Abstract
Whether animals may act as reservoirs for human caliciviruses is unclear. By sequence analysis of a short fragment of the RNA-dependent RNA polymerase (RdRp) region, porcine sapovirus (SaV) strains that genetically resemble human SaVs have been detected in piglets, but more-informative sequences (capsid gene) were not available for a precise characterization. In this study, the 3′ terminus (the 3′ end of open reading frame 1 [ORF1], including the polymerase complex and the complete capsid; ORF2; and the 3′ untranslated region) of one such human SaV-like strain, 43/06-18p3/2006/It, was determined, revealing that these viruses are more related genetically to human (47.4 to 54.9% amino acid identity) than to animal (35.2 to 44.7% amino acid identity) SaVs in the capsid gene. In addition, the recombination-prone RdRp-capsid junction region was highly conserved with those of human SaVs of genogroup GI. The presence of porcine viruses similar to human SaVs is a significant finding because of the potential for zoonotic infections or generation of porcine/human recombinants.
Sapoviruses (Sapporo-like viruses; Sapovirus genus) (SaVs) are important enteric pathogens that cause diarrhea in humans, pigs, and mink (5, 7, 9, 26). The prototype strain Sapporo virus was detected in an outbreak in an infants' home in Sapporo, Japan, in 1977 (17). The Sapovirus genus is classified in the family Caliciviridae, along with the genera Norovirus, Vesivirus, and Lagovirus and with unassigned caliciviruses of recent identification (7, 27, 32). The SaV genome is a polyadenylated, single-stranded, positive-sense RNA 7.3 to 7.5 kb in length and contains two main open reading frames (ORFs) (8, 15, 19, 30). ORF1 encodes a polyprotein that undergoes protease processing to produce several nonstructural proteins, including an RNA-dependent RNA polymerase (RdRp) and a capsid protein. ORF2 encodes a small basic protein with an unknown function. An additional ORF, overlapping with the 5′ end of the capsid gene, is present in some human SaVs, as suggested by the presence of a conserved translation motif, GCAAUGG (31).
Based on the capsid gene sequence, SaV can be classified into five distinct genogroups (GI to GV) (4). Human SaVs belong to GI, GII, GIV, and GV, whereas porcine SaVs belong to GIII. SaV GI, GIV, and GV possess three ORFs, whereas SaV GII and GIII have only two main ORFs (4). Human SaVs are genetically variable, and at least 13 genetic clusters, or genotypes (GI.1 to G1.5, GII.1 to GII.6, GIV.1, and GV.1), have been identified (4, 13, 31).
The porcine calicivirus strains Cowden and LL14/02/US, representatives of SaV genogroup GGIII, have been detected in diarrheic piglets and have been shown to induce enteric diseases and lesions in experimentally infected pigs (5, 6, 8, 10, 35). The majority of porcine enteric caliciviruses detected in weaning and postweaning piglets are characterized as GGIII SaVs (16, 24, 37). In addition, porcine caliciviruses related genetically to genogroup GII human noroviruses (NoVs) (GII.11, GII.18, and GII.19) have been identified, almost exclusively in older, asymptomatic pigs (20 to 24 weeks of age) (33, 36, 37), and do not appear to be associated with pathogenicity in pigs (34). Porcine SaVs distantly related to the porcine SaV prototype strain Cowden have been also described. The strains JJ681/00/US and K7/JP share 52% amino acid identity with each other but only 32 to 37% amino acid identity with each SaV genogroup (GI to GV) and are likely prototypes of novel SaV genogroups, namely, GVI (JJ681/00/US) and GVII (K7/JP) (35, 38). In addition, SaVs of a distinct new genogroup (prototype strain MEC151A) have been identified in minks (GenBank accession number AY144337).
Genetic recombination between genetically related caliciviruses within the same genogroup or genotype has been described frequently (29), but it may also have occurred between genetically unrelated strains (12). The human SaV GIV and GII strains exhibit the same polymerase gene but have completely different capsid genes, and SaV GIV strains (SW278 and Ehime 1107) are thought to have originated by intergenogroup recombination between human GII SaV and GIV SaVs of unidentified sources (12). Accordingly, intensification of the molecular epidemiology of animals is paramount for a more in-depth understanding of the dynamics of evolution of human caliciviruses.
In this study, we report the identification and the genetic characterization of an unusual porcine SaV strain, 43/06-18p3. The virus was identified during a large surveillance study for viral enteritis in piglets in Italy (21). By sequence analysis of a conserved fragment of the polymerase complex, strain 43/06-18p3 displayed high identity (97 to 98% amino acid identity) to the porcine strains QW19 (35), SWECII/VA103 (GenBank accession number AY615811), and SWECII/VA14 (GenBank accession number AY615810). This group of caliciviruses appears to be genetically similar to human SaVs (65 to 67% amino acid identity), but the complete sequence of the capsid gene has not been determined, and the exact genetic relationship with human SaVs is unclear. To establish firmly the genetic relationships of these viruses and achieve a definitive classification, the sequence of the genome of strain 43/06-18p3, from the 3′ end of ORF1 to the poly(A) tail, was determined, revealing that the virus was genetically more closely related to human SaVs than to porcine SaVs and providing evidence for recent meeting points during the evolution of SaVs in humans and pigs.
