Abstract
Norovirus genogroup II excretion during an outbreak of gastroenteritis was investigated in an aged-care facility. Viral shedding peaked in the acute stage of illness and continued for an average of 28.7 days. The viral decay rate was 0.76 per day, which corresponds to a viral half-life of 2.5 days.
Gastroenteritis of norovirus (NoV) etiology is the most common cause of epidemic nonbacterial gastroenteritis worldwide (1). NoV, a single-stranded, nonenveloped RNA virus, belongs to the family Caliciviridae. There are five genogroups within the Norovirus genus, and three are associated with human gastroenteritis (NoV genogroup I [GI], GII, and GIV). NoV is highly infectious, persists outside the host for long periods, and is resistant to disinfectants (6). Therefore, NoV outbreaks of gastroenteritis occur commonly in confined settings, including aged-care facilities, cruise ships, prisons, and hospitals (2, 4, 9-11, 24). Outbreaks in nursing homes and aged-care facilities account for between 27 and 80% of all NoV outbreaks and can result in patient death (2, 9, 15). The need for a rapid and definitive viral diagnosis is therefore imperative to provide a prompt public health response.
Viral loads in stools of patients in the acute stage of NoV infection have been measured using quantitative PCR assays (5, 12, 13, 19, 20). Using this technique, studies have reported median NoV GII viral loads in the range of 1.14 × 107 to 3.81 × 108 copies/g stool in patients with acute gastroenteritis (5, 12, 19, 20). NoV shedding can continue for long periods in the presence or absence of clinical symptoms (16, 21). Using molecular detection methods, clinical studies have reported the detection of NoV GII excretion in the stools for 10 days (12), 12 days (18), 22 days (21), and 47 days (16) after the onset of illness. In transplant and immunocompromised patients, NoV infection can lead to chronic diarrhea and prolonged viral excretion from 120 days to 2 years (7, 14, 17). However, all these clinical reports failed to continuously document NoV RNA detection until the cessation of viral excretion. Thus, a definitive period of viral excretion for NoV has not been determined, and consequently the period of infectivity is currently not clearly defined. In the current study we investigated the duration of viral excretion and the viral load decay rate in infected patients in an aged-care setting.
Outbreak of gastroenteritis.
In June 2003, an outbreak of vomiting and diarrhea affecting 28 (56%) of 50 patients and 43 (57%) of 75 staff members occurred in two out of three wards at an aged-care facility in New South Wales, Australia. The total duration of the outbreak in which clinical symptoms were present was 19 days. Stool specimens were collected every 3 to 7 days from 14 volunteers (six males and eight females) from the onset of illness until the second consecutive NoV RNA-negative specimen. Of the 14 volunteers (median, 85 years), 13 were patients (aged 63 to 93 years) and one was a staff member (58 years old). A single etiological agent was identified, a recombinant NoV strain termed NoV/Picton/2003/AU (GII.b RNA polymerase/GII.1 capsid, GenBank accession number AY919139) as described in the work of Bull et al. (3). Products derived from four separate individuals were sequenced. Subsequent alignments revealed greater than 99 to 100% nucleotide identity, confirming the idea that a common etiological agent was responsible for the outbreak.
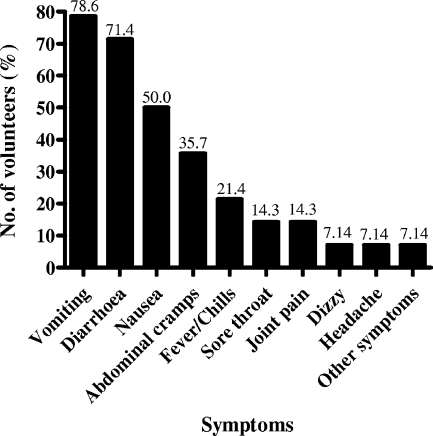
Symptoms in all 14 NoV GII RNA-positive volunteers were closely documented until symptoms ceased (Fig. 1). Gastrointestinal symptoms lasted on average 2.6 days (range, 1 to 4 days; median, 3 days) and were characteristic of a NoV GII infection (8, 16). Vomiting was the prominent symptom (78.6% of patients), while diarrhea (71.4%), nausea (50.0%), and abdominal cramps (35.7%) were the other common clinical manifestations (Fig. 1).
FIG. 1.
Distribution of clinical symptoms from NoV GII RNA-infected volunteers presenting with gastroenteritis. The most common clinical symptom experienced among the volunteers was vomiting in 11 (78.6%) and diarrhea in 10 (71.4%) of 14 volunteers. The percentage of volunteers presenting with each clinical symptom of gastroenteritis is shown on top of the respective bar.
Viral load measurements.
