Abstract
Candida spp. are important causes of nosocomial bloodstream infections. Around 80% of patients with candidemia have an indwelling central venous catheter (CVC). Determining whether the CVC is the source of candidemia has implications for patient management. We assessed whether the time to detection of Candida species in peripheral blood (time to positivity [TTP]) can serve as a marker for catheter-related candidemia. Prospective surveillance of Candida bloodstream infection was conducted in two medical centers. TTP was recorded by the BacT/Alert automated system. Sixty-four candidemia episodes were included. Fifty patients (78%) had an indwelling CVC. Thirteen patients (20.3%) had definite catheter-related candidemia. TTP was shorter for definite catheter-related candidemia (17.3 ± 2 h) than that for candidemia from other sources (38.2 ± 3 h; P < 0.001). A TTP cutoff of 30 h was 100% sensitive and 51.4% specific for catheter-related candidemia (area under the receiver-operator characteristic curve of 0.76). We conclude that TTP in peripheral blood is a sensitive but nonspecific marker for catheter-related candidemia and that a TTP of more than 30 h can help exclude an intravascular catheter as the possible source of candidemia.
Candida spp. are common causes of nosocomial bloodstream infections, ranking as the fourth most frequent pathogens involved in such infections overall and the third most frequent pathogens in intensive care units (7, 15, 18). The estimated attributable outcomes of candidemia include excess mortality of 14% to 49% (5, 19), prolongation of hospital stay by 10 days (19), and increased expenditure of around 40,000 dollars per case (19).
Central venous catheters (CVCs) are present in about 80% of patients with candidemia (2) and significantly increase the risk of candidemia in hospitalized patients (3). The role of catheters in neutropenic patients is less clear because the gastrointestinal tract is a likely source of candidemia in these patients. The Infectious Diseases Society of America guidelines for the treatment of candidiasis state that for candidemia, removal of existing intravascular catheters is desirable, if feasible, especially in nonneutropenic patients (13). However, removal of all intravascular catheters in patients with candidemia entails the need to insert new catheters and may therefore be associated with mechanical complications, bleeding, and increased expenditure. Catheter removal may be particularly complicated in patients with long-term tunneled catheters and in those with multiple catheters. These considerations are underscored by evidence showing that only 25% of catheters removed in patients with candidemia are in fact infected with Candida (2). Clearly, methods to safely reduce the unnecessary removal of uninfected catheters are required.
Several noninvasive methods for the diagnosis of catheter-related bloodstream infection have been put forth, none of which has been specifically assessed for candidemia. Quantitative cultures may be used to compare the concentrations of bacteria in samples of blood drawn simultaneously from the catheter lumen and peripheral blood; a 5- to 10-fold higher concentration in the catheter lumen is highly suggestive of catheter-related infection (14, 17). However, quantitative blood cultures are labor-intensive, expensive, and prone to contamination and therefore are not routinely performed by most microbiology laboratories. In automated blood culture systems that continuously monitor bacterial growth by colorimetric detection of CO2, the time to detection of growth correlates inversely with the bacterial load and may be a readily available substitute for quantitative blood cultures (2). However, to effectively compare time to positivity (TTP) from peripheral blood and catheter lumen (i.e., differential TTP), equal volumes of blood must be drawn simultaneously from both sources and entered into the culture system at the same time. More commonly in everyday practice, the clinician is faced with a peripheral blood culture growing a Candida species in a patient with one or more intravascular catheters, and the likelihood of a catheter-related infection must be determined according to clinical risk factors, such as the receipt of hyperalimentation through the catheter, isolation of Candida parapsilosis from blood, and the absence of an apparent alternative source of candidemia (12).
Recent retrospective studies on Staphylococcus aureus bacteremia have shown that TTP in peripheral blood alone may yield useful information regarding the source of bacteremia. A short TTP (less than 12 to 14 h) was more frequent if the source of bacteremia was endovascular, such as an infected catheter or endocarditis (8, 10). The usefulness of TTP in peripheral blood for the diagnosis of catheter-related candidemia (CRC) has not been previously studied.
