Abstract
In this study, the use of isothermal microcalorimetry (IMC) for differentiation between methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-susceptible S. aureus (MSSA) and MIC determination was evaluated. It was possible to differentiate between MRSA and MSSA within 4 h, whereas the standard method required 24 h. The MICs of cefoxitin were successfully determined for MRSA and MSSA by using IMC.
Methicillin-resistant Staphylococcus aureus (MRSA) infections continue to be associated with significant adverse outcomes and increased health care costs (7). One of the reasons for this is the time required for detection, which is still at least 48 h by conventional methods (4, 11). A faster MRSA detection method would allow faster interventions and rapid implementation of targeted infection control measures (3, 6, 9).
Isothermal microcalorimetry (IMC) is a universal tool for real-time evaluation of rate processes in small (e.g., 3- to 20-ml) ampoules, including processes involving cultured cells. Microcalorimetry has long been used to study the metabolism of cultured cells. James reviewed work in cellular microcalorimetry in 1987 (8) and reported a 1918 paper by Hill as the earliest employing microcalorimetry to study bacteria. Recent studies suggest that IMC may have clinical diagnostic potential. We have reported the potential use of IMC to detect Staphylococcus aureus or other microorganisms in blood products, i.e., platelet concentrates (12).
In this study, we evaluated the use of IMC to differentiate between MRSA and methicillin-susceptible S. aureus (MSSA) and to determine the MICs of cefoxitin for MRSA and MSSA.
The strains used in this study, S. aureus ATCC 43300 (MRSA) and S. aureus ATCC 25923 (MSSA), were both cultivated overnight at 37°C in brain heart infusion (BHI) broth. They were then adjusted using phosphate-buffered saline to an optical density at 600 nm (OD600) of 0.01 and used to inoculate the samples at 0.01% (vol/vol).
The IMC instrument used was a TAM III thermostat (TA Instruments, New Castle, DE) equipped with 48 microcalorimeters. The detection limit is ∼0.27 μW, which corresponds to approximately 135,000 CFU/ampoule (8). Samples were 4-ml sealed glass ampoules containing 3 ml BHI and the bacterial inoculum, leaving a headspace of 1 ml air. After equilibration of the microcalorimeters themselves for at least 45 min at 37°C, the samples were lowered into the equilibration position. After 15 min, the samples were further lowered into the measuring position, and after 45 min (when the heat flow signal was stable), the actual measurement began. This time was taken as time zero.
The calorimetric differentiation between MRSA and MSSA was performed according to the protocol described by the British Society for Antimicrobial Chemotherapy (BSAC) (1), modified in that we used BHI as the growth medium and 4 mg liter−1 oxacillin instead of 2 mg liter−1 as the breakpoint concentration. For comparison, BHI agar plates and BHI broth cultures supplemented with either cefoxitin or oxacillin were prepared and kept in an incubator or water bath. In these control samples, the MSSA strain was completely inhibited for 24 h of incubation at 37°C whereas the MRSA strain showed visible growth after only 18 h. For 24 h of incubation, oxacillin inhibited the growth of the MRSA strain much more than cefoxitin did (final OD600, 0.35 and 0.76, respectively).
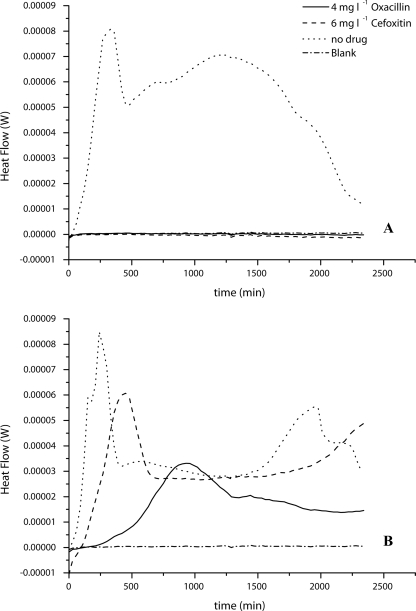
The calorimetric measurements showed the same results. For MSSA, only the culture without any antibiotics showed a measurable heat flow signal, and detection took only 60 min (Fig. 1A). The MRSA strain grew in every sample, but the increase in inhibition with increased antibiotic could be seen in the heat flow curves (Fig. 1B). It took 240 min for a detectable heat flow signal in the sample with oxacillin but only 100 min in the sample with cefoxitin. However, both antibiotics had metabolic inhibitory effects on the MRSA, which can be seen in the reduced maximum heat flow signal (Fig. 1B).
FIG. 1.
Heat flow rate curves for calorimetric measurements of MRSA-MSSA differentiation by use of the antibiotic cefoxitin or oxacillin. (A) S. aureus ATCC 25923 (MSSA) in BHI supplemented with the given concentrations of antibiotics; (B) S. aureus ATCC 43300 (MRSA) in BHI supplemented with the given concentrations of antibiotics. Blank, uninoculated BHI broth. Curves are averages for three experiments.
