Abstract
The performances of commercial enzyme-linked immunosorbent assays (ELISAs) in detecting herpes simplex virus type 2 (HSV-2) antibodies have been inconsistent for African and human immunodeficiency virus (HIV)-positive populations. We compared the performances of the HerpeSelect and Kalon glycoprotein G2 ELISAs for patients with genital ulcer disease in Ghana and the Central African Republic. Sera from 434 women were tested with the HerpeSelect assay, and a subsample (n = 199) was tested by the Kalon assay. Ulcer swabs and cervicovaginal lavage samples were tested for HSV-2 DNA by PCR. HSV-2-seronegative women with detectable genital HSV-2 DNA were retested for HSV-2 antibodies 14 and 28 days later by the two ELISAs. A total of 346 (80%) women were positive by HerpeSelect at baseline, and 225 (54%) had detectable genital (lesional or cervicovaginal) HSV-2 DNA. Sixty-six (19%) HerpeSelect-positive samples had low-positive index values (1.1 to 3.5), and 58% of these samples had detectable genital HSV-2 DNA. Global agreement between the two serological assays was 86%. Concordance was high (99%) for sera that were negative by HerpeSelect or had high index values (>3.5). Defining infection detected by HSV-2 DNA PCR and/or Kalon assay as true infection, 71% of sera with low-positive index values were associated with true HSV-2 infection. Twenty-five women were identified as having nonprimary first-episode genital HSV-2 infection. Rates of HSV-2 seroconversion at day 14 were 77% (10/13 patients) by HerpeSelect assay and 23% (3/13 patients) by Kalon assay, with four additional seroconversions detected by Kalon assay at day 28. HIV serostatus did not influence assay performance. Low index values obtained with the HerpeSelect assay may correspond to true HSV-2 infection, in particular to nonprimary first episodes of genital HSV-2 infection, and need to be interpreted in the context of clinical history.
Identification of individuals infected with herpes simplex virus type 2 (HSV-2) is critical for the management of genital herpes as well as for epidemiological studies and clinical trials. The performances of commercially available assays to detect HSV-2-specific glycoprotein G2 (gG2) antibodies vary significantly between study populations (2). For example, the FDA-approved and widely available HerpeSelect enzyme-linked immunosorbent assay (ELISA) (Focus Technologies) has a high sensitivity and specificity, ranging from 96 to 100%, against the reference HSV-2 Western blot (WB) assay for testing of sera from North American or Western European populations but significantly lower specificity for testing of sera from adult African populations (2, 7, 16). Moreover, concordance between WB and HerpeSelect assays was found to vary between countries in sub-Saharan Africa (2, 6, 7, 9) and according to human immunodeficiency virus (HIV) serostatus (16). HerpeSelect and other assays were compared in an evaluation study using African sera from population-based surveys, and the Kalon gG2-specific ELISA (Kalon Biologicals) was found to be as sensitive as and more specific than HerpeSelect, with WB as the reference method (16). Similar results were documented for Brazilian populations, using clinicovirological reference groups (13). To increase the specificity of HerpeSelect, some authors have suggested using a higher index value of 3.5 for the seropositivity cutoff instead of the manufacturer's recommended cutoff of 1.1 (2, 9, 13, 16).
A critical element in evaluating the performance of these assays is the stage of HSV-2 infection. One study reported that the sensitivity of HerpeSelect increased for patients suffering from genital ulcer disease (GUD) compared to that for blood donors (6), perhaps because HerpeSelect may detect primary genital herpes earlier than the Kalon assay or even WB does (1, 12).
In the present report, we assess the performance characteristics of the HerpeSelect and Kalon assays for African patients presenting with symptomatic and molecularly documented genital HSV-2 infection.