MATERIALS AND METHODS
RNA extraction.
RNA was extracted from 200 μl of 10% (wt/vol) fecal suspensions in phosphate-buffered saline, using the guanidinium isothiocyanate/silica method described by Boom et al. (1). RNA was eluted in 50 μl H2O-diethyl pyrocarbonate with RNasin (0.2 μg/μl; Promega) and used in reverse transcription-PCR (RT-PCR).
RT-PCR for calicivirus.
RT and PCR were performed in a one-step procedure, using the SuperScript III one-step system (Invitrogen, United Kingdom). p290 and p289, a pair of universal calicivirus primers that target the conserved motifs DYSKWDST and YGDD, respectively, of the RdRp region of caliciviruses, were used (14, 40).
Origins of the samples.
Strain 43/06-18p3 was detected in a swine farm in Brescia in 2005 during the use of a passive surveillance system for piglets with either weaning or postweaning diarrhea in Northern and Central Italy. Fresh fecal samples or intestinal contents were placed into sterile containers and stored frozen at −20 or −70°C until tested.
A total of 208 samples from 118 herds were analyzed, and calicivirus RNA was detected by RT-PCR in 68 samples (32.5%) and in 46 herds (38.9%) alone or in mixed infections with group A and C rotaviruses (21). By sequence analysis of the short fragment of the RdRp region, the majority of the calicivirus-positive samples were characterized as genogroup III SaVs, while unclassified caliciviruses, distantly related to the representatives of the other SaV genogroups, were identified in five herds. Unexpectedly, one outbreak was associated with a porcine SaV (strain 43/06-18p3) related genetically (97 to 98% amino acid identity in the RdRp region) to rare SaV strains (QW19, SWECII/VA103, and SWECII/VA14) that genetically resemble human GII and GIV SaVs. Sample 43/06-18p3 also contained group A rotavirus and picornavirus RNA. An additional two samples (43/06-18p1 and 43/06-18p2) from the same enteritis outbreak were available for analysis and tested positive for group A rotavirus, calicivirus, and picornavirus (21). The two calicivirus strains were closely related (>99% nucleotide identity) to strain 43/06-18p3 in the RdRp fragment, suggesting a clonal origin.
RT-PCR amplification of the 3′ genomic RNA.
Since the sequences of the capsid genes of such human SaV-like (QW19-like) porcine SaVs are unknown, attempts were made to determine the sequence of the 3′ end of SaV strain 43/06-18p3. To amplify the 3′ end, the cDNA of a 3.1-kb fragment of the porcine calicivirus 43/06-18p3 was synthesized by a SuperScript III first-strand cDNA synthesis kit (Invitrogen, United Kingdom) according to the manufacturer's instructions with primer VN3T20 (5′-GAG TGA CCG CGG CCG CT20-3′) (35). The first PCR was then performed by using TaKaRa La Taq polymerase (TaKaRa Bio Inc., Japan) with the primers p290 and VN3T20. A second PCR was necessary to produce a PCR product suitable for cloning, using internal forward primers selected on the basis of the obtained sequence fragment of the RdRp region of strain 43/06-18p3 and the reverse primer VN3T20. After initial denaturation at 94°C for 3 min, the first 5 cycles were performed at 94°C for 30 s, 50°C for 30 s, and 72°C for 5 min, followed by 30 cycles with a shorter elongation time of 3 min and a final extension at 72°C for 10 min. All RT-PCR products were analyzed on agarose gel electrophoresis gel and stained with ethidium bromide. The RT-PCR product bands were visualized by using UV light.
Cloning and sequencing.
The RT-PCR products were purified by using a QIAquick gel extraction kit (Qiagen, Inc.). The 3-kb RT-PCR product was cloned by using a PCR XL cloning kit (Invitrogen, United Kingdom). Plasmid DNA was extracted by using an alkaline lysis method. The insertion was confirmed by restriction enzyme digestion. Three clones were sequenced by a primer-walking strategy, using the internal primers p1F186/208 (CAATCACATGACTTACTTCGCTG), p2F502/522 (TGCTATTGGGTAAAGGGCTCG), p3F817/836 (CCCGTGGTCGGGACATCCAG), p4f1195/1213 (GTGCTGCCACCCGGGCTCA), p5f1680/1698 (TGGCCACGCGGAGCGTGTC), p6f2212/2232 (TACTCGTCTCAATTGGATTAC), p7f2641/2659 (GTGAAGCCATGTCCATGAC), and p8f2939/2955 (CAATTATGGGAAGATCACACT). DNA sequencing was done by using BigDye Terminator cycle chemistry and a 3730 DNA analyzer (Applied Biosystems, Foster, CA).
