Abstract
In 2000, we encountered cases of nosocomial infections with epidemic keratoconjunctivitis (EKC) at a university hospital in Kobe, in the western part of Japan. Two human adenovirus (HAdV) strains, Kobe-H and Kobe-S, were isolated from patients with nosocomial EKC infection. They were untypeable by existing neutralizing antisera; however, the isolate was neutralized with homologous antisera. We then encountered several cases of EKC due to nosocomial infections in eye clinics in different parts of Japan. A total of 80 HAdVs were isolated from patients with EKC at eight different hospitals. The partial hexon gene sequences of the isolates were determined and compared to those of the prototype strains of 51 serotypes. All isolates had identical partial hexon nucleotide sequences. Phylogenetic analysis classified these isolates into species of HAdV-D. The isolates showed 93.9 to 96.7% nucleotide identity with HAdV-D prototype strains, while all 32 HAdV-D prototype strains ranged from 93.2 to 99.2% identity. The sequences of the loop 2 and fiber knob regions from the representative strain, Kobe-H, were dissimilar in all prototype strains of 51 serotypes. We believe that this virus is a novel serotype of HAdV that causes EKC.
Human adenoviruses (HAdVs), which belong to the genus Mastadenovirus of the family Adenoviridae, infect billions of people worldwide and cause various diseases, including conjunctivitis, respiratory infectious disease, diarrhea in infants and young children, and hemorrhagic cystitis (41). HAdVs initially were grouped into six subgenera (A to F) on the basis of several biochemical and biophysical criteria (8, 41). In 1999, however, a reclassification on the basis of nucleotide and deduced amino acid sequences was approved by the International Committee on Taxonomy of Viruses; under this reclassification, the 51 serotypes of HAdVs were grouped into six species, HAdV-A to HAdV-F (8).
In Japan, roughly 8,500 HAdV isolates were reported in 2003 to 2007 (Infectious Agents Surveillance Report [http://idsc.nih.go.jp/iasr/index.html]). They were obtained from patients with epidemic conjunctivitis (979; 11.5%), upper and lower respiratory tract infections (1,582; 18.6%), and gastroenteritis (348; 4.1%). Virus isolation followed by a neutralization test (NT) using a type-specific antiserum has been the standard procedure for serotyping (39). To date, the amplification of the genome by PCR, the determination of the nucleotide sequences, and phylogenetic analysis have become generalized techniques for both the classification and identification of viruses. We recently determined the partial hexon sequences of all 51 prototype strains and have developed a rapid and reliable method of molecular diagnosis based on phylogenetic analysis (25, 33). This method has successfully classified the 51 prototype strains of HAdVs into the six designated species, as approved by the International Committee on Taxonomy of Viruses. Over the last 30 years, we have applied this molecular diagnosis to the identification of hundreds of isolates and swabs without virus isolation from patients from different parts of the world with EKC and lower respiratory tract infections (5, 6, 21, 25, 33).
In 2000, we encountered cases of nosocomial EKC infection at a university hospital in Kobe, in the western part of Japan. Two HAdV strains, Kobe-H and Kobe-S, were isolated from the patients and showed identical nucleotide sequences of partial hexon genes. Adenoviral conjunctivitis is caused primarily by HAdV-3 (of the HAdV-B species), HAdV-4 (of HAdV-E), and HAdV-8, HAdV-19, and HAdV-37 (all of HAdV-D). Among these, HAdV-8, HAdV-19, and HAdV-37 cause more severe EKC than the others (3, 4, 7). The more serious outcomes occur in all age groups and can trigger highly contagious nosocomial infections (3, 7, 20, 26, 38). Kobe-H and Kobe-S were untypeable by using existing neutralizing antisera, suggesting that they represent a novel adenovirus causing EKC. After the nosocomial infections of 2000, we encountered several cases of nosocomial EKC infection, including cases at eye clinics, in different parts in Japan. Surprisingly, the 80 isolates from patients with EKC at eight different hospitals shared partial nucleotide sequences that were identical.
In this paper, we characterize the isolates from the patients with EKC. The present phylogenetic analysis classified these isolates into the HAdV-D species, and the Kobe-H strain represents only 93.9 to 96.7% nucleotide identity between the 32 prototype strains of HAdV-D, while all prototype strains of HAdV-D ranged from 93.2 to 99.2% identity. Quantitative NT and genetic analysis of the main neutralization ɛ determinant, loops 1 (L1) and 2 (L2) in the hexon gene, clearly indicated that the representative strain Kobe-H is different from previously known HAdVs. We believe that the Kobe-H strain is a novel HAdV that causes nosocomial EKC infection.
MATERIALS AND METHODS
Viruses.
The isolates and conjunctival swabs were obtained from nosocomial or sporadic infections from 2000 to 2005 (Table 1). Kobe-H and -S were isolated from patients with nosocomial EKC infections at a university hospital in Kobe, Japan, in 2000. These strains were supplied from the Reference Center of Nosocomial Infection, Hokkaido University Graduate School of Medicine, Sapporo, Japan. The prototype strains of 51 HAdVs were obtained from the American Type Culture Collection and from the National Institute of Infectious Diseases, Tokyo, Japan. These viruses were used directly for DNA extraction without further propagation.