MATERIALS AND METHODS
Women presenting with GUD at study sites in Ghana and the Central African Republic were enrolled in a randomized placebo-controlled trial of acyclovir (400 mg three times daily for 5 days) given in addition to routine syndromic antibacterial GUD treatment (11) (ClinicalTrials register no. NCT00158483). Briefly, eligible and consenting women with clinically verified GUD (including blisters and sores) were interviewed and examined genitally, and samples were obtained and tested as follows. Two swabs from the ulcer base were used to determine GUD etiology and to detect lesional HIV type 1 (HIV-1) RNA, using molecular methods (5, 8, 11, 14); a cervicovaginal lavage (CVL) sample was collected to detect and quantify HSV-2 DNA and HIV-1 RNA in genital secretions, using real-time PCR (10); and blood samples were obtained for detection of HSV-2 antibodies (HerpeSelect; Focus Technologies, Cypress Hill, CA) and HIV-1 antibodies and for CD4 lymphocyte counts, as previously described (11). Serum samples obtained at days 14 and 28 from women who were HSV-2 seronegative at day 0 but positive for lesional and/or cervicovaginal HSV-2 DNA (i.e., with genital HSV-2 infection) were tested using HerpeSelect to detect possible HSV-2 seroconversion, and their enrollment (day 0) samples were tested for HSV-1 antibodies by HerpeSelect gG1 assay (Focus Technologies) to determine if these were cases of primary genital herpes (dually HSV-1- and HSV-2-seronegative samples) or nonprimary first-episode HSV-2 infection (HSV-1-seropositive and HSV-2-seronegative samples). All samples from a given woman were tested in the same ELISA run.
The Kalon gG2-specific assay (Kalon Biologicals, Aldershot, United Kingdom) was used to test all available sera with HerpeSelect index values ranging from >1.1 to 3.5 (n = 63 of 66 sera), a random selection of 88 sera with index values of >3.5, and 48 sera with index values of <0.9, including all cases (n = 25) of potential HSV-2 seroconverters (as defined above).
We calculated the percentage agreement between the two tests and their concordance by Cohen's kappa coefficient (κ), and we tested for differences in positive test results by using McNemar's χ2 test for paired samples. Chi-square tests were used to compare proportions for unpaired tests, the Wilcoxon rank sum test was used for comparison of medians, and Spearman's rank test was used for correlation of categorical variables.
RESULTS
Of 441 women with GUD enrolled in the trial, 434 had HSV-2 serological results (278 and 156 women from Ghana and the Central African Republic, respectively). HSV-2 seroprevalence was higher in the Central African Republic than in Ghana (95% versus 71%; P < 0.001). Among the 413 patients with genital samples, HSV-2 DNA was detected in 225 (54%) by PCR with the lesional (n = 208) or the CVL (n = 17) specimen (with 146 patients being positive for both samples).
Table 1 shows the distribution of HerpeSelect index values by genital HSV-2 DNA status, combining results from the two countries since no difference in the distributions of HerpeSelect index values was observed (not shown). Serum samples from 346 (80%) women were found to be HSV-2 seropositive by HerpeSelect (index values of >1.1), of which 66 (19%) had low-positive index values (≤3.5). Samples from another six women were found to be equivocal (index values of 0.9 to 1.1), with one being associated with positive detection of HSV-2 DNA (in the lesional sample). Overall, 58% (36/62 patients) of women with low-positive HerpeSelect index values and 60% (164/275 patients) of women with high-positive HerpeSelect index values had molecular evidence of genital HSV-2 infection (P = 0.8). Of the 200 HSV-2-seropositive women with genital HSV-2 DNA, 36 (18%) had index values of <3.5. Among HSV-2-seropositive women, there was little difference in median index values between women with (median = 6.8; interquartile range, 4.4 to 10.2) and without (median = 7.8; interquartile range, 4.4 to 10.1) detectable genital HSV-2 DNA (P = 0.50). The frequency of recent clinical episodes was significantly correlated with the HerpeSelect titer. An episode of GUD in the preceding 12 months was reported by 14%, 17%, 26%, and 40% of patients in the groups with negative, equivocal, low-positive, and high-positive HerpeSelect serology, respectively (Spearman's rank correlation; P < 0.0001) (Table 1).
TABLE 1.