Sequence editing and phylogenetic analysis.
Sequence editing and multiple alignments were performed with the BioEdit software package (v2.1) (11). BLAST (Basic Local Alignment Search Tool; http://www.ncbi.nlm.nih.gov) and FASTA (http://www.ebi.ac.uk/fasta33) with default values were used to find homologous hits. Phylogenetic analysis (neighbor joining) with bootstrap (1,000 replicates) was conducted by using the MEGA software package (v3.0) (18). The sequences were analyzed with SimPlot (20), using a window size of 200 and step size of 20, with gap strip off and Hamming correction on.
RESULTS
We successfully amplified and sequenced the 3′-end 3 kb of the genome associated with the partial RdRp (265 amino acids [aa]), the capsid protein, the ORF2 gene, and the 3′ untranslated region (UTR) of the strain 43/06-18p3 (GenBank accession number EU221477). The sizes of the deduced capsid and ORF2 proteins were 563 and 168 aa, respectively, while the 3′ UTR was 104 nucleotides (nt) in length (Table 1). The ORF1-ORF2 overlap region was identical in size and nucleotide sequence (ATGA) to those of the other SaV strains, while the size of the 3′ UTR was different from those for other porcine SaVs and more similar to that for human SaV.
TABLE 1.
Sizes of the predicted capsid proteins, the ORF1-ORF2 overlap regions, the ORF2 proteins, and the 3′ UTRs of human and porcine SaVs
| GenBank accession no. | Strain | Size of:
|
||||
|---|---|---|---|---|---|---|
| Capsid (aa) | ORF1-2 overlap (nt) | ORF3 (aa) | ORF2 (aa) | 3′ UTR (nt) | ||
| AF435813 | Hu/Mex14917/00/GI.3 | 566 | 4 | 162 | 165 | 77 |
| AF435809 | Hu/Mex340/90/GII.2 | 558 | 4 | None | 166 | 108 |
| AY157863 | Hu/Cruise ship/00/GII.3 | 559 | 1 | None | 166 | 98 |
| AF435814 | Hu/Hou7-1181/90/GIV | 553 | 4 | 161 | 167 | 93 |
| AY289803 | Hu/Arg39/GV | 569 | 4 | 155 | 166 | 86 |
| AF182760 | Po/Cowden/GIII | 544 | 4 | None | 164 | 55 |
| AY826426 | Po/QW270/GIII | 544 | 4 | None | 164 | 55 |
| AY823308 | Po/MM280/GIII | 544 | 4 | None | 164 | 55 |
| AY826423 | Po/OH-JJ259/GIII | 544 | 4 | None | 173 | 55 |
| AY974192 | Po/JJ681/GVI? | 554 | 4 | None | 168 | 28 |
| AB221130 | Po/K7/GVII? | 544 | 4 | None | 168 | 35 |
| EU221477 | Po/46/03-18p3/GVIII? | 563 | 4 | None | 168 | 104 |
The percent amino acid identities of the deduced complete capsid protein sequences of the newly identified porcine SaV, the previously reported porcine and human SaVs, and non-SaV calicivirus strains are summarized in Table 2.
TABLE 2.
Amino acid identities in the capsid protein of strain Po/SaV/GVIII?/43/06-18p3/06/It with human and animal SaVs and with non-SaV caliciviruses
| Straina | GenBank accession no. | % Identity |
|---|---|---|
| SaVs | ||
| Hu/SaV/GI.1/Dresden/01/DE | AY694184 | 53.8 |
| Hu/SaV/GI.2/Houston/90/US | U95644 | 50.6 |
| Hu/SaV/GIV/Hou7-1181/US | AF435814 | 51.1 |
| Hu/SaV/GIV/Chiba/000671/99/Jp | AJ786349 | 53.2 |
| Hu/SaV/GV/Arg39/Arg | AY289803 | 54.9 |
| Hu/SaV/GV/Ehime475/04/Jp | DQ366344 | 54.5 |
| Hu/SaV/GII.3Cruise ship/00/US | AY157863 | 49.3 |
| Hu/SaV/GII.4/Chiba/990763/99/Jp | AJ606690 | 47.4 |
| Po/SaV/GIII/Cowden/80/US | AF182760 | 40.9 |
| Po/SaV/GIII/JJ259/00/US | AY826423 | 39.1 |
| Po/SaV/GIII/LL14/02/US | AY425671 | 39.6 |
| Po/SaV/GIII/MM280/03/US | AY823308 | 41.1 |
| Po/SaV/GVI?/OH-JJ681/00/US | AY974192 | 35.2 |
| Po/SaV/GVII?/K7/Jp | AB221130 | 36.7 |
| Mink/SaV/G?/Canada151A/Can | AY144337 | 44.7 |
| Non-SaV caliciviruses | ||
| Fe/FCV | Z11536 | 29.6 |
| Ca/CaCV | AF053720 | 29.5 |
| Bo/Newbury agent-1 | DQ013304 | 29.6 |
| Hu/NoV/GGI.1/Norwalk/93/US | M87661 | 17.5 |
| La/RHDV/Rainham | AJ006019 | 23.5 |
The designations for the strains displaying the highest identities are in boldface.
