Abstract
Interpretive disk diffusion breakpoints for caspofungin are proposed by evaluating 762 isolates of Candida spp., representing 10 different species obtained as part of the caspofungin clinical trials. Standardized broth microdilution reference tests were compared to the zone diameters observed with 5-μg caspofungin disks produced by two different disk manufacturers. Disk diffusion breakpoints of ≥11 mm for susceptible are proposed. Compared to results from MIC testing, these zone diameters produced error rates that were ≤0.3% for all categories. In addition, an eight-laboratory disk diffusion quality control (QC) study was performed, and QC ranges are proposed for the four QC strains recommended by the CLSI.
The need for the early diagnosis and treatment of fungal infections has been well documented (13, 16, 19). Caspofungin, an FDA-approved antifungal agent of the echinocandin family, has been shown to be effective in the treatment of a variety of fungal infections, including those caused by Candida species (1, 2). It is the first drug of its class to be approved for use by the FDA. It also has been shown to be effective in vitro against isolates with decreased susceptibility to amphotericin B or to the azole compounds (15, 20). Although there are case reports of caspofungin resistance among clinical isolates of Candida (9, 11, 12, 14), surveillance studies have not demonstrated the widespread emergence of caspofungin resistance among clinical isolates causing invasive candidiasis (17). The CLSI has developed and approved standardized testing methodologies under which antifungal agents should be tested. These methodologies include both broth microdilution (6, 7) and disk diffusion techniques (5, 8). Methods for the development of MIC breakpoints have been previously described (18). MIC quality control (QC) ranges for caspofungin also have been published previously (3). Recently, caspofungin MIC breakpoints were approved by the CLSI (M. A. Pfaller, D. J. Diekema, J. H. Rex, B. D. Alexander, D. Andes, S. D. Brown, V. Chaturvedi, M. A. Ghannoum, C. C. Knapp, L. Ostrosky-Zeichner, D. J. Sheehan, and T. J. Walsh, submitted for publication) using the CLSI-recommended standard method for broth microdilution. The CLSI-approved MIC breakpoint for caspofungin susceptibility is ≤2 μg/ml. There is no intermediate or dose-dependent category.
The purpose of this study was to compare broth microdilution MICs to zone diameters produced by 5-μg caspofungin susceptibility disks using the CLSI standardized methodologies against Candida isolates obtained during the caspofungin clinical trials. Once the data were generated, the results were analyzed and disk diffusion breakpoints are proposed. In addition, after completing an eight-laboratory disk diffusion study, QC ranges are proposed.
MATERIALS AND METHODS
Isolates.
A total of 762 strains of Candida spp., representing 10 different species, were provided by Merck & Co. The isolates were obtained as part of the caspofungin clinical trials. The species included 530 C. albicans, 72 C. glabrata, 27 C. guilliermondii, 1 C. kefyr, 18 C. krusei, 2 C. lipolytica, 2 C. lusitaniae, 52 C. parapsilosis, 1 C. rugosa, and 57 C. tropicalis strains. Prior to being tested, all strains were passaged at least twice on Sabouraud dextrose agar to ensure viability and purity.
Antimicrobial agent.
Caspofungin (lot no. REK0070) was provided as a standardized powder by Merck & Co. Fluconazole powder (lot no. 02FLU-010-00) was obtained from Pfizer Pharmaceuticals and was used as the control drug for all testing. Stock solutions were prepared in water, and serial twofold dilutions were prepared as recommended by the CLSI (7).
Broth microdilution tests.
The reference broth microdilution method described by the CLSI (7) was utilized as the reference method in this study. The medium was RPMI 1640 broth (lot no. 014K8310; Sigma) supplemented with l-glutamine (lot no. 94H01931; Sigma) and buffered to pH 7.0 with morpholinepropanesulfonic acid organic buffer (lot no. 125K0010; Sigma). The concentrations of caspofungin tested were serial twofold dilutions ranging from 0.004 to 128 μg/ml. The fluconazole concentrations tested ranged from 0.008 to 256 μg/ml. After 24 h of incubation at 35°C, MICs were read as the lowest concentration that showed a marked decrease in the density of growth (approximately 50% inhibition, as judged by the unaided eye). Colony counts were performed on the suspension in the growth control well from randomly selected tests, and the results ranged from 5 × 102 to 3.7 × 103 CFU/ml.
Disk diffusion test.
