Abstract
The incidence of invasive group A streptococcal disease has increased in Norway since the 1980s. Analysis of 100 isolates recovered from 1988 to 2003 showed an increased genotypic diversity over time, while the prevalence of the strain that dominated in 1988, sequence type (ST)-28/emm-1, decreased. Necrotizing fasciitis was often associated with ST-15/emm-3.
Streptococcus pyogenes (group A streptococci [GAS]) is a common human pathogen responsible for a variety of clinical manifestations (4, 8, 16). Most common are superficial infections of the upper respiratory tract or skin, while invasive infections such as necrotizing fasciitis (NF) and streptococcal toxic shock syndrome (STSS) can be life-threatening (8, 11, 16).
A major virulence factor of GAS is the M protein. Different M-protein types are epidemiologically associated with particular syndromes; e.g., M-protein type 1 (M-1) and M-3 are associated with higher frequencies of severe invasive disease and increased rates of case fatality (21, 23, 27). Introduction of penicillin virtually eliminated GAS in developed countries until the 1980s. Then, increased invasiveness, reflected by an increased number of cases of septicemia, NF, and STSS, were reported from the United States and Europe (8, 12, 17).
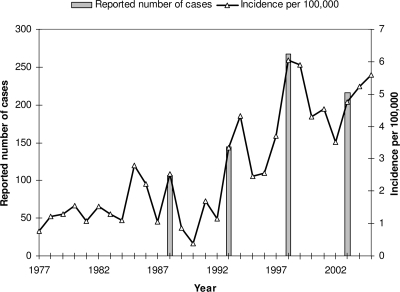
The Norwegian Notification System for Infectious Disease (www.msis.no) at the Norwegian Institute of Public Health (NIPH) has recorded cases of GAS bacteremia since 1977. Incidence rates were less than 1.5 per 100,000 population until 1985 and 1988, when two outbreaks occurred. This was followed by an unusually low incidence rate during the next winter (17). A new outbreak developed in 1993-1994, followed by high incidence rates in the years thereafter. In 1998, yet another outbreak occurred, and the incidence rate was 5.8 per 100,000 population. This is, to date, the highest incidence recorded in Norway (Fig. 1).
FIG. 1.
Incidence and occurrence rate (per 100,000 population per year) of invasive GAS infections in Norway (population, 4.5 million), 1977 to 2005. The number of reported cases is indicated for the 4 years of our study.
The National Reference Laboratory for Streptococci at NIPH receives most GAS isolates from invasive cases of infection that occur throughout Norway (NIPH received between 67% and 82% of all GAS isolates in the years during the period considered). To determine whether changes in the epidemiological situation reflected changes in the disease-causing strains, we characterized 100 isolates causing invasive disease using antibiotic susceptibility testing, T typing, testing for serum opacity factor (SOF), emm typing, and multilocus sequence typing (MLST). By using the random number generator “randbetween (bottom, top)” (Microsoft Excel software), 25 isolates from each of the years 1988, 1993, 1998, and 2003 were selected from among the isolates from invasive cases received at NIPH. These represented from 8% to 16% of the cases of which NIPH was notified in these 4 years and, to our knowledge, contain no systematic biases.
All isolates were grown in 5% CO2 at 35°C overnight and were examined for antibiotic resistance by Etest (AB Biodisk, Solna, Sweden), as recommended by the manufacturer. The MICs of benzylpenicillin, clindamycin, erythromycin, ofloxacin, tetracycline, and trimethoprim-sulfamethoxazole were determined. T typing and testing for SOF were performed as described previously (18, 19). Chromosomal DNA was isolated by boiling in Tris-EDTA buffer or, when that was not sufficient, by the protocol given at http://www.cdc.gov/ncidod/biotech/strep/protocol_emm-type.htm. MLST and emm typing was performed as described previously(3, 9) with a pipetting robotic platform (Epmotion 5075; Eppendorf, Hamburg, Germany). The PCR products were purified with exonuclease I and shrimp alkaline phosphatase (USB Corporation, Cleveland, OH). The sequencing products were cleaned by filtration with a Montage SEQ96 sequencing reaction cleanup kit (Millipore, Billerica, MA) and were sequenced on an ABI 3730 DNA analyzer (Applied Biosystems, Foster City, CA). New emm subtypes and new MLST alleles and sequence types (STs) were assigned by the curators of the databases (http://www.cdc.gov/ncidod/biotech/strep/strepblast.htm and http://Spyogenes.mlst.net, respectively).
