Abstract
Eight genotypes (A to H) and nine subtypes (adw2, adw4, ayw1, ayw2, ayw3, ayw4, adrq+, adrq−, and ayr) of hepatitis B virus (HBV) have been identified worldwide. They appear to be associated with geographical distribution, virological characteristics, and possibly clinical outcomes. We performed sequence analysis of part of the S gene and the entire precore/core gene of HBV isolates obtained from HBsAg-positive blood donors in Papua Province, Indonesia. Phylogenetic analysis of the S gene sequences revealed that 23 (85.2%) of the 27 HBV isolates tested belonged to genotype C (HBV/C) and 2 (7.4%) each to HBV/B and HBV/D. Interestingly, 19 (82.6%) of the 23 isolates of HBV/C clustered in a branch that was distinct from the previously reported subgenotypes C1 to C5 (HBV/C1 to HBV/C5). Similarly, two isolates of HBV/D clustered in a branch distinct from the reported subgenotypes HBV/D1 to HBV/D5. Phylogenetic analysis of the entire precore/core gene confirmed the consistent presence of the distinct branches in HBV/C and HBV/D. We therefore propose novel subgenotypes designated HBV/C6 and HBV/D6. The majority of HBV/C6 isolates in Papua had alanine at positions 159 and 177 (A159/A177) in the HBsAg. A159/A177 is different from the determinants for adrq+ (A159/V177), found throughout Asia, and adrq− (V159/A177), found in New Caledonia and Polynesia, possibly representing a unique antigenic group (provisionally referred to as adrq indeterminate). In conclusion, we have identified two novel HBV subgenotypes, HBV/C6 and HBV/D6, the first of which is the most prevalent subgenotype of HBV in Papua, Indonesia.
It is well known that Indonesia has a moderate to high endemicity of hepatitis B virus (HBV) infection. The carrier rates among voluntary blood donors in 11 large cities in Indonesia were reported to range from 2.1% to 9.5%, with that of Papua Province being 10.5% (12, 27).
At present, eight genotypes of HBV, designated A to H, have been identified worldwide (1, 2, 21, 23, 29). Genotypes B and C (HBV/B and HBV/C, respectively) are predominant in Asia (14, 26, 30). An earlier classification system divided HBV into nine subtypes, namely, adw2, adw4, ayw1, ayw2, ayw3, ayw4, adrq+, adrq−, and ayr (4, 18). HBV isolates of different genotypes and subtypes appear to show different geographical distributions (14, 25), virological characteristics, and, possibly, clinical outcomes (6, 10, 11, 13, 24, 25, 34). They can also provide historical information on the migration pattern of the ancestor of a local population (13, 23) and the possibility of cross-species transmission to or from chimpanzees (32).
Subgenotypes (subgroups) have been identified for certain HBV genotypes (7, 9, 15, 20, 22, 26). HBV/C has been classified into five subgenotypes, HBV/C1 to HBV/C5 (7, 9, 26, 30), as has HBV/D, into HBV/D1 to HBV/D5 (3, 5, 22, 28). Similarly, HBV/B was initially classified into two subgroups, Bj and Ba (31). Subgroup Bj is found mostly in East Asia, including Japan, while subgroup Ba is found throughout Asia and has recombination with HBV/C in the C gene (31). More recently, subgroup Ba has been further divided into four subgenotypes, HBV/B2 to HBV/B5, with subgroup Bj being renamed subgenotype HBV/B1 (20, 26, 30).
An epidemiological study on HBV genotypes and subtypes in Papua, Indonesia, was conducted more than a decade ago with a limited number of samples (19, 27). The objective of this study was to determine the distribution of HBV genotypes, subgenotypes, and subtypes among HBsAg-positive blood donors in Jayapura, Papua, Indonesia.
MATERIALS AND METHODS
Serum samples.