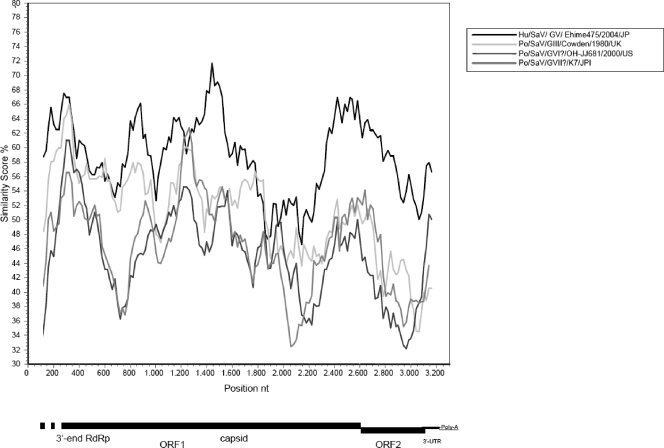
In the complete capsid region, 43/06-18p3 was genetically closest to the GV human SaV strain Arg39 (54.9% amino acid and 60.3% nucleotide identity). The amino acid identities with GV SaVs ranged from 54.5 to 54.9%, while they were 50.6 to 53.8% with GI SaVs, 51.1 to 53.2% with GIV SaVs, and 47.4 to 49.3% with GII SaV. The amino acid identities were 44.7% with mink SaV and only 39.1 to 41.1% with GIII (Cowden-like) SaVs, 35.2% with strain Po/OH-JJ681/00, and 36.7% with Po/K7/Jp. When the nucleotide sequence of the virus was plotted against those of human and porcine SaVs in SimPlot, the genetic relatedness of strain 43/06-18p3 to human SaV appeared clearly (Fig. 1). The regions most conserved between strain 43/06-18p3 and GV SaV were located close to the junction region, at the 5′ end of the capsid gene (a region corresponding to the highly conserved S domain of the capsid protein) and around the ORF1-ORF2 overlap.
FIG. 1.
Genome organization of the porcine SaV strain 43/06-18p3. The nucleotide positions of the 3′-end RdRp, the deduced capsid, ORF2, and the 3′-end UTR are indicated. A nucleotide identity plot comparing the genome of the porcine virus 43/06-18p3 [from the 3′ end of ORF1 to the poly(A) tail] with porcine viruses of the various genogroups (III, VI and VII) and with the GV strain Hu/Ehime475/2004/JP (GenBank accession number DQ366344) is shown. The sequences were analyzed with SimPlot (20), using a window size of 200 and step size of 20, with gap strip off and Hamming correction on.
Based on the full-length capsid protein, the SaV intragenus sequence identities range from 32.8 to 50.3% amino acid identity and from 43.6 to 54.2% nucleotide identity (28), while the intergenus amino acid identities of caliciviruses are 15 to 27% (7, 32). Based on these criteria, strain 43/06-18p3 could be classified as a highly divergent strain within GV SaVs or as a novel SaV genogroup outlier between GI and GV human SaVs.
