Abstract
Culture-based studies have shown that Streptococcus mutans and lactobacilli are associated with root caries (RC). The purpose of the present study was to assess the bacterial diversity of RC in elderly patients by use of culture-independent molecular techniques and to determine the associations of specific bacterial species or bacterial communities with healthy and carious roots. Plaque was collected from root surfaces of 10 control subjects with no RC and from 11 subjects with RC. The bacterial 16S rRNA genes from extracted DNA were PCR amplified, cloned, and sequenced to determine species identity. From a total of 3,544 clones, 245 predominant species or phylotypes were observed, representing eight bacterial phyla. The majority (54%) of the species detected have not yet been cultivated. Species of Selenomonas and Veillonella were common in all samples. The healthy microbiota included Fusobacterium nucleatum subsp. polymorphum, Leptotrichia spp., Selenomonas noxia, Streptococcus cristatus, and Kingella oralis. Lactobacilli were absent, S. mutans was present in one, and Actinomyces spp. were present in 50% of the controls. In contrast, the microbiota of the RC subjects was dominated by Actinomyces spp., lactobacilli, S. mutans, Enterococcus faecalis, Selenomonas sp. clone CS002, Atopobium and Olsenella spp., Prevotella multisaccharivorax, Pseudoramibacter alactolyticus, and Propionibacterium sp. strain FMA5. The bacterial profiles of RC showed considerable subject-to-subject variation, indicating that the microbial communities are more complex than previously presumed. The data suggest that putative etiological agents of RC include not only S. mutans, lactobacilli, and Actinomyces but also species of Atopobium, Olsenella, Pseudoramibacter, Propionibacterium, and Selenomonas.
The population of the elderly is increasing worldwide. Due to better dental health care, the elderly are experiencing a higher retention of teeth, which implies an increased number of exposed root surfaces susceptible to caries (24, 32). Several studies indicate that the oral microflora can change with advancing age, possibly due to impaired immune function and subsequent colonization with nonoral bacterial species such as staphylococci and enterobacteria (9, 22, 24). Other microbial consequences of aging, e.g., an increase in yeast colonization, are related to long-term medication, reduced salivary flow rate, and denture wearing (18).
Presently not much is known about the microbial etiology of root caries (RC) in the elderly, and there is no consensus as to which microbes might cause the disease (6, 8). In several animal studies, filamentous bacteria, such as species of Actinomyces, were implicated in RC etiology (12, 28). Other culture-based cross-sectional human studies have focused on comparing the bacterial floras of plaque associated with sound and carious root surfaces (2, 3, 7, 15, 30). Mutans streptococci alone or in combination with lactobacilli were detected more frequently in plaque overlying carious surfaces than on healthy root surfaces. Other culture-based studies, using nonselective media and anaerobic sampling, suggest that the microflora associated with RC is much more complex than previously assumed (3, 12, 27). Additional species, such as non-mutans streptococci, species of Bifidobacterium, Rothia, and Veillonella, enterococci, anaerobic gram-negative rods, and the yeast Candida albicans, have been detected (27).
More recently, the use of culture-independent methods has played a key role in the discovery of previously unrecognized species in the oral cavity as well as in redefining the pathogenesis of the major oral infections (1, 14, 19-21, 23, 31). The outcome of these studies indicates that the major oral infections are polymicrobial (11).
Culture-independent studies agree on the bacterial complexity of advanced coronal caries (4, 20). It has been suggested that the deep coronal lesions comprise similar bacterial species as in the deep layers in RC (10). However, there have not been any culture-independent studies so far to describe the bacterial community of RC in the elderly.
In the present study, healthy and carious roots of individuals aged 82 years and older were investigated. The aim of the study was to assess the bacterial diversity of RC in the elderly by use of culture-independent molecular techniques and to determine the associations of specific bacterial species or bacterial communities with healthy and carious roots.
MATERIALS AND METHODS
Subject population.
Twenty-one elderly patients from a nursing home in Oslo, Norway (2 males and 19 females) were included in the study. The mean age was 89 years, ranging from 82 to 98 (Table 1). All subjects were examined clinically 1 to 5 days before sampling and were subsequently divided into two groups: a control group (n = 10) and an RC group (n = 11). The control subjects were RC free and the RC subjects had one or more lesions of RC at the time of the clinical examination. RC definition and diagnosis were based on the criteria of the World Health Organization (33). A lesion on an exposed root surface was classified as carious when it felt soft or leathery on probing. All subjects were instructed not to clean their teeth the evening and the morning before sampling. Smoking, number of remaining teeth, and tooth-brushing habits were recorded (Table 1). Subjects did not have any clinical signs of mucosal diseases and were not on antibiotic therapy up to 10 days prior to sampling.
TABLE 1.
Characteristics of subject groups
| Subject group | No. of subjects | Mean (± SD) (range):
|
% Females | No. smoking | |
|---|---|---|---|---|---|
| Age | No. of remaining teeth | ||||
| Control | 10 | 85.6 ± 3.2 (82-92) | 22.9 ± 3.4 (17-28) | 90 | 1 |
| RC | 11 | 91.8 ± 4.2 (86-98) | 14.8 ± 6.7 (5-25) | 91 | 2 |
Samples.