A total of 587 serum samples were taken from blood donors who visited the Indonesian Red Cross Blood Center, Jayapura, Papua, Indonesia, during the 4 months from September 2004 to January 2005. All subjects signed an informed consent form and participated voluntarily in this study. The sera were screened for HBsAg by use of an immunochromatography method (entebe HBsAg strip; Hepatika Laboratory, Mataram, Indonesia). Twenty-seven (4.6%) of the 587 sera tested positive for HBsAg and were subsequently analyzed for HBV genotypes and subtypes as described below. The sera were also tested for the HBV viral load by use of a commercially available kit (Cobas Amplicor HBV monitor test; Roche Diagnostic). The study protocol was reviewed and approved by the Ethics Committee at Jayapura General Hospital.
DNA extraction and PCR amplification.
DNA extraction and amplification were done as described previously (17). In brief, HBV DNA was extracted from 60 μl of serum samples by use of DNAzol reagent (Invitrogen). Part of the viral S gene (nucleotides [nt] 256 to 796) was amplified by PCR with primers P7 and P8 (Table 1) as reported previously (17). When the PCR amplification was negative, a second-round (nested) PCR was carried out using primers HBS1 and HBS2. The sequence of this limited region has been shown to be sufficient for genotype analysis (17, 33). Both first-round and second-round PCRs were performed for 40 cycles, each consisting of 1 min at 94°C, 1 min at 55°C, and 2 min at 72°C. The entire precore and core regions of the viral genome were also amplified by PCR using primers HBC1 and HBC2 under the same condition. When the first-round PCR was negative, a second-round PCR was performed using primers HBC3 and HBC4 under the same condition. Amplification products were visualized on a 2% agarose gel stained with ethidium bromide.
TABLE 1.
Oligonucleotide primers used for PCR amplification of the HBV genome
| Primer | Gene (position) | Polarity | Sequencea |
|---|---|---|---|
| P7 | S (256 to 278) | Sense | 5′-GTGGTGGACTTCTCTCAATTTTC-3′ |
| P8 | S (796 to 776) | Antisense | 5′-CGGTAWAAAGGGACTCAMGAT-3′ |
| HBS1 | S (455 to 474) | Sense | 5′-CAAGGTATGTTGCCCGTTTG-3′ |
| HBS2 | S (713 to 694) | Antisense | 5′-AAAGCCCTGCGAACCACTGA-3′ |
| HBC1 | C (1650 to 1669) | Sense | 5′-TTACATAAGAGGACTCTTGG-3′ |
| HBC2 | C (2494 to 2475) | Antisense | 5′-TAAAGCCCAGTAAAGTTTCC-3′ |
| HBC3 | C (1744 to 1761) | Sense | 5′-GGGAGGAGATTAGGTTAA-3′ |
| HBC4 | S (2476 to 2457) | Antisense | 5′-CCCACCTTATGAGTCCAAGG-3′ |
M = A or C; W = A or T.
Analysis of HBV genotypes, subgenotypes, and subtypes.
Nucleotide sequences of the amplified fragments were determined with the BigDye deoxy Terminator v1.1 cycle sequencing kit (Applied Biosystems) and an ABI Prism 310 genetic analyzer (Perkin Elmer) as described previously (8, 16, 17). The sequences were compared to those from the international DNA data bank (DDBJ/EMBL/GenBank). HBV genotypes were determined based on the homology (>96%) in the S gene (1, 18) by use of the computer software Genetyx-Win v7.0 (Genetyx Corporation, Tokyo, Japan). Phylogenetic trees were constructed by means of unweighted-pair group method using arithmetic averages (UPGMA). Subgenotypes were assigned as described previously (3, 7, 9, 14, 20, 22, 26, 28, 30). HBV subtypes were deduced on the basis of the predicted amino acid sequences of HBsAg (13, 14, 21, 22).
Nucleotide sequence accession numbers.
The sequence data reported in this paper have been deposited in the DDBJ/EMBL/GenBank nucleotide sequence databases with the accession numbers AB334299 through AB334303, AB354882 through AB354908, and AB355452 through AB355456.
RESULTS
HBV genotypes and subgenotypes in Papua, Indonesia, and identification of novel subgenotypes of HBV/C and HBV/D.