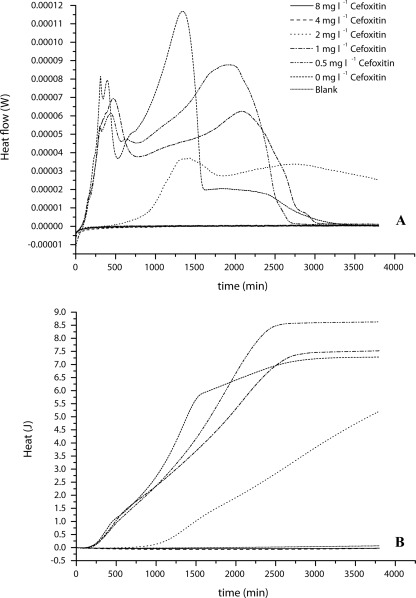
The determination of the MIC for cefoxitin was performed in cation-adjusted Mueller-Hinton broth, using a modified protocol for the broth dilution test described by the CLSI (formerly NCCLS) (2). Instead of 35°C, the samples were incubated at 37°C. Microcalorimetry was performed as described above. A set of ampoules was additionally incubated in a water bath, and the OD600 was determined after 24 h of incubation. The tested concentrations ranged from 0 to 64 mg liter−1 cefoxitin, as recommended by the BSAC (1). After 24 h of incubation, the MIC for the MSSA strain was determined as 4 mg liter−1 and that for the MRSA strain as 32 mg liter−1, using OD600 measurements as well as heat flow measurements (Fig. 2 and 3). Figure 2A shows that almost all concentrations below the MIC started to grow immediately, except the sample with 2 mg liter−1 cefoxitin, which exhibited a detectable heat flow signal only after 500 min. Otherwise, after 250 min of growth the shapes of the heat flow curves started changing, indicating a change in the metabolic activity of the MSSA strain related to the cefoxitin concentration.
FIG. 2.
MIC determination of cefoxitin for S. aureus ATCC 25923 (MSSA) in the range of 0.0 mg liter−1 to 8 mg liter−1. (A) Heat flow curve; (B) Heat curve. Blank, Mueller-Hinton plus 2% NaCl alone. Curves are averages for three experiments.
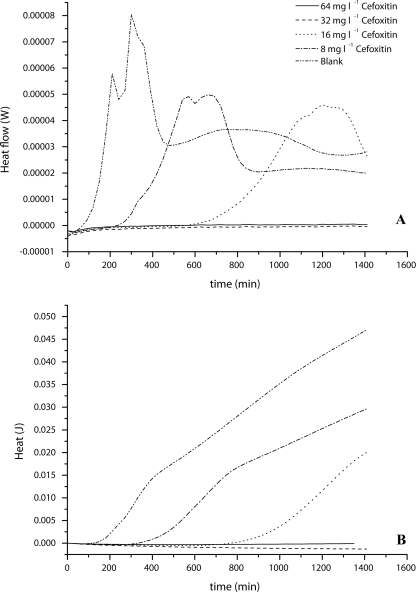
FIG. 3.
MIC determination of cefoxitin for S. aureus ATCC 43300 (MRSA) in the range of 8 mg liter−1 to 64 mg liter−1. (A) Heat flow curve; (B) Heat curve. Blank, Mueller-Hinton plus 2% NaCl alone. Curves are averages for three experiments.
The integration of the heat flow rate curves up to a given time x gives the heat produced up to time x. The plot of the heat produced (rather than the heat flow rate) against the time of the MIC determination results in the heat curves shown in Fig. 2B, which are analogous to cumulative colony or cell counts (data not shown). Although the sample with 2 mg liter−1 cefoxitin is delayed compared to the other samples with detectable growth of MSSA, the slope of the curve is almost parallel to the others. It is, however, not yet fully understood why the samples with 0.5 and 1 mg liter−1 produce more heat than the sample without antibiotic.
Similar to the results for MSSA, the MIC of cefoxitin for the MRSA strain was confirmed as 32 mg liter−1 by using IMC. However, the shape and especially the delay in detection time were different from those for MSSA. With increasing cefoxitin concentration, the delay in detection of the heat flow signal increased (Fig. 3A). Additionally, the maximum heat flow was reduced compared to that in the sample with no added cefoxitin. Growth in the sample with the highest concentration still allowing growth of MRSA (16 mg liter−1) could be detected only after approximately 600 min. In contrast to that for MSSA, the heat produced decreased with increasing cefoxitin concentration (Fig. 3B). This difference might be related to the resistance mechanism of the MRSA strain.