Distribution of HerpeSelect gG2 index values according to genital HSV-2 DNA detection in women with GUD in Ghana and the Central African Republic
| Clinical characteristic | No. (%) of patients in HerpeSelect index value group
|
||||
|---|---|---|---|---|---|
| <0.9 (negative) (n = 82) | 0.9-1.1 (equivocal) (n = 6) | >1.1-3.5 (low positive) (n = 66) | >3.5 (high positive) (n = 280) | Total (n = 434) | |
| Genital HSV-2 DNAa | |||||
| Negative | 49 (67) | 2 (67) | 26 (43) | 111 (41) | 188 (46) |
| Positive | 24 (33) | 1 (33) | 36 (58) | 164 (60) | 225 (54) |
| History of GUD in preceding 12 mo | 11 (14)b | 1 (17) | 17 (26) | 111 (40)b | 140 (32)b |
Defined as the presence of lesional and/or cervicovaginal HSV-2 DNA by real-time PCR. PCR results were undetermined for 9, 3, 4, and 5 patients in the groups with HerpeSelect index values of <0.9, 0.9 to 1.1, >1.1 to 3.5, and >3.5, respectively, i.e., for 21 patients in total.
PCR results were undetermined for one sample in each group, i.e., for two patients in total.
Table 2 shows results obtained by serological testing using both the HerpeSelect and Kalon assays, including those for samples from patients with suspected first episodes of genital HSV-2. Overall agreement between the two serological assays was 86% (171/199 samples) or 87% (173/199 samples), depending on the classification of equivocal results of the Kalon assay as negative or positive, respectively, and concordance defined by the kappa coefficient was good (κ = 0.68). All 48 samples found to be negative with HerpeSelect were negative with the Kalon assay. Similarly, all but 1 of the 88 samples with HerpeSelect index values of >3.5 were positive with the Kalon assay. The one discordant sample had a high HerpeSelect index value (6.8), with HSV-2 DNA detection in both lesional and cervicovaginal samples. The combined results of negative samples (index values of <0.9 with both assays) and high-positive samples (index values of >3.5 for HerpeSelect) gave an agreement of 99% (135/136 samples), corresponding to a high kappa coefficient (κ = 0.98).
TABLE 2.
Results of HerpeSelect and Kalon testing according to assay index values
| Kalon index value group | No. (%) of women in HerpeSelect index value groupa
|
||
|---|---|---|---|
| >3.5 | >1.1-3.5 | <0.9 | |
| >1.1b | 87 (99) | 36 (57) | 0 |
| HSV-2 DNA positive | 51 | 25 | 0 |
| HSV-2 DNA negative | 36 | 10 | 0 |
| 0.9-1.1 | 0 | 2 (3) | 0 |
| HSV-2 DNA positive | 0 | 1 | 0 |
| HSV-2 DNA negative | 0 | 1 | 0 |
| <0.9c | 1 (1) | 25 (40) | 48 (100) |
| HSV-2 DNA positive | 1 | 8 | 22 |
| HSV-2 DNA negative | 0 | 14 | 25 |
| Total (n = 199) | 88 | 63 | 48 |
Sera with HerpeSelect values of 0.9 to 1.1 (equivocal) were excluded from this analysis.
HSV DNA data are missing for one sample from the group with HerpeSelect values of >1.1 to 3.5.
HSV DNA data are missing for three samples from the group with HerpeSelect values of >1.1 to 3.5 and for one sample from the group with HerpeSelect values of <0.9.
Among the 63 samples with HerpeSelect index values ranging from >1.1 to 3.5, Kalon assay results were found to be negative for 25 (40%) (of which 8 were associated with detection of genital HSV-2 DNA), equivocal for 2 (3%) (with 1 being positive for HSV-2 DNA in the CVL sample only), and positive for 36 (57%).
Assuming detection of genital HSV-2 DNA (n = 34) and/or concordant seropositivity with the Kalon assay (n = 36) as confirmation of HSV-2 infection, 71% (45/63 samples) of samples with HerpeSelect index values between >1.1 and 3.5 should be considered true HSV-2 infections and would have been misclassified using the higher cutoff of 3.5 suggested by Hogrefe et al. (7) and Nascimento et al. (13). Among discordant samples tested for HSV-2 DNA, 40% (10/25 samples) were associated with positive detection of HSV-2 DNA (Table 2). Finally, among women found to be seropositive with HerpeSelect and to have concomitant genital HSV-2 DNA, only 88% (76/86 patients) were considered HSV-2 seropositive with the Kalon assay.