A total of 27 serum samples collected from HBsAg-positive blood donors (26 male and 1 female; mean age, 28.5 years; range, 18 to 46 years) were analyzed. Twenty-three (85.2%) subjects were of Papuan ethnic origin. HBV DNA of the S gene was detected in 17 (63.0%) samples after the first-round PCR and in 10 samples (37.0%) after the second-round PCR. Of the 17 samples that yielded amplified products after the first-round PCR, 15 sera (88%) had viral loads of >2 × 105 copies/ml, and the remaining 2 sera (12%) had 6.5 × 104 and <3.2 × 102 copies/ml. On the other hand, seven (87.5%) of the eight samples that yielded amplified products only after the second-round PCR had viral loads of 7 × 103 copies/ml or lower, while the remaining one (12.5%) had a high viral load. As shown in Table 2 and Fig. 1, 23 (85.2%) of the 27 isolates belong to HBV/C and 2 (7.4%) each to HBV/B and HBV/D.
TABLE 2.
HBV genotype and subtype distribution patterns in Papua, Indonesia
| Genotypea | Subtypea | No. of cases (%)
|
||
|---|---|---|---|---|
| Papuan | Non-Papuan | Total | ||
| HBV/B | adw2 | 1 | 1 | 2 (7.4) |
| HBV/Cb | adrc | 22 | 1 | 23 (85.2) |
| HBV/Dd | ayw2 | 0 | 2 | 2 (7.4) |
| Total | 23 (85.2) | 4 (14.8) | 27 (100) | |
Based on partial S gene analysis.
Subgenotypes HBV/C6 (19 isolates) and HBV/C2 (2 isolates) and undetermined ones (2 isolates) are included.
adrq+ (10 isolates) and adrq-indeterminate (13 isolates) are included.
HBV/D6 (two isolates).
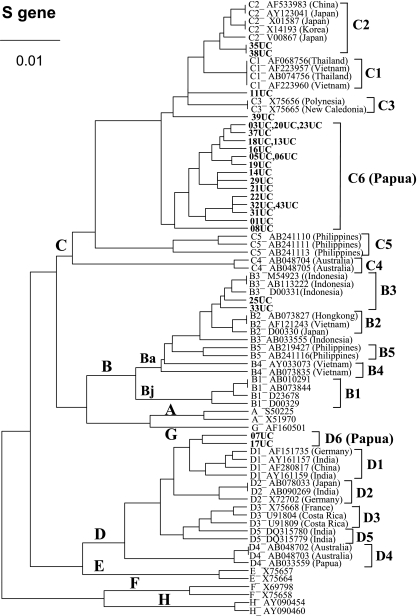
FIG. 1.
UPGMA phylogenetic analysis of HBV isolates from Papua, Indonesia (code UC; shown in bold), and those from the international DNA data bank (indicated with the accession numbers and countries of origin) on the basis of partial S gene sequences (nt 500 to 703). The genotypes and subgenogroups (Bj and Ba) are indicated on the branches, and the subgenotypes are on the right.
Phylogenetic analysis of part of the S region (nt 500 to 703) of the HBV genome revealed that 19 (82.6%) of the 23 isolates of HBV/C obtained in Papua formed a single cluster which is distinct from subgenotypes C1 (Vietnam/Myanmar/Thailand cluster), C2 (Japan/Korea/China), C3 (New Caledonia/Polynesia), C4 (Australia), and C5 (the Philippines) (Fig. 1). This result suggested the possible presence of a novel subgenotype, HBV/C6. Consistently, phylogenetic analysis of the entire precore/core gene (nt 1814 to 2452) also classified the unique Papuan isolates into a cluster that is distinct from HBV/C1 through HBV/C5 (Fig. 2). We therefore propose a novel subgenotype, HBV/C6, which is the most prevalent HBV subgenotype in Papua, Indonesia.
