in 0.05% uranyl acetate for 1 h in the dark, and dehydrated through an ethanol series. For transmission electron microscopy (TEM), dehydrated sample was infiltrated in 1:1 ethanol:LR White resin for 1 h and then in 100% resin for 1 h and embedded in resin at 60°C overnight. Sections were cut with a DuPont Sorvall ultramicrotome, collected on a collodion-coated copper grid, stained in Reynolds lead stain, and imaged with a JEOL 100CX TEM. For scanning electron microscopy (SEM), dehydrated sample was critical point dried, mounted on an aluminum specimen stub with carbon tape, coated with 60:40 gold:palladium in an Edwards S-150 sputter coater, and imaged with a Cambridge S-260 SEM.
RESULTS
Isolation of a novel temperature-sensitive mutant from a density-enriched population.
Conditional msbA temperature-sensitive mutant WD2 (17) was observed to sediment faster than parent strain W3110A E. coli on gradients of Ludox (14). We used this observation to devise an enrichment protocol for temperature-sensitive mutants with membrane assembly defects. Several new msbA and htrB mutants with temperature-sensitive growth defects were isolated from a randomly mutagenized population of E. coli (14).
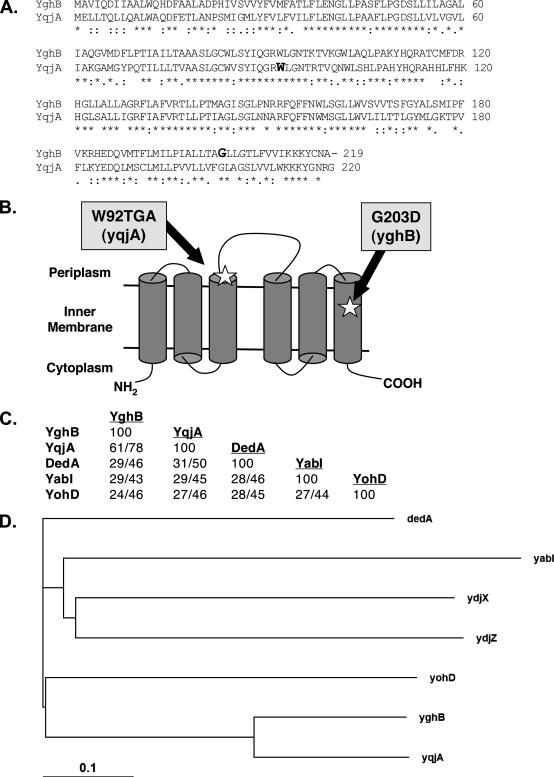
The growth of one mutant isolated from this screen that was not complemented at 42°C by expression of either msbA or htrB was named Lud135 and characterized further. Screening an E. coli genomic library (16) for genes that could restore growth to Lud135 at 42°C resulted in the isolation of several plasmids containing the gene yghB and one containing yabI. These genes are predicted to encode proteins with sequence identity (24 to 61%) with members of the dedA family encoding IM proteins that include yabI, yghB, dedA, yohD, and yqjA in E. coli (Fig. 1). These five genes were amplified and sequenced from Lud135 chromosomal DNA, and while yohD, yabI, and dedA contained no mutations, yghB contained a missense mutation resulting in the amino acid change G203D (GGC to GAC), and yqjA harbored a nonsense mutation in codon 92 resulting in a change from a tryptophan codon to a stop codon (TGG to TGA) (Fig. 1A and B). YghB and YqjA are predicted polytopic IM proteins that exhibit 61% amino acid identity to each other over the entire length of the proteins (Fig. 1A). Prediction programs and experimental assignment of the C terminus to the cytoplasm (8) suggest that both proteins likely have six membrane-spanning domains with a periplasmic loop between transmembrane helices three and four (Fig. 1B). However, other topologies are possible and this topology will need to be experimentally verified.
FIG. 1.
The E. coli DedA family of related predicted IM proteins. (A) Amino acid alignment between YqjA and YghB. W92 and G203, the sites of mutations in Lud135 in YqjA and YghB, respectively, are shown in bold. (B) Predicted IM topology of E. coli YghB and YqjA according to the Polyphobius prediction program (22) and experimental assignment of the C terminus to the cytoplasm (8). Both proteins are predicted to have six membrane spans and the indicated topology. Note that not all programs predict the same topology and alternate models are possible. The locations of the missense mutation in YghB (G203D) and the nonsense mutation in YqjA (W92TGA) in Lud135 are shown. (C) Percents identity and similarity between each DedA family member found in E. coli (45). All members display significant protein BLAST scores to each other (E values, <10−4), but there are no clearly conserved domains when all five proteins are aligned (not shown). YqjA and DedA display some similarity to YdjZ and YdjX, respectively (with 25 and 22% identity). However, other DedA family members are not significantly similar to YdjZ and YdjX, and so they are not included in this analysis for simplicity. (D) Phylogenetic tree showing evolutionary relationships between the E. coli proteins with similarity to DedA. The more distantly related YdjX and YdjZ were included for comparison. The analysis was performed using ClustalX (23).
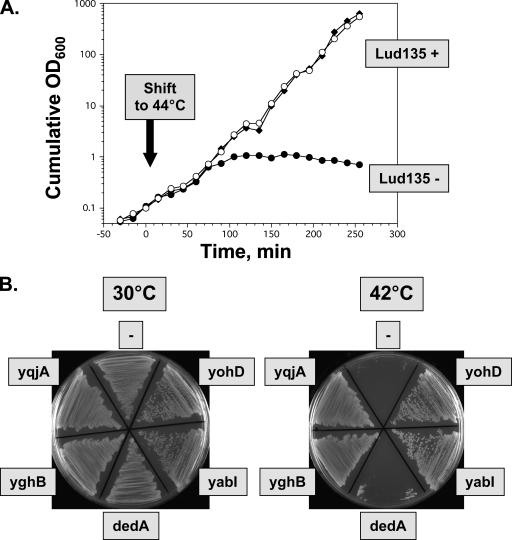
E. coli yghB and yqjA were amplified from parent strain W3110A genomic DNA and cloned into low-copy-number vector pACYC184 (6) to give vectors p-yghB and p-yqjA, respectively (Table 2). Lud135 was transformed with pACYC184, p-yghB, and p-yqjA, and growth of these strains was monitored in liquid culture following a temperature shift to 44°C (Fig. 2A). Lud135/pACYC184 grows for approximately 1.5 h at the elevated temperature and begins to lose viability, as determined by measurement of culture density. Lud135/p-yghB and Lud135/p-yqjA showed no such growth defect. yabI, yohD, and dedA were also cloned and expressed in Lud135 and growth was monitored on agar plates. Growth of Lud135 at 42°C is complemented by expression of yghB and yqjA, as expected, and also by yabI and yohD but not dedA, indicating some functional overlap between these family members (Fig. 2B).