In the control group, supragingival plaque from one healthy root was collected (referred to as control sample), while from each RC subject the following three samples were collected: supragingival plaque from one healthy root in the RC subjects (hrRC sample), plaque from one carious root (carious sample), and the underlying dentin from the same carious root (dentinal sample) (Fig. 1). The sampled healthy root sites were chosen randomly. All samples were taken by one examiner (D. P.). Plaque samples were taken by use of sterile Gracey curettes. The carious root surfaces were cleaned with distilled water and a sterile rubber cup (Turbo Prophy cup; Young Dental, Earth City, MO) after the supragingival plaque (carious sample) had been collected. The outer layer of infected dentin was removed with either a spoon excavator or by a round burr in a low-speed hand piece, and the inner part of the RC lesion was sampled. All samples were immediately suspended in 300 μl of TE buffer (50 mM Tris, 1 mM EDTA, pH 7.6), transported on ice to the laboratory, and stored at −80°C.
FIG. 1.
(A) Control subject. d, plaque, healthy root. (B and C) RC subject. (B) a, plaque, healthy root; b, plaque, carious root. (C) c, dentin, same carious root.
DNA extraction.
Bacterial DNA was extracted using the QIAamp DNA mini kit (Qiagen, GmbH, Hilden, Germany) according to the instructions of the manufacturer. The extracts were stored at −20°C.
Amplification of 16S rRNA genes.
The 16S rRNA genes were amplified under standard conditions by use of a universal forward primer (5′-GAG AGT TTG ATY MTG GCT CAG-3′) and a universal reverse primer (5′-GAA GGA GGT GWT CCA RCC GCA-3′) (23). PCR was performed in thin-walled tubes with GeneAmp PCR systems 2700 and 9700 (ABI, Foster City, CA). Two microliters of DNA template was added to a reaction mixture (final volume, 50 μl) containing 20 pmol of each primer, 40 nmol of deoxynucleoside triphosphates, 1.5 mmol of Mg2+, and 1 U of Platinum Taq polymerase (Invitrogen, San Diego, CA). The samples were preheated at 95°C for 4 min, followed by 30 cycles of amplification under the following conditions: denaturation at 95°C for 45 s, annealing at 60°C for 45 s, and elongation at 72°C for 1.5 min, with an additional 15 s for each cycle. A total of 30 cycles were performed, followed by a final elongation step at 72°C for 15 min. The results of the PCR amplification were examined by electrophoresis in a 1% agarose gel.
Cloning and sequencing.
Cloning of PCR products was performed using the TOPO TA cloning kit (Invitrogen) according to the manufacturer's instructions. Briefly, transformation was done with competent Escherichia coli TOP10 cells. The transformed cells were plated onto Luria-Bertani agar plates supplemented with kanamycin (50 μg/ml) and incubated overnight at 37°C. Colonies were transferred to 70 μl of 10 mM Tris-HCl. Amplification of inserts was performed with (M13) primers (forward, 5′-GTAAAACGACGGCCAG-3′; reverse, 5′-CAGGAAACAGCTATGAC-3). PCR products of the correct size (containing 16S rRNA genes) were purified with the QIAquick PCR purification kit (Qiagen) and sequenced with an ABI Prism cycle sequencing kit (BigDye Terminator cycle sequencing kit with AmpliTaq DNA polymerase FS and GeneAmp PCR systems 2700 and 9700; ABI). The primers used for sequencing have been described previously (23). Quarter-dye chemistry was used with 80 μM primers and 1.5 μl of PCR product in a final volume of 20 μl. Cycle sequencing was performed with a GeneAmp PCR system 9700 (ABI), with 25 cycles of denaturation at 96°C for 10 s, annealing at 55°C for 5 s, and extension at 60°C for 4 min. The sequencing reactions were run on an ABI 3730 DNA sequencer (ABI). The single-read sequences of 29 clones were obtained by Qiagen Genomic Services by use of the same M13 primers.
Sequence analysis.
A total of 3,544 clones each with an insert of approximately 1,500 bases were analyzed. The number of sequenced clones per sample ranged from 60 to 94. A sequence of approximately 500 bases was first obtained to determine identity or approximate phylogenetic position. For the identification of closest relatives, the sequences of the inserts were compared to the 16S rRNA gene sequences of over 10,000 microorganisms in our database and over 400,000 sequences in the Ribosomal Database Project (5), EMBL (http://www.ebi.ac.uk/embl/), GenBank (http://www.ncbi.nlm.nih.gov/GenBank/), and DDBJ (http://www.ddbj.nig.ac.jp/) nucleotide sequence databases. Corrections for similarity matrices (13) and chimeric sequences (Chimera Check program in The Ribosomal Database Project [RDP-II] [http://rdp.cme.msu.edu/]) and construction (25) and drawing (29) of phylogenetic trees were done according to the method described by Paster et al. (23).
Nucleotide sequence accession numbers.