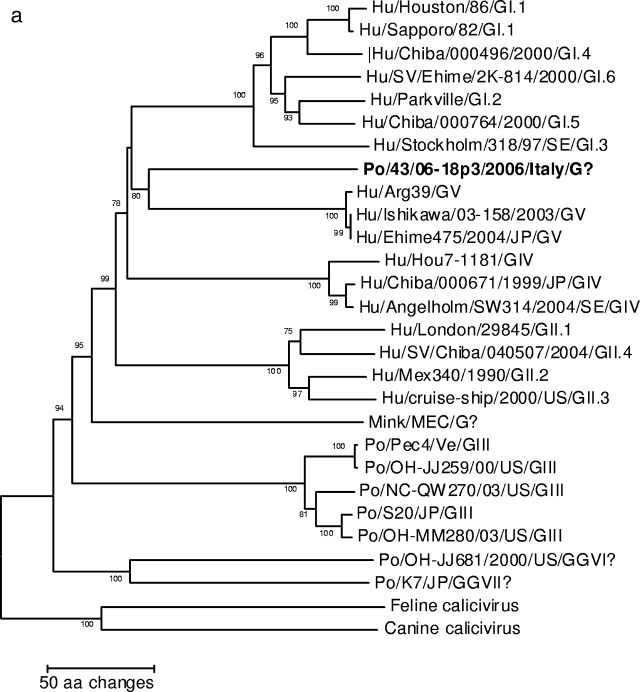
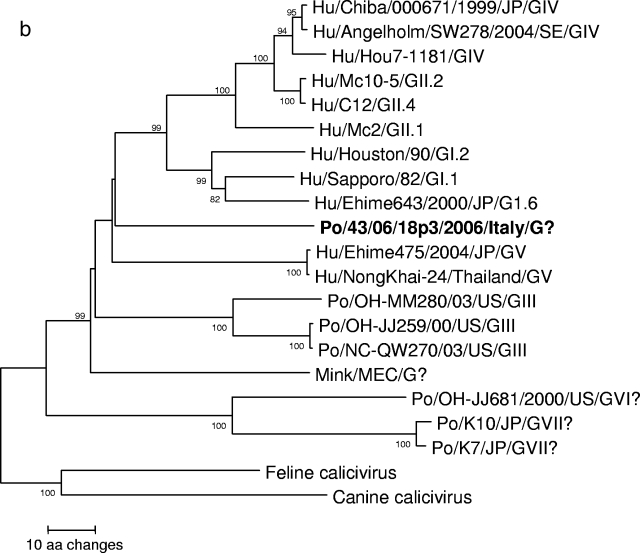
A neighbor-joining phylogenetic tree based on the deduced amino acid sequences of the complete capsid (Fig. 2a) revealed that strain 43/06-18p3 segregated with GV SaV (bootstrap value, 80%). Also, in the tree based on the 265-aa fragment at the COOH terminus of the polymerase complex, strain 43/06-18p3 segregated with human SaVs (Fig. 2b).
FIG. 2.
Neighbor-joining phylogenetic trees of members of the genus Sapovirus. Canine (CaCV) and feline (FCV) strains (Vesivirus) were used as an outgroup. The trees are based on the deduced amino acid sequences of the complete capsid protein (Fig. 2a) and of the 295-aa COOH terminus of the polymerase complex (Fig. 2b). The SaV genogroups (G plus roman numerals) and genotypes (Arabic numbers following genogroup numbers) are indicated. The designation for the newly identified porcine SaV strain is in boldface.
Nucleotide alignments around the RdRp-capsid junction region were also performed. This region appears to be a preferential recombination site for caliciviruses, including SaVs (12, 35). This region contains the 26-nt conserved genomic-subgenomic RNA motif (7). We found that the nucleotide sequences of strain 43/06-18p3 were highly conserved with GV and GI SaVs (Fig. 3). Only two nucleotide mismatches between the virus and the GI SaV prototype strain Sapporo virus were found within the 26-nt motif.
FIG. 3.
Sequence alignments of the RdRp-capsid junction region of SaVs. Dots indicate the residues identical to those in the sequence of the first line (Hu/Sapporo/82/JP/GI.1). The start codon for the capsid protein is highlighted, and the conserved genomic-subgenomic RNA nucleotide motif is underlined. Six SaV genogroups (GI to VI) are indicated. The designation for the newly sequenced porcine SaV strain is in boldface.
DISCUSSION
The development of molecular diagnostic techniques has allowed researchers to acquire exact data on the epidemiology and genetic heterogeneity of noncultivatable enteric viruses, such as rotaviruses and caliciviruses, in humans. In a similar fashion, the introduction of these technologies to veterinary diagnostics is allowing researchers to gather precious information on the evolutionary relationships of animal and human enteric viruses (22, 23). Based on analysis of a short polymerase fragment, porcine SaV strains that genetically resemble human SaVs rather than previously recognized porcine SaVs have been identified (35). In this study, analysis of the 3′ end of the genome of one such SaV strain was accomplished. Based on analysis of the capsid gene, the porcine SaV 43/06-18p3 appeared to be more related genetically to human SaVs, notably to human strains of genogroups GV and GI.