MATERIALS AND METHODS
Study design.
The data presented here are from a substudy of a national active surveillance of Candida bloodstream infections in Israel and include data from two of the participating medical centers. We included adult patients (≥18 years old) who had a Candida species growing from at least one blood culture bottle drawn from a peripheral vein. Patients were recruited from November 2005 to December 2006. The study was approved by the institutional review boards of both participating centers.
Microbiological methods.
Blood cultures were obtained by physicians on the basis of clinical suspicion of infection. Physicians were instructed to draw 10 ml of blood into each bottle and to send inoculated bottles to the microbiology laboratory without delay. Both centers used the BacT/Alert automated blood culture system (bioMerieux, Marcy l'Etoile, France). The system detects growth using a colorimetric sensor that changes its color as the level of carbon dioxide in the medium rises. TTP is routinely recorded by the BacT/Alert system and is defined as the time between the placement of each blood culture bottle in the incubation cabinet and the detection of growth. Blood culture bottles were excluded from our analysis if growth of more than one organism was detected. The earliest date on which Candida grew from blood was chosen for analysis. If more than one blood culture bottle grew Candida species on the same date, the bottle with the shortest TTP was chosen.
To correct for the volume of blood inoculated into the blood culture bottles, culture bottles from center A that were positive for growth of Candida species were weighed, and the volume of inoculated blood was calculated according to the following formula: volume (in milliliters) = [weight of bottle after inoculation (in grams) − 60]/1.05 (the weight of a culture bottle prior to blood inoculation was 60 g, and the specific gravity of blood is 1.05 [16]).
Candida species were identified by morphology, pigmentation on CHROMagar (Hardy Laboratories, Santa Monica, CA) and Vitek 2 using the ID-YST card (bioMerieux).
In both participating centers, the standard recommendation of the infectious diseases team is to extract indwelling vascular catheters in nonneutropenic patients with candidemia. However, the final decision was made at the discretion of the attending physician. The tips of extracted CVCs were cultured by the plate roll method as described by Maki et al. (9). The catheter tip was rolled over blood agar, and growth was assessed after overnight incubation. If 15 or more colonies were detected, the tip culture was considered positive.
Definitions.
Among patients with an indwelling CVC, we defined three groups according to the likelihood of CRC.
(i) Group 1.
Definite CRC was defined if both of the following conditions were satisfied: (i) growth of ≥15 colonies of a Candida species from a removed catheter tip; (ii) identification of the same Candida species from the catheter tip and peripheral blood (12).
(ii) Group 2.
Possible CRC was defined if a CVC was present at the time of candidemia, but the catheter tip was not available for culture, and no alternative source of candidemia was identified.
(iii) Group 3.
Non-CRC was defined if the tip of a removed catheter was negative for growth of Candida spp., or if the catheter tip was not available for culture and an alternative source of candidemia was established.
Diagnosis of candidiasis at a noncatheter site was defined based upon microbiological culture results. For example, Candida peritonitis was defined as a peritoneal-space culture growing Candida spp. in a clinical setting consistent with peritonitis. Candidemia was designated “without an apparent source” if no source was microbiologically documented.
Data collection.
Demographic and clinical data were entered into standardized forms by researchers at each center. A study coordinator, who had no knowledge of the TTP results, entered data into a central electronic database.
Statistical analysis.
TTP was compared between the patient groups using one-way analysis of variance with post hoc Bonferroni analysis. Kaplan-Meier survival analysis with the log rank (Mantel-Cox) test was used to compare the cumulative TTP in patients with and without CRC.
To test the diagnostic accuracy of a defined TTP cutoff value for CRC, a receiver-operator characteristic (ROC) curve was plotted, and the area under the curve was calculated. Bivariate correlation between the volume of blood in culture bottles and TTP was calculated using Pearson's coefficient. TTP values are presented as means ± standard errors. A P of ≤0.05 was considered significant. Statistics were calculated using SPSS version 13 for Windows (SPSS, Chicago, IL).