The complete 16S rRNA gene sequences of clones representing novel phylotypes defined in this study (OCG019 [EU669563], OCG080 [EU669564], OCH033 [EU669565], OCN091 [EU669566], OCT046 [EU669567], and OCV103 [EU669568]), sequences of species not previously reported, and published sequences are available for electronic retrieval from the EMBL, GenBank, and DDBJ nucleotide sequence databases under the accession numbers shown in Fig. 2 and in Tables S6 and S7 in the supplemental material.
FIG. 2.
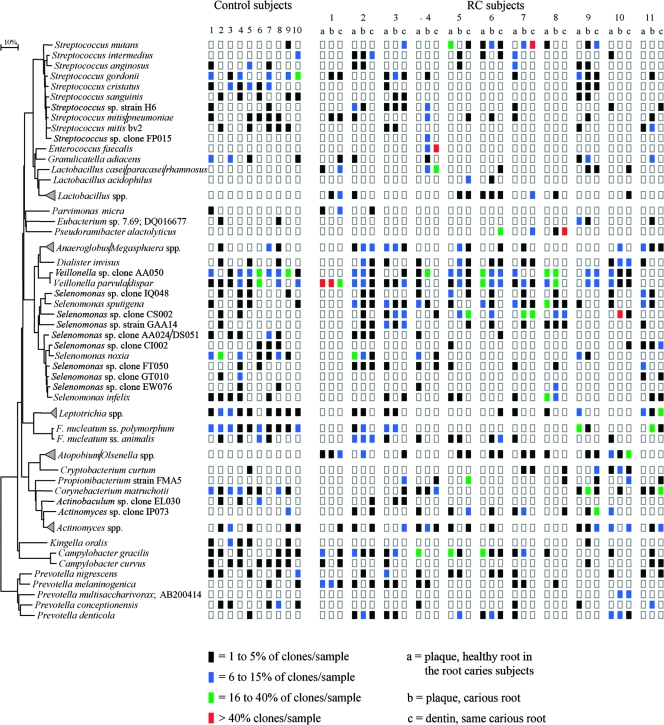
Phylogenetic tree and distribution between samples of the dominant species. The marker bar represents a 10% difference in nucleotide sequences. The samples are divided in the columns with those from the control subjects first, followed by the three different samples from subjects with RC. The prevalence of the different species is color coded. ss., subsp.
RESULTS
General results.
In the present study, bacterial profiles of healthy and diseased roots from 21 elderly subjects were investigated. A remarkably high diversity, 245 bacterial species representing eight bacterial phyla, was observed for the 3,544 clones analyzed (Table 2; also see Table 4 below). The overall bacterial profile had 112 (46%) cultivable species within known genera and 133 (54%) sequences from not-yet-cultivated phylotypes or species that are currently unrecognized (Table 4 below; also see Table S6 in the supplemental material). The number of species detected in control samples ranged from 16 to 41 per sample and from 8 to 35 in those from the RC patients (Table 3).
TABLE 2.
Number of clones per sample group
| Sample group | Total no. of clones | Mean | Range |
|---|---|---|---|
| Control | 834 | 83 | 70-94 |
| RC subject | |||
| hrRC | 910 | 83 | 60-94 |
| Carious | 942 | 86 | 68-94 |
| Dentinal | 858 | 78 | 68-90 |
| Total | 3,544 | 82 | 60-94 |
TABLE 4.
Bacterial phyla identified in sample groups
| Phylogenetic group | Totala
|
Control
|
RC subject sample
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| hrRC
|
Carious
|
Dentinal
|
||||||||
| Taxa (n = 245) | % Total clones | Taxa (n = 123) | % Total clones | Taxa (n = 129) | % Total clones | Taxa (n = 137) | % Total clones | Taxa (n = 117) | % Total clones | |
| Firmicutes | 102 | 64.1 | 49 | 58.3 | 67 | 65.3 | 73 | 68.8 | 55 | 63.2 |
| Bacteroidetes | 50 | 9.8 | 24 | 9.7 | 24 | 10.6 | 27 | 12.7 | 17 | 5.7 |
| Actinobacteria | 44 | 11.4 | 16 | 10.7 | 13 | 5.0 | 16 | 7.7 | 30 | 22.8 |
| Fusobacteria | 21 | 8.7 | 15 | 13.4 | 11 | 9.1 | 7 | 6.7 | 6 | 6.1 |
| Proteobacteria | 19 | 5.2 | 13 | 7.3 | 10 | 8.8 | 10 | 3.5 | 3 | 1.2 |
| Spirochaetales | 4 | 0.5 | 2 | 0.2 | 2 | 0.8 | 2 | 0.3 | 3 | 0.7 |
| TM7 | 3 | 0.1 | 0 | 0 | 2 | 0.3 | 1 | 0.1 | 1 | 0.1 |
| Synergistes | 2 | 0.1 | 1 | 1 | 0 | 0 | 1 | 0.1 | 2 | 0.2 |
The total number of clones was 3,544.
TABLE 3.