TABLE 1.
Type identification of clinical isolates from patients with EKC by phylogenetic analysis
| Representative isolate/origin (in Japan)/year | No. of isolates or swabs | Typing by phylogeny | Typing of partial hexon regions (916 and 350 bp) for:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Highest-scoring prototype
|
Next-highest-scoring prototype
|
|||||||||
| 916 bp
|
350 bp
|
916 bp
|
350 bp
|
|||||||
| Type | % Identity | Type | % Identity | Type | % Identity | Type | % Identity | |||
| Kobe-H/Kobe/2000 | 1 isolate | NTa | HAdV-8 | 96.7 | HAdV-8b | 95.4 | HAdV-22 | 95.4 | HAdV-22c | 95.1 |
| Kobe-S/Kobe/2000 | 1 isolate | NT | HAdV-8 | 96.7 | HAdV-8b | 95.4 | HAdV-22 | 95.4 | HAdV-22c | 95.1 |
| Hamamatsu/2000 | 5 isolates | NT | HAdV-8 | 96.7 | HAdV-8b | 95.4 | HAdV-22 | 95.4 | HAdV-22c | 95.1 |
| 33371/Sapporo/2001 | 6 isolates | NT | HAdV-8 | 96.7 | HAdV-8b | 95.4 | HAdV-22 | 95.4 | HAdV-22c | 95.1 |
| 33537/Kumamoto/2001 | 3 isolates | NT | HAdV-8 | 96.7 | HAdV-8b | 95.4 | HAdV-22 | 95.4 | HAdV-22c | 95.1 |
| C029/Matsuyama/2003 | 12 isolates | NT | HAdV-8 | 96.7 | HAdV-8b | 95.4 | HAdV-22 | 95.4 | HAdV-22c | 95.1 |
| 085/Itoman/2003 | 1 isolate | NT | HAdV-8 | 96.7 | HAdV-8b | 95.4 | HAdV-22 | 95.4 | HAdV-22c | 95.1 |
| 36876/Tokyo/2005 | 16 isolates | NT | HAdV-8 | 96.7 | HAdV-8b | 95.4 | HAdV-22 | 95.4 | HAdV-22c | 95.1 |
| 65041/Nagoya/2005d | 33 swabs | NT | HAdV-8 | 96.7 | HAdV-8b | 95.4 | HAdV-22 | 95.4 | HAdV-22c | 95.1 |
NT, not typed.
HAdV-8, HAdV-29, HAdV-38, HAdV-43, and HAdV-46.
HAdV-22, HAdV-24, HAdV-25, HAdV-30, HAdV-32, HAdV-33, HAdV-37, HAdV-45, and HAdV-47.
HAdV DNA was detected in conjunctival swabs.
Phylogeny-based classification using a partial hexon sequence.
A partial hexon sequence of HAdV was amplified from the isolates as described previously (25, 33). In brief, viral DNA was extracted from 100 μl of virus suspension using a Sumitest EX-R&D kit (Medical & Biological Laboratories Co., Ltd., Naggoya, Japan) by following the manufacturer's instructions. The DNA was dissolved in 100 μl of TE buffer (10 mM Tris [pH 8.0], 1 mM EDTA). The 1,004-bp fragment of the hexon gene was amplified with 50 pmol of a pair of primers, AdTU7 (nucleotide positions 20,734 to 20,753; 5′-GCCACCTTCTTCCCCATGGC-3′) and AdTU4′ (positions 21,718 to 21,737; 5′-GTAGCGTTGCCGGCCGAGAA-3′). The positions of the primers for PCR were numbered according to the complete nucleotide sequence of the HAdV-2 strain (GenBank accession no. J01917). Using 10 μl of the PCR product, nested PCR was performed to amplify the 956-bp DNA fragment with a pair of primers, AdnU-S′ (positions 20,743 to 20,762; 5′-TTCCCCATGGCNCACAACAC-3′) and AdnU-A (positions 21,679 to 21,698; 5′-GCCTCGATGACGCCGCGGTG-3′). PCR was carried out for 36 cycles in a Cetus 9600 Thermal Cycler (PE Applied Biosystems, Foster City, CA). Each cycle consisted of denaturation at 94°C for 1 min, annealing at 50°C for 1 min, and primer extension at 72°C for 2 min. After the last cycle, extension was continued at 72°C for 7 min. The PCR products were separated on 3% agarose gels and purified with a QIAquick gel extraction kit (Qiagen, Valencia, CA).
