Abstract
A randomized intervention study was conducted to determine if discontinuing use of calf milk replacer medicated with oxytetracycline results in increased tetracycline susceptibility in Salmonella and Campylobacter spp. and Escherichia coli in dairy calves over a 12-month period. Dairy herds with enteric bacteria with known low tetracycline susceptibility were enrolled for the study. Fecal samples from preweaned calves and environmental samples were collected from eight dairy herds in Michigan and New York State. Samples were collected monthly for 3 months prior to and 12 months after four of the eight herds discontinued medicated milk replacer feeding. Salmonella and Campylobacter spp. and E. coli were isolated, and antimicrobial susceptibility testing was conducted using automated broth microdilution. A total of 804 intervention and 1,026 control calf fecal samples and 122 intervention and 136 control environmental samples were collected for testing. No differences in owner-reported morbidity and mortality between treatment groups were seen. The intervention was significantly associated with increasing tetracycline susceptibility in E. coli and Salmonella. Tetracycline susceptibility increased in intervention herds for the first 3 months after switching to nonmedicated milk replacer but declined in subsequent months. Discontinuing the practice of feeding medicated milk replacers to calves increased tetracycline susceptibility in E. coli and Salmonella on dairy farms, without increasing cattle disease, but declines in effectiveness after 3 months suggest that other factors contribute to decreasing susceptibility on the farm.
The problem of human infections with antimicrobial-resistant pathogens has been recognized for decades, and the emergence of resistance in bacterial pathogens continues to be a great concern for public health (2, 9). Antimicrobial resistance or the lack of efficacy of a drug for treatment of a disease agent for which the drug was previously effective was recognized soon after the widespread use of antibiotics began. Antibiotic use does not directly cause the development of resistance but results in selective pressure that allows bacteria resistant to the antibiotic to flourish (26). Once resistance develops in nonpathogenic bacteria, it can be transferred to pathogenic organisms (27, 28). Plasmids coding for resistance to multiple antimicrobial agents may develop over time by the acquisition of integrons (8), and since integrons can carry genes for resistance to more than one agent, resistance to one of the agents will continue after its use has been discontinued on the farm, as long as selective pressure exists for the development of resistance to the other agents carried by the integron (17).
Food animals have been implicated as sources of antimicrobial-resistant bacteria for humans. Epidemiological investigations have provided evidence for the transmission of antimicrobial-resistant Salmonella spp. from cattle to humans through direct contact or consumption of contaminated beef (7, 18, 19, 39). Drug-resistant Campylobacter spp. have been increasing in humans (14, 33), and evidence suggests that foods of animal origin may be associated with human cases of antimicrobial-resistant campylobacteriosis (13).
While there are associations between food animals and human infections with antimicrobial-resistant bacteria, on-farm exposures to such drugs and development of resistance continue to warrant study. Studies have associated antimicrobial use with resistance in enteric pathogens on dairy farms (16, 21, 38, 40, 41), with various levels of resistance depending on the dosage of antimicrobial agents used (24), and declines in resistance after removing exposure to antimicrobial agents (21). However, some studies have found no difference in resistance between treated and untreated cattle (22, 35). Given the uncertainty in the literature as to the role of on-farm drug use in the development of antimicrobial resistance on the farm, the objective of this study was to determine if discontinuing feeding of milk replacer medicated with oxytetracycline and neomycin to preweaned calves results in increased susceptibility to select antimicrobial agents in Salmonella and Campylobacter spp. and Escherichia coli isolated from calves and the environment of dairy farms.
MATERIALS AND METHODS
Study design.
An intervention study was conducted to determine if discontinuing use of milk replacer medicated with oxytetracycline and neomycin resulted in increased susceptibility to tetracycline (Tet) in Salmonella, Campylobacter, and E. coli in dairy calves. The intervention in this study was changing from medicated to nonmedicated milk replacers on participating dairy farms. The study was divided into two time periods: a 3-month preintervention period (October 2003 to December 2003) and a 12-month postintervention period (January 2004 to December 2004). All herds were sampled monthly prior to intervention and monthly from intervention to the end of the study.
Study population.
Because the objective of the current study was to determine how removing oxytetracycline from preweaned-calf diets affects antimicrobial susceptibility, conventionally managed (not certified organic) dairy herds infected with laboratory-confirmed Salmonella, E. coli, or Campylobacter with low Tet susceptibility that were feeding on milk replacer containing oxytetracycline were solicited for participation. The herds included in this study were either enrolled in an earlier project designed to describe the prevalence and the antimicrobial susceptibility of Campylobacter and Salmonella isolates obtained from organic and conventional dairy farms across key animal management groups (15, 16) or were identified based on veterinary diagnostic laboratory submissions. A total of eight herds were enrolled for the study: four from Michigan, and four from New York. Two large (at least 200 adult cattle) and two small (<200 adult cattle) herds were selected from eligible herds in Michigan, and the members of each pair were randomly assigned to the intervention or control group. The same process was repeated for herds from New York, except that large herds in New York were those with at least 100 adult cattle and small herds had less than 100 adult cattle.
Data collection.
At the beginning of the study, a questionnaire was administered to describe the herd inventory and general farm management practices pertaining to calf management, animal housing, and antimicrobial use in calves and adult cows.
At each sampling visit, a short monthly questionnaire was completed to describe changes in herd inventory (numbers and types of cattle), animal housing and management, calf feeding practices, levels of calf diarrhea on the farm, and farm level antimicrobial drug use in calves and cows. Additionally, information on calves sampled (animal identification number, age in months, occurrence of diarrhea or other illnesses, and any antimicrobial treatments received prior to sampling) was collected.
Intervention.
Data and samples were collected from all farms for 3 months prior to initiation of the intervention phase of the study to establish baseline levels of bacterial prevalence and Tet susceptibility in E. coli, Salmonella, and Campylobacter. All cattle from farms in the preintervention phase were fed medicated milk replacer containing oxytetracycline and neomycin and not including ionophores. Intervention herds then began feeding on the same brand of milk replacer they had been using, except that the product no longer contained oxytetracycline or neomycin and transition rations did not contain any antimicrobial agents. None of the study diets after intervention contained ionophores. Other than the content of the intervention herd milk replacer, no other changes in calf management practices were undertaken in the intervention herds, and herd managers continued to treat calves and cattle for disease with antimicrobial agents when necessary.
Sample collection.
Calves were systematically selected from preweaned female dairy calves on the farm at the day of visit. Healthy calves were sampled before any calves with signs of diarrhea, respiratory disease, or other illness. Fecal samples were collected by placing approximately 10 grams of fecal material obtained by rectal retrieval into Whirl-Pak bags labeled with farm identification number, sampling date, and sample number. A separate glove was used for the collection of each sample.
Environmental samples were collected by swabbing sites with 10- by 10-cm gauze pads soaked in 30 ml sterile double-strength skim milk. A composite calf hutch sample was collected by combining individual swabs collected from at least four calf hutches, and a composite maternity pen sample was collected by swabbing four areas in the maternity pen. Gauzes were placed into Whirl-Pak bags labeled with farm identification number, sampling date, and sample location.
After collection, samples were shipped to a central laboratory at Michigan State University. Samples from New York were shipped on the day of collection, whenever possible, via overnight delivery in Styrofoam boxes with ice packs. If samples could not be shipped the same day, they were stored in a refrigerator for 12 to 36 h until the next shipping opportunity.
Laboratory isolation and identification. (i) E. coli.