To provide an insight into the period of infectivity in elderly individuals with acute NoV gastroenteritis, the viral load and its decay over time were determined. NoV GII RNA was quantified in all 14 volunteers from the onset of gastrointestinal illness until the second consecutive NoV GII RNA-negative specimen (total samples quantified [n] = 92). A 20% (wt/vol) stool suspension was made by weighing 0.2 g of stool and suspending it in a total volume of 1 ml water. Following centrifugation, viral RNA was extracted as described in the work of Bull et al. (4). A highly conserved region which included 55 bp at the 3′ end of the polymerase and 344 bp at the 5′ end of the capsid was targeted using primers described in the work of Bull et al. (4). NoV GII RNA was quantified by a real-time nested reverse transcriptase-PCR using a Bio-Rad MyiQ Single-Color real-time PCR detection system (Bio-Rad, CA) as described in the work of Tu et al. (23). Standards for quantification were derived from a 381-bp first-round PCR product that was cloned into pCRII-TOPO (Invitrogen), as described in the work of White et al. (25), and was quantified by spectrophotometry.
A viral load was defined as the number of NoV GII RNA copies per gram of stool (copies/g stool). The viral loads detected in patients were highly variable. For viral loads measured between days 0 and 7, 8 and 14, 15 and 21, and 22 and 28 the standard deviation was used to measure variance and the standard error of the mean (SEM) was used to measure the precision of the results. The average viral load for days 0 to 7 was 1.07 × 108 ± 3.29 × 108 copies/g stool (n = 12 specimens, SEM of 9.51 × 107 copies/g stool). Consecutively, the average viral load detected between days 8 and 14 was 1.32 × 106 ± 4.07 × 106 copies/g stool (n = 10 specimens, SEM of 1.29 × 106 copies/g stool). For days 15 to 21 it was 1.20 × 105 ± 2.00 × 105 copies/g stool (n = 14 specimens, SEM of 5.35 × 104 copies/g stool), and for days 22 to 28 it was 4.02 × 104 ± 8.43 × 104 copies/g stool (n = 14 specimens, SEM of 2.25 × 104 copies/g stool). The detection limit of the NoV GII amplification assay was 25 copies/reaction, equivalent to 8.93 × 103 copies/g stool.
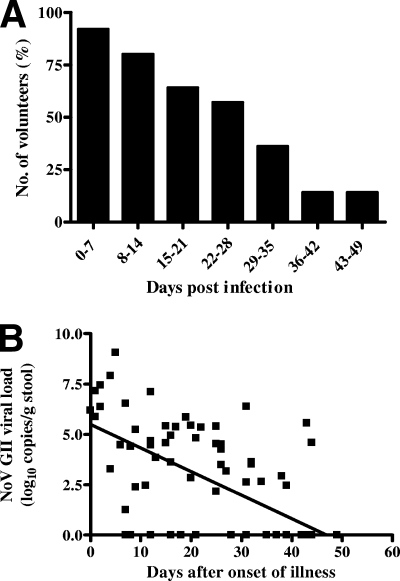
The duration of viral shedding was investigated in all 14 volunteers and determined as the midpoint between the last NoV GII RNA-positive specimen and subsequent NoV GII RNA-negative specimen (Fig. 2A). The period of viral excretion was on average 28.7 days (median, 28.5 days), with a range of 13.5 to 44.5 days (Fig. 2A). Thus, viral RNA was continually detected in the postrecovery stage of gastrointestinal illness. This is significant, as it was previously thought that following the resolution of symptoms viral shedding ceases within 100 h (22). It is difficult, however, to assess the infectivity of the excreted virus at these late time points, as there are currently no cell culture systems for human NoV. No correlation was observed between the following: initial viral load (i.e., viral load at t = 0) and duration of symptomatic illness, initial viral load and duration of NoV excretion, age and duration of symptomatic illness, age and initial viral load, and age and duration of viral excretion.
FIG. 2.
(A) The percentages of volunteers positive for the detection of NoV GII RNA in stool specimens over time. The median duration of viral excretion was 28.5 days (n = 14 volunteers, range of 13.5 to 44.5 days, mean of 28.7 days). The initial day of illness equaled day 0. (B) Rate of viral decay in NoV GII-infected volunteers. NoV GII RNA was quantified in all 14 volunteers from the onset of gastrointestinal illness until the second consecutive negative NoV GII RNA specimen. Assuming an exponential decay of viral load over time, the following equation was determined: V = V010−dt, where d is the viral decay rate (day−1), V is NoV GII RNA copies/g at time t (day), and V0 is the initial viral load. The rate of viral decay was obtained by fitting the above equation on a logarithmic (log10) scale (r2 = 0.4236, P < 0.0001), with the assumption that a measured viral load of 0.00 copies/g stool (defined as a cycle threshold value below the detection limit of the assay) was equivalent to 1 copy/g stool. The viral decay rate in NoV-infected individuals was 0.76 per day, which corresponds to a half-life (t1/2) of 2.5 days.