The nucleotide sequence of the PCR products was determined using the CEQ 2000XL DNA analysis system with a Dye Terminator cycle sequencing kit (Beckman Coulter, Inc., Fullerton, CA). The nucleotide sequences of the partial hexons were analyzed by a comparison to those of prototype strains of all 51 HAdV serotypes from GenBank using the SINCA software (Fujitsu, Ltd., Tokyo, Japan). Evolutionary distances were estimated using Kimura's two-parameter method (22), and unrooted phylogenetic trees were constructed using the neighbor-joining (N-J) method (30). Bootstrap analyses were performed by 1,000 resamplings of the data sets (16). Similarity plots depicting the relationships among the aligned hexon nucleotide sequences were generated using SimPlot (version 3.5.1) (23). The similarity of the hexon gene between the novel HAdV and the prototype strains of the 51 serotypes was calculated in each window of 200 nucleotides by the Kimura two-parameter method (22) with a transition/transversion ratio of 2.0. The window was successively advanced along the genome alignment in 20-nucleotide increments. We previously determined the nucleotide sequences of the partial hexon genes (916 bp) of all 32 prototype strains of HAdV-D and HAdV-E (33). In the previous study, we used the residual HAdV prototype strains, AdV-6 of HAdV-C, AdV-31 of HAdV-A, and AdV-50 of HAdV-B, to complete the database based on 350 bp of the hexon gene of HAdV; these sequences were not available from GenBank (25).
Serological analysis.
Antisera against the Kobe-H strain were raised in rabbits by following the conventional procedure (12). Serological analyses were performed by a quantitative NT with type-specific antisera (for HAdV-8, HAdV-9, HAdV-19, and HAdV-37) purchased from Denka Seiken Co., Ltd. (Tokyo, Japan), or from the American Type Culture Collection (for HAdV-8). NTs were performed with A549 human lung cancer cells in 96-well microplates. The 50% tissue culture infectious doses of challenge virus that caused a cytopathic effect after 7 days of incubation at 37°C were used. Duplicates of the twofold serial-diluted antisera were used in the HAdV NTs (32).
Genome typing.
Viral DNA was extracted from infected cells and placed in a 75-cm2 plastic flask using 3 ml of Hirt lysis solution (10 mM Tris, 1 mM EDTA, 0.6% sodium dodecyl sulfate, pH 8.0) (18). Proteinase K was added at a final concentration of 50 μg/ml, and the samples were incubated at 37°C for 1 h. Cellular DNA was precipitated with 1 M of NaCl (final concentration) overnight at 4°C. After phenol-chloroform extraction, the supernatant was treated with a mixture of ribonucleases A (25 mg/ml) and T1 (80 U/ml) (Sigma, St. Louis, MO), and phenol-chloroform extraction was performed. Viral DNA was precipitated with isopropanol and suspended in 50 μl of TE buffer (1 mM Tris-HCl, pH 8.0, 0.1 mM EDTA). One microgram of viral genomic DNA was digested with 5 U of each of the following restriction enzymes: BamHI, HindIII, and SmaI (Takara Shuzo Co., Ltd., Kyoto, Japan). The digested viral DNA was loaded onto 1% agarose gels containing 1 μg/ml ethidium bromide. DNA bands were photographed with a UV transilluminator and a Polaroid camera. The migration patterns of the DNA fragments were compared to those of previously reported genome types (1, 11, 17, 31, 40).
Nucleotide sequence accession numbers.
The GenBank accession numbers of the nucleotide sequences presented in this study are AB333801 and AB359056. GenBank sequences AB330082 to AB330132 were used to generate alignments of the hexon gene.
RESULTS
Phylogeny-based classification using a partial hexon sequence.
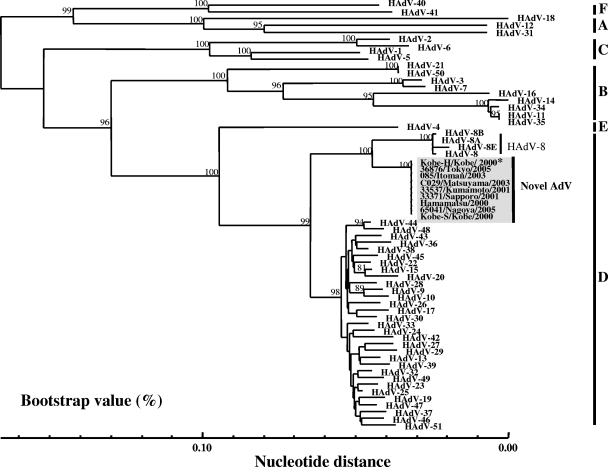
In 2000, we encountered cases of nosocomial EKC at a university hospital in Kobe, in the western part of Japan. Two HAdVs, the Kobe-H and Kobe-S strains, were isolated from the EKC patients. These strains had identical partial hexon sequences. After the Kobe episode, several studies reported an HAdV in different parts of Japan that was similar to the Kobe-H and Kobe-S strains and that caused nosocomial EKC (Table 1). To assess the genetic constellation of the HAdV that caused the nosocomial infections, Kobe-H was selected as a representative isolate, and the alignment of the partial hexon nucleotide sequences was performed with 51 prototype strains and three HAdV-8 variants, including HAdV-8A, HAdV-8B, and HAdV-8E, using the SINCA genetic software program (34).