Number of different species per sample
| Subject group | Sample | No. of species per sample
|
|
|---|---|---|---|
| Range | Mean | ||
| Control | Control | 16-41 | 28 |
| RC | hrRC | 9-35 | 25 |
| Carious | 8-31 | 24 | |
| Dentinal | 10-34 | 21 | |
Firmicutes was the predominant phylogenetic group followed, in all sample groups, except for the dentinal samples, by Bacteroidetes. In dentin, Actinomyces was the second most dominant genus (Tables 4 and 5). Species of Veillonella (20%), Selenomonas (20%), and Streptococcus (12%) were the most dominant bacterial species in terms of the total number of clones. It was noteworthy that recognized caries-associated taxa, such as species of Actinomyces (4.2%) and lactobacilli (2%), constituted only a minor part of the number of bacterial species detected (see Table S6 in the supplemental material). Selenomonas sputigena, Veillonella parvula/Veillonella dispar, and Veillonella sp. clone AA050 were the most commonly detected. The most prevalent species among the streptococci were Streptococcus mutans, S. gordonii, and Streptococcus intermedius (see Table S6 in the supplemental material). Streptococcus spp. were detected at similarly high levels in all categories, while Veillonella and Prevotella spp. were less prevalent in the dentinal samples. The levels of Selenomonas spp. in both plaque samples of the RC subjects were twice as high as in the control and dentinal samples.
TABLE 5.
Distribution of major species in the sample groups
| Species | Control subjects (n = 10)
|
RC subjects (n = 11)
|
||||||
|---|---|---|---|---|---|---|---|---|
| No. of clones | No. of subjects with species | hrRC samplese
|
Carious samples
|
Dentinal samples
|
||||
| No. of clones | No. of subjects with species | No. of clones | No. of subjects with species | No. of clones | No. of subjects with species | |||
| Propionibacterium sp. strain FMA5a | 43 | 7 | ||||||
| Pseudoramibacter alactolyticusa | 1 | 1 | 76 | 3 | ||||
| Prevotella multisaccharivorax AB200414a | 5 | 1 | 6 | 1 | ||||
| Enterococcus faecalisa | 5 | 1 | 49 | 1 | ||||
| Lactobacillus spp.a | 4 | 3 | 14 | 6 | 57 | 5 | ||
| Atopobium spp. Olsenella spp.a | 7 | 3 | 16 | 7 | 32 | 7 | ||
| Lactobacillus casei/Lactobacillus paracasei/Lactobacillus rhamnosusa | 2 | 1 | 7 | 2 | 31 | 5 | ||
| Actinomyces spp.a | 25 | 5 | 30 | 5 | 22 | 4 | 70 | 8 |
| Selenomonas sp. clone CS002a | 4 | 2 | 2 | 1 | 92 | 7 | 48 | 6 |
| Streptococcus mutansa | 3 | 1 | 25 | 3 | 17 | 3 | 52 | 5 |
| Actinomyces sp. clone IP073a | 7 | 1 | 12 | 5 | 10 | 3 | 36 | 4 |
| Dialister invisusa | 7 | 3 | 7 | 4 | 24 | 5 | 16 | 5 |
| Selenomonas sp. strain GAA14a | 1 | 1 | 8 | 3 | 10 | 5 | 11 | 4 |
| Prevotella denticolab | 3 | 1 | 9 | 5 | 33 | 5 | 8 | 3 |
| Prevotella melaninogenicab | 2 | 2 | 14 | 5 | 20 | 5 | 4 | 2 |
| Selenomonas sp. clone FT050b | 3 | 2 | 19 | 6 | 2 | 2 | 2 | 2 |
| Anaeroglobus spp./Megasphaera spp.b | 9 | 3 | 17 | 3 | 26 | 6 | 13 | 5 |
| Selenomonas sputigenab | 5 | 4 | 61 | 10 | 12 | 7 | 8 | 4 |
| Veillonella parvula/Veillonella disparb | 58 | 9 | 103 | 9 | 126 | 9 | 42 | 8 |
| Veillonella sp. clone AA050b | 82 | 9 | 74 | 7 | 78 | 7 | 23 | 6 |
| Corynebacterium matruchotiic | 41 | 8 | 7 | 3 | 25 | 4 | 20 | 4 |
| Campylobacter gracilisc | 13 | 6 | 63 | 9 | 22 | 6 | 7 | 3 |
| Selenomonas sp. clone IQ048c | 9 | 4 | 16 | 4 | 8 | 5 | 4 | 2 |
| Prevotella nigrescensc | 13 | 5 | 16 | 6 | 6 | 3 | 4 | 2 |
| Selenomonas infelixc | 12 | 5 | 24 | 4 | 13 | 3 | 2 | 2 |
| Selenomonas sp. clone AA024/DS051c | 14 | 5 | 3 | 2 | 1 | 1 | 6 | 2 |
| Streptoccocus gordoniic | 36 | 6 | 14 | 3 | 9 | 5 | 5 | 3 |
| Campylobacter curvusc | 10 | 7 | 4 | 2 | 3 | 2 | 2 | 2 |
| Leptotrichia spp.c | 33 | 9 | 24 | 6 | 7 | 3 | 16 | 2 |
| Granulicatella adiacensd | 26 | 4 | 5 | 2 | 16 | 3 | 1 | 1 |
| Streptococcus sanguinisd | 6 | 5 | 1 | 1 | 2 | 2 | 1 | 1 |
| Streptococcus cristatusd | 22 | 6 | 6 | 2 | 2 | 1 | 1 | 1 |
| Selenomonas noxiad | 45 | 7 | 25 | 3 | 15 | 2 | 5 | 1 |
| Fusobacterium nucleatum subsp. polymorphumd | 50 | 10 | 22 | 3 | 34 | 4 | 1 | 1 |
| Streptococcus anginosusd | 7 | 3 | 9 | 3 | 8 | 3 | ||
| Streptococcus mitis bv. 2d | 11 | 5 | 4 | 3 | 11 | 2 | ||
| Prevotella conceptionensisd | 19 | 5 | 8 | 3 | 7 | 1 | ||
| Kingella oralisd | 11 | 5 | 1 | 1 | ||||
Species associated with RC subjects.