FIG. 2.
Lud135, a temperature-sensitive yghB yqjA mutant. (A) Lud135 transformed with vector pACYC184 (Lud135−, black circles), p-yghB (Lud135+, black diamonds), or p-yqjA (Lud135+, white circles) was grown at 30°C to an OD600 of ∼1.0 in LB-chloramphenicol and then diluted 10-fold into prewarmed medium (44°C); growth was subsequently monitored at 15-min intervals. Cultures were diluted 10-fold into fresh prewarmed medium when the culture density reached an OD600 of 0.3 to 0.4 to maintain logarithmic growth. Cumulative growth was calculated by correcting for dilution. (B) Growth of Lud135/pACYC184 (labeled “−”), Lud135/p-yqjA, Lud135/p-yghB, Lud135/p-dedA, Lud135/p-yohD, and Lud135/p-yabI on LB-chloramphenicol agar plates at 30 and 42°C.
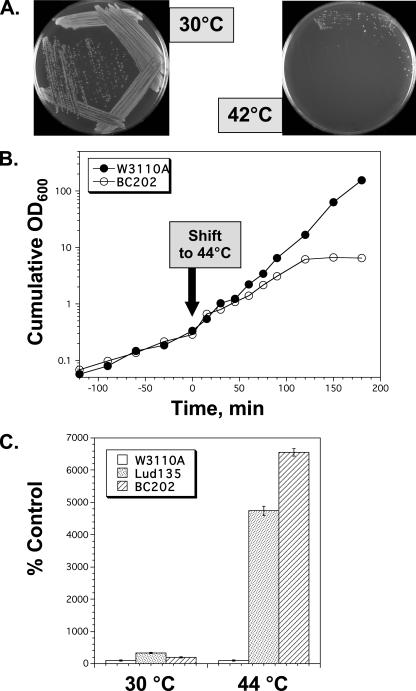
The identification of mutations in both genes suggests that the growth defect of Lud135 may be due to either of these mutations alone or both mutations exhibiting a synthetic phenotype. yghB is located near minute 67.9 of the E. coli chromosome and yqjA is located near minute 70 (4), which is not close enough for cotransduction of these alleles by P1 phage (33). P1 transduction of an antibiotic resistance marker adjacent to yghB (metC::Kanr) or yqjA (exuR::Kanr) (Table 2) into Lud135 (to independently correct one or the other mutation) results each time in a strain that is not temperature sensitive for growth (data not shown). Individual deletion mutants of each of these genes have been made as part of the Keio collection (1), and both strains grow normally at all temperatures in our hands (Table 2 and data not shown). yghB could be deleted from Lud135 (which contains the yqjA nonsense mutation), and the resulting strain displays the same growth phenotype as Lud135 (data not shown), indicating that the yghB missense mutation is phenotypically indiscernible from the null mutation in the ΔyqjA null background. To confirm that the effects seen in Lud135 are not due to other unlinked mutations in this mutagenized background, targeted deletion of YqjA (ΔyqjA::Tetr) was made in strain JW2976 (1), which harbors the ΔyghB781::Kanr deletion (BC201) (Table 2). Both mutations were also introduced into the W3110 background for better comparison with Lud135 (BC202) (Table 2). BC202 also displays a temperature-sensitive growth phenotype when grown on LB agar plates (Fig. 3A). In contrast to Lud135, which stops growing after approximately 90 min at 42°C in liquid culture (Fig. 2A), BC202 grows for nearly 3 hours before growth arrest (Fig. 3B). The differences between Lud135 and BC202 in this respect are most likely due to the presence of additional unlinked mutations in Lud135, isolated from a chemically mutagenized population. These data confirm that the combination of mutations of yghB and yqjA results in a synthetic growth defect at elevated temperatures. YghB and YqjA therefore play redundant but essential roles in some aspect of cellular physiology required for growth at 42°C.
FIG. 3.
BC202, an engineered yghB yqjA double-deletion strain. (A) Growth of BC202 (ΔyghB::Kanr ΔyqjA::Tetr) on LB agar plates at 30 and 42°C. (B) Growth of W3110 and BC202 in liquid medium at 44°C. Cell growth was monitored as described in the legend to Fig. 2. (C) Lud135 and BC202 undergo lysis during growth at 44°C. W3110A, Lud135, and BC202 were grown in LB-Tet medium containing 1 mM IPTG at 30°C to OD600s of 0.80, 0.91, and 0.76, respectively. One milliliter of each culture was added to 25 ml of LB-Tet-IPTG prewarmed to 44°C and cultures were grown with shaking for 3 hours at this temperature. Final OD600s of the 44°C-grown cultures were 1.33, 0.31, and 0.36, respectively. Cell-free medium was recovered by centrifuging the cultures and filtering supernatants through a 0.45-μm filter. β-Galactosidase activity in the medium was assayed as described previously (33). Values represent the averages of three determinations and error bars indicate standard deviations.
Both Lud135 and BC202 undergo lysis at 44°C (Fig. 3C). During growth at 44°C for 3 hours, Lud135 and BC202 released approximately 40- and 60-fold more cytoplasm, respectively, than the parent strain, as measured by the presence of β-galactosidase in the culture supernatant (cells were grown in the presence of 1 mM IPTG to induce the lac operon). β-Galactosidase activity was low but detectable in cell-free medium from cultures grown at 30°C or W3110A grown at 44°C for the same amount of time.
Lud135 and BC202 display the same temperature-sensitive growth phenotype when grown in M9 minimal medium (not shown). The mutants also grow at 37°C on plates and in liquid medium (not shown), although we define 30°C as the permissive growth condition in the experiments reported herein.