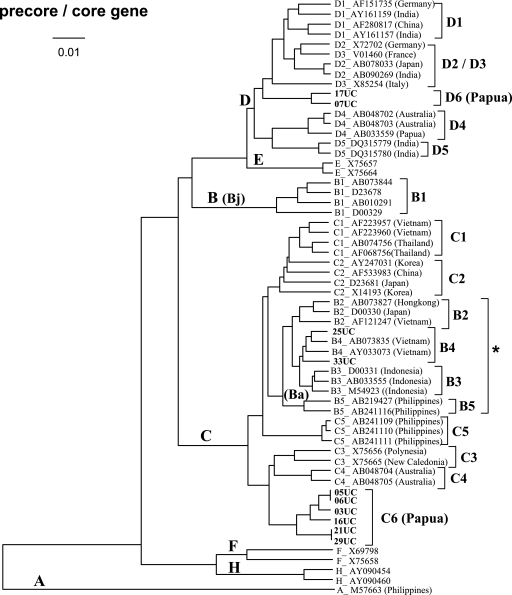
FIG. 2.
UPGMA phylogenetic analysis of HBV isolates from Papua, Indonesia (code UC; shown in bold), and those from the international DNA data bank (indicated with the accession numbers and countries of origin) on the basis of precore/core gene sequences (nt 1814 to 2452). Only 10 isolates from Papua that could be amplified by first-round PCR have been included in this analysis. HBV/G has been excluded to illustrate a more detailed phylogenetic relationship among the other genotypes and subgenotypes, as the precore/core gene of HBV/G is 36 nt longer than that of the other genotypes except for HBV/A. The genotypes and subgenogroups (Bj and Ba) are indicated on the branches, and the subgenotypes are on the right. *, HBV/B2 to HBV/B5 (Ba subgenogroups) cluster in the same branch as HBV/C due to the genetic recombination with HBV/C over the precore/core gene (31).
Two isolates of HBV/C (35UC and 38UC) were classified into subgenotype C2, and the remaining two (11UC and 39UC) were not definitely classifiable on the basis of partial S gene sequencing alone.
Apart from the new subgenotype of HBV/C, we found unique HBV/D isolates (07UC and 17UC), which formed a cluster distinct from the five subgenotypes so far reported, D1 to D5, on the basis of partial S (Fig. 1) and the entire precore/core region (Fig. 2) sequences. Therefore, we provisionally propose another novel subgenotype, HBV/D6, which is circulating in Papua, Indonesia, though less prevalently than HBV/C6.
Two HBV/B isolates obtained in this study (25UC and 33UC) were shown to belong to subgroup Ba, which consists of HBV/B2 through HBV/B5 on the basis of the partial S and the entire precore/core region sequences (Fig. 1 and 2). However, the subgenotype classification of those isolates is still ambiguous, as they appear to belong to HBV/B3 on the basis of the partial S gene sequences but are closer to HBV/B4 than HBV/B3 on the basis of the entire precore/core region sequences. Whether they belong to HBV/B3 or B4 or whether they represent a novel subgenotype of HBV/B awaits further analysis, including sequence determination of the entire virus genome.
HBV subtypes in Papua, Indonesia.
Figure 3 depicts the multiple alignment of amino acid sequences at positions 116 to 183 (nt 500 to 703) in HBsAg of the HBV isolates analyzed in this study and the 36 reference sequences of the eight HBV genotypes (A to H) obtained from the international DNA data bank. Based on the amino acid substitutions at positions 122, 127, 134, and 160, it was found that the subtype collectively called “adr” (adrq+ and adrq indeterminate found in this study [see below]) was predominant (85.2%), followed by adw2 (7.4%) and ayw2 (7.4%) in Papua, Indonesia (Table 2). The isolates of adw2 belong to HBV/B (either subgenotype B3 or subgenotype B4) and that of ayw2 to the novel subgenotype HBV/D6.
FIG. 3.
Multiple alignment of amino acid sequences of HBsAg (positions 116 to 183) of HBV isolates from Papua, Indonesia (code UC; shown in bold) and those from the international DNA data bank (indicated with the accession numbers and countries of origin). Genotypes, subgenotypes, and subtypes are also indicated. *, residues that determine subtypes (13, 14, 21, 22).