Species associated with both control and RC subjects.
Species associated more with control than with RC subjects.
Species associated with control subjects.
Microflora of control subjects.
On the sound root surfaces of the control subjects, most bacteria were present in low or moderate numbers (Fig. 2). Only Veillonella spp., Selenomonas noxia, and S. gordonii were detected at high clone levels in a few healthy subjects (between 16 and 40% of the clones). Health-associated bacterial species, such as Kingella oralis, Fusobacterium nucleatum subsp. polymorphum, Leptotrichia spp., Streptococcus cristatus, Campylobacter curvus, Corynebacterium matruchotii, and Selenomonas noxia, were rare in the majority of samples from RC subjects (Fig. 2; Table 5). K. oralis, Prevotella conceptionensis, Streptococcus mitis bv. 2, and Streptococcus anginosus were absent in the dentinal samples. Lactobacilli were not detected, S. mutans was present in only 1, and Actinomyces was present in 5 of the 10 healthy subjects, with clone levels of less than 5% (except for healthy subject no. 3) (Fig. 2). Plaque from the control subjects consisted of a diverse bacterial flora with the predominance of a few species and an average number of 28 species per sample (Table 3).
Microflora of hrRC samples.
The microflora of plaque overlying the healthy root surfaces in the RC subjects (hrRC samples) had lower diversity than the control samples, i.e., 9 to 35 versus 16 to 41 species detected per sample (Table 3). Compared to the predominant species in control subjects, the occurrence of Fusobacterium nucleatum subsp. polymorphum, S. cristatus, S. gordonii, C. curvus, C. matruchotii, and S. noxia was reduced, and other bacterial species like Campylobacter gracilis, Selenomonas sp. clone FT050, S. sputigena, P. melaninogenica, and S. mutans (Fig. 2; Table 5) were predominant. The most prevalent species described above differed from subject to subject in this category (Fig. 2). Lactobacilli were detected for three subjects. S. mutans levels increased, while the presence of Actinomyces was at the same level in healthy and control samples (five subjects). The levels of Actinomyces presence in the control and hrRC samples were similar.
Microflora of carious samples.
The microflora of plaque covering RC lesions (carious samples) had even lower diversity than the microflora of the sound root surfaces in this subject group (Table 3). Seven of the 11 subjects (subjects 1, 4, and 7 to 11) had species with clone levels of 16 to over 40% (Fig. 2). The levels of V. parvula/V. dispar and Selenomonas sp. clone CS002 were more than 40% in these two subjects. In only two subjects (no. 1 and 8), the same microorganisms (V. parvula/V. dispar and Veillonella sp. clone AA050) exhibited the same levels in plaque samples from both healthy and diseased root surfaces of the same subject. Selenomonas sp. clone CS002 (no. 7 and 10), C. matruchotii (no. 9), and F. nucleatum subsp. polymorphum (no. 11) were other dominant bacterial taxa in this category (Fig. 2). Selenomonas sp. clone CS002 and C. matruchotii were rare in the hrRC and healthy subjects. S. mutans was detected in three carious samples. The phylotype Selenomonas sp. clone CS002 showed a strikingly high prevalence and the highest level among the bacterial species detected in the plaque of the diseased root surfaces.
Only one subject had S. mutans both in the hrRC and in the carious sample. Actinomyces was found in 4 and lactobacilli in 6 of the 11 carious samples (Fig. 2; Table 5). The lactobacilli showed unchanged levels but prevalence higher (6 of 11 subjects) than in the hrRC samples.
Microflora of dentinal samples.
The bacterial profiles of the carious dentin (dentinal samples) differed in diversity as well as in bacterial dominance from the other categories. The number of species per sample ranged from 10 to 34 (Table 3). Nine of the 11 dentinal samples (no. 1 and 4 to 11) had at least one species with clone levels of 16 to 40% or higher (Fig. 2). In three subjects (no. 4, 7, and 8), Enterococcus faecalis, S. mutans, and Pseudoramibacter alactolyticus were found with levels higher than 40%. V. parvula/V. dispar (no. 1), Lactobacillus casei/Lactobacillus paracasei/Lactobacillus rhamnosus (no. 4), Propionibacterium sp. strain FMA5 (no. 5), Selenomonas sp. clone CS002 (no. 5 and 7), P. alactolyticus (no. 6), Actinomyces sp. clone IP073 (no. 9), Atopobium and Olsenella spp. (no. 10), C. matruchotii, and Leptotrichia spp. (no. 11) had clone levels of 16 to 40% (Fig. 2). V. parvula/V. dispar, Selenomonas sp. clone CS002, and C. matruchotii were also prevalent in the plaque overlying the carious lesion.