Restoration of growth of Lud135/BC201 with divalent cations and high concentrations of NaCl.
The growth of Lud135 and BC201 on plates at 42°C is completely restored by the addition of >5 mM divalent cations such as Mg2+, Ba2+, Sr2+, and Ca2+ (Table 3). The growth of Lud135/BC201 was also analyzed in the presence of different concentrations of sodium chloride, and a complex pattern, nearly identical for both strains, was revealed (Table 3). The mutants did not grow at 30°C on LB plates containing less than 100 mM or greater than 600 mM NaCl. The mutants were not sensitive to hypo-osmotic shock per se, in that they could survive for 1 hour as well as the parent strain a shift from 170 mM to 0 mM NaCl (data not shown). Very small colonies were visible following an overnight incubation at 42°C on LB plates containing 300 to 500 mM NaCl (the normal concentration being 10 g/liter or approximately 170 mM NaCl). These colonies, while small, were viable in that they could be regrown under permissive conditions on normal LB plates. The inclusion of 400 mM sucrose in LB plates did not support the growth of the mutants at 42°C, ruling out an osmotic contribution by the salts.
TABLE 3.
Visible colony formation of Lud135 and BC201 when grown on LB media containing different supplements
| LB medium supplement (concn in mM)a | Colony formation at indicated temp seen forb:
|
|||
|---|---|---|---|---|
| Lud135
|
BC201
|
|||
| 30°C | 42°C | 30°C | 42°C | |
| None | + | − | + | − |
| Ca2+ (10) | + | + | + | + |
| Sr2+ (10) | + | + | + | + |
| Mg2+ (10) | + | + | + | + |
| Ba2+ (5) | + | + | + | + |
| NaCl (0) | − | − | − | − |
| NaCl (100) | − | − | + | − |
| NaCl (170)c | + | − | + | − |
| NaCl (200) | + | − | + | −/+ |
| NaCl (300) | + | + | + | + |
| NaCl (400) | + | + | + | + |
| NaCl (500) | + | + | + | + |
| NaCl (600) | + | − | + | −/+ |
| Sucrose (400) | + | − | + | − |
Calcium, magnesium, strontium, barium, sodium chloride, or sucrose was included in LB agar plates at the indicated concentrations.
Lud135 or BC201 (∼500 CFU) was plated and incubated at 30 and 42°C overnight. Parent strain W3110A grew under all conditions (not shown). A plus sign (+) indicates clearly visible colony formation, a minus sign (−) indicates no visible colony formation, and minus/plus (−/+) indicates barely visible colony formation after the overnight incubation.
LB normally contains ∼170 mM NaCl (10 g/liter).
Analysis of phospholipid and lipid A synthesis by Lud135/BC202.
Since Lud135 was isolated using a technique that enriches for msbA mutants, it was of interest to investigate lipid topology, lipid biosynthesis, and lipid export to the OM. The topology of newly synthesized PE was determined using TNBS and was not grossly altered in Lud135/BC202 (data not shown), with the majority being internally oriented and not accessible to the dye (15). Additionally, it was found that Lud135/BC202 does not display the same lipid export defect seen for WD2 (W3110A, msbA2) (17). No accumulation in the IM of lipid A or phospholipids is observed using sucrose gradients following growth at any temperature (data not shown).
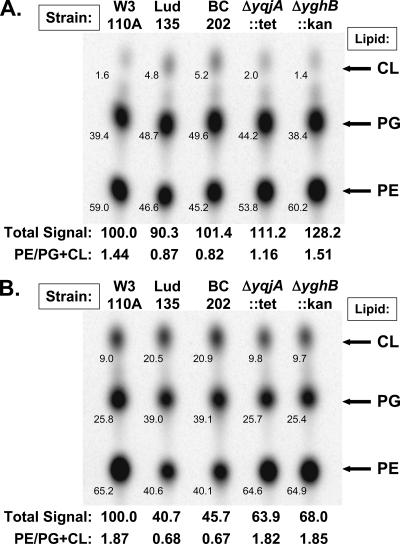
We analyzed the phospholipid composition in Lud135 and its parent strain W3110A as well as in the double-deletion mutant BC202 (ΔyqjA::Tetr ΔyghB781::Kanr) and each single mutant BC203 (ΔyqjA::Tetr) and BC204 (ΔyghB781::Kanr) (Table 2). Cells were grown at 30°C until late log phase, diluted threefold into fresh medium at 30°C or 44°C, grown for 30 minutes, and then labeled with 32Pi for 10 minutes. Lipids were extracted from equal cell numbers (volumes of cells adjusted according to final OD600) without prior washing due to concerns that material might be lost due to cell lysis of mutants during repeated centrifugation. At 30°C, phospholipids were synthesized at near-normal levels in the double mutants, but the composition of individual lipid species was altered (Fig. 4A). In parent strain W3110A and BC204, PE comprised ∼60% of total membrane phospholipids. In Lud135 and BC202, PE comprised <50% of the total membrane phospholipids and there were elevated levels of acidic phospholipids phosphatidylglycerol (PG) and cardiolipin (CL). BC203 (ΔyqjA::Tetr), interestingly, displayed a reproducible “intermediate” phenotype between double mutants and the parent strain in terms of percentage of acidic phospholipids at this growth temperature.
FIG. 4.
Membrane phospholipid analysis of mutant Lud135, BC202, and single-deletion strains. W3110A, Lud135, BC202 (W3110; ΔyghB::Kanr ΔyqjA::Tetr), BC203 (W3110; ΔyqjA::Tetr), and BC204 (W3110; ΔyghB::Kanr) were grown at 30°C (A) and 44°C (B) for 30 min and labeled with 32Pi for 10 minutes. Equal numbers of cells, based on final OD600, were extracted directly from growth media without washing (out of concerns that multiple rounds of centrifugation may cause cell lysis of the mutants), and phospholipids were resolved by TLC in the solvent chloroform:methanol:acetic acid at a 65:25:10 ratio. The small number on the lower left hand side of each spot gives the percent contribution of each lipid species to the total phospholipid composition for each strain. The value obtained by adding the signal strength for each lipid species from each strain and dividing by the value obtained for W3110A at each temperature (arbitrarily set to 100) was defined as the total relative signal. This is a representative experiment, with the numbers corresponding to the data shown. Nearly identical data were obtained on four separate occasions.