Fecal samples were streaked onto MacConkey agar, incubated for 18 to 24 h at 37°C, and inspected for lactose-positive, bile salt precipitate-positive colonies. Three isolates demonstrating morphology indicative of E. coli were selected for identification from the initial MacConkey agar plate. If more than one morphological variation of suspect E. coli colonies was present, isolates were selected from each variation. A sterile needle was used to inoculate an isolated colony onto triple-sugar-iron (TSI) and urea slants, which were incubated for 24 h at 37°C. If the TSI slant was glucose, sucrose, and/or lactose positive (red slant and yellow butt or yellow throughout) and the urea slant was negative (yellow throughout), methyl red (MR), Voges-Proskauer (VP), indole, and Simmons citrate media were inoculated to confirm E. coli. If the TSI slant showed a red slant and yellow butt, an oxidase test was performed. Cultures testing oxidase negative were considered typical of E. coli and inoculated as above (MR, VP, indole, citrate) to confirm E. coli identification. Positive MR (red color change), negative VP (no color change), positive indole (red ring at top), and negative Simmons citrate (no color change) media were used to confirm that a colony was E. coli. If there were multiple E. coli colonies available from a single sample, up to three different isolates were collected from the sample. Each isolate was stabbed onto a tryptic soy agar (TSA) slant and stored at room temperature prior to antimicrobial susceptibility testing, and the remainder of the isolates were suspended in tryptic soy broth (TSB), 0.5 ml of the suspension was added to 0.5 ml 65% glycerol solution, and the mixture was frozen at −70°C.
(ii) Salmonella.
For Salmonella, samples were enriched by adding tetrathionate broth at a 1:10 dilution to the sample and incubated for 48 h at 37°C. The enriched sample was streaked onto XLT4 agar and incubated for 24 h at 37°C. Three suspect colonies (red or yellow with black centers) were inoculated onto TSI and urea agar slants and incubated for 24 h at 37°C. If the TSI slant was not alkaline/acid/H2S positive (red slant, yellow/black butt) and the urea slant was not negative (yellow throughout), the test was considered negative for Salmonella. Potential Salmonella colonies were then inoculated onto lysine-iron agar and Simmons citrate agar slants; colonies that were lysine decarboxylase and hydrogen sulfide positive in lysine-iron agar (purple slant, purple-black butt) as well as positive in Simmons citrate (blue) were considered positive for Salmonella. If there were multiple positive colonies from a single sample, up to three different isolates were collected. Each isolate was stabbed onto a TSA slant and stored at room temperature prior to antimicrobial susceptibility testing, and the remainder of the isolates were suspended in TSB, 0.5 ml of the suspension was added to 0.5 ml 65% glycerol solution, and the mixture was frozen at −70°C.
(iii) Campylobacter.
Fecal samples were diluted with phosphate-buffered saline at 1:4, swabbed into 1 ml of Bolton broth in a tissue culture plate, and incubated microaerophilically for 48 h at 42°C. Environmental samples were added to Bolton broth at a 1:10 dilution and incubated for 48 h at 42°C. The enriched sample was streaked onto Campy Blaser agar and incubated for 48 h at 42°C. Three suspect colonies (bluish gray color) were streaked onto sheep blood agar and incubated for 48 h at 42°C, and colonies with off-white/yellowish growth on sheep blood agar were inoculated in Mueller-Hinton broth. A portion also received oxidase testing and Gram staining. Gram-negative and oxidase-positive spiral-shape rods demonstrating growth and motility in Mueller-Hinton broth were identified as Campylobacter, and the hippurate test was performed to identify isolates as Campylobacter jejuni or as non-C. jejuni. If there were multiple colonies available from a single sample, up to three different isolates were collected from the sample. After an isolate was confirmed, it was suspended in TSB, 0.5 ml of the suspension was added to 0.5 ml 65% glycerol solution, and the mixture was frozen at −70°C.
Antimicrobial susceptibility testing.
Commercially prepared broth microdilution antimicrobial panels, with a prepared range of concentrations for Tet and other agents, were used for antimicrobial susceptibility testing for E. coli (CMV7CNCD), Salmonella (CMV7CNCD), and Campylobacter (CAMPY) isolates. For all plates, E. coli ATCC 25922 was used as the quality control. Quality control results were reviewed with each batch of tests run, and, if these were not within acceptable limits, all tests were rerun. All quality control results were in the expected range for results reported in this paper.
Samples of Campylobacter and samples of E. coli and Salmonella isolates from TSA stabs that failed to grow or were contaminated were regrown from frozen stock for susceptibility testing. An appropriate selective agar plate (Campy Blaser agar for Campylobacter, XLT4 for Salmonella, MacConkey for E. coli) was inoculated from frozen stock culture using a heat-sterilized inoculating loop. The loop was used to loosen a piece of frozen culture, which was transferred to the selective agar, streaked for isolation, and incubated in conditions specific to the bacteria.
Isolates of confirmed E. coli and Salmonella from TSA slants or frozen stocks were streaked onto Mueller-Hinton agar and incubated for 18 to 24 h at 37°C, and a bacterial suspension was prepared according to the instructions from the manufacturer of the automated broth microdilution system (Trek Diagnostics, Inc.). Each panel was inoculated by adding 50 μl of bacterial suspension by use of an autoinoculator, covered with a seal, and incubated at 37°C for 18 h.
Isolates of confirmed Campylobacter from frozen stocks were streaked onto brucella agar plates and incubated microaerophilically at 42°C for 24 h. A bacterial suspension was prepared according to the manufacturer's instructions (Trek Diagnostics, Inc.), and the CAMPY panel was inoculated by adding 100 μl of the bacterial suspension to each well using an autoinoculator. The panels were covered with a gas-permeable seal and incubated microaerophilically for 48 h at 37°C. Panels for E. coli and Salmonella were read with an autoreader, and Campylobacter panels were read manually.
The MIC at which no bacterial growth occurred was read from each plate for each isolate. The MIC50 and MIC90 for Tet from a given herd were calculated as the antimicrobial concentrations of Tet that inhibited 50% and 90% of the growth of isolates, respectively, from each herd. Breakpoint MICs less than or equal to 8 (for E. coli and Salmonella spp.) or less than or equal to 4 (for Campylobacter) were used to classify isolates as susceptible, based on recommendations by the Clinical and Laboratory Standards Institute for E. coli and Salmonella spp. and the National Antimicrobial Resistance Monitoring System for Campylobacter in retail meat (30). Isolates that were classified as intermediate or resistant (MIC greater than 8 μg/ml for E. coli and Salmonella and greater than 4 μg/ml for Campylobacter) were considered to be not susceptible for the purposes of analysis.
Statistical analysis.
All statistical analyses were conducted separately for E. coli, Salmonella, and Campylobacter. Descriptive statistics were generated to describe patterns of susceptibility to Tet between intervention and control herds. In cases where MICs were identified as being greater than the maximum dilution available from the susceptibility testing panel, the next greater dilution value was used in calculation of mean MICs for descriptive purposes. Susceptibility, measured as the percentage of isolates demonstrating susceptibility and numbers of isolates with specific dilutions, was assessed for significance using Fisher's exact test for categorical outcomes.
To test the hypothesis that discontinuation of the use of oxytetracycline-supplemented milk replacers resulted in increased susceptibility to Tet, data analysis was conducted at two levels: herd and individual animal. Multivariable analyses were conducted at both herd and individual animal levels by matching intervention herds with control herds within each state and herd size category.
(i) Herd level analysis.