Of the 23 isolates of HBV/C, 10 isolates (43.5%) (35UC, 38UC, 11UC, 39UC, 08UC, 22UC, 01UC, 31UC, 32UC, and 43UC) had alanine at position 159 (A159) and valine at position 177 (V177) in HBsAg (Fig. 3). This combination (A159/V177) is considered to be important for the expression of the q determinant (21, 22). Therefore, those HBV isolates (two isolates of HBV/C2, one isolate of undetermined subgenotype [Fig. 1], and seven isolates of HBV/C6) were classified into subtype adrq+. On the other hand, subtype adrq−, which has a different combination (V159/A177) and is found mostly in Polynesia and New Caledonia (4, 18, 22), was not found in Papua, Indonesia. Interestingly, the remaining 13 (56.5%) of the HBV/C isolates in Papua (14UC, 19UC, 21UC, 29UC, 03UC, 05UC, 06UC, 13UC, 16UC, 18UC, 20UC, 23UC, and 37UC) had another combination (A159/A177) that is different from what is seen for both adrq+ and adrq− (Fig. 3). Therefore, we provisionally refer to those isolates as adrq indeterminate. All isolates of adrq indeterminate were shown to belong to the novel subgenotype HBV/C6 (Fig. 1 and 2).
DISCUSSION
The present study demonstrates that HBV/C (85.2%) is the most predominant HBV genotype, followed by HBV/B (7.4%) and HBV/D (7.4%), among HBsAg-positive blood donors in Jayapura, Papua, Indonesia (Table 2). This result is consistent with a previous observation that all HBV isolates from blood donors in the same area belonged to HBV/C (27). Our result also demonstrates that while people of Papuan ethnic origin are infected preferentially with HBV/C, those of non-Papuan ethnic origin in Papua appear to be infected almost equally with HBV/B, HBV/C, and HBV/D. In other cities in Indonesia, such as Jakarta, Padang, Manado, Bajawa, and Balikpapan, HBV/B, HBV/C, and HBV/D have been isolated, with HBV/B being the most prevalent (27). We previously reported that all 54 HBV isolates from Surabaya, East Java Province, Indonesia, belonged to HBV/B (17). Thus, it is likely that HBV/C is predominant in the easternmost part of Indonesia, whereas HBV/B is predominant in other parts of Indonesia.
Five subgenotypes of HBV/C (HBV/C1 to HBV/C5) have been reported so far, each of which shows distinct geographical clustering, i.e., C1 (Vietnam/Myanmar/Thailand), C2 (Japan/Korea/China), C3 (New Caledonia/Polynesia), C4 (Australia), and C5 (the Philippines) (9, 22, 26, 30). In this study, we found that the majority of HBV/C isolates in Papua, Indonesia, clustered in a novel phylogenetic branch that is distinct from HBV/C1 to HBV/C5 on the basis of both the partial S and the entire precore/core gene sequences (Fig. 1 and 2). We propose that those isolates represent a new subgenotype, HBV/C6.
In addition to the novel subgenotype HBV/C6, we have identified a possible new HBV/D subgenotype in Papua, Indonesia (Fig. 1 and 2). It was reported that for some HBV strains, analysis of S gene sequences alone might be insufficient to classify HBV/D isolates into subgenotypes (22). In our study, however, the results of both S region and precore/core region sequences were consistent and the result with the precore/core region sequences showed even more distinct divergence from the known subgenotypes HBV/D1 to HBV/D5. Although we need to identify more isolates of close resemblance to draw a definitive conclusion, we provisionally propose the novel subgenotype HBV/D6. A larger scale of surveillance is planned to obtain more isolates of HBV/D6, since HBV/D is not so frequently found in Papua (Table 2). Also, sequence analysis of the entire virus genome of the isolates of HBV/D6 as well as HBV/C6 and the indeterminate ones (11UC and 39UC) is now under way in our laboratory to better understand the phylogenetic positions of those HBV isolates.
Sugauchi et al. (31) classified HBV/B isolates into two groups: Bj (“j” stands for Japan), mostly found in Japan, and Ba (“a” stands for Asia), found throughout Asia. Subgroup Ba has undergone genetic recombination with HBV/C over the precore/core gene of the HBV genome. We observed in this study that HBV/B isolates in Papua, Indonesia, have undergone the same genetic recombination to be classified as subgroup Ba. Subgroup Ba can be further divided into a number of subgenotypes, i.e., HBV/B2 to HBV/B5 (20, 22, 26). It should also be noted, however, that the HBV/B2-to-HBV/B5 subgenotype classification may not be well applicable for some HBV strains on the basis of the S region sequence alone (20, 22). In fact, our two samples from Papua (25UC and 33UC) were classified as HBV/B3 on the basis of partial S gene sequences (Fig. 1) but as HBV/B4 on the basis of the precore/core gene sequences (Fig. 2). The entire genome sequence analysis is needed to determine whether those isolates belong to either HBV/B3 or HBV/B4 or whether they represent a novel subgenotype of HBV/B.