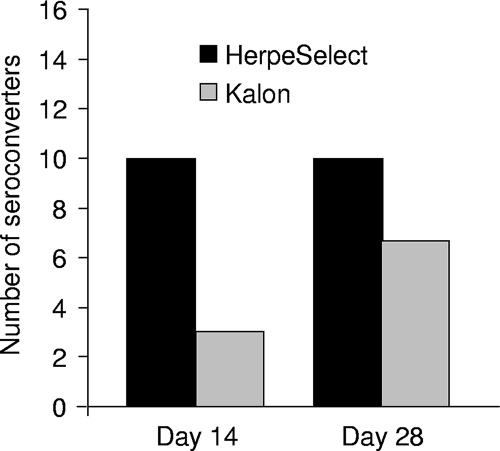
Among the 76 women classified as HSV-2 seronegative or equivocal by HerpeSelect at day 0 who had genital samples, 25 (6% of all women in the trial) had genital HSV-2 DNA detected by PCR (Table 1). These patients presented to the clinics after a median of 6 days (range, 3 to 18 days) following symptom onset. All of these women were HSV-1 seropositive by HerpeSelect gG1 assay and thus were classified as having nonprimary first episodes of genital HSV-2 infection. All day 0 sera were also found to be negative by Kalon assay. Among these women, 15 were tested on either day 14 or day 28 (10 were tested on both days, 3 were tested only on day 14, and 2 were tested only on day 28), and 11 cases of HSV-2 seroconversion were detected, with 10 detected by HerpeSelect and 7 detected by Kalon assay. HSV-2 seroconversion was detected earlier with HerpeSelect than with the Kalon assay (Fig. 1). At day 14, 77% (10/13 samples) of sera were positive with HerpeSelect, compared with only 23% (3/13 sera) with the Kalon assay (P = 0.01). Four additional seroconversions were observed with the Kalon assay at day 28, and none were detected by HerpeSelect. The median HerpeSelect index value for these 10 seroconversion cases was 4.3 (range, 2.1 to 15.9) at day 14 and 8.6 (range, 1.5 to 16.6) at day 28. Among patients with nonprimary first episodes of genital HSV-2 infection, four were HIV seropositive at baseline and one woman seroconverted to HIV seropositive within 28 days. All three HIV-seropositive samples which were tested at day 28 showed HSV-2 seroconversion.
FIG. 1.
Number of cases and timing of HSV-2 seroconversion by HSV-2 serological assay among 15 patients with first episodes of genital HSV-2 infection in Ghana and the Central African Republic. On day 14, 13 women were tested. For day 28, the number of seroconversion cases corresponds to the cumulative number of seroconverters on days 14 and 28. Results of HerpeSelect are presented in black bars, and results of the Kalon assay are shown with gray bars.
Forty-seven percent (202/432 women) of women included in this study were HIV-1 seropositive. The analysis of HSV-2 serological results according to HIV-1 serostatus did not show any effect of HIV-1 infection on the performance of HerpeSelect. Samples giving discordant HSV-2 assay results were less frequently HIV-1 seropositive than were those giving concordant results (14% versus 48%; McNemar's P = 0.01). The median HerpeSelect index values were similar for HIV-1-seropositive and -seronegative samples (7.2 versus 7.5; P = 0.46).
DISCUSSION
To our knowledge, this is the first report of an evaluation of HSV-2 seroassays among cases of genital herpes documented through molecular methods, including cases of nonprimary first episodes of genital HSV-2 infection in Africa. Previous studies have suggested that the specificity of the HerpeSelect assay for detecting HSV-2 serum antibodies may be lower for African sera or sera from HIV-1-seropositive patients (2, 7, 16). A higher positivity cutoff index value of 3.5 (instead of the manufacturer's recommended cutoff of 1.1) has therefore been suggested as a way to increase assay specificity. However, the clinical stage of HSV-2 infection had not been ascertained in these studies, which may have interfered with the correct interpretation of results. In the present study, we aimed to reevaluate the significance of low HerpeSelect index values among African patients presenting with GUD. We compared the results obtained with HerpeSelect with those of the Kalon serological assay, which is known to have a high specificity (13, 16).