Herd level Tet susceptibility outcomes were calf fecal sample MIC50 and MIC90 values and the percentage of fecal sample isolates demonstrating Tet susceptibility at each sampling visit. Multivariable analyses using repeated-measure generalized linear mixed models (GLMM) with binomial linkages (PROC GLIMMIX, SAS 9.3.1) were developed for the proportion of isolates with susceptibility to Tet at each sampling visit after the start of the intervention. The GLMM included random-effect terms for herd (for repeated measures) and state with herd size (for matching intervention herds with control herds within each state and herd size category). The main risk factor of interest included in the model was intervention status (intervention/control). Covariates considered for inclusion in the multivariable analysis included time of sampling (visit number relative to start of intervention); rates of diarrhea in preweaned calves, weaned calves, and adult cattle at time of sampling; Tet MICs of isolates collected from calf pen and maternity pen at time of sampling; and use of oxytetracycline (yes/no) in calves and cows. Separate “univariable” repeated-measure GLMM were developed for each risk factor, and those with P values of <0.20 were considered for inclusion in the multivariable model. Possible interaction terms were generated for all combinations of eligible risk factors, and interaction terms with P values of <0.20 were identified for inclusion in the analysis. All eligible factors and interaction terms were entered into the multivariable analysis, and a hierarchical backwards model-building approach was used to develop the final model, using the best combination of model term P values and model generalized χ2 values.
(ii) Animal level analysis.
Animal level outcomes were calf fecal isolate Tet MICs and antimicrobial susceptibility status (yes/no). The primary risk factor of interest was intervention status, and additional covariates in the multivariable analysis included herd, time, and whether the calf had diarrhea (yes/no) or received oxytetracycline (yes/no) in the month preceding sample collection. Calf level susceptibility was tested using multivariable logistic regression, with a two-level ordinal outcome (susceptible/not susceptible), and the not-susceptible outcome as baseline. The logistic regression models included random-effect terms for the herd to account for clustering within each herd and state/herd size to match intervention herds with appropriate controls in the analysis. Risk factors included in the model were intervention status; visit number; rates of diarrhea in preweaned calves, weaned calves, and adult cattle at time of sampling; the use of oxytetracycline in calves and cows; calf age in days; and calf health status. The model-building approach used was identical to the process described for the herd level models. In addition to risk factor analysis, individual calf MIC data were tested using multivariable analysis of variance (ANOVA) and nested multivariable regression (stratified by herd) to determine if there were associations between MIC and time of sampling.
RESULTS
Eight herds were enrolled in the study, with a combined population of 126 preweaned calves, 1,120 weaned calves, and 1,517 adult cows at the beginning of the study (Table 1). For all herds, calves were housed in individual pens and group housing was uncommon: only the large Michigan intervention herd and the small New York control herd reported using indoor group pens for calves. Two herds (Michigan large control and New York large intervention) kept calves in outdoor hutches, while all other herds used indoor hutches. Three herds (both Michigan intervention herds and the Michigan large control herd) reported introducing adult cattle from off-farm sources in the 60-day period prior to the beginning of the study. For the large intervention herds from Michigan and New York and the one large Michigan control herd, weaned calves were imported, and for only one herd (Michigan large control) were preweaned calves imported. The highest levels of diarrhea and animal mortality (Table 1) seen during the study period were in preweaned calves. There were no statistically significant trends in diarrhea or mortality levels over time in either intervention or control herds.
TABLE 1.
Study herd populations, prevalence of diarrhea, and overall mortality from the 60-day period prior to the beginning of the study by treatment group, state, and herd sizea
| Treatment group | State | Herd size | Preweaned calves
|
Weaned calves
|
Adult cows
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Diar (%) | Mort (%) | n | Diar (%) | Mort (%) | n | Diar (%) | Mort (%) | |||
| Intervention | MI | Small | 6 | 33.3 | 16.7 | 35 | 0 | 0 | 165 | 2.4 | 0.6 |
| Large | 30 | 0 | 0 | 400 | 0 | 0 | 450 | 0 | 1.3 | ||
| NY | Small | 5 | 40 | 20 | 6 | 83.3 | 0 | 52 | 3.8 | 0 | |
| Large | 11 | 100 | 0 | 67 | 0 | 0 | 100 | 0 | 0 | ||
| Control | MI | Small | 12 | 0 | 16.7 | 162 | 0 | 0 | 164 | 0 | 0.6 |
| Large | 45 | 6.7 | 6.7 | 310 | 0 | 0 | 399 | 0.3 | 0.5 | ||
| NY | Small | 3 | 0 | 0 | 40 | 0 | 0 | 60 | 0 | 1.7 | |
| Large | 14 | 35.7 | 0 | 100 | 10 | 0 | 127 | 1.6 | 0 | ||
n, number of animals; Diar, diarrhea; Mort, overall mortality.
All farms fed colostrum to each calf at least two or three times, while for the large Michigan intervention herd calves were given six colostrum feedings. All herds were fed at least 2 quarts of colostrum per feeding, with the small Michigan intervention and large Michigan control herds fed 4 quarts of colostrum per feeding. One-half of all farms reported feeding whole milk prior to starter feeds: all Michigan herds except the large intervention herd and the small New York intervention herd. Only one herd (New York small intervention) reported feeding waste milk to calves. All herds were fed medicated calf grain; one-half of the herds (both Michigan control herds and both New York intervention herds) were fed calf crumbles with antibiotics (Tets and sulfa drugs). Both Michigan intervention herds were fed calf grain with decoquinate, while all New York control herds, the small New York intervention herd, and both large Michigan herds were fed grain with Bovatech.
The most commonly reported antimicrobial agents used in calves at the beginning of the study were penicillin (three of eight herds: small and large New York intervention and large Michigan control), ampicillin (two of eight: large New York intervention and control), and florfenicol (small Michigan intervention and large Michigan control). In adult cows, the most commonly reported drugs were ampicillin (five of eight: large Michigan and New York intervention, small and large Michigan intervention and large New York intervention), cephalosporins (five of eight: small Michigan and New York intervention, large New York intervention, small Michigan, and New York control), and penicillin (four of eight: small Michigan and large New York intervention, small New York, and large Michigan control).
A total of 1,492 calf fecal samples and 232 environmental samples (calf pens and maternity pens) were collected on control and intervention farms from Michigan and New York (Table 2). The recovery rates for E. coli, Salmonella, and Campylobacter from fecal samples were 98%, 10%, and 4%, respectively, while recovery rates from environmental samples were 69%, 10%, and 4%, respectively. There were E. coli isolates from all sampling dates, but Salmonella was only intermittently isolated. All 3 preintervention sampling dates, but only 5 of 12 postintervention sampling dates, yielded Salmonella on intervention farms. None of the 3 preintervention sampling dates and 11 of 12 postintervention sampling dates yielded Salmonella from control farms. Intervention herd samples yielded Campylobacter at each sampling date, but three of five postintervention samples did not yield any Campylobacter isolates.
TABLE 2.
Numbers of samples collected and recovery rates for E. coli, Salmonella, and Campylobacter prior to freezing before antimicrobial susceptibility testing
| Sample type and treatment group | State | Herd size | No. of samples | Recovery of:
|
|||||
|---|---|---|---|---|---|---|---|---|---|
|
E. coli
|
Salmonella
|
Campylobacter
|
|||||||
| n | % | n | % | n | % | ||||
| Animal samples | |||||||||
| Intervention | MI | Small | 151 | 146 | 96.7 | 12 | 7.9 | 9 | 6.0 |
| Large | 531 | 518 | 97.6 | 13 | 2.4 | 34 | 6.4 | ||
| NY | Small | 56 | 54 | 96.4 | 0 | 0 | 4 | 7.1 | |
| Large | 66 | 65 | 98.5 | 0 | 0 | 7 | 10.6 | ||
| Control | MI | Small | 134 | 134 | 100 | 0 | 0 | 6 | 4.5 |
| Large | 640 | 619 | 96.7 | 143 | 22.3 | 2 | 0.3 | ||
| NY | Small | 72 | 72 | 100 | 0 | 0 | 10 | 13.9 | |
| Large | 180 | 177 | 98.3 | 0 | 0 | 6 | 3.3 | ||
| All herds | 1,830 | 1,785 | 97.5 | 168 | 9.2 | 78 | 4.3 | ||
| Environmental samples | |||||||||
| Intervention | MI | Small | 38 | 30 | 78.9 | 2 | 5.3 | 3 | 7.9 |
| Large | 38 | 31 | 81.6 | 12 | 31.6 | 2 | 5.3 | ||
| NY | Small | 30 | 16 | 53.3 | 0 | 0 | 0 | 0 | |
| Large | 16 | 6 | 37.5 | 0 | 0 | 2 | 12.5 | ||
| Control | MI | Small | 38 | 29 | 76.3 | 0 | 0 | 0 | 0 |
| Large | 38 | 33 | 86.8 | 12 | 31.6 | 1 | 2.6 | ||
| NY | Small | 30 | 15 | 50.0 | 0 | 0 | 3 | 10.0 | |
| Large | 30 | 14 | 46.7 | 0 | 0 | 1 | 3.3 | ||
| All herds | 258 | 174 | 67.4 | 26 | 10.1 | 12 | 4.7 | ||
After isolates from frozen stocks were regrown, a total of 1,930 E. coli, 192 Salmonella, and 44 Campylobacter isolates were available for antimicrobial susceptibility testing. Given the low numbers of isolates recovered, multivariable analyses were not conducted for Campylobacter.