Disk diffusion tests were performed by following the procedure outlined by the CLSI for yeasts (5) using Mueller-Hinton agar supplemented with 2% glucose and 0.5% methylene blue (MHA-GMB). Caspofungin-impregnated paper disks were provided by Merck. The disks were prepared to contain 5 μg of caspofungin and were manufactured by BBL (lot no. 6150854) and Oxoid (lot no. 372738). Twenty-five-microgram fluconazole disks (lot no. 6093051; BBL) were used as the internal control. Inhibitory zone diameters were measured after 24 h of incubation, at which point there was a sharp decline in the amount of growth (approximately 50% inhibition, as judged by the unaided eye).
Daily QC.
Daily QC testing was performed using the CLSI-recommended strains of C. parapsilosis ATCC 22019 and C. krusei ATCC 6258 for the MIC portion of the study and C. parapsilosis ATCC 22019, C. krusei ATCC 6258, C. albicans ATCC 90028, and C. tropicalis ATCC 750 for the disk diffusion portion of the study. The fluconazole MIC and disk diffusion QC ranges utilized in this study were those recommended by the CLSI (6, 8).
Disk diffusion QC ranges.
An eight-laboratory collaborative study was performed in order to define control limits for disk diffusion tests using 5-μg caspofungin disks. The study was designed to meet or exceed the guidelines established by the CLSI (4). MHA-GMB was used. Three lots of MHA-GMB (lot nos. 100,967 [Acumedia], 6179147 [Difco], and 4223266 [BDMS/BBL]) were tested in each participating laboratory. Repetitive disk diffusion tests were performed in all eight collaborating laboratories, each using the proposed method and four standard reference or QC strains. Two lots of 5-μg disks (lot nos. 6150854 [BDMS/BBL] and 372738 [Oxoid]) were used for each test. A single lot of 25-μg fluconazole disks (lot no. 6093051; BDMS/BBL) served as the internal control. Readings were made at 24 h of incubation, as recommended by the CLSI (5).
The eight laboratories that participated in this study were the following: S. Brown, Clinical Microbiology Institute, Wilsonville, OR; D. Diekema, University of Iowa, Iowa City; M. Ghannoum, Case Western Reserve University, Cleveland, OH; G. Hall, Cleveland Clinic, OH; D. Hardy, University of Rochester Medical Center, NY; C. Knapp, TREK Diagnostic Systems, Cleveland, OH; L. Ostrosky-Zeichner, University of Texas-Houston Medical School; and R. Rennie, University of Alberta Hospital, Edmonton, Alberta, Canada. QC ranges are proposed based upon the method of Gavan et al. (10).
RESULTS AND DISCUSSION
Disk diffusion breakpoints.
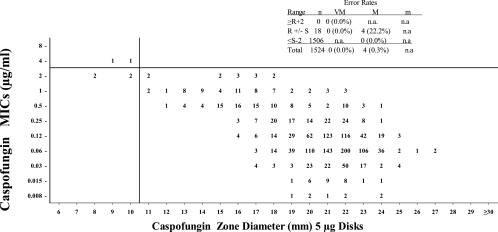
The MIC and disk diffusion results obtained as part of this investigation are presented in Fig. 1. Disk diffusion zone diameters were recorded for all strains using both BBL and Oxoid prepared disks (i.e., two disks per strain). There was good correlation between the zone diameters observed with the disks provided by the two different manufacturers, in that 99.1% of the values observed were ±3 mm of the mode. Fully 83.5% of the values observed were ±1 mm of the mode. The average zone of inhibition observed with the BBL disks was 20.47 mm; an average of 20.95 mm was observed with the Oxoid disks. The differences in zones of inhibition between the two manufacturers were consistently small for all species under study.
FIG. 1.
Caspofungin MIC at the 24-h endpoint versus caspofungin zone diameters at 24 h using 5-μg BBL and Oxoid disks combined (two disks per strain). The MIC breakpoints were ≤2 μg/ml for susceptible and >2 μg/ml for nonsusceptible. R, resistant; S, susceptible; m, minor; M, major; VM, very major; n.a., not applicable.
MIC breakpoints of ≤2 μg/ml for susceptible and >2 μg/ml for nonsusceptible have been previously described by Pfaller et al. (submitted) and approved by the CLSI. Figure 1 shows the 24-h MIC reading using these breakpoints. All of the MIC and disk diffusion values were able to be read at 24 h, and none required additional incubation.