This study also demonstrated that the majority of HBV isolates in blood donors in Papua, Indonesia, belonged to the subtype collectively called “adr” (adrq+ and adrq indeterminate) (85.2%), while the remaining isolates belonged to adw2 (7.4%) and ayw2 (7.4%) (Table 2). This result is consistent with the previous observations that “adr” was predominant in Papua, Indonesia (19, 27). On the other hand, it was reported that subtype “adw” (adw2 and adw4) was most prevalent in Sumatra, Java, the southern part of Kalimantan, Bali, Lombok, Ternate, and Morotai (17, 19) and that subtype “ayw” (ayw1 to ayw4) was most prevalent in the eastern part of Nusa Tenggara and Moluccas (19). Subtype ayr was not found in this study and was rarely found, if at all, in Indonesia as a whole (19) or in other countries except Vietnam (14). Thus, this study has confirmed that, unlike the other regions of Indonesia, Papua is within the “adr” zone.
HBV genotypes and subtypes have been used as an instrument to identify migration patterns of the ancestors of local populations in certain geographical areas. Mulyanto et al. (19) speculated that the ancestors of the inhabitants of the easternmost part of Indonesia, like Papua, came most likely from Melanesia and Polynesia, where HBV subtype “adr” was predominant. In Papua, however, C/adrq−, which is the most typical genotype and subtype for Melanesia and Polynesia, was not found; instead, C/adrq+ and C/adr indeterminate were prevalent (Fig. 3). Phylogenetically, most of those HBV isolates belong to the novel subgenotype HBV/C6 (Fig. 1 and 2). These results suggest the possibility of unique HBV evolution in Papua during the long history of viral persistence and transmission through many generations.
In conclusion, we have identified novel HBV subgenotypes, HBV/C6 and HBV/D6, in Papua, Indonesia. We have also confirmed that Papua belongs to the C/adr zone on the basis of HBV genotype and subtype classification systems. However, the identification of the novel subgenotypes (HBV/C6 and HBV/D6) and the C/adrq-indeterminate isolates suggests the possibility that HBV has undergone unique evolution in Papua, Indonesia, compared to that in Melanesia and Polynesia and other parts of the world.
Acknowledgments
We are grateful to N. M. Mertaniasih for her constant support throughout this study; to R. H. Hutabarat, D. E. Rangan, B. Sandjaja, and Y. A. Porotuo for their cooperation; and to K. Poedjiati and M. Amin for their technical assistance.
This study was supported in part by the government of the Province of Papua and University of Cendrawasih School of Medicine, Papua, Indonesia, and also by the Program of Founding Research Centers for Emerging and Reemerging Infectious Diseases, the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
Footnotes
Published ahead of print on 7 May 2008.