Overall susceptibility to Tet.
Susceptibility to Tet was low (<33%) among all three bacterial agents from fecal samples collected in the preintervention period, regardless of intervention status (Table 3). Low levels of susceptibility were seen in calf pen isolates of E. coli and Campylobacter from preintervention samples, while high levels of susceptibility were seen in Salmonella from calf pen samples and E. coli and Salmonella from preintervention samples. Susceptibility increased significantly after the intervention began in E. coli from the intervention herd and all herd fecal samples (Fisher's exact P < 0.05) and Salmonella from all herd fecal samples (Table 3). When the mean MIC of bacterial isolates from different sources was considered (Table 4), the average MICs for intervention herd E. coli from maternity pens and intervention herd Salmonella from calf fecal samples were statistically significantly lower than those for control isolates. Additionally, calf pen E. coli and Salmonella isolates from intervention herds had lower mean MICs than those from control herds, but these differences were not statistically significant.
TABLE 3.
Numbers and percentages of isolates demonstrating tetracycline susceptibility before and after the initiation of the intervention from isolates regrown from frozen stock
| Sample source and agent | Type of herd | Preintervention
|
Postintervention
|
Fisher's exact P | OR for susceptibility (95% CI) | ||
|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||
| Fecal matter | |||||||
| E. coli | Control | 156 | 1.3 | 834 | 2.2 | 0.7559 | 1.1 (0.9-1.2) |
| Intervention | 197 | 2.0 | 578 | 9.3 | 0.0004 | 1.3 (1.2-1.4) | |
| Salmonella | Control | 0 | 144 | 0 | |||
| Intervention | 14 | 21.4 | 11 | 45.4 | 0.3892 | 1.8 (0.8-4.1) | |
| Campylobacter | Control | 2 | 0 | 12 | 8.3 | 1.0 | 1.2 (0.9-1.5) |
| Intervention | 7 | 0 | 13 | 7.7 | 1.0 | 1.6 (1.1-2.2) | |
| Calf pen | |||||||
| E. coli | Control | 8 | 0 | 30 | 23.3 | 0.3074 | 1.3 (1.1-1.6) |
| Intervention | 7 | 0 | 33 | 24.2 | 0.3087 | 1.3 (1.1-1.6) | |
| Salmonella | Control | 0 | 6 | 33.3 | |||
| Intervention | 3 | 66.7 | 4 | 100 | 0.4286 | 3.8 (0.2-69.4) | |
| Campylobacter | Control | 0 | 1 | 100 | |||
| Intervention | 2 | 0 | 0 | ||||
| Maternity pen | |||||||
| E. coli | Control | 7 | 85.7 | 41 | 65.8 | 0.4087 | 0.4 (0.1-2.7) |
| Intervention | 7 | 100 | 32 | 84.4 | 0.5628 | 0.4 (0.02-6.1) | |
| Salmonella | Control | 1 | 100 | 5 | 80 | 1.0 | 1.0 (0.1-16.0) |
| Intervention | 2 | 100 | 2 | 100 | |||
| Campylobacter | Control | 1 | 0 | 2 | 50 | 1.0 | 2.0 (0.5-8.0) |
| Intervention | 1 | 0 | 3 | 66.7 | 1.0 | 3.0 (0.6-14.9) | |
TABLE 4.
Mean tetracycline MICs for E. coli, Salmonella, and Campylobacter after initiation of the intervention, by type of sample and intervention status, from isolates regrown from frozen stock
| Agent | Sample type | Intervention
|
Control
|
Kruskal-Wallis result
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| No. of isolates | MIC (μg/ml)
|
No. of isolates | MIC (μg/ml)
|
||||||
| Mean | SD | Mean | SD | χ2 | P | ||||
| E. coli | Fecal | 578 | 55.2 | 19.8 | 834 | 57.8 | 14.4 | 1.1 | 0.2952 |
| Calf pen | 33 | 45.8 | 26.9 | 30 | 46.8 | 25.9 | 0.0 | 0.9604 | |
| Maternity pen | 32 | 9.9 | 17.7 | 41 | 21.0 | 26.7 | 3.5 | 0.0615 | |
| Salmonella | Fecal | 11 | 36.7 | 31.3 | 144 | 64.0 | 14.2 | 67.2 | <0.0001 |
| Calf pen | 4 | 4.0 | 0 | 6 | 44.0 | 31.0 | 4.0 | 0.0455 | |
| Maternity pen | 2 | 4.0 | 0 | 5 | 16.0 | 26.8 | 0.4 | 0.5271 | |
| Campylobacter | Fecal | 13 | 108.5 | 39.9 | 12 | 106.8 | 41.2 | 0.0 | 0.9130 |
| Calf pen | 0 | 1 | 4.0 | ||||||
| Maternity pen | 3 | 21.4 | 36.9 | 2 | 48.0 | 22.6 | 1.4 | 0.2386 | |
The proportions of isolates susceptible to Tet before and after the start of intervention were assessed for all bacterial isolates from calf fecal samples (Table 3). While there were no significant differences in levels of susceptibility in E. coli after the start of intervention for control herds, there was a statistically significant increase in the proportion of E. coli isolates demonstrating susceptibility after the start of the intervention. There were no significant differences in levels of susceptibility before and after the start of intervention for Salmonella and Campylobacter, regardless of intervention status. Considering the distribution of fecal MICs before and after the start of intervention (Table 5), the only significant difference in the MIC distribution identified was for E. coli isolates from intervention herds.
TABLE 5.
Percentages of calf fecal isolates with tetracycline MICs, before and after the start of intervention, with the Cochran-Mantel-Haenszel statistic test for differences in distributions of MICs between pre- and postintervention isolates
| Agent | MIC (μg/ml) | Control herds
|
Intervention herds
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| % of isolatesa
|
Cochran-Mantel-Haenszel result
|
% of isolates
|
Cochran-Mantel-Haenszel result
|
||||||
| Pre-Intb | Post-Intc | χ2 | P | Pre-Intd | Post-Inte | χ2 | P | ||
| E. colif | 0.6 | 0.4494 | 10.1 | 0.0015 | |||||
| 4 | 1.3 | 2.1 | 2.0 | 9.3 | |||||
| 8 | 0 | 0.4 | 0 | 1.4 | |||||
| 16 | 0 | 0.7 | 2.0 | 1.7 | |||||
| 32 | 14.1 | 13.6 | 5.6 | 5.0 | |||||
| >32 | 84.6 | 83.2 | 90.4 | 81.7 | |||||
| Salmonellaf | 1.1 | 0.2889 | |||||||
| 4 | 0 | 0 | 21.4 | 45.4 | |||||
| 8 | 0 | 0 | 0 | 0 | |||||
| 16 | 0 | 0 | 0 | 0 | |||||
| 32 | 0 | 0 | 7.1 | 0 | |||||
| >32 | 0 | 100.0 | 71.4 | 54.5 | |||||
| Campylobacterg | 0.1 | 0.7238 | 1.6 | 0.2083 | |||||
| 2 | 0 | 8.3 | 0 | 7.7 | |||||
| 4 | 0 | 0 | 0 | 0 | |||||
| 8 | 0 | 0 | 0 | 0 | |||||
| 16 | 0 | 0 | 0 | 0 | |||||
| 32 | 0 | 0 | 0 | 0 | |||||
| 64 | 50.0 | 16.7 | 0 | 15.4 | |||||
| >64 | 50.0 | 75.0 | 100 | 76.9 | |||||
Pre-Int, preintervention; Post-Int, postintervention.