Disk diffusion breakpoints of ≥11 mm for susceptible are proposed. These breakpoints are proposed on the basis of the combined data from both the BBL and Oxoid disks (i.e., two disks per isolate). The error rates are calculated for the combined disk data but not for the individual disk manufacturers. The proposed disk diffusion breakpoints produced 0 very major errors and 4 (0.3%) major errors. All error rates were well within the acceptable limits proposed by the CLSI (4). Unfortunately, isolates that were truly resistant to caspofungin were rarely encountered during the caspofungin clinical trials. The lack of these isolates severely limits our ability to propose reliable MIC or disk diffusion breakpoints. As with all other drugs, the accuracy of these breakpoints must be continually monitored in order to ensure that they continue to be effective in the event that organisms with decreased susceptibility are encountered.
QC ranges.
QC ranges are proposed according to the method of Gavan et al. (10), which is based upon the all-laboratory median plus or minus half the range of medians for each laboratory. The two lots of caspofungin disks gave essentially identical results, in that the eight laboratory means for the two disks were within 0.1 mm (data not shown). The proposed QC limits include an 8- to 10-mm range of zone diameters for each of the control strains (Table 1).
TABLE 1.
Caspofungin disk diffusion QC
| QC strain | No. of occurrences at the following zone diameter (mm)a
|
% in rangeb | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | ≥31 | ||
| C. albicans ATCC 90028 | 2 | 6 | 14 | 22 | 36 | 74 | 84 | 83 | 57 | 46 | 24 | 20 | 8 | 3 | 96.0 | ||||||
| C. parapsilosis ATCC 20019 | 1 | 1 | 10 | 74 | 99 | 82 | 78 | 60 | 29 | 22 | 14 | 9 | 1 | 97.7 | |||||||
| C. tropicalis ATCC 750 | 4 | 18 | 56 | 94 | 112 | 104 | 59 | 21 | 9 | 2 | 1 | 98.5 | |||||||||
| C. krusei ATCC 6258 | 1 | 5 | 36 | 66 | 101 | 115 | 71 | 54 | 19 | 5 | 3 | 4 | 98.3 | ||||||||
Recommended QC ranges are in boldface.
Percentage of results that fall within the recommended range.
Conclusions.
Disk diffusion breakpoints of ≥11 mm of inhibition for susceptible and ≤10 mm of inhibition for nonsusceptible and the proposed QC limits have now been approved by the CLSI subcommittee for antifungal susceptibility testing and will be presented in an upcoming printing of their documents. It is hoped that the addition of disk diffusion methodology will place antifungal susceptibility testing within the grasp of even the smallest clinical laboratory.
Acknowledgments
This study was made possible by a grant from Merck & Co., Rahway, NJ.
Footnotes
Published ahead of print on 9 April 2008.
REFERENCES
- 1.Abruzzo, G. K., A. M. Flattery, C. J. Gill, L. Kong, J. G. Smith, V. B. Pikounis, J. M. Balkovec, A. F. Dropinski, H. Rosen, H. Kropp, and K. Bartizal. 1997. Evaluation of the echinocandin antifungal MK-0991 (L-743,872): efficacies in mouse models of disseminated aspergillosis, candidiasis, and cryptococcosis. Antimicrob. Agents Chemother. 412333-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abruzzo, G. K., C. J. Gill, A. M. Flattery, L. Kong, C. Leighton, J. G. Smith, V. B. Pikounis, K. Bartizal, and H. Rosen. 2000. Efficacy of the echinocandin caspofungin against disseminated aspergillosis and candidiasis in cyclophosphamide-induced immunosuppressed mice. Antimicrob. Agents Chemother. 452310-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barry, A. L., P. C. Fuchs, and S. D. Brown. 2000. Quality control limits for broth microbiology susceptibility tests of ten antifungal agents. J. Clin. Microbiol. 383457-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute. 2001. Development of in vitro susceptibility testing criteria and quality control parameters; approved guideline. Document M23-A2. Clinical and Laboratory Standards Institute, Wayne, PA.
- 5.Clinical and Laboratory Standards Institute. 2004. Method for antifungal disk diffusion susceptibility testing of yeasts; approved guideline. Document M44. Clinical and Laboratory Standards Institute, Wayne, PA.
- 6.Clinical and Laboratory Standards Institute. 2006. Quality control minimal inhibitory concentration (MIC) limits for broth microdilution and MIC interpretive breakpoints. Supplement. M27-S2. Clinical and Laboratory Standards Institute, Wayne, PA.