For the dentinal samples, more taxa with high levels were detected than for the other sample categories. Most dentinal samples had one or few predominant species in their bacterial profiles, but the species differed from subject to subject. Interestingly, L. casei/L. paracasei/L. rhamnosus and Atopobium and Olsenella spp. were absent from the control subjects but increased their presence in hrRC and carious samples (Table 5). The uncharacterized Propionibacterium sp. strain FMA5 was the only dominant species found exclusively in the carious dentin.
S. mutans was present in 5, lactobacilli in 7, and Actinomyces spp. in 9 of the 11 dentinal samples (Table 5). S. mutans (no. 7), lactobacilli (L. casei/L. paracasei/L. rhamnosus [no. 4]), and Actinomyces spp. (Actinomyces sp. clone IP073 [no. 9]) were each present in only one dentinal sample (Fig. 2). One dentinal sample (no. 6) had S. mutans, lactobacilli, and Actinomyces spp. together. Six dentinal samples showed no signs of S. mutans (no. 1, 2, 4, 8, 10, and 11).
In subject no. 11, no lactobacilli and only moderate levels of Actinomyces spp. were detected. Subject no. 2 did not have dominant species or any lactobacilli. The bacterial profile of this dentinal sample showed a combination of moderate levels (5 to 16% of clones) of S. intermedius, Anaeroglobus and Megasphaera spp., Fusobacterium nucleatum subsp. animalis, and many other bacterial species at low levels, including Actinomyces spp. (1 to 5% of the clones) (Fig. 2).
Three dentinal samples (2, 8, and 11) had low or moderate Actinomyces levels and no S. mutans or lactobacilli. Two dentinal samples showed Actinomyces (no. 6 and 7); one of these samples had high levels of S. mutans (no. 7), while the other sample had only low levels of S. mutans and lactobacilli. Actinomyces spp. were detected in 9 of 11 dentinal samples. The phylotype Actinomyces sp. clone IP073 was found in four dentinal samples at high levels (Table 5).
DISCUSSION
The present study offers the first description of the microflora associated with root surfaces in elderly subjects based on culture-independent methods. Among the 21 subjects examined, different bacterial profiles were observed. The overall bacterial diversity of RC was considerable; 245 species or phylotypes were identified, of which 54% have not yet been cultivated. This breadth of diversity was also observed in another study of advanced carious dentinal lesions (20).
Overall, the bacterial profiles exhibited reduced diversity when moving from healthy to diseased subjects. A similar observation was made for childhood caries (1, 16) and for saliva samples from caries-free and caries-active individuals (17). In the control subjects, the bacterial profiles showed high bacterial diversity and no dominance of particular bacterial species. In the RC subjects, the plaque of the sound root surfaces exhibited a lower bacterial diversity, and the diversity decreased further when moving to plaque from affected teeth. The lowest diversity was observed for the dentinal samples, which might be expected, as this is a more remote and specialized environment.
Another interesting characteristic of the profiles was the considerable subject-to-subject variation. The bacterial profiles differed as to the presence of dominant bacterial species and in the shift of dominance from one category to the other. Certain bacterial species appeared to be strongly associated with health, as they were rarely detected for (e.g., Leptotrichia spp. and S. noxia) or were absent from (K. oralis, P. conceptionensis, S. mitis bv. 2 and S. anginosus) the RC subjects but were commonly found in the control subjects. F. nucleatum subsp. polymorphum was found in all 10 control subjects, but it was also present at high concentrations in certain plaque samples from carious roots. In the hrRC samples, several species, such as Veillonella sp. clone AA050, V. parvula/V. dispar, S. noxia, C. gracilis, S. mutans, S. sputigena, S. infelix, and F. nucleatum subsp. polymorphum, were found at high levels. The high prevalence and levels of phylotype Veillonella sp. clone AA050 and Actinomyces sp. clone IP073 were notable. The phylotype Selenomonas sp. clone CS002 showed a strikingly high prevalence and the highest concentration in the carious samples. Lactobacilli (L. casei/L. paracasei/L. rhamnosus), E. faecalis, P. alactolyticus, and Propionibacterium sp. strain FMA5 appeared to be associated with disease, as they were common in dentinal samples while rare or absent in other categories. Hoshino (10) described the dominance of Propionibacterium spp. in deep dentin layers, but the study was culture based, which may explain the absence of the other bacterial species mentioned above.
The most predominant bacterial species in terms of health or disease association were as follows: F. nucleatum subsp. polymorphum (healthy root in control subject), S. sputigena (healthy root in RC subject), Selenomonas sp. clone CS002 (plaque on carious root), and Propionibacterium sp. strain FMA5 (dentin from carious root).