RESULTS
A total of 105 patient-specific episodes of Candida bloodstream infection occurred within the study period. Forty-one episodes were excluded from this analysis for the following reasons: patient age of <18 years (7 episodes), bloodstream infection with multiple organisms (11 episodes), blood drawn exclusively through a catheter lumen (14 episodes), and TTP data not recorded (11 episodes). The remaining 64 episodes (45 from center A and 19 from center B) comprised the study cohort.
Demographic and clinical data are summarized in Table 1. Patients from center B were more frequently hospitalized within the previous 90 days compared to patients from center A (62.2% versus 26.3%; P = 0.013); there were no other significant differences between the patient populations for the two participating centers.
TABLE 1.
Demographic and clinical characteristics of study group (64 patients)
| Characteristic | Value (%)a |
|---|---|
| Age, yr [median (interquartile range)] | 70 (52-81) |
| Sex | |
| Male | 28 (43.8) |
| Female | 36 (56.2) |
| Charlson comorbidity index [median (interquartile | |
| range)]b | 3.0 (1-4) |
| Debilitated state | 13 (20.3) |
| Long-term care facility | 6 (9.4) |
| Intravascular catheter | 50 (78.1) |
| Nontunneled | 40 |
| Tunneled | 7 |
| PICCc | 3 |
| Intravenous drug use | 1 (1.6) |
| Solid organ transplantation | 1 (1.6) |
| Stem cell transplantation | 1 (1.6) |
| Hemodialysis | 8 (12.5) |
| Stay in an intensive care unit | 23 (35.9) |
| Neutropenia | 2 (3.1) |
| Parenteral nutrition | 17 (26.6) |
| Anticancer chemotherapy | 6 (9.4) |
| Surgery | 30 (46.9) |
| Abdominal | 18 |
| Other | 12 |
| Burns | 1 (1.6) |
| Corticosteroids | 0 (0) |
Values represent number of patients (percentage of total) unless specified otherwise.
The Charlson comorbidity index represents the number and severity of comorbid conditions (4).
PICC, peripherally inserted central catheter. Comorbid conditions were included if present within 30 days prior to the onset of candidemia.
The most common risk factors for candidemia in this study population were recent surgery (46.9%), parenteral nutrition (26.6%), and stay in the intensive care unit (35.9%). Only two patients had been neutropenic in the 30 days prior to the onset of candidemia. Blood isolates included Candida albicans (31 isolates), Candida glabrata (15 isolates), Candida tropicalis (10 isolates), Candida parapsilosis (6 isolates), and Candida krusei (2 isolates).
Fifty patients (78.1%) had an indwelling CVC at the time of candidemia. These included 40 nontunneled catheters, 7 tunneled catheters, and 3 peripherally inserted central catheters. Thirty-five (70%) catheters were extracted and cultured, and of these, 13 catheter tips were positive for growth of Candida species by the plate roll method. Failure to extract catheters occurred most commonly due to rapid clinical deterioration and death of the patient before blood culture results were received (12 of 15 cases).