This genetic relatedness between human SaVs and the porcine strain 43/06-18p3 was also observed in the 26-nt conserved genomic-subgenomic RNA motif. This region tends to be highly conserved among strains within the same genogroup (12, 35). In this region, there were only two nucleotide mismatches between strain 43/06-18p3 and GI SaVs. Genetic recombination between genetically related caliciviruses within the same genogroup or genotype has been described frequently (29, 35). Almost all the recombination events have been mapped to the RdRp-capsid junction region and occur exclusively between members of the same genogroup. This is consistent with the nucleotide conservation seen among strains within a genogroup in this junction region and facilitating homologous recombination. However, recombination may also have occurred between genetically unrelated strains (12). The human SaV GIV and GII strains exhibit the same polymerase gene but have completely different capsid genes, and the SaV GIV strains (SW278 and Ehime 1107) are hypothesized to have originated by intergenogroup recombination between human GII SaV and yet-unrecognized GIV SaVs of animal or human origins (12). The detection of animal SaVs genetically related to human SaVs and possessing a potential recombination site gives strong support to this hypothesis. The contemporaneous presence of homologous and heterologous enteric caliciviruses in the same host may generate favorable conditions for exchange of genetic material between heterologous and homologous enteric caliciviruses. Since heterologous infections by enteric caliciviruses are permitted (2) and human and animal enteric caliciviruses may contemporaneously contaminate foodstuff for human consumption (3), such favorable conditions may occur on more occasions.
The detection of human SaV-like SaVs in pigs has raised the question of whether these viruses are genuine porcine caliciviruses or human enteric viruses incidentally transmitted from humans to animals. There is evidence that enteric caliciviruses can infect heterologous species, resulting in mild or unapparent infections, and human like GII.4 NoVs have been identified recently in piglets (2, 25). Porcine SaVs resembling the strain 43/06-18p3 have been identified in the United States (35) and in The Netherlands (GenBank accession numbers AY615811 and AY615810). This indicates that such human like porcine SaVs circulate in pigs in distinct geographical settings, although infrequently. Attempts to passage one such SaV strain, QW19, in two gnotobiotic pigs orally and intranasally inoculated with fecal filtrates were unsuccessful (Q.-H. Wang and L. J. Saif, unpublished). Also, the load of virus QW19 in the original fecal sample was low and similar viruses were not detected in other pigs, thus suggesting a nonporcine origin for the virus (35, 37). Serological investigations with pigs and with other animal species, including humans, using recombinant, antigen-based immunoenzyme assays, will allow researchers to gather more-precise information and to address the zoonotic potential of these porcine SaVs.
For GI, GIV, and GV human SaVs, another ORF, overlapping the 5′ end of the capsid gene, was proposed because there is a conserved translation initiation motif, GCAATGG, at the 5′ end of this ORF (4, 31). This motif was disrupted in the capsid sequence of strain 43/06-18p3 by two nucleotide mutations (GTAACGG), while it is not recognizable at all in the capsid sequences of GII human SaVs and of porcine SaVs. The retention of the original motif, slightly altered, in strain 43/06-18p3 may suggest that such viruses lost the usage of ORF3 in recent times and that the motif, no longer under functional constraints, is now accumulating mutations.
In summary, we identified a porcine SaV that was genetically more similar to human SaVs, suggesting the possibility of a pig reservoir for human strains or vice versa. We also identified a potential recombinational site between the porcine virus and GI human SaVs. In order to assess whether animal SaVs have emerged over time in humans by direct interspecies transmission or by exchange of genetic material via recombination with human viruses, it is pivotal to explore the genetic diversity of animal SaVs. Increased surveillance for enteric pathogens in humans and animals will allow detection of numerous yet-unidentified caliciviruses, giving more-in-depth insights into the evolution and ecology of these viruses.
Acknowledgments
This study was supported by grants from the research project Fondi di Ateneo per l'anno 2007, Studio dei calicivirus animali ed implicazioni zoonosiche.
We thank S. Arista for her critical comments and suggestions and A. Bellacicco, S. Lucente, and M. Tempesta for their technical support.
Footnotes
Published ahead of print on 16 April 2008.
REFERENCES
- 1.Boom, R., C. J. Sol, M. M. Salimans, C. L. Jansen, P. M. Wertheim-van Dillen, and J. van der Noordaa. 1990. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 28495-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheetham, S., M. Souza, T. Meulia, S. Grimes, M. G. Han, and L. J. Saif. 2006. Pathogenesis of a genogroup II human norovirus in gnotobiotic pigs. J. Virol. 8010372-10381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costantini, V., F. Loisy, L. Joens, F. S. Le Guyader, and L. J. Saif. 2006. Human and animal enteric caliciviruses in oysters from different coastal regions of the United States. Appl. Environ. Microbiol. 721800-1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farkas, T., W. M. Zhong, Y. Jing, P. W. Huang, S. M. Espinosa, N. Martinez, A. L. Morrow, G. M. Ruiz-Palacios, L. K. Pickering, and X. Jiang. 2004. Genetic diversity among sapoviruses. Arch. Virol. 1491309-1323. [DOI] [PubMed] [Google Scholar]
- 5.Flynn, W. T., and L. J. Saif. 1988. Serial propagation of porcine enteric calicivirus-like virus in primary porcine kidney cell cultures. J. Clin. Microbiol. 26206-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flynn, W. T., L. J. Saif, and P. D. Moorhead. 1988. Pathogenesis of porcine enteric calicivirus-like virus in four-day-old gnotobiotic pigs. Am. J. Vet. Res. 49819-825. [PubMed] [Google Scholar]
- 7.Green, K. Y., R. M. Chanock, and A. Z. Kapikian. 2001. Human caliciviruses, p. 841-874. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott/The Williams & Wilkins Co., Philadelphia, PA.