At 44°C, W3110A and the single-deletion mutants synthesized similar levels of PE, elevated levels of CL, and reduced levels of PG compared to the levels produced at 30°C (Fig. 4B). Importantly, the ratios of PE to acidic phospholipids (CL plus PG) were similar in these strains regardless of growth temperature. Phospholipid levels were severely reduced in Lud135/BC202 at 44°C, and both mutants synthesized a proportion of acidic phospholipids PG and CL even higher than that for PE. PE accounted for only ∼40% of the total phospholipids synthesized under these growth conditions. Incorporation of 32Pi into phospholipids was decreased at 44°C, but this was probably a result of the loss of membrane potential at this temperature, since incorporation of 32Pi into nucleic acids was reduced by ∼50% in the mutants under these growth conditions as well (data not shown). The structure and amounts of lipid A in the mutants at 30°C were unchanged from the parent strain, with the majority hexa-acylated glucosamine disaccharide with two or three phosphates (data not shown). Similar results were obtained regardless of labeling protocol (steady-state labeling versus pulse-labeling) or extraction procedure (acidic versus neutral Bligh-Dyer extraction). Levels of incorporation of [35S]methionine into protein were similar in all strains at 30°C but were repressed following growth at 44°C (not shown).
Lud135 and BC202 were not found to be any more sensitive than the parent strain at 30°C to any antibiotic (polymyxin, chloramphenicol, kanamycin, rifampin, neomycin, ampicillin, vancomycin) or detergent {SDS, deoxycholic acid, 3-[(3-cholamidopropyl)-dimethyammonio]-1-propanesulfonate (CHAPS), Triton X-100 } tested (data not shown), suggesting a largely intact OM when the cells are grown under permissive conditions. All our dense Ludox mutants were screened for SDS resistance to remove deep rough mutants, which were frequently coisolated with dense mutants (see Materials and Methods). Lud135 was therefore not expected to be hypersensitive to this detergent.
Lud135 and BC201 display defects in cell division.
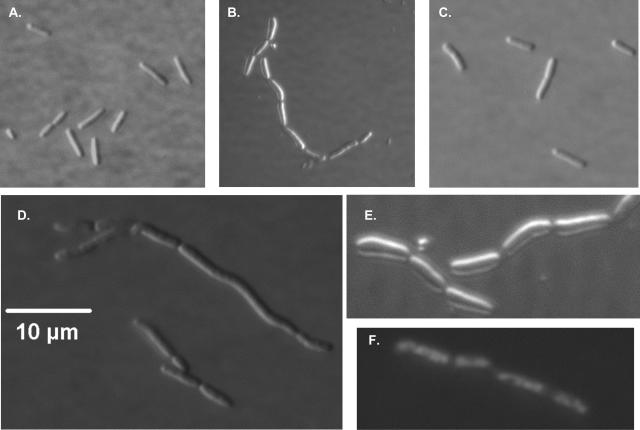
Examination of W3110A, Lud135, and BC201 grown at 30°C using differential interference contrast microscopy, TEM, and SEM is shown in Fig. 5 to 7. Lud135/pACYC184 displays a phenotype similar to what is observed for certain cell division mutants, especially those blocked at a late stage of cell division, such as the FtsK mutants (13). While the cells are generally not filamentous, they appear to begin but cannot complete constriction at the septal ring (Fig. 5B and E). Chains composed of six to nine cells are commonly observed, while shorter chains and individual cells are observed but less common. Expression of wild-type yghB from a plasmid (Fig. 5C) or inclusion of 10 mM Mg2+ in the growth medium (data not shown) restores normal cell division to Lud135. The engineered double-knockout strain BC201 displays a very similar phenotype to Lud135, with many chains apparent and occasional signs of filamentation (Fig. 5D). Nucleoid segregation is not affected in Lud135, as demonstrated by DAPI (4′,6′-diamidino-2-phenylindole) staining (Fig. 5F). It is likely that YghB and YqjA play essential but redundant roles in cell division, possibly through an effect on membrane lipid composition. The single-deletion mutants BC203 and BC204 (Table 2) appear normal in this analysis (data not shown). Lud135 and BC201 grown briefly at 44°C display a phenotype similar to that observed when the cells are grown at 30°C (data not shown).
FIG. 5.
Differential interference contrast microscopy of Lud135/BC201. Cells were grown at 30°C in liquid LB medium to mid-log phase and visualized using a Nikon Microphot-FXA phase-contrast microscope. (A) Parent strain W3110A. (B) Lud135/pACYC184. (C) Lud135/p-yghB. (D) BC201. (E) Lud135/pACYC184 (close-up). (F) DAPI-stained Lud135/pACYC184 illustrating nucleoid segregation. Lud135 cells grown briefly at 42°C display a similar phenotype (not shown).
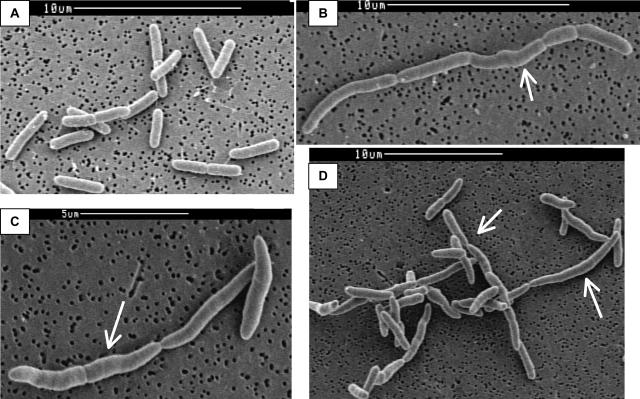
FIG. 7.
SEM of W3110A, Lud135, and BC201. W3110A (A), Lud135 (B), and BC201 (C and D) cells were grown at 30°C in liquid LB medium to mid-log phase, treated as described in Materials and Methods, and imaged with a Cambridge S-260 SEM. Lud135/BC201 shows cell division defects, including the formation of chains of incompletely divided cells and signs of filamentation and membrane bulging (arrows).