For the control subjects S. mutans was rare and lactobacilli were absent, while for the hrRC and carious samples the prevalence and levels of S. mutans and lactobacilli increased. Actinomyces prevalence and levels in the hrRC and carious samples were similar to what was seen for the control subjects. The most prevalent Actinomyces species, phylotype Actinomyces sp. clone IP073, was an exception, as it was detected in the plaque of only one control subject but for four RC subjects. The numbers of S. mutans and Actinomyces decreased slightly, while lactobacilli increased both in prevalence and levels from hrRC to carious samples. S. mutans was present in only 50% of the dentinal samples, which causes the role of S. mutans in the development of RC to be questioned (7) and contradicts culture studies implicating S. mutans as the major putative agent causing RC (26). The prevalences of S. mutans alone or in combination with lactobacilli were similar in hrRC and carious samples (i.e., present in half the samples), which is in line with previous observations (17, 26). Lactobacilli were absent in the healthy subjects but highly represented in carious dentin, supporting the suggestion that lactobacilli might not play a significant role in the initiation of RC but could be important in its progression or cause (12). Similarly, the observation that Actinomyces was found in half of the control, hrRC, and carious samples at similar low levels and in nine dentinal samples at high levels might indicate that they are not involved in the initiation but in the progression of the RC process. Overall, the present results suggest that not all the three classical bacterial species associated with RC need to be present for the disease to develop.
The microbial flora associated with RC was far more complex than previously assumed. Bacterial species typically associated with RC were detected, such as S. mutans, lactobacilli, and Actinomyces; however, additional species, such as Atopobium spp., Olsenella spp., Pseudoramibacter alactolyticus, and Propionibacterium sp. strain FMA5, were also commonly found. The data suggest that these last species may also be involved in the development of RC.
Supplementary Material
Acknowledgments
We thank the Cathinka Guldberg-Centre in Oslo, Norway, particularly Marianne Wenaasen, Sabah Tariq, and Torunn Birkeland, for patient management. Dominique A. Caugant and Jan Oksnes (Norwegian Institute of Public Health, Oslo, Norway) are kindly acknowledged for the use of laboratory facilities and for technical support in sequencing.
The study was supported by the Faculty of Dentistry, University of Oslo, Oslo, Norway.
Footnotes
Published ahead of print on 2 April 2008.
Supplemental material for this article may be found at http://jcm.asm.org/.
REFERENCES
- 1.Becker, M. R., B. J. Paster, E. J. Leys, M. L. Moeschberger, S. G. Kenyon, J. L. Galvin, S. K. Boches, F. E. Dewhirst, and A. L. Griffen. 2002. Molecular analysis of bacterial species associated with childhood caries. J. Clin. Microbiol. 401001-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Billings, R. J., L. R. Brown, and A. G. Kaster. 1985. Contemporary treatment strategies for root surface dental caries. Gerodontics 120-27. [PubMed] [Google Scholar]
- 3.Bowden, G. H., J. Ekstrand, B. McNaughton, and S. J. Challacombe. 1990. Association of selected bacteria with the lesions of root surface caries. Oral Microbiol. Immunol. 5346-351. [DOI] [PubMed] [Google Scholar]
- 4.Chhour, K. L., M. A. Nadkarni, R. Byun, F. E. Martin, N. A. Jacques, and N. Hunter. 2005. Molecular analysis of microbial diversity in advanced caries. J. Clin. Microbiol. 43843-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cole, J. R., B. Chai, R. J. Farris, Q. Wang, S. A. Kulam, D. M. McGarrell, G. M. Garrity, and J. M. Tiedje. 2005. The Ribosomal Database Project (RDP-II): sequences and tools for high-throughput rRNA analysis. Nucleic Acids Res. 33D294-D296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellen, R. P., D. W. Banting, and E. D. Fillery. 1985. Longitudinal microbiological investigation of a hospitalized population of older adults with a high root surface caries risk. J. Dent. Res. 641377-1381. [DOI] [PubMed] [Google Scholar]
- 7.Ellen, R. P., D. W. Banting, and E. D. Fillery. 1985. Streptococcus mutans and Lactobacillus detection in the assessment of dental root surface caries risk. J. Dent. Res. 641245-1249. [DOI] [PubMed] [Google Scholar]
- 8.Emilson, C. G., N. Ravald, and D. Birkhed. 1993. Effects of a 12-month prophylactic programme on selected oral bacterial populations on root surfaces with active and inactive carious lesions. Caries Res. 27195-200. [DOI] [PubMed] [Google Scholar]