To obtain the rate of viral decay, viral load measurements were plotted against days after onset of illness for each volunteer (Fig. 2B). Assuming an exponential decay of viral load over time, let V = V010−dt, where d is the viral decay rate (day−1), V is NoV GII RNA copies/g at time t (day), and V0 is the initial viral load. To estimate V0 and d from our data, we fitted a line on a logarithmic (log10) scale (r2 = 0.4236, P < 0.0001) (Fig. 2B), obtaining V0 = 105.49 and d = −0.12 (day−1). Thus, the viral decay rate was 0.76 per day, which corresponds to a viral half-life (t1/2) of 2.5 days.
In conclusion, this study reports the duration and quantity of NoV GII RNA excretion in human stools to provide a clearer insight into the period of NoV infectivity in an aged-care setting. In the presence of rapid viral decay, prolonged (∼4 weeks) asymptomatic shedding of NoV was detected in the elderly. Such data would be useful for risk assessment models, implementing quarantine strategies, and preventing dissemination of the disease.
Acknowledgments
E. T.-V. Tu was supported by a University of New South Wales postgraduate award, and R. A. Bull was supported by an Australian postgraduate award.
We thank the 14 volunteers and Mark M. Tanaka for his help with statistical analyses.
Footnotes
Published ahead of print on 16 April 2008.
REFERENCES
- 1.Atmar, R. L., and M. K. Estes. 2006. The epidemiologic and clinical importance of norovirus infection. Gastroenterol. Clin. N. Am. 35275-290. [DOI] [PubMed] [Google Scholar]
- 2.Blanton, L. H., S. M. Adams, R. S. Beard, G. Wei, S. N. Bulens, M. A. Widdowson, R. I. Glass, and S. S. Monroe. 2006. Molecular and epidemiologic trends of caliciviruses associated with outbreaks of acute gastroenteritis in the United States, 2000-2004. J. Infect. Dis. 193413-421. [DOI] [PubMed] [Google Scholar]
- 3.Bull, R. A., G. S. Hansman, L. E. Clancy, M. M. Tanaka, W. D. Rawlinson, and P. A. White. 2005. Norovirus recombination in ORF1/ORF2 overlap. Emerg. Infect. Dis. 111079-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bull., R. A., E. T.-V. Tu, C. J. McIver, W. D. Rawlinson, and P. A. White. 2006. Emergence of a new norovirus genotype II.4 variant associated with global outbreaks of gastroenteritis. J. Clin. Microbiol. 44327-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan, M. C., J. J. Sung, R. K. Lam, P. K. Chan, N. L. Lee, R. W. Lai, and W. K. Leung. 2006. Fecal viral load and norovirus-associated gastroenteritis. Emerg. Infect. Dis. 121278-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doultree, J. C., J. D. Druce, C. J. Birch, D. S. Bowden, and J. A. Marshall. 1999. Inactivation of feline calicivirus, a Norwalk virus surrogate. J. Hosp. Infect. 4151-57. [DOI] [PubMed] [Google Scholar]
- 7.Gallimore, C. I., D. Lewis, C. Taylor, A. Cant, A. Gennery, and J. J. Gray. 2004. Chronic excretion of a norovirus in a child with cartilage hair hypoplasia (CHH). J. Clin. Virol. 30196-204. [DOI] [PubMed] [Google Scholar]
- 8.Graham, D. Y., X. Jiang, T. Tanaka, A. R. Opekun, H. P. Madore, and M. K. Estes. 1994. Norwalk virus infection of volunteers: new insights based on improved assays. J. Infect. Dis. 17034-43. [DOI] [PubMed] [Google Scholar]
- 9.Green, K. Y., G. Belliot, J. L. Taylor, J. Valdesuso, J. F. Lew, A. Z. Kapikian, and F. Y. Lin. 2002. A predominant role for Norwalk-like viruses as agents of epidemic gastroenteritis in Maryland nursing homes for the elderly. J. Infect. Dis. 185133-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansman, G. S., K. Katayama, N. Maneekarn, S. Peerakome, P. Khamrin, S. Tonusin, S. Okitsu, O. Nishio, N. Takeda, and H. Ushijima. 2004. Genetic diversity of norovirus and sapovirus in hospitalized infants with sporadic cases of acute gastroenteritis in Chiang Mai, Thailand. J. Clin. Microbiol. 421305-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho, E. C., P. K. Cheng, A. W. Lau, A. H. Wong, and W. W. Lim. 2007. Atypical norovirus epidemic in Hong Kong during summer of 2006 was caused by a new genogroup II/4 variant. J. Clin. Microbiol. 452205-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hohne, M., and E. Schreier. 2004. Detection and characterization of norovirus outbreaks in Germany: application of a one-tube RT-PCR using a fluorogenic real-time detection system. J. Med. Virol. 72312-319. [DOI] [PubMed] [Google Scholar]