The 100 isolates were from patients whose ages ranged from 1 to 98 years. Five patients had been diagnosed with STSS (one 11-year-old and four adults 25 to 63 years old) and 12 patients with NF (all but one were adults 39 to 74 years old). Meningitis cases were detected in children or young adults, while pneumonia usually occurred in children or elderly individuals.
The most prevalent T types were T-1/SOF negative (48%), T-3/SOF negative (14%), and T-28/SOF positive (9%) (Table 1). emm typing revealed 19 emm types and 25 emm subtypes, including 6 new subtypes (subtypes 3,38; 6,60; 5,62; 53,8; st106M,3; and 90,4). emm-1 was the most prevalent emm type (48%) and included three subtypes. emm-3 (15%) included two subtypes, and six isolates were emm-28,0.
TABLE 1.
ST, emm type, T type, presence of SOF, and type of disease for 100 S. pyogenes isolates from invasive cases, Norway, 1988 to 2003
| STa | No. of isolates | emmb | T type | Presence of SOF | Disease manifestationd |
|---|---|---|---|---|---|
| ST-5 | 1 | 83,1 | 13,B3264 | − | Pne |
| ST-15 | 11 | 3,1 | 3 | − | Bac (6), Men, NF (4) |
| ST-15 | 2 | 3,1 | NTc | − | Bac, NF |
| ST-15 | 2 | 3,38 | 3 | − | Bac, STSS |
| ST-28 | 45 | 1,0 | 1 | − | Bac (31), Men (2), NF (4), Pne (4), STSS (4) |
| ST-28 | 1 | 1,18 | 1 | − | Bac |
| ST-28 | 1 | 1,22 | 1 | − | Bac |
| ST-36 | 3 | 12,0 | 12 | − | Bac |
| ST-36 | 1 | 12,0 | NT | − | NF |
| ST-37 | 1 | 6,60 | 6 | − | Bac |
| ST-37 | 1 | 6,60 | NT | − | Bac |
| ST-38 | 1 | 4,0 | 4 | + | Bac |
| ST-39 | 3 | 4,0 | 4 | + | Bac |
| ST-46 | 1 | 22,0 | 12 | + | Bac |
| ST-46 | 1 | 22,0 | 3,13,B3264,12 | + | Bac |
| ST-52 | 6 | 28,0 | 28 | + | Bac (4), NF (2) |
| ST-62 | 1 | 87,0 | 28 | + | Bac |
| ST-63 | 1 | 77,0 | 13 | + | Bac |
| ST-63 | 2 | 77,0 | 28 | + | Bac |
| ST-99 | 1 | 5,62 | 5,27,44 | − | Men |
| ST-150 | 1 | st1815,0 | 8,25,imp19 | + | Bac |
| ST-168 | 1 | 120,0 | 3 | − | Bac |
| ST-253 | 1 | 78,0 | 11 | − | Bac |
| ST-312 | 1 | 92,0 | 8,imp19 | − | Bac |
| ST-340 | 1 | 53,2 | 8 | − | Bac |
| ST-382 | 2 | 6,0 | 6 | − | Bac, Men |
| ST-382 | 1 | 6,0 | 13 | − | Bac |
| ST-415 | 1 | 1,0 | 1 | − | Bac |
| ST-416 | 1 | 53,8 | NT | − | Bac |
| ST-417 | 1 | st106M,3 | 8,imp19 | + | Bac |
| ST-418 | 1 | 90,4 | 23 | − | Bac |
| ST-419 | 1 | 22,0 | 13 | + | Bac |
| ST-420 | 1 | 80,0 | 14 | − | Bac |
STs in boldface are STs new to the MLST database.
emm types with the st prefix are relatively new to the emm database and have not yet been assigned to a distinct emm type. emm types in boldface are new subtypes of the said emm type to the emm database.
NT, not typeable.
Bac, bacteremia; Men, meningitis; Pne, pneumonia. The numbers in parentheses indicate the numbers of cases.