Numbers of isolates: E. coli, 156; Salmonella, 0; Campylobacter, 2.
Numbers of isolates: E. coli, 834; Salmonella, 144; Campylobacter, 12.
Numbers of isolates: E. coli, 197; Salmonella, 14; Campylobacter, 7.
Numbers of isolates: E. coli, 578; Salmonella, 11; Campylobacter, 13.
MIC: susceptible, <8 μg/ml; resistant, ≥32 μg/ml.
MIC: susceptible, <8 μg/ml; resistant, ≥16 μg/ml.
The effects of intervention were also assessed for samples from calf pens and maternity pens (Table 3). There were no significant associations (P ≤ 0.05) between the onset of intervention and levels of susceptibility in maternity pen samples and calf pen samples. Calf pen samples of E. coli and Salmonella and maternity pen samples of Salmonella and Campylobacter showed tendencies (0.3 < P < 0.5) to increased Tet susceptibility after the initiation of the intervention, and maternity pen samples of E. coli showed weak tendencies (0.4 < P < 0.5) to decreased susceptibility.
Tetracycline susceptibility in E. coli. (i) Herd level calf fecal samples.
Since the MIC90 for Tet in E. coli was 32 μg/ml for all herds at all time points, statistical analysis of the MIC90 was not possible. The Tet MIC50 for control herd E. coli was 32 μg/ml throughout the study period, while the MIC50 values for intervention herds declined to 13.6 μg/ml in the sixth month after intervention and subsequently rose to values from 23 to 32 μg/ml in the remaining months of the study (F = 9.9, P = 0.002). There were no associations between time in the study and MIC50 for either group.
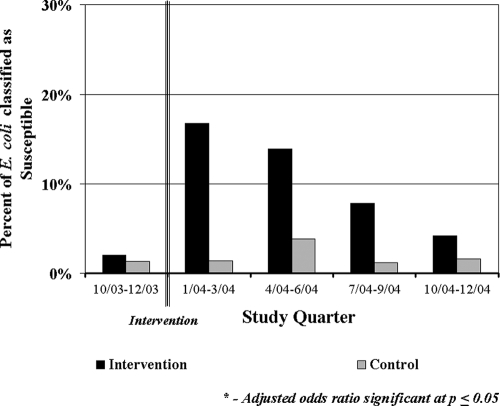
An evaluation of Tet susceptibility as the proportion of isolates demonstrating susceptibility over time showed that intervention herds had increases in the proportion of susceptible isolates after the intervention was started and that these proportions remained higher than the proportion of susceptible isolates in control herds (Fig. 1). This difference was statistically significant from the second to the fourth month postintervention. Univariable logistic regression models, with random-effect terms for herd and state/herd size, were generated for calf fecal isolates in 3-month periods to investigate the association between intervention and isolate susceptibility. The odds of increasing susceptibility in the intervention group rose in the first quarter after the start of the intervention and declined in subsequent quarters. These odds ratios (OR) were statistically significant at a P of ≤0.05 in the first two quarters postintervention, while the OR of the third quarter approached significance (P = 0.0675). When intervention herds were viewed alone, there was a significant difference between Tet susceptibility in calf fecal isolates from the preintervention phase to the postintervention phase (OR = 5.0, 95% confidence interval [CI] = 1.7 to 10.0).
FIG. 1.
Proportion of calf fecal E. coli isolates from control and intervention herds demonstrating susceptibility, by study quarter, adjusted for herd, herd size, and state.
The results of the multivariable logistic regression model for herd level Tet susceptibility after the intervention was started showed significant associations between the proportion of susceptible isolates with intervention (OR = 6.6, 95% CI = 2.0 to 21.2) and use of oxytetracycline in adult cattle (OR = 0.2, 95% CI = 0.1 to 0.6). The model also included levels of preweaned-calf diarrhea (OR for 10% change = 0.7, 95% CI = 0.5 to 1.1), visit number (OR = 0.9, 95% CI = 0.9 to 1.0), and the interaction between visit number and use of oxytetracycline in adult cattle (P = 0.0001).
(ii) Animal level calf fecal samples.
Nested analysis found significant associations between Tet MIC level and herd (F = 11.8, P < 0.0001), time (F = 5.2, P < 0.0001), and the interaction between herd and time (F = 3.7, P < 0.0001) for the entire study period. When multivariable ANOVA was used, significant associations were seen between intervention (estimated marginal mean MIC = 31.6 μg/ml, 95% CI = 29.0 to 34.3) and control herds (mean = 33.2 μg/ml, 95% CI = 30.6 to 35.7), seasons, oxytetracycline use in calves (mean = 34.6 μg/ml, 95% CI = 30.6 to 38.5) versus nonuse (mean = 30.1 μg/ml, 95% CI = 28.3 to 31.9), and the MIC of calf pen isolates (calf isolate MIC decreases with decreasing calf pen isolate MIC, and the calf isolate MIC is lower when calf pen E. coli is susceptible or intermediate).
The results of the multivariable logistic regression model for calf level Tet susceptibility after the intervention was started showed significant associations between susceptibility (yes or no) and intervention (OR = 9.1, 95% CI = 3.2 to 26.5), visit number (OR = 0.86, 95% CI = 0.76 to 0.98), and calf age (OR for 1-week change = 1.1, 95% CI = 1.0 to 1.4), and interaction terms for intervention with visit number (type III F = 6.0, P = 0.0140), intervention with calf age (type III F = 11.9, P = 0.0009), and visit number with calf age (type III F = 9.7, P = 0.0018).
(iii) Environmental samples.
For E. coli from calf pen samples, there were no significant differences between intervention and control herds in the MICs from calf pen samples over time but there was a slight decline in MICs postintervention for both control and intervention herds (Table 3). In the multivariable repeated-measure analysis, there was an association between calf pen MICs over time (P = 0.0246) but no overall trend in effect was seen. Maternity pen samples had E. coli with significantly lower MICs for intervention herds than for control herds (F = 6.5, P = 0.012), but time was not significantly associated with MIC for either control or intervention herds.
Tetracycline susceptibility in Salmonella. (i) Herd level calf fecal samples.
The rates of isolation of Salmonella were much lower than those for E. coli (Table 2); there were no isolates recovered from New York herds and one herd from Michigan (small control herd), making comparisons between states impossible. Additionally, Salmonella was not isolated continuously from the three herds, so temporal analyses were not possible. There were lower levels of susceptible isolates from control herds than intervention herds for fecal, calf pen, and maternity pen samples (Fig. 1), but this difference was statistically significant only for calf fecal samples.
The MIC50 and MIC90 for Tet in Salmonella isolates in control herds were both 32 μg/ml, while intervention herds had MIC50 and MIC90 of 4 μg/ml and 32 μg/ml, respectively. The Tet MIC50 and MIC90 values were significantly different between control and intervention herds (multivariable ANOVA P = 0.004 and 0.033, respectively), but there were no significant differences in MIC50 and MIC90 values from the preintervention to postintervention time periods.
(ii) Animal level calf fecal samples.