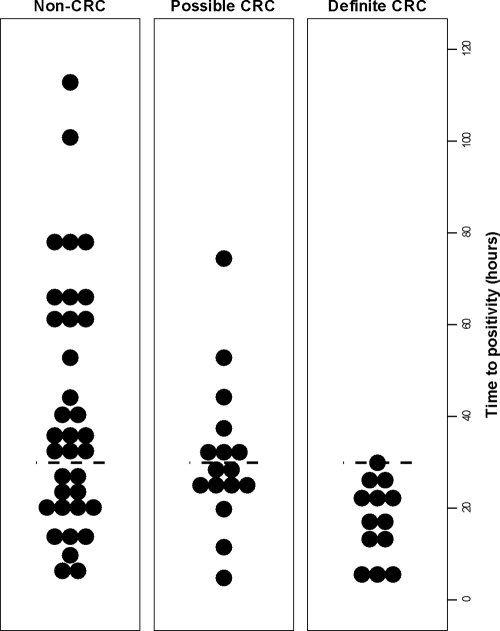
Using the criteria defined above, the 64 study patients were divided into definite CRC (13 patients), possible CRC (16 patients), and non-CRC (35 patients) groups (Table 2). The overall mean TTP was 33.9 ± 3 h (range, 4.1 to 112.8 h). There was no difference in TTP between the two participating centers (33.8 ± 3 h in center A and 34.4 ± 7 h in center B; P = 0.9). TTP was significantly shorter in cultures from the definite CRC group (17.3 ± 2 h; range, 4.1 to 29.9 h) than for cultures from all other candidemia episodes (38.2 ± 3 h; range, 4.7 to 112.8 h; P < 0.001). Cultures from patients with no identifiable source of candidemia exhibited significantly longer TTPs than those for all other episodes of candidemia (45.0 ± 6 h versus 27.8 ± 3 h; P = 0.004). In patients with an indwelling CVC, cultures from the definite CRC group exhibited significantly shorter TTP than cultures from the non-CRC group (17.3 ± 2 h versus 37.7 ± 5 h; P = 0.009). Candida cultures in the possible CRC group exhibited TTP values intermediate between those of patients with definite CRC and non-CRC (Table 2 and Fig. 1). There was no significant difference in the volume of blood inoculated into culture bottles in the three patient groups (P = 0.6), and no significant correlation between TTP and the volume of inoculated blood (P = 0.4).
TABLE 2.
TTP according to the site of Candida infection in 64 patient-specific episodes
| Patient condition and/or diagnosisa | No. of patients (%) | TTP (h) (mean ± SE) | Pb |
|---|---|---|---|
| Patients with indwelling CVC | 50 (78.1) | 30.2 ± 3 | 0.08 |
| Definite CRC | 13 (20.3) | 17.3 ± 2 | 0.009 |
| Possible CRC | 16 (25) | 30.9 ± 4 | |
| Non-CRC | 21 (42) | 37.7 ± 5 | |
| Patients without indwelling CVC | 14 (21.8) | 47.3 ± 9 | 0.08 |
| All non-catheter-associated episodesc | 35 (54.7) | 41.5 ± 5 | 0.003 |
| Peritonitis | 11 (17.2) | 33.3 ± 7 | 0.9 |
| Candidemia without an apparent source | 20 (31.2) | 45 ± 6 | 0.004 |
| Disseminated candidiasis | 2 (3.1) | 29.8 ± 8 | 0.8 |
| Endocarditis | 1 (1.6) | 23 |
See the text for definitions. There were no cases of Candida endophthalmitis, osteomyelitis, arthritis, meninigitis, pneumonia, or empyema.
P values are for the comparison of TTP in each diagnostic category with all other episodes of candidemia (one-way analysis of variance).
Includes patients with and without an indwelling catheter.
FIG. 1.
Time to positivity among patient populations with different likelihoods of catheter-related candidemia. The short horizontal dashed lines mark the 30-h cutoff.
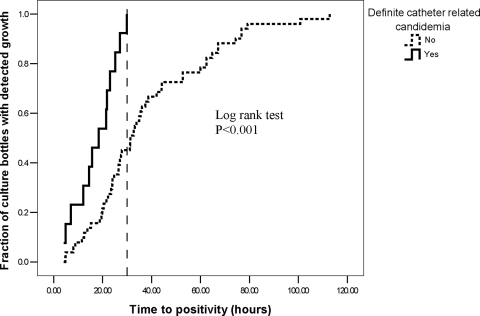
By 30 h from the start of incubation, growth of a Candida species was detected in all 13 episodes of definite CRC, but in only 23 of 51 episodes (45.1%) of candidemia from other sources. Kaplan-Meier analysis revealed significantly earlier detection of Candida growth in patients with definite CRC (log rank test, P < 0.001) (Fig. 2). Therefore, we examined the performance of a TTP cutoff of 30 h for the diagnosis of CRC among a subgroup of 50 patients with indwelling CVCs. In this group, using a TTP of ≤30 h displayed a sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of 100%, 51.4%, 41.9%, and 100%, respectively, for the detection of definite CRC. For the detection of definite and possible CRC combined, the sensitivity and NPV using a TTP of ≤30 h decreased to 75.9% and 63.2%, respectively, but specificity and PPV increased to 57.1% and 71%, respectively (Table 3). Analysis of ROC curves at different TTP cutoff levels confirmed that the highest diagnostic accuracy for definite CRC is obtained at a cutoff level of 30 h (area under the ROC curve of 0.76).