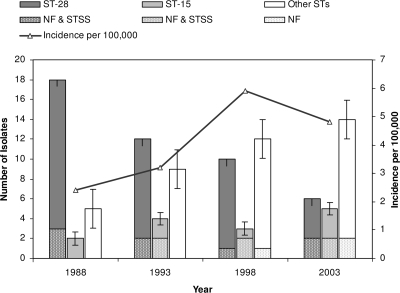
MLST identified 24 STs, 7 of which were new (Table 1). ST-28 was the most prevalent (47%), followed by ST-15 (15%); each of the other STs was represented by only 1 to 6% of the isolates. The prevalence of ST-28 declined from 72% in 1988 to 24% in 2003, while that of ST-15 increased from 8% in 1988 to 20% in 2003 (Fig. 2). The number of STs encountered among the 25 isolates from each year increased from 6 in 1988 to 13 in 2003. Simpson's index of diversity increased from 0.43 in 1988 (95% confidence interval [CI], 0.17 to 0.69) to 0.90 in 2003 (95% CI, 0.79 to 1.01). Seven STs were new to the MLST database, and these were seen more often in the later years. There were five single-locus variants (SLVs): ST-28 and ST-415 (emm-1), ST-340 and ST-416 (emm-53), ST-37 and ST-382 (emm-6), ST-38 and ST-39 (emm-4), and finally, ST-5 and ST-340. The last pair, however, did not share emm types. For three of the five SLVs, the differences were single nucleotide changes that occurred at different positions in the gtr locus.
FIG. 2.
Distributions of ST-28, ST-15, and other STs as well as the distributions of cases of NF and STSS caused by these STs during the 4 years investigated.
Susceptibility testing revealed two erythromycin-resistant isolates, one of which was also resistant to tetracycline. Tetracycline-resistant isolates were genetically diverse (five STs) and were mainly emm-22, emm-53, or emm-77. All five emm-6 isolates were ofloxacin resistant (MIC range, 16 to 32 μg/ml), and finally, two ST-28/emm-1 isolates were resistant to trimethoprim-sulfamethoxazole. While macrolide resistance rates of over 20% were reported from Italy (6) and both macrolide and tetracycline resistance rates of over 90% have been found in China (10), antibiotic resistance in GAS is still rare in Norway. This finding is consistent with previous reports from both Norway and Denmark (13, 14). Only one ofloxacin-resistant emm-6 isolate has been reported previously (15); however, the major part of fluoroquinolone nonsusceptibility is found in emm-6 strains (1, 15).
By randomly selecting 25 isolates from our strain collection for each of the 4 years, isolates from only a small and variable proportion (8 to 16%) of the total number of cases that occurred during those years was analyzed. While the number of cases produced by the identified strains may also vary between the years that we selected, our study shows a significant trend of increasing genetic diversity among GAS isolates in Norway between 1988 and 2003.
The results of MLST correlated well with those of T typing and emm typing and also resolved a new SLV of ST-28 (ST-415) that was otherwise indistinguishable by emm typing or T typing. Nearly two-thirds of our isolates were ST-28 and ST-15, which have been associated with the dissemination of M-1 and M-3 strains around the world (20, 21, 26). It must be stressed, however, that substantial levels of genetic diversity may exist among strains of the same MLST and/or emm type. In 1988, T-1 strains caused an outbreak in Norway with a more than doubling of the incidence in comparison with that in the previous year (Fig. 1). Neighboring countries, Sweden and Denmark, were also affected, and case fatality rates of 48% were reported (2, 25). At the same time, England and the United States experienced marked increases in M-1 and M-3 cases, and these were associated with increased case fatality rates (5, 22). In recent years, emm-28 dominated in countries neighboring Norway, Denmark and Finland (14, 24), while emm-89 dominated in Sweden (7). In Norway, ST-28/emm-1 still remained the most prevalent strain in 2003, even though its frequency had dropped considerably in the previous 15 years (Fig. 2). This trend is apparently still continuing, as in 2006 only 13 of 158 (8%) isolates received at NIPH were T type T-1. It might be speculated that the ST-28/emm-1 strain has changed genetically and is somewhat less virulent than it was in the late 1980s.
Of the 15 cases associated with ST-15/emm-3 strains, 6 (40%) were cases of NF or STSS, while only 11 additional cases of NF or STSS were caused by strains of other STs (chi-square = 6.62; P < 0.02). This suggests that ST-15 isolates are more virulent than other strains, as also reported in a study from Canada, where M-3 isolates more often caused NF and death than M-1 isolates did (23).