As shown in Fig. 1, the prototype strains were segregated into six major clusters, which corresponded well with the six newly designated HAdV species A to F (8). Kobe-H was segregated into the D cluster with 32 prototype strains and clustered with the HAdV-8 prototype strain and its genome type strains, HAdV-8A, HAdV-8B, and HAdV-8E (Fig. 1). Within this cluster, however, Kobe-H had a lineage that was different from that of the prototype HAdV-8 and the three genome type strains. The genetic relationships between Kobe-H and the HAdV-D prototype strains were further compared one by one. The nucleotide identity among the 32 HAdV-D serotypes ranged from 93.2 (between HAdV-8 and HAdV-51) to 99.2% (between HAdV-23 and HAdV-25), with an average of 97.3%, and those between the prototype HAdV-8 strain and the three genome type strains were 99.3 to 99.9%. Interestingly, Kobe-H showed only 96.7% nucleotide identity with HAdV-8 and 96.3 to 96.8% identity with the three genome type strains. These data suggest that Kobe-H is a novel serotype in the HAdV-D strains.
FIG. 1.
Phylogenetic analyses of the representative strain Kobe-H and isolates from patients with EKC in different parts of Japan. The 916-bp sequence of a partial hexon gene of the representative samples was analyzed by the N-J method together with the prototype strains of all 51 HAdV serotypes and three genome type strains of HAdV-8. HAdV-8 is the prototype strain (Trim). HAdV-8A, HAdV-8B, and HAdV-E are the genome type strains of HAdV-8. The numbers at the nodes are percentages of 1,000 bootstrap pseudoreplicates containing the cluster distal to the node.
Serological analysis.
The clinical isolates including Kobe-H formed a monophyletic cluster with the HAdV-8 prototype strain of HAdV-D. To examine the serological reactivity between the Kobe-H and HAdV-D strains, a quantitative neutralization assay was performed with the antiserum against HAdV-8 along with the antisera against the HAdV-9, HAdV-19, and HAdV-37 prototype strains, which are the causative agents of conjunctivitis. As shown in Table 2, no prototype-specific antiserum reacted with Kobe-H at a titer higher than 1:64 of homologous titer, except for the HAdV-8 antiserum. Conversely, the anti-Kobe-H serum did not react with any of the six prototype strains, except that a low-NT titer was found in the reaction mixture with HAdV-9 (Table 2). As the serotype has been defined on the basis of its immunological distinctiveness, the new serotype should show a homologous/heterologous titer ratio of >16 in either direction (39). Therefore, these results again suggest that Kobe-H is a novel serotype of the species HAdV-D.
TABLE 2.
Quantitative NT with Kobe-H against type-specific antisera
| Virus strain | Quantitative neutralization titer of antisera for strain:
|
||||||
|---|---|---|---|---|---|---|---|
| Kobe-H | HAdV-3p | HAdV-4p | HAdV-8p | HAdV-9p | HAdV-19a | HAdV-37p | |
| Kobe-H | 128 | <1 | <1 | 64 | 4 | <1 | <1 |
| HAdV-3pa | <1 | 4,096 | 4 | <1 | <1 | <1 | <1 |
| HAdV-4p | <1 | <1 | 32,768 | 1 | <1 | <1 | 8 |
| HAdV-8p | <1 | <1 | 1 | 4,096 | <1 | <1 | <1 |
| HAdV-9p | 1 | <1 | <1 | 4 | 256 | <1 | <1 |
| HAdV-19ab | <1 | <1 | 1 | 2 | <1 | 1,024 | 2 |
| HAdV-37p | <1 | <1 | <1 | <1 | <1 | <1 | 256 |
The prototype strains are HAdV-3p (GB), HAdV-4p (RI-67), HAdV-8p (Trim), HAdV-9p (Hicks), and HAdV-37p (GW).
Isolate from EKC.
Genome typing.
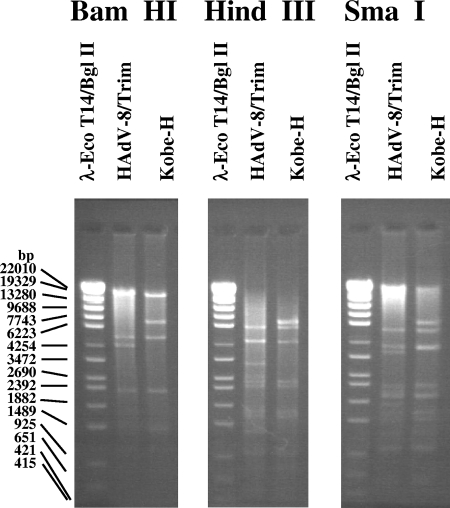
To determine the genotype of Kobe-H, its genomic DNA was digested with three restriction endonucleases: BamHI, HindIII, and SmaI. Each restriction pattern was different from that of known prototype strains (Fig. 2).
FIG. 2.
Genome type of Kobe-H. Viral genomic DNA was digested with three restriction enzymes: BamHI, HindIII, and SmaI.
Full-length hexon and fiber knob gene nucleotide sequences.