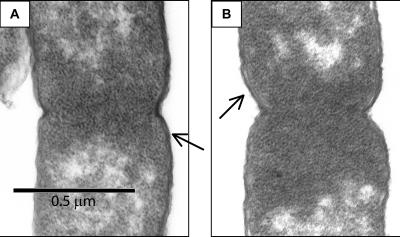
TEM was conducted to investigate envelope structure in the mutants. We were especially interested in any visible changes that might occur at the septal ring. BC201 (Fig. 6A and B) and Lud135 (data not shown) are not grossly different from the parent strain in this analysis, although we cannot rule out subtle effects due to the mutations on membrane and septal ring structure. SEM (Fig. 7A to D) reveals a greater level of detail with regard to the cell structure. In addition to the chaining phenotype, septums are only partially constricted, with the septation process appearing halted at a stage following initial constriction. Additionally, Lud135 (Fig. 7B) and BC201 (Fig. 7C and D) show signs of bulging along portions of the cell wall.
FIG. 6.
TEM of W3110A, Lud135, and BC201. W3110A (A) and BC201 (B) cells were grown at 30°C in liquid LB medium to mid-log phase, treated as described in Materials and Methods, and imaged with a JEOL 100CX TEM. Both cells are visualized at the same stage of septation. Arrows point to regions where IMs and OMs can be distinguished.
DISCUSSION
We have reported previously that Ludox density gradients were used to enrich for msbA and htrB mutants from a population of chemically mutagenized E. coli (14). One intriguing mutant that did not fit this category (Lud135) is temperature sensitive due to two serendipitous mutations: a missense mutation in the gene yghB (G203D) and a nonsense mutation in yqjA (W92TGA). These genes encode proteins with high amino acid identity that are members of the conserved and widely distributed dedA gene family. Plasmid copies of either gene restore growth at elevated temperatures to Lud135 (Fig. 2A and B) and neither mutation alone is sufficient for the observed growth phenotype. A strain containing targeted deletions of yghB and yqjA (BC201/BC202) is phenotypically similar to Lud135 (Fig. 3). Millimolar concentrations of divalent cations restore growth to the mutant at 42°C (Table 3). Lud135/BC202 has an altered composition of membrane phospholipids with increased ratios of acidic (CL plus PG) to the zwitterionic phospholipids (PE) regardless of growth temperature (Fig. 4). At the permissive temperature of 30°C, Lud135/BC201 is defective in the completion of cell division (Fig. 5 to 7) but is not hypersensitive to antibiotics and detergents.
The E. coli genome contains a number of dedA family members encoding proteins of ∼20 to 60% amino acid identity that are all annotated as being nonessential predicted IM proteins, including dedA, yghB, yqjA, yabI, and yohD (Fig. 1) (4). There is little reported on the function of DedA family members in the literature, and the functions of YghB or YqjA have never been addressed to our knowledge. The primary sequences of the E. coli DedA family offer few clues to their function.
dedAs comprise a large gene family found widespread in eubacteria and some archaea but are not present in most sequenced eukaryotic genomes. The exception to these generalizations is the presence of dedAs in the genomes of eukaryotic green algae Ostreococcus tauri and Chlamydomonas reinhardtii (12, 27) (E value for protein BLAST scores against E. coli YqjA, <1 × 10−10) and their absence from certain prokaryotic hyperthermophiles, including the Thermotogae, Thermodesulfobacteria, and Aquificae lineages. The significance of the phylogenetic distribution of this gene family is not clear at this time, but the high level of evolutionary conservation speaks to their functional importance. Currently, there are more than 1,000 genes in the online database annotated as being dedA family members or possessing significant amino acid identity to E. coli DedA (E value for protein BLAST scores against E. coli YqjA, <0.02). Of course, it remains to be seen whether they function similarly in their respective organisms.
Hints as to the function of this widely distributed gene family have come from high-throughput or DNA microarray studies. A DedA orthologue from Ralstonia metallidurans was shown to be required for resistance to selenium (24). Another DedA family member isolated from an environmental library has been shown to support the growth of E. coli on 4-hydroxybutyrate (19). YqjA expression is regulated by σE in E. coli (9) and by PhoP in Salmonella enterica (43). A DedA gene is required for resistance to cationic antimicrobial peptides in Neisseria meningitidis (46) and S. enterica (43). The expression of E. coli YghB was significantly induced (11-fold) by the quorum-sensing molecule AI-2 in a DNA microarray study (11). These studies collectively suggest that DedA family members may function in envelope biogenesis and maintenance.
It is possible that some aspect of lipid asymmetry or lipid domain formation is compromised in Lud135/BC201. The septal membrane is an example of a bacterial membrane microdomain and is enriched in CL (26, 30, 31). Perhaps YghB/YqjA in some way promotes lipid domain formation (a bacterial “caveolin”?) by assisting in the formation of CL-enriched domains at the septum. It is also possible that YghB/YqjA serves a “scaffolding” function to recruit proteins to a membrane complex. Analysis of protein-protein interactions may provide clues to the function of these proteins. A recent large-scale analysis of the interaction network of roughly 20% of E. coli proteins by use of tandem affinity purification was largely biased toward essential proteins and did not include YghB or YqjA as “bait,” nor were they found to partner with any of the baited proteins (5).
Another possibility is that YghB and YqjA play a role in driving membrane hemifusion at the septal ring, playing a role analogous to eukaryotic SNARE (SNAP receptor) proteins (2, 7). The cell division phenotype reported here supports such a role, and bioinformatics provides some interesting clues. It is common to find dedA family members annotated as “SNARE-associated Golgi protein” in many genome databases where they are found. However, Tvp38, the Saccharomyces cerevisiae protein in question that was found to colocalize with tSNARE in Tlg2-containing Golgi subcompartments (20), is more similar to E. coli YdjX than to YghB or YqjA. YdjX is in turn 25% identical to YqjA, with less identity to YghB. Since none of the other components of the eukaryotic vesicle fusion machinery, including SNARE, have obvious prokaryotic homologues, this may or may not turn out to be significant. In any case, the mutants reported here, Lud135 and BC201, represent valuable tools to study the functions of these genes in a well-characterized model organism.