Our study demonstrated a significant change in the genotype distribution of GAS strains associated with invasive disease in Norway over a period of 15 years. It is of outmost importance to continue to carefully monitor the epidemiological trends of this pathogen. Because of the severity of the clinical presentation, the increase in ST-15/emm-3 cases is of concern.
Acknowledgments
The excellent technical assistance of Gro Lermark, Jan Oksnes, Torill Alvestad, and Anne-Marie Klem is acknowledged.
This publication made use of the MLST website (http://www.mlst.net) at Imperial College London, developed by David Aanensen and funded by the Wellcome Trust. Funding was provided by Norwegian Research Council grant 166001/v40 to D.A.C.
Footnotes
Published ahead of print on 16 April 2008.
REFERENCES
- 1.Albertí, S., G. Cortés, C. García-Rey, C. Rubio, F. Baquero, J. Á. García-Rodríguez, E. Bouza, and L. Aguilar. 2005. Streptococcus pyogenes pharyngeal isolates with reduced susceptibility to ciprofloxacin in Spain: mechanisms of resistance and clonal diversity. Antimicrob. Agents Chemother. 49418-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen, M. M., and T. Rønne. 1995. Group A streptococcal bacteraemias in Denmark 1987-89. J. Infect. 3133-37. [DOI] [PubMed] [Google Scholar]
- 3.Beall, B., R. Facklam, and T. Thompson. 1996. Sequencing emm-specific PCR products for routine and accurate typing of group A streptococci. J. Clin. Microbiol. 34953-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carapetis, J. R., A. C. Steer, E. K. Mulholland, and M. Weber. 2005. The global burden of group A streptococcal diseases. Lancet Infect. Dis. 5685-694. [DOI] [PubMed] [Google Scholar]
- 5.Colman, G., A. Tanna, A. Efstratiou, and E. T. Gaworzewska. 1993. The serotypes of Streptococcus pyogenes present in Britain during 1980-1990 and their association with disease. J. Med. Microbiol. 39165-178. [DOI] [PubMed] [Google Scholar]
- 6.Creti, R., M. Imperi, L. Baldassarri, M. Pataracchia, S. Recchia, G. Alfarone, and G. Orefici. 2007. emm types, virulence factors, and antibiotic resistance of invasive Streptococcus pyogenes isolates from Italy: what has changed in 11 years? J. Clin. Microbiol. 452249-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darenberg, J., B. Luca-Harari, A. Jasir, A. Sandgren, H. Pettersson, C. Schalen, M. Norgren, V. Romanus, A. Norrby-Teglund, and B. H. Normark. 2007. Molecular and clinical characteristics of invasive group A streptococcal infection in Sweden. Clin. Infect. Dis. 45450-458. [DOI] [PubMed] [Google Scholar]
- 8.Efstratiou, A. 2000. Group A streptococci in the 1990s. J. Antimicrob. Chemother. 453-12. [DOI] [PubMed] [Google Scholar]
- 9.Enright, M. C., B. G. Spratt, A. Kalia, J. H. Cross, and D. E. Bessen. 2001. Multilocus sequence typing of Streptococcus pyogenes and the relationships between emm type and clone. Infect. Immun. 692416-2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jing, H. B., B. A. Ning, H. J. Hao, Y. L. Zheng, D. Chang, W. Jiang, and Y. Q. Jiang. 2006. Epidemiological analysis of group A streptococci recovered from patients in China. J. Med. Microbiol. 551101-1107. [DOI] [PubMed] [Google Scholar]
- 11.Keefer, C. S., F. J. Ingelfinger, and W. W. Spink. 1937. Significance of hemolytic streptococcic bacteremia: a study of two hundred and forty-six patients. Arch. Intern. Med. 501084-1097. [Google Scholar]
- 12.Lamagni, T. L., A. Efstratiou, J. Vuopio-Varkila, A. Jasir, and C. Schalén. 2005. The epidemiology of severe Streptococcus pyogenes associated disease in Europe. Euro. Surveill. 10179-184. [PubMed] [Google Scholar]