Our results show 99% concordance between the HerpeSelect and Kalon assays for negative or high-positive (>3.5) HerpeSelect results, which confirms previous reports of excellent agreement between these two assays (13, 16). In contrast, the concordance was moderate when HerpeSelect positivity included index values between >1.1 and 3.5. However, we found that 40% (10/25 samples) of discordant samples were associated with detection of genital HSV-2 DNA, and 71% of samples with low-positive HerpeSelect index values could be classified as having established HSV-2 infection when results of molecular testing or seroconcordance were included. Overall, nearly 20% of the study population with positive HerpeSelect serology had index values comprising between >1.1 and 3.5, 58% of whom had evidence of genital HSV-2 infection. This is consistent with data published by Ashley-Morrow et al. showing that 22% of U.S. patients with established HSV-2 infection had low HerpeSelect index values (3). Reciprocally, 18% of women testing positive by HerpeSelect and carrying genital HSV-2 DNA had low HerpeSelect index values. These findings demonstrate that a nonnegligible proportion of true genital herpes cases would be missed by using a higher cutoff for HerpeSelect.
The analysis of the kinetics of HSV-2 antibodies in sera associated with nonprimary first clinical episodes of HSV-2 infection showed that HerpeSelect detected HSV-2 seroconversion more frequently and earlier than did the Kalon assay and that HerpeSelect index values increased rapidly. These results corroborate data from the work of Ashley-Morrow et al. (3) demonstrating that for newly infected patients, HerpeSelect index values start low, often in the negative range, and peak a median of 9 to 10 weeks later. Our results also confirm the higher sensitivity of HerpeSelect than that of the Kalon assay in detecting nonprimary first HSV-2 episodes, as previously reported (12). In a U.S. study including 14 patients with nonprimary first episodes with culture-positive HSV-2 lesions, the sensitivity of HerpeSelect was 77%, in contrast to 43% for the Kalon assay, and the median time for seroconversion was significantly longer by Kalon assay (149 days) than by HerpeSelect (22 days) (12). In our study, patients with nonprimary first episodes of HSV-2 infection presented to the clinics after a median of 6 days (range, 3 to 18 days) following symptom onset, and thus the maximum follow-up time to assess seroconversion was 46 days postonset, which explains the higher detection rate obtained with HerpeSelect. We also found that HerpeSelect seropositivity and index values correlated with reported GUD symptoms in the preceding year. These results suggest that frequent viral reactivations may sustain specific humoral responses and, conversely, that low titers may be related to insufficient antigenic stimulation secondary to infrequent viral replication, as suggested by Ashley-Morrow et al. (3).
Thus, the ability of methods to detect early HSV-2 infection may account for some of the assay discrepancies found in our and other studies. It is even possible that some recent HSV-2 infections may have been accompanied by a lack of HSV-2 DNA detection, since the delay between the onset of symptoms and presentation at the clinic for study enrollment was quite long for some women, allowing some patients to have already cleared the virus. Such misclassifications would have contributed to underestimating the true sensitivity of HerpeSelect.
We did not use the reference WB HSV assay in this study to help resolve some of the assays' discrepancies. However, it has been shown that although it is highly sensitive and specific, WB may have less ability to detect recent cases of genital herpes, as detection of seroconversion among first episodes of genital HSV-2 infection was slower than that of HerpeSelect (1). Moreover, it has been shown that some discordant results between HerpeSelect and WB on African sera were eventually classified as true positive results by HerpeSelect after testing with an inhibition assay (7).
Finally, our data did not support a differential performance of HerpeSelect according to HIV serostatus. On the contrary, we observed less discrepancy between the HerpeSelect and Kalon assays for samples from HIV-infected women. This may be related to the higher rate of HSV-2 reactivation in such patients, which might sustain index values of HSV-2-specific antibodies in the high range.