- 9.Fure, S. 1998. Five-year incidence of caries, salivary and microbial conditions in 60-, 70- and 80-year-old Swedish individuals. Caries Res. 32166-174. [DOI] [PubMed] [Google Scholar]
- 10.Hoshino, E. 1985. Predominant obligate anaerobes in human carious dentin. J. Dent. Res. 641195-1198. [DOI] [PubMed] [Google Scholar]
- 11.Jenkinson, H. F., and R. J. Lamont. 2005. Oral microbial communities in sickness and in health. Trends Microbiol. 13589-595. [DOI] [PubMed] [Google Scholar]
- 12.Jordan, H. V., and B. F. Hammond. 1972. Filamentous bacteria isolated from human root surface caries. Arch. Oral Biol. 171333-1342. [DOI] [PubMed] [Google Scholar]
- 13.Jukes, T. H., and C. R. Cantor. 1969. Evolution of protein molecules, p. 21-132. In H. N. Munro (ed.), Mammalian protein metabolism, vol. 3. Academic Press, Inc., New York, NY. [Google Scholar]
- 14.Kazor, C. E., P. M. Mitchell, A. M. Lee, L. N. Stokes, W. J. Loesche, F. E. Dewhirst, and B. J. Paster. 2003. Diversity of bacterial populations on the tongue dorsa of patients with halitosis and healthy patients. J. Clin. Microbiol. 41558-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keltjens, H. M., M. J. Schaeken, J. S. van der Hoeven, and J. C. Hendriks. 1987. Microflora of plaque from sound and carious root surfaces. Caries Res. 21193-199. [DOI] [PubMed] [Google Scholar]
- 16.Li, Y., Y. Ge, D. Saxena, and P. W. Caufield. 2007. Genetic profiling of the oral microbiota associated with severe early-childhood caries. J. Clin. Microbiol. 4581-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li, Y., C. Y. Ku, J. Xu, D. Saxena, and P. W. Caufield. 2005. Survey of oral microbial diversity using PCR-based denaturing gradient gel electrophoresis. J. Dent. Res. 84559-564. [DOI] [PubMed] [Google Scholar]
- 18.Marsh, P. D., and M. V. Martin. 2000. Acquisition, adherence, distribution and functions of the oral microflora, p. 59. In Oral microbiology, 3rd ed. Chapman & Hall, London, United Kingdom.
- 19.Martin, F. E., M. A. Nadkarni, N. A. Jacques, and N. Hunter. 2002. Quantitative microbiological study of human carious dentine by culture and real-time PCR: association of anaerobes with histopathological changes in chronic pulpitis. J. Clin. Microbiol. 401698-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munson, M. A., A. Banerjee, T. F. Watson, and W. G. Wade. 2004. Molecular analysis of the microflora associated with dental caries. J. Clin. Microbiol. 423023-3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munson, M. A., T. Pitt-Ford, B. Chong, A. Weightman, and W. G. Wade. 2002. Molecular and cultural analysis of the microflora associated with endodontic infections. J. Dent. Res. 81761-766. [DOI] [PubMed] [Google Scholar]
- 22.Nyvad, B. 1993. Microbial colonization of human tooth surfaces. APMIS Suppl. 321-45. [PubMed] [Google Scholar]
- 23.Paster, B. J., S. K. Boches, J. L. Galvin, R. E. Ericson, C. N. Lau, V. A. Levanos, A. Sahasrabudhe, and F. E. Dewhirst. 2001. Bacterial diversity in human subgingival plaque. J. Bacteriol. 1833770-3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Percival, R. S., S. J. Challacombe, and P. D. Marsh. 1991. Age-related microbiological changes in the salivary and plaque microflora of healthy adults. J. Med. Microbiol. 355-11. [DOI] [PubMed] [Google Scholar]
- 25.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4406-425. [DOI] [PubMed] [Google Scholar]
- 26.Schupbach, P., V. Osterwalder, and B. Guggenheim. 1996. Human root caries: microbiota of a limited number of root caries lesions. Caries Res. 3052-64. [DOI] [PubMed] [Google Scholar]
- 27.Shen, S., L. P. Samaranayake, H. K. Yip, and J. E. Dyson. 2002. Bacterial and yeast flora of root surface caries in elderly, ethnic Chinese. Oral Dis. 8207-217. [DOI] [PubMed] [Google Scholar]
- 28.Sumney, D. L., and H. V. Jordan. 1974. Characterization of bacteria isolated from human root surface carious lesions. J. Dent. Res. 53343-351. [DOI] [PubMed] [Google Scholar]
- 29.Van de Peer, Y., and R. De Wachter. 1994. TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Comput. Appl. Biosci. 10569-570. [DOI] [PubMed] [Google Scholar]
- 30.Van Houte, J., H. V. Jordan, R. Laraway, R. Kent, P. M. Soparkar, and P. F. DePaola. 1990. Association of the microbial flora of dental plaque and saliva with human root-surface caries. J. Dent. Res. 691463-1468. [DOI] [PubMed] [Google Scholar]
- 31.Wade, W. G., D. A. Spratt, D. Dymock, and A. J. Weightman. 1997. Molecular detection of novel anaerobic species in dentoalveolar abscesses. Clin. Infect. Dis. 25(Suppl. 2)S235-S236. [DOI] [PubMed] [Google Scholar]
- 32.WHO. 2002. Active ageing: a policy framework, p. 6-9. WHO, Geneva, Switzerland.
- 33.WHO. 1997. Oral health surveys: basic methods, 4th ed., p.41. World Health Organization, Geneva, Switzerland.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.