- 7.Clinical and Laboratory Standards Institute. 2002. Reference method for broth dilution antifungal susceptibility tests of yeasts. Approved standard. Document M27-A2. Clinical and Laboratory Standards Institute, Wayne, PA.
- 8.Clinical and Laboratory Standards Institute. 2006. Zone diameter interpretive standards and corresponding minimal inhibitory concentration (MIC) interpretive breakpoints. Supplement. M44-S2. Clinical and Laboratory Standards Institute, Wayne, PA.
- 9.Dodgson, K. J., A. R. Dodgson, C. Pujol, S. A. Messer, D. R. Soll, and M. A. Pfaller. 2005. Caspofungin-resistant C. glabrata. Clin. Microbiol. Infect. 11(Suppl. 2)364. [Google Scholar]
- 10.Gavan, T. L., R. N. Jones, A. L. Barry, P. C. Fuchs, E. H. Gerlach, J. M. Matsen, L. B. Reller, C. Thornsberry, and L. D. Thrupp. 1981. Quality control limits for ampicillin, carbenicillin, mezlocillin, and piperacillin disk diffusion susceptibility tests: a collaborative study. J. Clin. Microbiol. 1467-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hernandez, S., J. L. Lopez-Ribot, L. K. Najvar, D. I. McCarthy, R. Bocanegra, and J. R. Graybill. 2004. Caspofungin resistance in Candida albicans: correlating clinical outcome with laboratory susceptibility testing of three isogenic isolates serially obtained from a patient with progressive candida esophagitis. Antimicrob. Agents Chemother. 481382-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krogh-Madsen, M., M. C. Arendrup, L. Heslet, and J. D. Knudsen. 2006. Amphotericin B and caspofungin resistance in Candida glabrata isolates recovered from a critically ill patient. Clin. Infect. Dis. 42938-944. [DOI] [PubMed] [Google Scholar]
- 13.MacCallum, D. M., and F. C. Odds. 2004. Need for early antifungal treatment confirmed in experimental disseminated Candida albicans infection. Antimicrob. Agents Chemother. 484911-4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moudgal, V., T. Little, D. Boikov, and J. A. Vazquez. 2005. Multiechinocandin- and multiazole-resistant Candida parapsilosis isolates serially obtained during therapy for prosthetic valve endocarditis. Antimicrob. Agents Chemother. 49767-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson, P. W., M. Lozano-Chiu, and J. H. Rex. 1997. In vitro growth-inhibitory activity of pneumocandins L-733,560 and K-743,872 against putatively amphotericin B- and fluconazole-resistant Candida isolates: influence of assay conditions. J. Med. Vet. Mycol. 35585-587. [PubMed] [Google Scholar]
- 16.Pappas, P. G., J. H. Rex, J. D. Sobel, S. G. Filler, W. E. Dismukes, T. J. Walsh, and J. E. Edwards. 2004. Guidelines for treatment of candidiasis. Clin. Infect. Dis. 38161-189. [DOI] [PubMed] [Google Scholar]
- 17.Pfaller, M. A., L. Boyken, R. J. Hollis, S. A. Messer, S. Tendolkar, and D. J. Diekema. 2006. In vitro susceptibilities of Candida spp. to caspofungin: four years of global surveillance. J. Clin. Microbiol. 44760-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rex, J., M. Pfaller, J. Galgiani, M. Bartlett, A. Espinel-Ingroff, M. Ghannoum, M. Lancaster, F. Odds, M. Rinaldi, T. Walsh, and A. Barry. 1997. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro and in vivo correlation data for caspofungin and itraconazole and Candida infections. Clin. Infect. Dis. 24235-247. [DOI] [PubMed] [Google Scholar]
- 19.Rex, J. H., T. J. Walsh, J. D. Sobel, S. G. Filler, P. G. Pappas, W. E. Dismukes, and J. E. Edwards. 2000. Practice guidelines for treatment of candidiasis. Clin. Infect. Dis. 30662-678. [DOI] [PubMed] [Google Scholar]
- 20.Vazquez, J. A., M. Lynch, D. Boikov, and J. D. Sobel. 1997. In vitro activity of a new pneumocandin antifungal, L-743,872, against azole-susceptible and -resistant Candida species. Antimicrob. Agents Chemother. 411612-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]