Our results confirm that some low-positive HerpeSelect index values correspond to true HSV-2 infection and that some may be related to recent infection. Depending on population composition, this would affect the evaluation of the performance of the various HSV-2 serological assays, and this has to be taken into consideration in the interpretation of study findings. Moreover, the choice of HSV-2 serological assays needs to be dictated by circumstances, whether for an epidemiological study examining risk factors for HSV-2 or a clinical trial seeking to enroll HSV-2-seropositive patients, for which highly specific assays would be more desirable, or for the diagnosis and management of patients with possible early HSV-2 infection, for which a sensitive assay would be required. Our results have particular resonance for the management of GUD in countries where HSV-2 infection is highly prevalent and where clinical or subclinical primary infection may occur frequently. The use of a sensitive assay comparable to HerpeSelect may help to detect recent herpesvirus infection and offer appropriate advice on management and counseling about the risk of HIV acquisition, which could increase in the presence of recent HSV-2 infection (4, 15).
Acknowledgments
The ANRS 12-12 Study Group is comprised of the following persons: Thomas Agyarko-Poku, Comfort Asamoah-Adu, Agnes Dzokoto, and Nzambi Khonde, West African Project To Combat AIDS and STDs, Accra, Ghana; Laurent Bélec, Hicham Bouhlal, Cécile Chemin, Jérôme LeGoff, and Ali Si-Mohamed, Université Paris V, Equipe Immunité et Biothérapie Muqueuse, Unité INSERM Internationale U743 (Immunologie Humaine), Centres de Recherches Biomédicales des Cordeliers, and Laboratoire de Virologie, Hôpital Européen Georges Pompidou, Paris, France; Sylvie Deslandes, Eric Frost, and Jacques Pépin, Centre for International Health, University of Sherbrooke, Sherbrooke, Canada; Gérard Grésenguet and Jean-De-Dieu Longo, Centre National de Référence des Maladies Sexuellement Transmissibles et du SIDA de Bangui and Unité de Recherches et d'Intervention sur les Maladies Sexuellement Transmissibles et du SIDA, Faculté des Sciences de la Santé, Bangui, Central African Republic; Richard Hayes, David Mabey, Philippe Mayaud, and Helen A. Weiss, Clinical Research Unit, Department of Infectious and Tropical Diseases, and Infectious Diseases Epidemiology Unit, Department of Epidemiology and Population Health, London School of Hygiene and Tropical Medicine, London, United Kingdom; and Jean-Elie Malkin, Centre Médical, Institut Pasteur, Paris, France.
Footnotes
Published ahead of print on 2 April 2008.
REFERENCES
- 1.Ashley-Morrow, R., E. Krantz, and A. Wald. 2003. Time course of seroconversion by HerpeSelect ELISA after acquisition of genital herpes simplex virus type 1 (HSV-1) or HSV-2. Sex. Transm. Dis. 30310-314. [DOI] [PubMed] [Google Scholar]
- 2.Ashley-Morrow, R., J. Nollkamper, N. J. Robinson, N. Bishop, and J. Smith. 2004. Performance of Focus ELISA tests for herpes simplex virus type 1 (HSV-1) and HSV-2 antibodies among women in ten diverse geographical locations. Clin. Microbiol. Infect. 10530-536. [DOI] [PubMed] [Google Scholar]
- 3.Ashley-Morrow, R., E. Krantz, D. Friedrich, and A. Wald. 2006. Clinical correlates of index values in the Focus HerpeSelect ELISA for antibodies to herpes simplex virus type 2 (HSV-2). J. Clin. Virol. 36141-145. [DOI] [PubMed] [Google Scholar]
- 4.Brown, J. M., A. Wald, A. Hubbard, K. Rungruengthanakit, T. Chipato, S. Rugpao, F. Mmiro, D. D. Celentano, R. S. Salata, C. S. Morrison, B. A. Richardson, and N. S. Padian. 2007. Incident and prevalent herpes simplex virus type 2 infection increases risk of HIV acquisition among women in Uganda and Zimbabwe. AIDS 211515-1523. [DOI] [PubMed] [Google Scholar]