- 8.Guo, M., K. O. Chang, M. E. Hardy, Q. Zhang, A. V. Parwani, and L. J. Saif. 1999. Molecular characterization of a porcine enteric calicivirus genetically related to Sapporo-like human caliciviruses. J. Virol. 739625-9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo, M., J. F. Evermann, and L. J. Saif. 2001. Detection and molecular characterization of cultivable caliciviruses from clinically normal mink and enteric caliciviruses associated with diarrhea in mink. Arch. Virol. 146479-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo, M. J., K. O. Hayes, A. Cho, V. Parwani, L. M. Lucas, and L. J. Saif. 2001. Comparative pathogenesis of tissue culture-adapted and wild-type Cowden porcine enteric calicivirus (PEC) in gnotobiotic pigs and induction of diarrhea by intravenous inoculation of wild-type PEC. J. Virol. 759239-9251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall, T. A. 1999. BioEdit: a user-friendly biological sequence alignment and analysis program for Windows 95/98/NT. Nucleic Acids Symp. 4195-98. [Google Scholar]
- 12.Hansman, G. S., N. Takeda, T. Oka, M. Oseto, K. O. Hedlund, and K. Katayama. 2005. Intergenogroup recombination in sapoviruses. Emerg. Infect. Dis. 111916-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansman, G. S., T. Oka, K. Katayama, and N. Takeda. 2007. Human sapoviruses: genetic diversity, recombination, and classification. Rev. Med. Virol. 17133-141. [DOI] [PubMed] [Google Scholar]
- 14.Jiang, X., P. W. Huang, W. M. Zhong, T. Farkas, D. W. Cubitt, and D. O. Matson. 1999. Design and evaluation of a primer pair that detects both Norwalk- and Sapporo-like caliciviruses by RT-PCR. J. Virol. Methods 83145-154. [DOI] [PubMed] [Google Scholar]
- 15.Katayama, K., T. Miyoshi, K. Uchino, T. Oka, T. Tanaka, N. Takeda, and G. S. Hansman. 2004. Novel recombinant sapovirus. Emerg. Infect. Dis. 101874-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim, H. J., H. S. Cho, K. O. Cho, and N. Y. Park. 2006. Detection and molecular characterization of porcine enteric calicivirus in Korea, genetically related to sapoviruses. J. Vet. Med. B 53155-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kogasaka, R., S. Nakamura, S. Chiba, Y. Sakuma, H. Terashima, T. Yokoyama, and T. Nakao. 1981. The 33- to 39-nm virus-like particles, tentatively designated as Sapporo agent, associated with an outbreak of acute gastroenteritis. J. Med. Virol. 8187-193. [DOI] [PubMed] [Google Scholar]
- 18.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Brief. Bioinform. 5150-163. [DOI] [PubMed] [Google Scholar]
- 19.Liu, B. L., I. N. Clarke, E. O. Caul, and P. R. Lambden. 1995. Human enteric caliciviruses have a unique genome structure and are distinct from the Norwalk-like viruses. Arch. Virol. 1401345-1356. [DOI] [PubMed] [Google Scholar]
- 20.Lole, K. S., R. C. Bollinger, R. S. Paranjape, D. Gadkari, S. S. Kulkarni, N. G. Novak, R. Ingersoll, H. W. Sheppard, and S. C. Ray. 1999. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J. Virol. 73152-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martella, V., K. Bányai, E. Lorusso, A. L. Bellacicco, N. Decaro, V. Mari, L. Saif, V. Costantini, S. De Grazia, G. Pezzotti, A. Lavazza, and C. Buonavoglia. 2008. Genetic heterogeneity of porcine enteric caliciviruses identified from diarrhoeic piglets. Virus Genes 36365-373. [DOI] [PubMed] [Google Scholar]
- 22.Martella, V., K. Banyai, E. Lorusso, N. Decaro, A. L. Bellacicco, C. Desario, M. Corrente, G. Greco, P. Moschidou, M. Tempesta, S. Arista, M. Ciarlet, A. Lavazza, and C. Buonavoglia. 2007. Genetic heterogeneity in the VP7 of group C rotaviruses. Virology 367358-366. [DOI] [PubMed] [Google Scholar]
- 23.Martella, V., K. Banyai, M. Ciarlet, M. Iturriza-Gomara, E. Lorusso, S. De Grazia, S. Arista, N. Decaro, G. Elia, A. Cavalli, M. Corrente, A. Lavazza, R. Baselga, and C. Buonavoglia. 2006. Relationships among porcine and human P[6] rotaviruses: evidence that the different human P[6] lineages have originated from multiple interspecies transmission events. Virology 344509-519. [DOI] [PubMed] [Google Scholar]