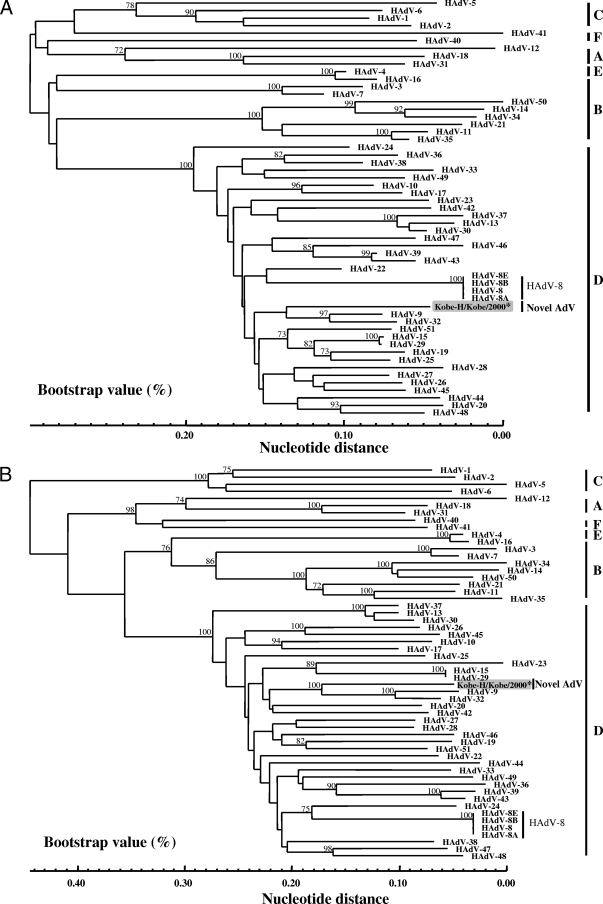
Recently, the main neutralization ɛ determinant encoded in L1 and L2 in hexon genes was determined for all HAdV prototype strains, and criteria for typing based on the L1 and L2 nucleotide sequences have been proposed (24). These criteria may facilitate the identification of new HAdV prototypes, and we thus determined the full-length hexon sequence of Kobe-H as previously described by Ebner et al. (14), as well as those of the prototype strains, which were not available from GenBank. When the full-length hexon nucleotide sequence of Kobe-H was compared to those of the 51 prototype strains, the L2 nucleotide sequence of Kobe-H showed 56.9 (HAdV-41 of HAdV-F) to 85.8% (HAdV-9 of HAdV-D) identity and showed only 78.5% identity to the HAdV-8 prototype strain (Table 3). The phylogenetic analysis showed that Kobe-H does not form a monophylic cluster with any of the 51 prototype strains (Fig. 3A). Therefore, we also analyzed the L1 region because of its higher contribution to the ɛ determinant (24). Kobe-H showed 40.7 (HAdV-1 of HAdV-C) to 75.9% (HAdV-9 of HAdV-D) identity with the 51 prototype strains (Table 3). The phylogenetic analysis showed that Kobe-H does not form a monophylic cluster with any of the 51 prototype strains (Fig. 3B). These results clearly indicate that Kobe-H is a new HAdV prototype strain.
TABLE 3.
Nucleotide sequence analysis of the L1 and L2 regions in the hexon gene and fiber knob region
| Kobe-H strain region | Highest-scoring prototype
|
Next-highest-scoring prototype
|
Lowest-scoring prototype
|
|||
|---|---|---|---|---|---|---|
| Type | % Identity | Type | % Identity | Type | % Identity | |
| Hexon L1 | HAdV-9 | 75.9 | HAdV-32 | 75.3 | HAdV-1 | 40.7 |
| Hexon L2 | HAdV-9 | 85.8 | HAdV-32 | 84.3 | HAdV-41 | 56.9 |
| Fiber knob | HAdV-8 | 98.7 | HAdV-9 | 93.4 | HAdV-50 | 31.5 |
FIG. 3.
Phylogenetic analyses of the nucleic acid sequences of the ɛ determinant in L2 (A) and L1 (B). The nucleotide sequence of Kobe-H was analyzed by the N-J method together with the 51 prototype strains of HAdV and the three genome type strains of HAdV-8. The numbers at the nodes are percentages of 1,000 bootstrap pseudoreplicates containing the cluster distal to the node.
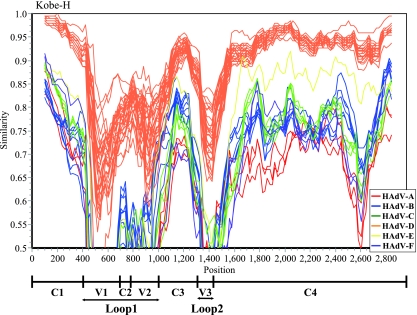
The inconsistent constellation of Kobe-H in the L2-based phylogeny and a partial hexon gene-based phylogeny suggest that recombination played a role in the evolution of the Kobe strains. To clarify the recombination events, we compared the complete hexon gene of the Kobe-H strain to those of all prototype strains by similarity plot analysis with a sliding window of 200 residues (Fig. 4). When Kobe-H was used as the query sequence and compared to the 51 prototype strains, the resultant 51 similarity plots indicated that the L1 and L2 regions were highly dissimilar, while 5′ and 3′ parts of the hexon gene were well conserved among some of the strains (Fig. 4). Therefore, we conclude that there was no recombination in the hexon gene between Kobe-H and the 51 prototype strains.
FIG. 4.