The cell division defect of Lud135/BC201 is especially striking. It is important to restate that this cell division defect occurs under permissive growth conditions, implying a central role for YghB/YqjA during normal cell division, and is not just an artifact of cell death taking place at elevated temperatures. It is likely that YghB and YqjA play essential but redundant roles in membrane biology necessary for the completion of cell division. Cytokinesis in bacteria occurs following the correct positioning of the septal ring by the MinCDE proteins. There occurs a recruitment in a hierarchical and linear manner of at least 10 critical proteins (18, 25), beginning with FtsZ, a conserved tubulin homologue. The block in cell division in Lud135/BC201 appears to occur at a step following FtsZ ring formation and is thus not likely to be an effect of an induction of SOS response-induced FtsZ assembly inhibitors such as SulA (3) but may be due to FtsZ mislocalization. YghB and YqjA may play a role in recruitment of a critical cell division protein or may play an essential role on their own in cytokinesis. A similar phenotype has been reported for other mutants with pleiotropic envelope defects, including lpxC (envA) point mutants (35, 36), mutants lacking components of the twin arginine transport pathway (44), and the murein hydrolase EnvC mutant (28, 40). Like Lud135/BC201, the envC mutant PM61 did display intriguing alterations in phospholipid composition (28, 29), so perhaps the cell division defect seen here is secondary to the observed phospholipid alterations. Unlike these mutants, Lud135/BC201 is not hypersensitive to detergents or antibiotics, indicating the presence of an intact OM, at least under permissive growth conditions.
It is known that bacteria precisely control their membrane lipid composition and that alterations in composition due to mutation have effects upon membrane protein folding and numerous cell functions, including cell division (reviewed in reference 48). While it is not clear if YghB and YqjA play direct or indirect roles in determining membrane lipid composition, in several respects Lud135/BC201 resembles the PE-deficient mutant AD93 (pss93::Kanr) (10, 32). PE is not required for the growth of E. coli under all conditions, and cells containing null or temperature-sensitive mutations in pssA are devoid of PE and have cell division defects (10, 32, 39). The growth of AD93 entirely dependent upon the presence of high levels of Mg2+ or Ca2+, which promote the formation of nonbilayer phases of CL (37). PE is a membrane lipid that has the natural potential to undergo a bilayer-to-nonbilayer transition at temperatures near but above the growth temperature. Such behavior is important for membrane fusion to take place, as occurs during cell division. Both Lud135 and AD93 therefore display a dependence upon divalent cations for growth and display cell division defects. Important differences between Lud135 and AD93 are that Ba2+ is effective at supporting growth of Lud135 at 42°C and that divalent cations are not required for growth at 30°C (Table 3). In addition, AD93 shows no dependence upon NaCl for growth, while Lud135/BC201 grows at 42°C on LB plates containing 300 to 500 mM NaCl. Of course, Lud135 does synthesize significant amounts of PE under all growth conditions and the lipid does appear to be oriented topologically correctly, with most being internally oriented and inaccessible to TNBS, as has been reported previously (15, 41).
A puzzling aspect of this work is that yghB and yqjA are both nonessential genes in E. coli, and individual in-frame deletion mutants have been made as part of the Keio collection and grow at all temperatures (1). It is perhaps a serendipitous finding of mutations in both genes in the same strain. YqjA and YghB are 61% identical at the amino acid level and plasmid copies of either gene restore growth to Lud135, showing that the proteins can function redundantly. Our P1 transduction experiments and construction of the targeted double-deletion strain BC201 show that both mutations are required for the growth phenotype of Lud135. More may be learned about the dedA family by studying multiple-deletion mutants rather than single mutants due to their apparent built-in redundancy.
In summary, we have utilized a new protocol for enriching for temperature-sensitive E. coli msbA mutants and other mutants with membrane defects. This procedure is based upon altered migration on a Ludox density gradient and is highly specific for conditional msbA missense mutants. We have isolated and provided preliminary characterization of a temperature-sensitive strain containing a nonsense mutation in yqjA and a missense mutation in yghB, two members of the widely distributed dedA gene family encoding polytopic IM proteins of unknown function. The isolation of Lud135 would not have been possible without prior Ludox enrichment of mutants. These results indicate that this procedure may be useful in identifying other genes involved in membrane biology in gram-negative bacteria.
The exact roles of yqjA and yghB remain to be determined, but this is to our knowledge the first report of a requirement for growth at elevated temperatures of any dedA family members or for a role in lipid synthesis or cell division. Lud135/BC201 provides an important tool to understand the functions of this highly conserved and mysterious gene family.
Acknowledgments
We thank Christian R. H. Raetz for his past and continuing support of our work. Lud135 was isolated in a screen carried out in his laboratory.
Work in the laboratory of Christian R. H. Raetz was supported partly by his National Institute of Health grant, GM-51310. Additional financial support came from the NIH (1 F32 AI-10613-01; W.T.D.) and the Louisiana Board of Regents LEQSF (2005-08)-RD-A-04 (W.T.D.).
Footnotes
Published ahead of print on 2 May 2008.