FIG. 2.
Cumulative time to positivity in blood cultures from 50 patients with intravascular catheters. The vertical dashed line marks the 30-h cutoff.
TABLE 3.
Accuracy of a TTP cutoff of 30 h for the diagnosis of CRC in 50 patients with indwelling CVCs
| Patient group | No. of patients with CRC diagnosis with the following TTP cutoff:
|
Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Area under ROC curve | |
|---|---|---|---|---|---|---|---|
| TTP ≤ 30 h | TTP > 30 h | ||||||
| Definite CRC | 13 | 0 | 100 | 51.4 | 41.9 | 100 | 0.76 |
| Possible + non-CRC | 18 | 19 | |||||
| Definite + possible CRC | 22 | 7 | 75.9 | 57.1 | 71.0 | 63.2 | 0.66 |
| Non-CRC | 9 | 12 | |||||
TTP for C. glabrata was significantly longer than for other Candida species (61.3 ± 7 h versus 25.6 ± 2 h; P < 0.001). Of eight episodes of C. glabrata infection that occurred in patients with CVCs, there were none in the definite CRC group, two in the possible CRC group, and six in the non-CRC group. TTP in the definite CRC group remained significantly shorter than for other candidemia episodes in patients with indwelling CVC after the exclusion of episodes involving C. glabrata from the analysis (17.3 ± 2 h versus 29.6 ± 2 h; P = 0.005).
In-hospital mortality was 54.6% (35/64). Death, shock, respiratory failure, and acute renal failure occurred with similar frequency in patients with TTP of ≤30 h and those with TTP of >30 h. There was no significant correlation between the TTP and the duration of candidemia (P = 0.4).
DISCUSSION
The diagnosis of CRC has important implications for patient management. A nonneutropenic patient with candidemia who has an indwelling intravascular catheter with no other apparent source of candidemia will often receive a diagnosis of CRC. This approach may result in the unnecessary removal of indwelling catheters, as well as diverting attention from other sites that may be responsible for candidemia, most importantly the gut.
High-grade candidemia (>5 CFU/ml) detected in peripheral blood has previously been shown to correlate with the presence of an infected intravascular catheter (1). Time to positivity is a readily available marker for high-grade bloodstream infection that bypasses the need for cumbersome quantitative cultures. In patients with S. aureus infection, a short TTP was shown to correlate with high-grade bacteremia as measured by quantitative blood culture methods and an intravascular source of infection (8, 10). To our knowledge, no previous study has examined the utility of TTP in patients with candidemia.
In our cohort of 64 patients, a TTP of less than 30 h was 100% sensitive for definite CRC. Thus, our results demonstrate that a Candida TTP of >30 h strongly suggests a noncatheter source of candidemia. Of 50 patients in our study who had an indwelling CVC, 19 (38%) had a Candida TTP of >30 h, which might have supported a decision not to remove the catheter.
The specificity of a TTP of >30 h for the diagnosis of definite CRC was low (51%), indicating that high-grade candidemia may result from non-catheter-related sources. Of note, since not all catheters in this study were removed and cultured, at least some patients in the possible CRC group may have had infected catheters. A broader definition for CRC, which included both definite and possible cases, resulted in improved positive predictive value (71%) at the cost of reduced sensitivity.