Of the 25 intervention and 144 control Salmonella isolates from calf fecal samples after the start of intervention, controls had lower susceptibility than intervention samples (Fig. 1). Repeated-measure analysis and nested analysis failed to yield results for Salmonella, given the intermittent nature of Salmonella recovery in this study. Multivariable ANOVA found significant differences in Tet MICs between control (all MICs = 32 μg/ml) and intervention (mean MIC, 21.4 μg/ml, 95% CI = 17.9 to 24.9 μg/ml) herds (ANOVA F = 59.3, P < 0.001) and differences over time (ANOVA F = 7.4, P < 0.001).
(iii) Environmental samples.
It was found that, when looking at Salmonella isolates from calf and maternity pen samples together, there were differences in Tet MICs between intervention and control herds when viewed as percentages of isolates susceptible to Tet (Fig. 1) and as MICs (Table 4), with trends demonstrating higher levels of susceptibility in intervention herds.
Tetracycline susceptibility in Campylobacter.
Tetracycline susceptibility in Campylobacter was low in all sample types, with slightly but not statistically significantly higher levels of susceptible isolates in intervention herds for fecal and maternity pen samples (Fig. 1). Given the low numbers of Campylobacter isolates recovered and the low levels of Tet susceptibility (8% of intervention and control fecal isolates), multivariable analyses were not conducted.
DISCUSSION
The goal of this study was to determine whether an intervention designed to increase antimicrobial susceptibility on dairy farms, by removing one source of potential selective pressure (subtherapeutic levels of oxytetracycline and neomycin in medicated calf milk replacer fed to calves) on farm microflora, would be effective. Results indicate that stopping the feeding of medicated milk replacer did have an effect on antimicrobial susceptibility in E. coli and Salmonella. The intervention was associated with increasing susceptibility in E. coli and Salmonella from fecal and calf pen samples, but not in maternity pen sample isolates.
Intervention and animal health.
There were no statistically significant trends in diarrhea or mortality levels over time in herds with medicated milk replacers eliminated, which has been reported in other studies (12, 24, 29), and this suggests that unmedicated milk replacers do not increase the risk of disease in preweaned or weaned calves. Other studies have found benefits of feeding antimicrobial agents for growth and health, (4, 34), but since our study did not measure growth and since owner-reported morbidity and mortality at monthly visits are a crude measure of health effects, we cannot directly compare the results of this study with those of Quigley et al. (34). However, it was noted in one study that passive immune transfer through colostrum was a more important factor than the use of subtherapeutic antimicrobial agents in feed for affecting morbidity and mortality (4). Given the lack of negative impacts on calf health in our study, the potential farm savings in feed costs, and changes seen in antimicrobial susceptibility on the farm, eliminating medicated milk replacers for calves may benefit dairy farms in terms of antimicrobial susceptibility as long as calf-rearing practices are modified to optimize the natural protection provided by passive immune transfer to prevent negative health outcomes.
Bacterial recovery rates.
The recovery rates for Salmonella in this study were in agreement with findings from other field studies of dairy cattle (36). The sporadic nature of Salmonella shedding by cattle, which unfortunately made inferences about changes in resistance in Salmonella over time difficult, has also been found in other studies (15, 42).
The low levels of Campylobacter found in this study (less than 5%) agreed with some studies (10), but other studies reported higher levels (from 30% to over 60%) of Campylobacter in dairy cattle (3, 4, 43). The lower levels of Campylobacter recovery from environmental samples were not surprising, given that these bacteria do not thrive outside living hosts, and have been seen in Campylobacter from other studies (32).
Antimicrobial susceptibility. (i) Levels of susceptibility.
Fecal samples from calves in intervention and control herds in this study had low proportions of isolates susceptible to Tet, which are comparable to proportions of susceptible isolates reported in dairy calves in Pennsylvania (2%) (11), preweaned dairy calves in Washington State (21%) (22), and calves with diarrhea in Spain (32%) (31). Tetracycline susceptibility was uncommon in bacteria on all farms in this study, but this finding was not unexpected. Demonstration of resistance was a selection requirement for this study: if herds showed little or no Tet resistance, changes in susceptibility due to the intervention might not be evident.
(ii) Diarrhea and susceptibility.
Rates of preweaned-calf diarrhea were slightly associated with decreased risk for Tet susceptibility in E. coli in the herd level analysis. Neonatal calf diarrhea can be the result of viral or bacterial infection of the gastrointestinal system, but only 2% (37) of 1,830 samples collected in this study came from calves with diarrhea, so results of bacterial cultures from these calves cannot be considered representative of all diarrheal pathogens on these farms.
(iii) Calf age and susceptibility.
Univariable analyses of Tet susceptibility found older calves to be significantly associated with decreased proportions of susceptible isolates, while multivariable analyses of Tet susceptibility in E. coli from calf fecal samples found increasing calf age to be slightly associated with increased risk in the calf level analysis. For the univariable analysis, other studies have found age to be positively associated with decreasing antimicrobial drug susceptibility in young calves (5, 6, 20, 22). Conversely, as suggested by the multivariable analysis, older calves may be able to resist infection with nonsusceptible strains of E. coli, and one study has found that nonsusceptible E. coli had a greater selective advantage in newborn calves than susceptible strains, regardless of exposure to antimicrobial agents in milk replacer (22). The strength of association between age and susceptibility in the multivariable and univariable analyses indicates that, although age may be an important factor contributing to the presence of susceptible organisms among calf enteric bacteria, it is a minor factor in comparison to the other factors that influence levels of drug susceptibility.
Antimicrobial susceptibility and the intervention.
Our findings concerning antimicrobial susceptibility and the intervention agree with earlier studies where removal of antimicrobial agents from cattle management resulted in increased susceptibility in enteric bacteria (1) and in another study where increasing calf exposure to penicillin in milk was associated with decreasing susceptibility (24). In an early study using therapeutic and subtherapeutic levels of chlortetracycline in swine feed, investigators found that feeding subtherapeutic levels of chlortetracycline (27.5 μg/g feed) for longer periods (84 days) decreased Tet susceptibility in fecal coliforms in comparison to using therapeutic levels (220 μg/g feed) for shorter periods (14 days) and that susceptibility increased after therapeutic levels were discontinued (25). The finding that oxytetracycline use in adult cattle decreased the risk of susceptibility in the herd level multivariable analysis also supports the theory that drug resistance will be sustained in the presence of selective pressure (26).
Strength of association through OR.
Herds that stopped feeding milk replacers containing oxytetracycline demonstrated increased susceptibility to Tet among E. coli isolates. This effect was significant in the multivariable analyses for Tet susceptibility at the herd (percentage of isolates demonstrating susceptibility) and individual calf levels, and OR from these analyses were comparable (OR = 6.6 for herd level model; OR = 9.1 for calf level model).
Study time.
Matching intervention and control herds in the multivariable analyses showed that time modestly decreased the risk for Tet susceptibility in E. coli from calf fecal samples at both herd and calf levels (OR = 1.1 and 1.2, respectively). There were initial increases in susceptibility after intervention was implemented (Fig. 1), but susceptibility returned to preintervention levels 3 to 4 months after medicated milk replacers were discontinued. This suggests that the intervention had a relatively quick effect on Tet susceptibility but that these changes were not permanent. It is possible that, in the intervention herds, the lack of long-term selective pressure by the use of medicated milk replacers allowed nonsusceptible bacteria surviving in the calf population to compete more successfully against susceptible bacteria for reasons beyond selection pressure (23).
Strength of association over time.