REFERENCES
- 1.Baba, T., T. Ara, M. Hasegawa, Y. Takai, Y. Okumura, M. Baba, K. A. Datsenko, M. Tomita, B. L. Wanner, and H. Mori. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 22006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barr, F. A., and U. Gruneberg. 2007. Cytokinesis: placing and making the final cut. Cell 131847-860. [DOI] [PubMed] [Google Scholar]
- 3.Bi, E., and J. Lutkenhaus. 1993. Cell division inhibitors SulA and MinCD prevent formation of the FtsZ ring. J. Bacteriol. 1751118-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 2771453-1474. [DOI] [PubMed] [Google Scholar]
- 5.Butland, G., J. M. Peregrin-Alvarez, J. Li, W. Yang, X. Yang, V. Canadien, A. Starostine, D. Richards, B. Beattie, N. Krogan, M. Davey, J. Parkinson, J. Greenblatt, and A. Emili. 2005. Interaction network containing conserved and essential protein complexes in Escherichia coli. Nature 433531-537. [DOI] [PubMed] [Google Scholar]
- 6.Chang, A. C., and S. N. Cohen. 1978. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J. Bacteriol. 1341141-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chernomordik, L. V., and M. M. Kozlov. 2005. Membrane hemifusion: crossing a chasm in two leaps. Cell 123375-382. [DOI] [PubMed] [Google Scholar]
- 8.Daley, D. O., M. Rapp, E. Granseth, K. Melen, D. Drew, and G. von Heijne. 2005. Global topology analysis of the Escherichia coli inner membrane proteome. Science 3081321-1323. [DOI] [PubMed] [Google Scholar]
- 9.Dartigalongue, C., D. Missiakas, and S. Raina. 2001. Characterization of the Escherichia coli sigma E regulon. J. Biol. Chem. 27620866-20875. [DOI] [PubMed] [Google Scholar]
- 10.DeChavigny, A., P. N. Heacock, and W. Dowhan. 1991. Sequence and inactivation of the pss gene of Escherichia coli. Phosphatidylethanolamine may not be essential for cell viability. J. Biol. Chem. 2665323-5332. [PubMed] [Google Scholar]
- 11.DeLisa, M. P., C. F. Wu, L. Wang, J. J. Valdes, and W. E. Bentley. 2001. DNA microarray-based identification of genes controlled by autoinducer 2-stimulated quorum sensing in Escherichia coli. J. Bacteriol. 1835239-5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Derelle, E., C. Ferraz, S. Rombauts, P. Rouze, A. Z. Worden, S. Robbens, F. Partensky, S. Degroeve, S. Echeynie, R. Cooke, Y. Saeys, J. Wuyts, K. Jabbari, C. Bowler, O. Panaud, B. Piegu, S. G. Ball, J. P. Ral, F. Y. Bouget, G. Piganeau, B. De Baets, A. Picard, M. Delseny, J. Demaille, Y. Van de Peer, and H. Moreau. 2006. Genome analysis of the smallest free-living eukaryote Ostreococcus tauri unveils many unique features. Proc. Natl. Acad. Sci. USA 10311647-11652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diez, A. A., A. Farewell, U. Nannmark, and T. Nystrom. 1997. A mutation in the ftsK gene of Escherichia coli affects cell-cell separation, stationary-phase survival, stress adaptation, and expression of the gene encoding the stress protein UspA. J. Bacteriol. 1795878-5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doerrler, W. T. 2007. Density gradient enrichment of Escherichia coli conditional msbA mutants. Appl. Environ. Microbiol. 737992-7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doerrler, W. T., H. S. Gibbons, and C. R. H. Raetz. 2004. MsbA-dependent translocation of lipids across the inner membrane of Escherichia coli. J. Biol. Chem. 27945102-45109. [DOI] [PubMed] [Google Scholar]
- 16.Doerrler, W. T., and C. R. H. Raetz. 2005. Loss of outer membrane proteins without inhibition of lipid export in an Escherichia coli YaeT mutant. J. Biol. Chem. 28027679-27687. [DOI] [PubMed] [Google Scholar]
- 17.Doerrler, W. T., M. C. Reedy, and C. R. H. Raetz. 2001. An Escherichia coli mutant defective in lipid export. J. Biol. Chem. 27611461-11464. [DOI] [PubMed] [Google Scholar]
- 18.Goehring, N. W., and J. Beckwith. 2005. Diverse paths to midcell: assembly of the bacterial cell division machinery. Curr. Biol. 15R514-R526. [DOI] [PubMed] [Google Scholar]
- 19.Henne, A., R. Daniel, R. A. Schmitz, and G. Gottschalk. 1999. Construction of environmental DNA libraries in Escherichia coli and screening for the presence of genes conferring utilization of 4-hydroxybutyrate. Appl. Environ. Microbiol. 653901-3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inadome, H., Y. Noda, Y. Kamimura, H. Adachi, and K. Yoda. 2007. Tvp38, Tvp23, Tvp18 and Tvp15: novel membrane proteins in the Tlg2-containing Golgi/endosome compartments of Saccharomyces cerevisiae. Exp. Cell Res. 313688-697. [DOI] [PubMed] [Google Scholar]
- 21.Inoue, H., H. Nojima, and H. Okayama. 1990. High efficiency transformation of Escherichia coli with plasmids. Gene 9623-28. [DOI] [PubMed] [Google Scholar]
- 22.Kall, L., A. Krogh, and E. L. Sonnhammer. 2004. A combined transmembrane topology and signal peptide prediction method. J. Mol. Biol. 3381027-1036. [DOI] [PubMed] [Google Scholar]
- 23.Larkin, M. A., G. Blackshields, N. P. Brown, R. Chenna, P. A. McGettigan, H. McWilliam, F. Valentin, I. M. Wallace, A. Wilm, R. Lopez, J. D. Thompson, T. J. Gibson, and D. G. Higgins. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 232947-2948. [DOI] [PubMed] [Google Scholar]
- 24.Ledgham, F., B. Quest, T. Vallaeys, M. Mergeay, and J. Coves. 2005. A probable link between the DedA protein and resistance to selenite. Res. Microbiol. 156367-374. [DOI] [PubMed] [Google Scholar]
- 25.Lutkenhaus, J. 2007. Assembly dynamics of the bacterial MinCDE system and spatial regulation of the Z ring. Annu. Rev. Biochem. 76539-562. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto, K., J. Kusaka, A. Nishibori, and H. Hara. 2006. Lipid domains in bacterial membranes. Mol. Microbiol. 611110-1117. [DOI] [PubMed] [Google Scholar]