Our patient cohort comprised mainly of intensive care unit patients, many of whom had undergone recent surgery. Only two patients were neutropenic. Therefore, our results should not be generalized to the neutropenic patient population. Further limitations of this study are the small sample size and observational nature, which meant that the volume of blood collected for culture was not controlled. However, we did not find differences in the volume of blood inoculated into bottles from patients with or without CRC, and there was no correlation between TTP and the volume of inoculated blood. Finally, since not all catheters were available for culture, it was necessary to define a group of patients with possible CRC, in whom catheter infection could neither be excluded or confirmed.
In studies of S. aureus bacteremia, a short TTP correlated with an increased risk of death, persistent bacteremia, and metastatic infection. In our study, TTP did not correlate with either mortality, duration of candidemia, or the development of organ failure. These differing results may be attributed to the relatively high prevalence of infective endocarditis in patients with S. aureus bacteremia and a short TTP (8). Infective endocarditis due to Candida species is a much rarer disease and was diagnosed in only one patient in our cohort. In patients with candidemia, a short TTP correlates with the presence of an intravascular catheter, and thus, with an easily removable nidus of infection. Therefore, it is not surprising that a short TTP did not portend a worse prognosis in this cohort of patients with candidemia.
The time to detection of C. glabrata was significantly longer than for other Candida species. A similar observation has been reported by Horvath et al. in in vitro experiments of simulated candidemia (6). Since there were no cases of CRC caused by C. glabrata in our study, the observed differences in TTP between CRC and non-CRC may be attributed at least in part to the causative species. However, since only 8 out of 50 patients (16%) with CVCs had C. glabrata infection, it is unlikely that the species alone accounted for the differences in TTP. Indeed, the TTP of patients with definite CRC remained significantly shorter than that of other patients with indwelling CVCs, even after the exclusion of C. glabrata cases from the analysis. Nonetheless, a TTP cutoff of 30 h may not apply to C. glabrata, and additional study is warranted to define appropriate TTP standards for this species.
In conclusion, our results suggest that the TTP may be a useful tool in the evaluation of patients with candidemia who have an indwelling CVC, and in selected cases, it may support a decision to retain the catheter. Further study is required to assess the significance of Candida TTP in different patient populations, such as neutropenic patients. In addition, the utility of differential TTP, which compares the time to detection of Candida in catheter lumen blood and in peripheral blood, should be evaluated in patients with candidemia.
Acknowledgments
This study was supported in part by MSD Israel Ltd. All authors had no conflict of interest.
Footnotes
Published ahead of print on 14 May 2008.
REFERENCES
- 1.Bille, J., R. S. Edson, and G. D. Roberts. 1984. Clinical evaluation of the lysis-centrifugation blood culture system for the detection of fungemia and comparison with a conventional biphasic broth blood culture system. J. Clin. Microbiol. 19126-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blot, F., G. Nitenberg, E. Chachaty, B. Raynard, N. Germann, S. Antoun, A. Laplanche, C. Brun-Buisson, and C. Tancrede. 1999. Diagnosis of catheter-related bacteraemia: a prospective comparison of the time to positivity of hub-blood versus peripheral-blood cultures. Lancet 3541071-1077. [DOI] [PubMed] [Google Scholar]