In this study, we presented a potential calorimetric method for decreasing the time for differentiation of MRSA from MSSA. Although one can argue that the overall time for detection is 3 h (1 h for equilibration and 2 h until signal detection), the method is still much faster than traditional culture methods. Also, there are calorimetric techniques which could greatly reduce the equilibration time. Taking into account that S. aureus ATCC 43300 is a low-level-resistance strain (5, 10), high-level-resistance strains may be detectable even earlier. This hypothesis and the direct detection of MRSA are currently being evaluated in our laboratory.
This study also showed that calorimetry works for the MIC determination of cefoxitin for the tested strains. Because calorimetry provides continuous real-time data, it was even possible to see effects of cefoxitin concentration on the growth behavior of the strains. However, the time reduction for the evaluation was relatively small, 10 h compared to 24 h with the traditional broth dilution method. Nevertheless, IMC is a suitable method for MIC determination of cefoxitin for MRSA or MSSA.
Acknowledgments
This work was supported mainly by grant no. 301 from the Velux Foundation, Zurich, Switzerland. We have also received support for microorganism and other cultured cell microcalorimetry from the Department of Orthopedic Surgery, University of Basel Faculty of Medicine. Our laboratory receives general support from the Hardy & Otto Frey-Zünd Foundation, Basel, Switzerland.
We thank A. Trampuz of the Infectiology Department, University of Basel Hospital, for his collaboration in some initial experiments, which one of us (D. Wirz) proposed and which led to the work described here. Finally, we are extremely grateful to J. Meyer and his team (Institute for Oral Microbiology and Preventive Dentistry, University of Basel Dental Clinics) for generously giving us access to their level II biosafety laboratory and equipment for our preparation of microcalorimetry specimens and performance of standard microbiological evaluations.
Footnotes
Published ahead of print on 16 April 2008.
REFERENCES
- 1.Brown, D. F. J., D. I. Edwards, P. M. Hawkey, D. Morrison, G. L. Ridgway, K. J. Towner, and M. W. D. Wren. 2005. Guidelines for the laboratory diagnosis and susceptibility testing of methicillin-resistant Staphylococcus aureus (MRSA). J. Antimicrob. Chemother. 561000-1018. [DOI] [PubMed] [Google Scholar]
- 2.Clinical Laboratory and Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard—7th Edition. Clinical and Laboratory Standards Institute, Wayne, PA.
- 3.de San, N., O. Denis, M.-F. Gasasira, R. De Mendonca, C. Nonhoff, and M. J. Struelens. 2007. Controlled evaluation of the IDI-MRSA assay for detection of colonization by methicillin-resistant Staphylococcus aureus in diverse mucocutaneous specimens. J. Clin. Microbiol. 451098-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang, H., and G. Hedin. 2006. Use of cefoxitin-based selective broth for improved detection of methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 44592-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Felten, A., B. Grandry, P. H. Lagrange, and I. Casin. 2002. Evaluation of three techniques for detection of low-level methicillin-resistant Staphylococcus aureus (MRSA): a disk diffusion method with cefoxitin and moxalactam, the Vitek 2 system, and the MRSA-screen latex agglutination test. J. Clin. Microbiol. 402766-2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gurran, C., M. G. Holliday, J. D. Perry, M. Ford, S. Morgan, and K. E. Orr. 2002. A novel selective medium for the detection of methicillin-resistant Staphylococcus aureus enabling result reporting in under 24 h. J. Hosp. Infect. 52148-151. [DOI] [PubMed] [Google Scholar]
- 7.Harbarth, S., C. Masuet-Aumatell, J. Schrenzel, P. Francois, C. Akakpo, G. Renzi, J. Pugin, B. Ricou, and D. Pittet. 2006. Evaluation of rapid screening and pre-emptive contact isolation for detecting and controlling methicillin-resistant Staphylococcus aureus in critical care: an interventional cohort study. Crit. Care. 10R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.James, A. M. 1987. Calorimetry past, present and future. In A. M. James (ed.), Thermal and energetic studies of cellular biological systems. IOP Publishing Ltd., Bristol, United Kingdom.
- 9.Johnson, G., M. Millar, S. Matthews, M. Skyrme, P. Marsh, E. Barringer, S. O'Hara, and M. Wilks. 2006. Evaluation of BacLite rapid MRSA, a rapid culture based screening test for the detection of ciprofloxacin and methicillin resistant S. aureus (MRSA) from screening swabs. BMC Microbiol. 683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swedish Reference Group for Antibiotics. 8 June 2006, posting date. S. aureus ATCC 29213: target MIC ranges. http://www.srga.org/refstam/sa29213.htm.
- 11.Tai, C. C., A. A. Nirvani, A. Holmes, and S. P. F. Hughes. 2004. Methicillin-resistant Staphylococcus aureus in orthopaedic surgery. Int. Orthop. 2832-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trampuz, A., S. Salzmann, J. Antheaume, and A. U. Daniels. 2007. Microcalorimetry: a novel method for detection of microbial contamination in platelet products. Transfusion 471643-1650. [DOI] [PubMed] [Google Scholar]