- 13.Littauer, P., D. A. Caugant, M. Sangvik, E. A. Høiby, A. Sundsfjord, and G. S. Simonsen. 2006. Macrolide-resistant Streptococcus pyogenes in Norway: population structure and resistance determinants. Antimicrob. Agents Chemother. 501896-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luca-Harari, B., K. Ekelund, M. van der Linden, M. Staum-Kaltoft, A. M. Hammerum, and A. Jasir. 2008. Clinical and epidemiological aspects of invasive Streptococcus pyogenes infections in Denmark during 2003 and 2004. J. Clin. Microbiol. 4679-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malhotra-Kumar, S., C. Lammens, S. Chapelle, C. Mallentjer, J. Weyler, and H. Goossens. 2005. Clonal spread of fluoroquinolone non-susceptible Streptococcus pyogenes. J. Antimicrob. Chemother. 55320-325. [DOI] [PubMed] [Google Scholar]
- 16.Martin, J. M., and M. Green. 2006. Group A streptococcus. Semin. Pediatr. Infect. Dis. 17140-148. [DOI] [PubMed] [Google Scholar]
- 17.Martin, P. R., and E. A. Høiby. 1990. Streptococcal serogroup A epidemic in Norway 1987-1988. Scand. J. Infect. Dis. 22421-429. [DOI] [PubMed] [Google Scholar]
- 18.Maxted, W. R., J. P. Widdowson, C. A. M. Fraser, L. C. Ball, and D. C. J. Bassett. 1973. Use of the serum-opacity reaction in the typing of group-A streptococci. J. Med. Microbiol. 613. [DOI] [PubMed] [Google Scholar]
- 19.Moody, M. D., J. Padula, D. Lizana, and C. T. Hall. 1965. Epidemiologic characterization of group A streptococci by T-agglutination and M-precipitation tests in the public health laboratory. Health Lab. Sci. 2149-162. [PubMed] [Google Scholar]
- 20.Musser, J. M., V. Kapur, J. Szeto, X. Pan, D. S. Swanson, and D. R. Martin. 1995. Genetic diversity and relationships among Streptococcus pyogenes strains expressing serotype M1 protein: recent intercontinental spread of a subclone causing episodes of invasive disease. Infect. Immun. 63994-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Brien, K. L., B. Beall, N. L. Barrett, P. R. Cieslak, A. Reingold, M. M. Farley, R. Danila, E. R. Zell, R. Facklam, B. Schwartz, and A. Schuchat. 2002. Epidemiology of invasive group a streptococcus disease in the United States, 1995-1999. Clin. Infect. Dis. 35268-276. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz, B., R. R. Facklam, and R. F. Breiman. 1990. Changing epidemiology of group A streptococcal infection in the USA. Lancet 3361167-1171. [DOI] [PubMed] [Google Scholar]
- 23.Sharkawy, A., D. E. Low, R. Saginur, D. Gregson, B. Schwartz, P. Jessamine, K. Green, and A. McGeer. 2002. Severe group A streptococcal soft-tissue infections in Ontario: 1992-1996. Clin. Infect. Dis. 34454-460. [DOI] [PubMed] [Google Scholar]
- 24.Siljander, T., M. Toropainen, A. Muotiala, N. P. Hoe, J. M. Musser, and J. Vuopio-Varkila. 2006. emm typing of invasive T28 group A streptococci, 1995-2004, Finland. J. Med. Microbiol. 551701-1706. [DOI] [PubMed] [Google Scholar]
- 25.Strömberg, A., V. Romanus, and L. G. Burman. 1991. Outbreak of group A streptococcal bacteremia in Sweden: an epidemiologic and clinical study. J. Infect. Dis. 164595-598. [DOI] [PubMed] [Google Scholar]
- 26.Svensson, N., S. Öberg, B. Henriques, S. Holm, G. Källenius, V. Romanus, and J. Giesecke. 2000. Invasive group A streptococcal infections in Sweden in 1994 and 1995: epidemiology and clinical spectrum. Scand. J. Infect. Dis. 32609-614. [DOI] [PubMed] [Google Scholar]
- 27.Veasy, L. G., L. Y. Tani, J. A. Daly, K. Korgenski, L. Miner, J. Bale, E. L. Kaplan, J. M. Musser, and H. R. Hill. 2004. Temporal association of the appearance of mucoid strains of Streptococcus pyogenes with a continuing high incidence of rheumatic fever in Utah. Pediatrics 113e168-e172. [DOI] [PubMed] [Google Scholar]