- 27.Merchant, S. S., S. E. Prochnik, O. Vallon, E. H. Harris, S. J. Karpowicz, G. B. Witman, A. Terry, A. Salamov, L. K. Fritz-Laylin, L. Marechal-Drouard, W. F. Marshall, L. H. Qu, D. R. Nelson, A. A. Sanderfoot, M. H. Spalding, V. V. Kapitonov, Q. Ren, P. Ferris, E. Lindquist, H. Shapiro, S. M. Lucas, J. Grimwood, J. Schmutz, P. Cardol, H. Cerutti, G. Chanfreau, C. L. Chen, V. Cognat, M. T. Croft, R. Dent, S. Dutcher, E. Fernandez, H. Fukuzawa, D. Gonzalez-Ballester, D. Gonzalez-Halphen, A. Hallmann, M. Hanikenne, M. Hippler, W. Inwood, K. Jabbari, M. Kalanon, R. Kuras, P. A. Lefebvre, S. D. Lemaire, A. V. Lobanov, M. Lohr, A. Manuell, I. Meier, L. Mets, M. Mittag, T. Mittelmeier, J. V. Moroney, J. Moseley, C. Napoli, A. M. Nedelcu, K. Niyogi, S. V. Novoselov, I. T. Paulsen, G. Pazour, S. Purton, J. P. Ral, D. M. Riano-Pachon, W. Riekhof, L. Rymarquis, M. Schroda, D. Stern, J. Umen, R. Willows, N. Wilson, S. L. Zimmer, J. Allmer, J. Balk, K. Bisova, C. J. Chen, M. Elias, K. Gendler, C. Hauser, M. R. Lamb, H. Ledford, J. C. Long, J. Minagawa, M. D. Page, J. Pan, W. Pootakham, S. Roje, A. Rose, E. Stahlberg, A. M. Terauchi, P. Yang, S. Ball, C. Bowler, C. L. Dieckmann, V. N. Gladyshev, P. Green, R. Jorgensen, S. Mayfield, B. Mueller-Roeber, S. Rajamani, R. T. Sayre, P. Brokstein, et al. 2007. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 318245-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michel, G., D. Di Savino, and J. Starka. 1977. Phospholipid composition and phenotypic correction of an envC division mutant of Escherichia coli. J. Bacteriol. 129145-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michel, G. P., D. Karibian, N. Bonnaveiro, and J. Starka. 1985. Is there a correlation between membrane phospholipid metabolism and cell division? Ann. Inst. Pasteur Microbiol. 136A111-118. [DOI] [PubMed] [Google Scholar]
- 30.Mileykovskaya, E., and W. Dowhan. 2005. Role of membrane lipids in bacterial division-site selection. Curr. Opin. Microbiol. 8135-142. [DOI] [PubMed] [Google Scholar]
- 31.Mileykovskaya, E., and W. Dowhan. 2000. Visualization of phospholipid domains in Escherichia coli by using the cardiolipin-specific fluorescent dye 10-N-nonyl acridine orange. J. Bacteriol. 1821172-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mileykovskaya, E., Q. Sun, W. Margolin, and W. Dowhan. 1998. Localization and function of early cell division proteins in filamentous Escherichia coli cells lacking phosphatidylethanolamine. J. Bacteriol. 1804252-4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 34.Nishijima, M., and C. R. H. Raetz. 1979. Membrane lipid biogenesis in Escherichia coli: identification of genetic loci for phosphatidylglycerophosphate synthetase and construction of mutants lacking phosphatidylglycerol. J. Biol. Chem. 2547837-7844. [PubMed] [Google Scholar]
- 35.Normark, S., H. G. Boman, and G. D. Bloom. 1971. Cell division in a chain-forming envA mutant of Escherichia coli K12. Fine structure of division sites and effects of EDTA, lysozyme and ampicillin. Acta Pathol. Microbiol. Scand. B 79651-664. [DOI] [PubMed] [Google Scholar]
- 36.Normark, S., H. G. Boman, and E. Matsson. 1969. Mutant of Escherichia coli with anomalous cell division and ability to decrease episomally and chromosomally mediated resistance to ampicillin and several other antibiotics. J. Bacteriol. 971334-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ortiz, A., J. A. Killian, A. J. Verkleij, and J. Wilschut. 1999. Membrane fusion and the lamellar-to-inverted-hexagonal phase transition in cardiolipin vesicle systems induced by divalent cations. Biophys. J. 772003-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poole, R. K. 1977. Fluctuations in buoyant density during the cell cycle of Escherichia coli K12: significance for the preparation of synchronous cultures by age selection. J. Gen. Microbiol. 98177-186. [DOI] [PubMed] [Google Scholar]
- 39.Raetz, C. R. H. 1976. Phosphatidylserine synthetase mutants of Escherichia coli. Genetic mapping and membrane phospholipid composition. J. Biol. Chem. 2513242-3249. [PubMed] [Google Scholar]
- 40.Rodolakis, A., P. Thomas, and J. Starka. 1973. Morphological mutants of Escherichia coli. Isolation and ultrastructure of a chain-forming envC mutant. J. Gen. Microbiol. 75409-416. [DOI] [PubMed] [Google Scholar]
- 41.Rothman, J. E., and E. P. Kennedy. 1977. Rapid transmembrane movement of newly synthesized phospholipids during membrane assembly. Proc. Natl. Acad. Sci. USA 741821-1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sawitzke, J. A., L. C. Thomason, N. Costantino, M. Bubunenko, S. Datta, and D. L. Court. 2007. Recombineering: in vivo genetic engineering in E. coli, S. enterica, and beyond. Methods Enzymol. 421171-199. [DOI] [PubMed] [Google Scholar]
- 43.Shi, Y., M. J. Cromie, F. F. Hsu, J. Turk, and E. A. Groisman. 2004. PhoP-regulated Salmonella resistance to the antimicrobial peptides magainin 2 and polymyxin B. Mol. Microbiol. 53229-241. [DOI] [PubMed] [Google Scholar]
- 44.Stanley, N. R., K. Findlay, B. C. Berks, and T. Palmer. 2001. Escherichia coli strains blocked in Tat-dependent protein export exhibit pleiotropic defects in the cell envelope. J. Bacteriol. 183139-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tatusova, T. A., and T. L. Madden. 1999. BLAST 2 Sequences, a new tool for comparing protein and nucleotide sequences. FEMS Microbiol. Lett. 174247-250. [DOI] [PubMed] [Google Scholar]
- 46.Tzeng, Y. L., K. D. Ambrose, S. Zughaier, X. Zhou, Y. K. Miller, W. M. Shafer, and D. S. Stephens. 2005. Cationic antimicrobial peptide resistance in Neisseria meningitidis. J. Bacteriol. 1875387-5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu, D., H. M. Ellis, E. C. Lee, N. A. Jenkins, N. G. Copeland, and D. L. Court. 2000. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. USA 975978-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang, Y. M., and C. O. Rock. 2008. Membrane lipid homeostasis in bacteria. Nat. Rev. Microbiol. 6222-233. [DOI] [PubMed] [Google Scholar]