When the OR for the effect of intervention on Tet susceptibility of E. coli from calf fecal samples were evaluated by quarter over the study period, the highest OR were seen in the first quarter of the study period, followed by a decline in the second quarter (Fig. 3). Even though there was an apparent increase in the OR in subsequent quarters, these OR were not statistically significant. We were unable to conclusively determine a trend toward decreasing odds of susceptibility linked with the intervention over time, but the changes in levels of susceptibility in the intervention group over time suggest that this may be occurring. This strong association immediately after the intervention was implemented indicates that the strategy did work to increase Tet susceptibility, but the subsequent decline in significant OR suggests that the intervention itself was not sufficient to continue increasing susceptibility over time.
Extrinsic sources of resistance.
Extrinsic sources of resistance factors are another most likely reason for the lack of long-term effect of the intervention in E. coli. Calves on intervention farms still had exposures to external sources of bacteria without restricted exposure to oxytetracycline, and the finding that maternity pen E. coli Tet MICs were slightly associated with increased risk in the herd level analysis suggests that this may be occurring. Studies have indicated that healthy dairy calves were rapidly colonized by resistant strains of E. coli shortly after birth (11). If contamination of maternity or calf housing areas occurs, outside bacteria could introduce resistance factors to the calf environment, where the calf could acquire the resistance factors. In an experimental study of E. coli in calves using supplements with and without oxytetracycline (22), the authors determined that environmental factors beyond the presence or absence of selective pressure through antimicrobial use maintains relatively constant levels of antimicrobial susceptibility on dairy farms. While our study was not designed to monitor possible extrinsic sources of resistance, the results support the hypothesis that extrinsic factors play a part in sustaining resistance in bacterial populations beyond antimicrobial exposure through the host itself.
The results of this study have demonstrated the impact of removing medicated calf milk replacer on Tet susceptibility in dairy calves without increases to calf morbidity or mortality, but additional work is needed to conclusively confirm these findings. Increasing the power of the study would be necessary to determine if there are longer-term effects of discontinuing medicated milk replacers, by increasing the numbers of herds in the analysis and following these herds for longer periods of time to determine if there are any long-term effects of the intervention on herd susceptibility levels. This would be particularly important in the cases of Salmonella and Campylobacter, as we were unable to collect sufficient numbers of isolates of these for better analyses.
In addition to testing bacteria from calves, calf housing, and maternity pens, other possible extrinsic sources of resistance factors should be investigated. The intervention appeared to decrease the levels of susceptibility in fecal samples more than in calf pen environmental samples (Table 3), which suggests that the intervention first affects calf gut bacteria which contaminate calf housing and that there are other factors contributing to the levels of susceptibility seen in calf pen bacteria. Monitoring of other sources of resistance factors for calves, such as dairy cows or manure storage areas, might provide additional information about the impact of the intervention in light of other sources of resistant bacteria or oxytetracycline. In conclusion, information from this study can be used to begin to design interventions to reduce exposure of bacteria in calves to antimicrobial agents to reduce the development and transmission of antimicrobial resistance among bacteria on dairy farms.
Acknowledgments
This research was funded by USDA-IREEGCP 2002-5110-01980.
We thank the participating farms for their cooperation.
Footnotes
Published ahead of print on 16 April 2008.
REFERENCES
- 1.Aarestrup, F. M., A. M. Seyfarth, H. D. Emborg, K. Pedersen, R. S. Hendriksen, and F. Bager. 2001. Effect of abolishment of the use of antimicrobial agents for growth promotion on occurrence of antimicrobial resistance in fecal enterococci from food animals in Denmark. Antimicrob. Agents Chemother. 452054-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angulo, F. J., N. L. Baker, S. J. Olsen, A. Anderson, and T. J. Barrett. 2004. Antimicrobial use in agriculture: controlling the transfer of antimicrobial resistance to humans. Semin. Pediatr. Infect. Dis. 1578-85. [DOI] [PubMed] [Google Scholar]
- 3.Bae, W., K. N. Kaya, D. D. Hancock, D. R. Call, Y. H. Park, and T. E. Besser. 2005. Prevalence and antimicrobial resistance of thermophilic Campylobacter spp. from cattle on Washington State farms. Appl. Environ. Microbiol. 71169-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beach, J. C., E. A. Murano, and G. R. Acuff. 2002. Prevalence of Salmonella and Campylobacter in beef cattle from transport to slaughter. J. Food Prot. 651687-1693. [DOI] [PubMed] [Google Scholar]
- 5.Berge, A. C. B., P. Lindeque, D. A. Moore, and W. Sischo. 2005. A clinical trial evaluating prophylactic and therapeutic antibiotic use on health and performance of preweaned calves. J. Dairy Sci. 882166-2177. [DOI] [PubMed] [Google Scholar]
- 6.Berge, A. C. B., D. A. Moore, and W. Sischo. 2006. Field trial evaluating the influence of prophylactic and therapeutic antimicrobial administration on antimicrobial resistance of fecal Escherichia coli in dairy calves. Appl. Environ. Microbiol. 723872-3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Besser, T. E., M. Goldoft, L. C. Pritchett, R. Khakhria, D. D. Hancock, D. H. Rice, J. M. Gay, W. Johnson, and C. C. Gay. 2000. Multi-resistant Salmonella typhimurium DT104 infections of humans and domestic animal in the Pacific northwest of the United States. Epidemiol. Infect. 124193-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carattoli, A., L. Villa, C. Pezzalla, E. Bordi, and P. Visca. 2001. Expanding drug resistance through integron acquisition by IndFl plasmids of Salmonella enterica Typhimurium. Emerg. Infect. Dis. 7444-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox, L. A., and D. A. Popken. 2006. Quantifying potential human health impacts of animal antibiotic use: enrofloxacin and macrolides in chickens. Risk Anal. 26135-146. [DOI] [PubMed] [Google Scholar]
- 10.Dodson, K., and J. LeJeune. 2005. Escherichia coli O157:H7, Campylobacter jejuni, and Salmonella prevalence in cull dairy cows marketed in northeastern Ohio. J. Food Prot. 68927-931. [DOI] [PubMed] [Google Scholar]
- 11.Donaldson, S. C., B. A. Straley, N. V. Hegde, A. A. Sawant, C. DebRoy, and B. Jayarao. 2006. Molecular epidemiology of ceftiofur-resistant Escherichia coli isolates from dairy calves. Appl. Environ. Microbiol. 723940-3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donovan, D. C., S. T. Franklin, C. C. L. Chase, and A. R. Hippen. 2002. Growth and health of Holstein calves fed milk replacers supplemented with antibiotics or Enteroguard. J. Dairy Sci. 85947-950. [DOI] [PubMed] [Google Scholar]