- 5.Carter, J., F. J. Bowden, K. S. Sriprakash, I. Bastian, and D. J. Kemp. 1999. Diagnostic polymerase chain reaction for donovanosis. Clin. Infect. Dis. 281168-1169. [DOI] [PubMed] [Google Scholar]
- 6.Gorander, S., J. Mbwana, E. Lyamuya, T. Lagergard, and J. A. Liljeqvist. 2006. Mature glycoprotein G presents high performance in diagnosing herpes simplex virus type 2 infection in sera of different Tanzanian cohorts. Clin. Vaccine Immunol. 13633-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hogrefe, W., X. Su, J. Song, R. Ashley, and L. Kong. 2002. Detection of herpes simplex virus type 2-specific immunoglobulin G antibodies in African sera by using recombinant gG2, Western blotting, and gG2 inhibition. J. Clin. Microbiol. 403635-3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson, G., S. Nelson, M. Petric, and R. Tellier. 2000. Comprehensive PCR-based assay for detection and species identification of human herpesviruses. J. Clin. Microbiol. 383274-3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laeyendecker, O., C. Henson, R. H. Gray, R. H. Nguyen, B. J. Horne, M. J. Wawer, D. Serwadda, N. Kiwanuka, R. A. Morrow, W. Hogrefe, and T. C. Quinn. 2004. Performance of a commercial, type-specific enzyme-linked immunosorbent assay for detection of herpes simplex virus type 2-specific antibodies in Ugandans. J. Clin. Microbiol. 421794-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Legoff, J., H. Bouhlal, G. Gresenguet, H. Weiss, N. Khonde, H. Hocini, N. Desire, A. Si-Mohamed, J. de Dieu Longo, C. Chemin, E. Frost, J. Pepin, J. E. Malkin, P. Mayaud, and L. Belec. 2006. Real-time PCR quantification of genital shedding of herpes simplex virus (HSV) and human immunodeficiency virus (HIV) in women coinfected with HSV and HIV. J. Clin. Microbiol. 44423-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Legoff, J., H. A. Weiss, G. Gresenguet, K. Nzambi, E. Frost, R. J. Hayes, D. C. Mabey, J. E. Malkin, P. Mayaud, and L. Belec. 2007. Cervicovaginal HIV-1 and herpes simplex virus type 2 shedding during genital ulcer disease episodes. AIDS 211569-1578. [DOI] [PubMed] [Google Scholar]
- 12.Morrow, R. A., D. Friedrich, and E. Krantz. 2003. Performance of the Focus and Kalon enzyme-linked immunosorbent assays for antibodies to herpes simplex virus type 2 glycoprotein G in culture-documented cases of genital herpes. J. Clin. Microbiol. 415212-5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nascimento, M. C., S. Ferreira, E. Sabino, I. Hamilton, J. Parry, C. S. Pannuti, and P. Mayaud. 2007. Performance of the HerpeSelect (Focus) and Kalon enzyme-linked immunosorbent assays for detection of antibodies against herpes simplex virus type 2 by use of monoclonal antibody-blocking enzyme immunoassay and clinicovirological reference standards in Brazil. J. Clin. Microbiol. 452309-2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orle, K. A., C. A. Gates, D. H. Martin, B. A. Body, and J. B. Weiss. 1996. Simultaneous PCR detection of Haemophilus ducreyi, Treponema pallidum, and herpes simplex virus types 1 and 2 from genital ulcers. J. Clin. Microbiol. 3449-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reynolds, S. J., A. R. Risbud, M. E. Shepherd, J. M. Zenilman, R. S. Brookmeyer, R. S. Paranjape, A. D. Divekar, R. R. Gangakhedkar, M. V. Ghate, R. C. Bollinger, and S. M. Mehendale. 2003. Recent herpes simplex virus type 2 infection and the risk of human immunodeficiency virus type 1 acquisition in India. J. Infect. Dis. 1871513-1521. [DOI] [PubMed] [Google Scholar]
- 16.van Dyck, E., A. Buve, H. A. Weiss, J. R. Glynn, D. W. Brown, B. De Deken, J. Parry, and R. J. Hayes. 2004. Performance of commercially available enzyme immunoassays for detection of antibodies against herpes simplex virus type 2 in African populations. J. Clin. Microbiol. 422961-2965. [DOI] [PMC free article] [PubMed] [Google Scholar]