- 3.Blumberg, H. M., W. R. Jarvis, J. M. Soucie, J. E. Edwards, J. E. Patterson, M. A. Pfaller, M. S. Rangel-Frausto, M. G. Rinaldi, L. Saiman, R. T. Wiblin, R. P. Wenzel, and the NEMIS Study Group. 2001. Risk factors for candidal bloodstream infections in surgical intensive care unit patients: the NEMIS prospective multicenter study. Clin. Infect. Dis. 33177-186. [DOI] [PubMed] [Google Scholar]
- 4.Charlson, M. E., P. Pompei, K. L. Ales, and C. R. MacKenzie. 1987. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 40373-383. [DOI] [PubMed] [Google Scholar]
- 5.Gudlaugsson, O., S. Gillespie, K. Lee, B. J. Vande, J. Hu, S. Messer, L. Herwaldt, M. Pfaller, and D. Diekema. 2003. Attributable mortality of nosocomial candidemia, revisited. Clin. Infect. Dis. 371172-1177. [DOI] [PubMed] [Google Scholar]
- 6.Horvath, L. L., D. R. Hospenthal, C. K. Murray, and D. P. Dooley. 2003. Detection of simulated candidemia by the BACTEC 9240 system with Plus Aerobic/F and Anaerobic/F blood culture bottles. J. Clin. Microbiol. 414714-4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jarvis, W. R. 1995. Epidemiology of nosocomial fungal infections, with emphasis on Candida species. Clin. Infect. Dis. 201526-1530. [DOI] [PubMed] [Google Scholar]
- 8.Khatib, R., K. Riederer, S. Saeed, L. B. Johnson, M. G. Fakih, M. Sharma, M. S. Tabriz, and A. Khosrovaneh. 2005. Time to positivity in Staphylococcus aureus bacteremia: possible correlation with the source and outcome of infection. Clin. Infect. Dis. 41594-598. [DOI] [PubMed] [Google Scholar]
- 9.Maki, D. G., C. E. Weise, and H. W. Sarafin. 1977. A semiquantitative culture method for identifying intravenous-catheter-related infection. N. Engl. J. Med. 2961305-1309. [DOI] [PubMed] [Google Scholar]
- 10.Marra, A. R., M. B. Edmond, B. A. Forbes, R. P. Wenzel, and G. M. Bearman. 2006. Time to blood culture positivity as a predictor of clinical outcome of Staphylococcus aureus bloodstream infection. J. Clin. Microbiol. 441342-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reference deleted.
- 12.Mermel, L. A., B. M. Farr, R. J. Sherertz, I. I. Raad, N. O'Grady, J. S. Harris, and D. E. Craven. 2001. Guidelines for the management of intravascular catheter-related infections. Clin. Infect. Dis. 321249-1272. [DOI] [PubMed] [Google Scholar]
- 13.Pappas, P. G., J. H. Rex, J. D. Sobel, S. G. Filler, W. E. Dismukes, T. J. Walsh, and J. E. Edwards. 2004. Guidelines for treatment of candidiasis. Clin. Infect. Dis. 38161-189. [DOI] [PubMed] [Google Scholar]
- 14.Raucher, H. S., A. C. Hyatt, A. Barzilai, M. B. Harris, M. A. Weiner, N. S. LeLeiko, and D. S. Hodes. 1984. Quantitative blood cultures in the evaluation of septicemia in children with Broviac catheters. J. Pediatr. 10429-33. [DOI] [PubMed] [Google Scholar]
- 15.Richards, M. J., J. R. Edwards, D. H. Culver, and R. P. Gaynes. 2000. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect. Control Hosp. Epidemiol. 21510-515. [DOI] [PubMed] [Google Scholar]
- 16.Trudnowski, R. J., and R. C. Rico. 1974. Specific gravity of blood and plasma at 4 and 37°C. Clin. Chem. 20615-616. [PubMed] [Google Scholar]
- 17.Wing, E. J., C. W. Norden, R. K. Shadduck, and A. Winkelstein. 1979. Use of quantitative bacteriologic techniques to diagnose catheter-related sepsis. Arch. Intern. Med. 139482-483. [PubMed] [Google Scholar]
- 18.Wisplinghoff, H., T. Bischoff, S. M. Tallent, H. Seifert, R. P. Wenzel, and M. B. Edmond. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39309-317. [DOI] [PubMed] [Google Scholar]
- 19.Zaoutis, T. E., J. Argon, J. Chu, J. A. Berlin, T. J. Walsh, and C. Feudtner. 2005. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin. Infect. Dis. 411232-1239. [DOI] [PubMed] [Google Scholar]