- 13.Endtz, H. P., G. J. Ruijs, B. van Klingeren, W. H. Jansen, T. Van der Reyden, and R. P. Mouton. 1991. Quinolone resistance in Campylobacter isolated from man and poultry following the introduction of fluoroquinolones in veterinary medicine. J. Antimicrob. Chemother. 27199-208. [DOI] [PubMed] [Google Scholar]
- 14.Engberg, J., F. M. Aarestrup, D. E. Taylor, P. Gerner-Smidt, and I. Nachamkin. 2001. Quinolone and macrolide resistance in Campylobacter jejuni and C. coli: resistance mechanisms and trends in human isolates. Emerg. Infect. Dis. 724-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fossler, C. P., S. J. Wells, J. B. Kaneene, P. L. Ruegg, L. D. Warnick, J. B. Bender, S. M. Godden, L. W. Halbert, A. M. Campbell, and A. M. Geiger. 2004. Prevalence of Salmonella spp. on conventional and organic dairy farms. J. Am. Vet. Med. Assoc. 225567-573. [DOI] [PubMed] [Google Scholar]
- 16.Halbert, L. W., J. B. Kaneene, P. L. Ruegg, L. D. Warnick, S. J. Wells, L. S. Mansfield, C. P. Fossler, A. M. Campbell, and A. M. Geiger-Zwald. 2006. Evaluation of antimicrobial susceptibility patterns in Campylobacter spp. isolated from dairy cattle and farms managed organically and conventionally in the midwestern and northeastern United States. J. Am. Vet. Med. Assoc. 2281074-1081. [DOI] [PubMed] [Google Scholar]
- 17.Harada, K., T. Asai, A. Kojima, K. Ishihara, and T. Takahashi. 2006. Role of coresistance in the development of resistance to chloramphenicol in Escherichia coli isolated from sick cattle and pigs. Am. J. Vet. Res. 76230-235. [DOI] [PubMed] [Google Scholar]
- 18.Holmberg, S. D., M. T. Osterholm, K. A. Senger, and M. L. Cohen. 1984. Drug-resistant Salmonella from animals fed antimicrobials. N. Engl. J. Med. 311617-622. [DOI] [PubMed] [Google Scholar]
- 19.Holmberg, S. D., J. G. Wells, and M. L. Cohen. 1984. Animal-to-man transmission of antimicrobial resistant Salmonella: investigations of U.S. outbreaks, 1971-1983. Science 225833-835. [DOI] [PubMed] [Google Scholar]
- 20.Hoyle, D. V., H. I. Knight, D. J. Shaw, K. Hillman, M. C. Pearce, J. C. Low, G. J. Gunn, and M. E. J. Woolhouse. 2004. Acquisition and epidemiology of antibiotic-resistant Escherichia coli in a cohort of newborn calves. J. Antimicrob. Chemother. 53867-871. [DOI] [PubMed] [Google Scholar]
- 21.Inglis, G. D., T. A. McAllister, H. W. Busz, L. J. Yanke, D. W. Morck, M. E. Olson, and R. R. Read. 2005. Effects of subtherapeutic administration of antimicrobial agents to beef cattle on the prevalence of antimicrobial resistance in Campylobacter jejuni and Campylobacter hyointestinalis. Appl. Environ. Microbiol. 713872-3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khachatryan, A. R., T. E. Besser, D. D. Hancock, and D. R. Call. 2006. Use of a nonmedicated dietary supplement correlates with increased prevalence of streptomycin-sulfatetracycline-resistant Escherichia coli on a dairy farm. Appl. Environ. Microbiol. 724583-4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khachatryan, A. R., D. D. Hancock, T. E. Besser, and D. R. Call. 2006. Antimicrobial drug resistance genes do not convey a secondary fitness advantage to calf-adapted Escherichia coli. Appl. Environ. Microbiol. 72443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langford, F. M., D. M. Weary, and L. Fisher. 2003. Antibiotic resistance in gut bacteria from dairy calves: a dose response to the level of antibiotics fed in milk. J. Dairy Sci. 863963-3966. [DOI] [PubMed] [Google Scholar]
- 25.Langlois, B. E., K. A. Dawson, T. S. Stahly, and G. L. Cromwell. 1984. Antibiotic resistance of fecal coliforms from swine fed subtherapeutic and therapeutic levels of chlortetracycline. J. Anim. Sci. 58666-674. [DOI] [PubMed] [Google Scholar]
- 26.Lederberg, J. 1997. Infectious disease as an evolutionary paradigm. Emerg. Infect. Dis. 3417-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levy, S. B. 1986. Environmental dissemination of microbes and their plasmids. Swiss Biotechnol. 532-37. [Google Scholar]
- 28.Marsik, F. J., and J. T. Parisi. 1975. Transmissible drug resistance of Escherichia coli and Salmonella from humans, animals, and their rural environments. J. Infect. Dis. 132296-302. [DOI] [PubMed] [Google Scholar]
- 29.Morrill, J. L., J. M. Morrill, A. M. Feyerherm, and J. F. Laster. 1995. Plasma proteins and a probiotic as ingredients in milk replacer. J. Dairy Sci. 78902-907. [DOI] [PubMed] [Google Scholar]
- 30.National Antimicrobial Resistance Monitoring System. 2004. NARMS retail meat annual report. http://www.fda.gov/cvm/2004NARMSCampylobacter.htm.
- 31.Orden, J. A., J. A. Ruiz-Santa-Quiteria, S. Garcia, D. Cid, and R. de la Fuente. 2000. In vitro susceptibility of Escherichia coli strains isolated from diarrhoeic dairy calves to 15 antimicrobial agents. J. Vet. Med. Ser. B 47329-335. [DOI] [PubMed] [Google Scholar]
- 32.Padungtod, P., and J. B. Kaneene. 2005. Campylobacter in food animals and humans in northern Thailand. J. Food Prot. 682519-2526. [DOI] [PubMed] [Google Scholar]
- 33.Pickering, L. K. 2004. Antimicrobial resistance among enteric pathogens. Semin. Pediatr. Infect. Dis. 1571-77. [DOI] [PubMed] [Google Scholar]
- 34.Quigley, J. D., J. J. Drewry, L. M. Murray, and S. J. Ivey. 1997. Body weight gain, feed efficiency, and fecal scores of dairy calves in response to galactosyl-lactose or antibiotics in milk replacers. J. Dairy Sci. 801751-1754. [DOI] [PubMed] [Google Scholar]
- 35.Ray, K. A., L. D. Warnick, R. M. Mitchell, J. B. Kaneene, P. L. Ruegg, S. J. Wells, C. P. Fossler, L. W. Halbert, and K. May. 2006. Antimicrobial susceptibility of Salmonella from organic and conventional dairy farms. J. Dairy Sci. 892038-2050. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez, A., P. Pangloli, H. A. Richards, J. R. Mount, and F. A. Draughon. 2006. Prevalence of Salmonella in diverse environmental farm samples. J. Food Prot. 692576-2580. [DOI] [PubMed] [Google Scholar]
- 37.Royal, W. A., R. A. Robinson, and K. I. Loken. 1970. The influence of chlortetracycline feeding on Salmonella typhimurium excretion in young calves. Vet. Rec. 8667-69. [DOI] [PubMed] [Google Scholar]
- 38.Sato, K., P. C. Bartlett, J. B. Kaneene, and F. P. Downes. 2004. Comparison of prevalence and antimicrobial susceptibilities of Campylobacter sp. isolates from organic and conventional dairy herds in Wisconsin. Appl. Environ. Microbiol. 701442-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spika, J. S., S. H. Waterman, G. W. Soo Hoo, M. E. St. Louis, R. E. Pacer, S. M. James, M. L. Bissett, L. W. Mayer, J. Y. Chiu, B. Hall, K. Green, M. E. Potter, M. L. Cohen, and P. A. Blake. 1987. Chloramphenicol-resistant Salmonella Newport traced through hamburger to dairy farms. N. Engl. J. Med. 316565-570. [DOI] [PubMed] [Google Scholar]
- 40.Timoney, J. F. 1978. The epidemiology and genetics of antibiotic resistance of Salmonella typhimurium isolated from diseased animals in New York. J. Infect. Dis. 13767-73. [DOI] [PubMed] [Google Scholar]
- 41.Timoney, J. F. 1981. R plasmids in the pathogenic enterbacteriaceae from calves, p. 708. In S. B. Levy, R. C. Clowes, and E. L. Koenig (ed.), Molecular biology, pathogenicity and ecology of bacterial plasmids. Plenum Press, New York, NY.
- 42.Wells, S. J., P. J. Fedorka-Cray, D. A. Dargatz, K. Ferris, and A. Green. 2001. Fecal shedding of Salmonella spp. by dairy cows on farm and at cull cow markets. J. Food Prot. 643-11. [DOI] [PubMed] [Google Scholar]
- 43.Wesley, I. V., S. J. Wells, K. M. Harmon, A. Green, L. Schroeder-Tucker, M. Glover, and I. Siddique. 2000. Fecal shedding of Campylobacter and Arcobacter spp. in dairy cattle. Appl. Environ. Microbiol. 661994-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]