Similarity plots of the nucleotide sequences of the full-length hexon gene calculated by SimPlot 3.5.1. The full length of the hexon nucleotide sequence of the Kobe-H strain was compared to those of the 51 prototype strains. Each point represents the similarity of the nucleotide sequence between the Kobe-H strain and the 51 prototype strains, within a sliding window of 200 nucleotides centered on the position plotted and with a step of 20 residues between points. Positions containing gaps were excluded from analysis. The vertical axis indicates the nucleotide identities between Kobe-H and the 51 prototype strains, expressed as percentages. The horizontal axis indicates the nucleotide positions of the hexon gene. The horizontal axis at the bottom indicates the position of conserved regions (C1 to C4), variable regions (V1 to V3), and L1 and L2.
To date, several intermediate strains have been reported, and it is thought that they emerged due to recombination events between the hexon and fiber knob genes of the different serotype strains (15, 24, 27, 28). It is well known that the fiber knob plays an important role in HAdVs attaching to a target cell (9, 10). Therefore, we determined the nucleotide sequence of the fiber knob region of Kobe-H and the 51 prototype strains, as described previously by Madisch et al. (24). These nucleotide sequences were compared to each other in order to clarify whether Kobe-H acquired cell tropism to conjunctiva by recombination with known HAdVs. The comparison of the sequence of the fiber knob of Kobe-H showed 31.5 (HAdV-50 of HAdV-B) to 98.7% (HAdV-8 of HAdV-D) identity, while the three genome type strains of HAdV-8 showed 99.6 to 100% identity to the HAdV-8 prototype strain (Table 3). These results strongly support the hypothesis that Kobe-H is a novel serotype of HAdV.
DISCUSSION
To date, 51 HAdV serotypes have been identified and grouped into six species, HAdV-A to HAdV-F (8). The nine most recent serotypes, HAdV-43 to HAdV-51, were isolated from human immunodeficiency virus-infected patients, and all except HAdV-50 belong to HAdV-B (11, 17, 31). The pathogenesis of these isolates was not clear. EKC typically is caused by one of three HAdV-D serotypes, HAdV-8, HAdV-19, or HAdV-37, and these strains frequently cause nosocomial outbreaks (29, 34-36). These viruses originally were isolated from patients with EKC in 1951, 1955, and 1976 (12, 13, 19). To date, several variants of these viruses have been reported (2, 6, 37), but none of the new serotypes causes EKC. The serotype of any strain isolated from a patient is defined on the basis of its immunological distinctiveness, such as that determined by quantitative neutralization with type-specific antisera. A serotype shows a homologous/heterologous titer ratio of >16 in both directions (39). Kobe-H was shown by NT to be weakly reactive to HAdV-8 and HAdV-9, probably due to the similarity of the Kobe-H hexon gene, including the L1 and L2 regions, to that of the HAdV-8 and HAdV-9 strains. Phylogenetic analysis of the 916-bp partial hexon gene showed that Kobe-H is close to HAdV-8, although its L1 and L2 regions were different from those of HAdV-8. These results led us to perform a similarity plot analysis with the full-length hexon gene; however, no recombination event was found in the Kobe-H hexon gene. Recently, an outbreak of EKC caused by a new intermediate adenovirus, type 22/H8, was reported (15). The fiber knob mediates the attachment of the virion to the primary receptor on the cell membrane (9, 10). The nucleotide sequence of the fiber knob region of Kobe-H was not identical to that of the 51 HAdV prototype strains, indicating that Kobe-H is not an intermediate HAdV.
In conclusion, we have identified a novel HAdV on the basis of quantitative NT and the phylogenetic analysis of the hexon and fiber knob genes. PCR using a set of primers to amplify a partial hexon gene was found to be efficient in amplifying not only all of the prototype strains but also the novel HAdVs. We believe that the present molecular diagnosis on the basis of the partial hexon sequence will be of use not only for the rapid diagnosis of HAdVs but also in molecular epidemiology. To date, only three HAdVs, HAdV-8, HAdV-19, and HAdV-37, are known to cause severe EKC (4). The origin and mechanism of transmission of the novel HAdV are not yet clear. Our phylogenetic analysis based on the partial hexon gene indicates that the novel HAdV had already appeared in 1995 as the causative agent of EKC (data not shown). The novel HAdV has sometimes been identified as HAdV-8 because of its weak cross-reaction with anti-HAdV-8 serum, possibly delaying its recognition as a novel HAdV. It also is possible that this virus is circulating in humans with asymptomatic infection; however, no asymptomatic infection was detectable in the present surveillance. The virus might gain pathogenesis by some unknown mechanism, becoming causative of large nosocomial EKC infections. The novel HAdV has caused nosocomial EKC infections in at least eight hospitals in Japan during the past 5 years, and it should be monitored as an emerging adenoviral infection.
Footnotes
Published ahead of print on 2 April 2008.