- 13.Kageyama, T., S. Kojima, M. Shinohara, K. Uchida, S. Fukushi, F. B. Hoshino, N. Takeda, and K. Katayama. 2003. Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. J. Clin. Microbiol. 411548-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufman, S. S., N. K. Chatterjee, M. E. Fuschino, M. S. Magid, R. E. Gordon, D. L. Morse, B. C. Herold, N. S. LeLeiko, A. Tschernia, S. S. Florman, G. E. Gondolesi, and T. M. Fishbein. 2003. Calicivirus enteritis in an intestinal transplant recipient. Am. J. Transplant. 3764-768. [DOI] [PubMed] [Google Scholar]
- 15.Marshall, J. A., A. Dimitriadis, and P. J. Wright. 2005. Molecular and epidemiological features of norovirus-associated gastroenteritis outbreaks in Victoria, Australia in 2001. J. Med. Virol. 75321-331. [DOI] [PubMed] [Google Scholar]
- 16.Murata, T., N. Katsushima, K. Mizuta, Y. Muraki, S. Hongo, and Y. Matsuzaki. 2007. Prolonged norovirus shedding in infants ≤6 months of age with gastroenteritis. Pediatr. Infect. Dis. J. 2646-49. [DOI] [PubMed] [Google Scholar]
- 17.Nilsson, M., K. O. Hedlund, M. Thorhagen, G. Larson, K. Johansen, A. Ekspong, and L. Svensson. 2003. Evolution of human calicivirus RNA in vivo: accumulation of mutations in the protruding P2 domain of the capsid leads to structural changes and possibly a new phenotype. J. Virol. 7713117-13124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Neill, H. J., C. McCaughey, D. E. Wyatt, F. Mitchell, and P. V. Coyle. 2001. Gastroenteritis outbreaks associated with Norwalk-like viruses and their investigation by nested RT-PCR. BMC Microbiol. 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozawa, K., T. Oka, N. Takeda, and G. S. Hansman. 2007. Norovirus infections in symptomatic and asymptomatic food-handlers in Japan. J. Clin. Microbiol. 453996-4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pang, X., B. Lee, L. Chui, J. K. Preiksaitis, and S. S. Monroe. 2004. Evaluation and validation of real-time reverse transcription-PCR assay using the LightCycler system for detection and quantitation of norovirus. J. Clin. Microbiol. 424679-4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rockx, B., M. de Wit, H. Vennema, J. Vinje, E. De Bruin, Y. Van Duynhoven, and M. Koopmans. 2002. Natural history of human calicivirus infection: a prospective cohort study. Clin. Infect. Dis. 35246-253. [DOI] [PubMed] [Google Scholar]
- 22.Thornhill, T., E. Kalica, R. G. Wyatt, A. Z. Kapikian, and R. M. Chanock. 1975. Pattern of shedding of the Norwalk particles in stools during experimentally induced gastroenteritis in volunteers as demonstrated by immune electron microscopy. J. Infect. Dis. 13228-34. [DOI] [PubMed] [Google Scholar]
- 23.Tu, E. T.-V., R. A. Bull., G. E. Greening, J. Hewitt, M. J. Lyon, J. A. Marshall, C. J. McIver, W. D. Rawlinson, and P. A. White. 2008. Epidemics of gastroenteritis during 2006 were associated with the spread of norovirus GII.4 variants 2006a and 2006b. Clin. Infect. Dis. 46413-420. [DOI] [PubMed] [Google Scholar]
- 24.White, P. A., G. S. Hansman, A. Li, J. Dable, M. Isaacs, M. Ferson, C. J. McIver, and W. D. Rawlinson. 2002. Norwalk-like virus 95/96-US strain is a major cause of gastroenteritis outbreaks in Australia. J. Med. Virol. 68113-118. [DOI] [PubMed] [Google Scholar]
- 25.White, P. A., Y. Pan, A. J. Freeman, G. Marinos, R. A. Ffrench, A. R. Lloyd, and W. D. Rawlinson. 2002. Quantification of hepatitis C virus in human liver and serum samples by using LightCycler reverse transcriptase PCR. J. Clin. Microbiol. 404346-4348. [DOI] [PMC free article] [PubMed] [Google Scholar]