- 24.Martinez, M. A., A. C. Alcala, G. Carruyo, L. Botero, F. Liprandi, and J. E. Ludert. 2006. Molecular detection of porcine enteric caliciviruses in Venezuelan farms. Vet. Microbiol. 11677-84. [DOI] [PubMed] [Google Scholar]
- 25.Mattison, K., A. Shukla, A. Cook, F. Pollari, R. Friendship, D. Kelton, S. Bidawid, and J. M. Faber. 2007. Human noroviruses in swine and cattle. Emerg. Infect. Dis. 131184-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayo, M. A. 2002. A summary of taxonomic changes recently approved by ICTV. Arch. Virol. 1471655-1656. [DOI] [PubMed] [Google Scholar]
- 27.Oliver, S. L., E. Asobayire, A. M. Dastjerdi, and J. C. Bridger. 2006. Genomic characterization of the unclassified bovine enteric virus Newbury agent-1 (Newbury1) endorses a new genus in the family Caliciviridae. Virology 350240-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phan, T. G., P. Khamrin, T. D. Quang, S. K. Dey, S. Takanashi, S. Okitsu, N. Maneekarn, and H. Ushijima. 2007. Emergence of intragenotype recombinant sapovirus in Japan. Infect. Genet. Evol. 7542-546. [DOI] [PubMed] [Google Scholar]
- 29.Reuter, G., K. Krisztalovics, H. Vennema, M. Koopmans, and G. Szucs. 2005. Evidence of the etiological predominance of norovirus in gastroenteritis outbreaks-emerging new-variant and recombinant noroviruses in Hungary. J. Med. Virol. 76598-607. [DOI] [PubMed] [Google Scholar]
- 30.Robinson, S., I. N. Clarke, I. B. Vipond, E. O. Caul, and P. R. Lambden. 2002. Epidemiology of human Sapporo-like caliciviruses in the South West of England: molecular characterisation of a genetically distinct isolate. J. Med. Virol. 67282-288. [DOI] [PubMed] [Google Scholar]
- 31.Schuffenecker, I., T. Ando, D. Thouvenot, B. Lina, and M. Aymard. 2001. Genetic classification of “Sapporo-like viruses”. Arch. Virol. 1462115-2132. [DOI] [PubMed] [Google Scholar]
- 32.Smiley, J. R., K. O. Chang, J. Hayes, J. Vinje, and L. J. Saif. 2002. Characterization of an enteropathogenic bovine calicivirus representing a potentially new calicivirus genus. J. Virol. 7610089-10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sugieda, M., and S. Nakajima. 2002. Viruses detected in the caecum contents of healthy pigs representing a new genetic cluster in genogroup II of the genus “Norwalk-like viruses”. Virus Res. 87165-172. [DOI] [PubMed] [Google Scholar]
- 34.Wang, Q. H., V. Costantini, and L. J. Saif. 2007. Porcine enteric caliciviruses: genetic and antigenic relatedness to human caliciviruses, diagnosis and epidemiology. Vaccine 255453-5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang, Q. H., M. G. Han, J. A. Funk, G. Bowman, D. A. Janies, and L. J. Saif. 2005. Genetic diversity and recombination of porcine sapoviruses. J. Clin. Microbiol. 435963-5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang, Q. H., M. G. Han, S. Cheetham, M. Souza, J. A. Funk, and L. J. Saif. 2005. Porcine noroviruses related to human noroviruses. Emerg. Infect. Dis. 111874-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang, Q. H., M. Souza, J. A. Funk, W. Zhang, and L. J. Saif. 2006. Prevalence of noroviruses and sapoviruses in swine of various ages determined by reverse transcription-PCR and microwell hybridization assays. J. Clin. Microbiol. 442057-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yin, Y., Y. Tohya, Y. Ogawa, D. Numazawa, K. Kato, and H. Akashi. 2006. Genetic analysis of calicivirus genomes detected in intestinal contents of piglets in Japan. Arch. Virol. 1511749-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reference deleted.
- 40.Zintz, C., K. Bok, E. Parada, M. Barnes-Eley, T. Berke, M. A. Staat, P. Azimi, X. Jiang, and D. O. Matson. 2005. Prevalence and genetic characterization of caliciviruses among children hospitalized for acute gastroenteritis in the United States. Infect. Genet. Evol. 5281-290. [DOI] [PubMed] [Google Scholar]