REFERENCES
- 1.Adrian, T., G. Wadell, J. C. Hierholzer, and R. Wigand. 1986. DNA restriction analysis of adenovirus prototype 1 to 41. Arch. Virol. 91277-290. [DOI] [PubMed] [Google Scholar]
- 2.Adrian, T., U. Wolf, H. J. Lauer, and R. Wigand. 1990. Restriction site mapping of adenovirus type 8 genome types. Res. Virol. 141611-624. [DOI] [PubMed] [Google Scholar]
- 3.Aoki, K., M. Kato, H. Ohtsuka, K. Ishii, N. Nakazono, and H. Sawada. 1982. Clinical and aetiological study of adenoviral conjunctivitis, with special reference to adenovirus type 4 and 19 infections. Br. J. Ophthalmol. 66776-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aoki, K., and Y. Tagawa. 2002. A twenty-one year surveillance of adenoviral conjunctivitis in Sapporo, Japan. Int. Ophthalmol. Clin. 4249-54. [DOI] [PubMed] [Google Scholar]
- 5.Ariga, T., Y. Shimada, K. Ohgami, Y. Tagawa, H. Ishiko, K. Aoki, and S. Ohno. 2004. New genome type of adenovirus serotype 4 caused nosocomial infections associated with epidemic conjunctivitis in Japan. J. Clin. Microbiol. 423644-3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ariga, T., Y. Shimada, K. Shiratori, K. Ohgami, S. Yamazaki, Y. Tagawa, M. Kikuchi, Y. Miyakita, K. Fujita, H. Ishiko, K. Aoki, and S. Ohno. 2005. Five new genome types of adenovirus type 37 caused epidemic keratoconjunctivitis in Sapporo, Japan, for more than 10 years. J. Clin. Microbiol. 43726-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becroft, D. M. 1967. Histopathology of fatal adenovirus infection of the respiratory tract in young children. J. Clin. Pathol. 20561-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benko, M., B. Harrach, G. W. Bothe, and W. C. Russel. 2005. Family Adenoviridae, p. 213-228. In C. M. Fauquet, M. A. Mayo, J. Maniloff, U. Desselberger, and L. A. Ball (ed.), Virus taxonomy: eighth report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press, San Diego, CA.
- 9.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for coxsackie b viruses and adenoviruses 2 and 5. Science 2751320-1323. [DOI] [PubMed] [Google Scholar]
- 10.Burmeister, W. P., D. Guilligay, S. Cusack, G. Wadell, and N. Arnberg. 2004. Crystal structure of species D adenovirus fiber knobs and their sialic acid binding sites. J. Virol. 787727-7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Jong, J. C., A. G. Wermenbol, M. W. Verweij-Uijterwaal, K. W. Slaterus, P. Wertheim-Van Dillen, G. J. Van Doornum, S. H. Khoo, and J. C. Hierholzer. 1999. Adenoviruses from human immunodeficiency virus-infected individuals, including two strains that represent new candidate serotypes Ad50 and Ad51 of species B1 and D, respectively. J. Clin. Microbiol. 373940-3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Jong, J. C., R. Wigand, G. Wadell, D. Keller, C. J. Muzerie, A. G. Wermenbol, and G. J. Schaap. 1981. Adenovirus 37: identification and characterization of a medically important new adenovirus type of subgroup D. J. Med. Virol. 7105-118. [DOI] [PubMed] [Google Scholar]
- 13.Desmyter, J., J. C. De Jong, K. W. Slaterus, and H. Verlaeckt. 1974. Keratoconjunctivitis caused by adenovirus type 19. Br. Med. J. 4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ebner, K., W. Pinsker, and T. Lion. 2005. Comparative sequence analysis of the hexon gene in the entire spectrum of human adenovirus serotypes: phylogenetic, taxonomic, and clinical implications. J. Virol. 7912635-12642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engelmann, I., I. Madisch, H. Pommer, and A. Heim. 2006. An outbreak of epidemic keratoconjunctivitis caused by a new intermediate adenovirus 22/H8 identified by molecular typing. Clin. Infect. Dis. 43e64-e66. [DOI] [PubMed] [Google Scholar]
- 16.Felsenstein, J. 1985. Confidence limits on phylogeneies: an approach using the bootstrap. Evolution 39783-791. [DOI] [PubMed] [Google Scholar]
- 17.Hierholzer, J. C., R. Wigand, L. J. Anderson, T. Adrian, and J. W. M. Gold. 1988. Adenovirus from patient with AIDS: a plethora of serotypes and a description of five new serotypes of subgenus D (types 43-47). J. Infect. Dis. 154804-813. [DOI] [PubMed] [Google Scholar]
- 18.Hirt, B. 1967. Selective extraction of polyoma DNA. J. Mol. Biol. 26365-369. [DOI] [PubMed] [Google Scholar]
- 19.Jawetz, E., S. J. Kimura, A. N. Nicholas, P. Thygeson, and L. Hanna. 1955. New type of APC virus from epidemic keratoconjunctivitis. Science 1221190-1191. [DOI] [PubMed] [Google Scholar]
- 20.Jernigan, J. A., B. S. Lowry, F. G. Hayden, S. A. Kyger, B. P. Conway, D. H. Groschel, and B. M. Farr. 1993. Adenovirus type 8 epidemic keratoconjunctivitis in an eye clinic: risk factors and control. J. Infect. Dis. 1671307-1313. [DOI] [PubMed] [Google Scholar]
- 21.Jin, X. H., H. Ishiko, T. H. Nguyen, T. Ohguchi, M. Akanuma, K. Aoki, and S. Ohno. 2006. Molecular epidemiology of adenoviral conjunctivitis in Hanoi, Vietnam. Am. J. Ophthalmol. 1421064-1066. [DOI] [PubMed] [Google Scholar]
- 22.Kimura, M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequence. J. Mol. Evol. 16111-120. [DOI] [PubMed] [Google Scholar]
- 23.Lole, K. S., R. C. Bollinger, R. S. Paranjape, D. Gadkari, S. S. Kulkarni, N. G. Novak, R. Ingersoll, H. W. Sheppard, and S. C. Ray. 1999. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J. Virol. 73152-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madisch, I., G. Harste, H. Pommer, and A. Heim. 2005. Phylogenetic analysis of the main neutralization and hemagglutination determinants of all human adenovirus prototypes as a basis for molecular classification and taxonomy. J. Virol. 7915265-15276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miura-Ochiai, R., Y. Shimada, T. Konno, S. Yamazaki, K. Aoki, S. Ohno, E. Suzuki, and H. Ishiko. 2007. Quantitative detection and rapid identification of human adenoviruses. J. Clin. Microbiol. 45958-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nauheim, R. C., E. G. Romanowski, T. Araullo-Cruz, R. P. Kowalski, P. W. Turgeon, S. S. Stopak, and Y. J. Gordon. 1990. Prolonged recoverability of desiccated adenovirus type 19 from various surfaces. Ophthalmology 971450-1453. [DOI] [PubMed] [Google Scholar]
- 27.Noda, M., Y. Miyamoto, Y. Ikeda, T. Matsuishi, and T. Ogino. 1991. Intermediate human adenovirus type 22/H10,19,37 as a new etiological agent of conjunctivitis. J. Clin. Microbiol. 291286-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pring-Akerblom, P., and T. Adrian. 1995. Characterization of adenovirus subgenus D fiber genes. Virology 206564-571. [DOI] [PubMed] [Google Scholar]
- 29.Richmond, S., R. Burman, E. Crosdale, L. Cropper, D. Longson, B. E. Enoch, and C. L. Dodd. 1984. A large outbreak of keratoconjunctivitis due to adenovirus type 8. J. Hyg. (London) 93285-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4406-425. [DOI] [PubMed] [Google Scholar]
- 31.Schnurr, D., and M. E. Dondero. 1993. Tow new candidate adenovirus serotypes. Intervirology 3679-83. [DOI] [PubMed] [Google Scholar]
- 32.Schrader, E., and R. Wigand. 1981. Neutralization of adenovirus infectivity and cytotoxin in various cell cultures. J. Virol. Methods 2321-330. [DOI] [PubMed] [Google Scholar]
- 33.Shimada, Y., T. Ariga, Y. Tagawa, K. Aoki, S. Ohno, and H. Ishiko. 2004. Molecular diagnosis of human adenoviruses D and E by a phylogeny-based classification method using a partial hexon sequence. J. Clin. Microbiol. 421577-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tabery, H. M. 1995. Two outbreaks of adenovirus type 8 keratoconjunctivitis with different outcome. Acta Ophthalmol. Scand. 73358-360. [DOI] [PubMed] [Google Scholar]
- 35.Takeuchi, R., Y. Nomura, M. Kojima, E. Uchio, N. Kobayashi, and M. Matumoto. 1990. A nosocomial outbreak of epidemic keratoconjunctivitis due to adenovirus type 37. Microbiol. Immunol. 34749-754. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka-Yokogui, K., N. Itoh, N. Usui, S. Takeuchi, E. Uchio, K. Aoki, M. Usui, and S. Ohno. 2001. New genome type of adenovirus serotype 19 causing nosocomial infections of epidemic keratoconjunctivitis in Japan. J. Med. Virol. 65530-533. [PubMed] [Google Scholar]
- 37.Wadell, G., and J. C. De Jong. 1980. Restriction endonucleases in identification of a genome type of adenovirus 19 associated with keratoconjunctivitis. Infect. Immun. 27292-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Warren, D., K. E. Nelson, J. A. Farrar, E. Hurwitz, J. Hierholzer, E. Ford, and L. J. Anderson. 1989. A large outbreak of epidemic keratoconjunctivitis: problems in controlling nosocomial spread. J. Infect. Dis. 160938-943. [DOI] [PubMed] [Google Scholar]
- 39.Wigand, R., A. Bartha, R. S. Dreizin, H. Esche, H. S. Ginsberg, M. Green, J. C. Hierholzer, S. S. Kalter, J. B. McFerran, U. Pettersson, W. C. Russell, and G. Wadell. 1982. Adenoviridae: second report. Intervirology 18169-176. [DOI] [PubMed] [Google Scholar]
- 40.Wigand, R., T. H. Adrian, and F. Bricout. 1987. A new human adenovirus of subgenus D: candidate adenovirus type 42. Arch. Virol. 94283-286. [DOI] [PubMed] [Google Scholar]
- 41.World, W. S. M., and M. S. Howrwitz. 2007. Adenoviridae, p. 2395-2436. In D. M. H. Knipe and P. M. Howley (ed.), Fields virology, 5th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]