REFERENCES
- 1.Arauz-Ruiz, P., H. Norder, K. A. Visoná, and L. O. Magnius. 1997. Molecular epidemiology of hepatitis B virus in Central America reflected in the genetic variability of the small S gene. J. Infect. Dis. 176851-858. [DOI] [PubMed] [Google Scholar]
- 2.Arauz-Ruiz, P., H. Norder, B. H. Robertson, and L. O. Magnius. 2002. Genotype H: a new Amerindian genotype of hepatitis B virus revealed in Central America. J. Gen. Virol. 832059-2073. [DOI] [PubMed] [Google Scholar]
- 3.Banerjee, A., F. Kurbanov, D. Sibnarayan, P. K. Chandra, Y. Tanaka, M. Mizokami, and R. Chakravarty. 2006. Phylogenetic relatedness and genetic diversity of hepatitis B virus isolates in Eastern India. J. Med. Virol. 781164-1174. [DOI] [PubMed] [Google Scholar]
- 4.Blitz, L., F. H. Pujol, P. D. Swenson, L. Porto, R. Atencio, M. Araujo, L. Costa, D. C. Monsalve, J. R. Torres, H. A. Fields, S. Lambert, C. Van Geyt, H. Norder, L. O. Magnius, J. M. Echevarría, and L. Stuyver. 1998. Antigenic diversity of hepatitis B virus strains of genotype F in Amerindians and other population groups from Venezuela. J. Clin. Microbiol. 36648-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bozdayi, G., A. R. Türkyilmaz, R. Idilman, E. Karatayli, S. Rota, C. Yurdaydin, and A. M. Bozdayi. 2005. Complete genome sequence and phylogenetic analysis of hepatitis B virus isolated from Turkish patients with chronic HBV infection. J. Med. Virol. 76476-481. [DOI] [PubMed] [Google Scholar]
- 6.Chan, H. L. Y., M. L. Wong, A. Y. Hui, L. C. T. Hung, F. K. L. Chan, and J. J. Y. Sung. 2003. Hepatitis B virus genotype C takes a more aggressive disease course than hepatitis B virus genotype B in hepatitis B e antigen-positive patients. J. Clin. Microbiol. 411277-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan, H. L. Y., S. K. W. Tsui, C. H. Tse, E. Y. T. Ng, T. C. C. Au, L. Yuen, A. Bartholomeusz, K. S. Leung, K. H. Lee, S. Locarnini, and J. J. Y. Sung. 2005. Epidemiological and virological characteristics of 2 subgroups of hepatitis B virus genotype C. J. Infect. Dis. 1912022-2033. [DOI] [PubMed] [Google Scholar]
- 8.Handajani, R., Soetjipto, M. I. Lusida, P. Suryohudoyo, P. Adi, P. B. Setiawan, C. A. Nidom, R. Soemarto, Y. Katayama, M. Fujii, and H. Hotta. 2000. Prevalence of GB virus C/hepatitis G virus infection among various populations in Surabaya, Indonesia, and identification of novel groups of sequence variants. J. Clin. Microbiol. 38662-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huy, T. T.-T., H. Ushijima, V. X. Quang, K. M. Win, P. Luengrojanakul, K. Kikuchi, T. Sata, and K. Abe. 2004. Genotype C of hepatitis B virus can be classified into at least two subgroups. J. Gen. Virol. 85283-292. [DOI] [PubMed] [Google Scholar]
- 10.Kao, J. H., P. J. Chen, M. Y. Lai, and D. S. Chen. 2000. Hepatitis B genotypes correlate with clinical outcomes in patients with chronic hepatitis B. Gastroenterology 118554-559. [DOI] [PubMed] [Google Scholar]
- 11.Kao, J.-H. 2002. Hepatitis B viral genotypes: clinical relevance and molecular characteristics. J. Gastroenterol. Hepatol. 17643-650. [DOI] [PubMed] [Google Scholar]
- 12.Khan, M., J. J. Dong, S. K. Acharya, Y. Dhagwahdorj, Z. Abbas, W. Jafri, D. H. Mulyono, N. Tozun, and S. K. Sarin. 2004. Hepatology issues in Asia: perspective from regional leaders. J. Gastroenterol. Hepatol. 19S419-S430. [Google Scholar]
- 13.Kidd-Ljunggren, K., Y. Miyakawa, and A. H. Kidd. 2002. Genetic variability in hepatitis B viruses. J. Gen. Virol. 831267-1280. [DOI] [PubMed] [Google Scholar]
- 14.Kramvis, A., M. Kew, and G. François. 2005. Hepatitis B virus genotypes. Vaccine 232409-2423. [DOI] [PubMed] [Google Scholar]
- 15.Kurbanov, F., Y. Tanaka, K. Fujiwara, F. Sugauchi, D. Mbanya, L. Zekeng, N. Ndembi, C. Ngansop, L. Kaptue, T. Miura, E. Ido, M. Hayami, H. Ichimura, and M. Mizokami. 2005. A new subtype (subgenotype) Ac (A3) of hepatitis B virus and recombination between genotypes A and E in Cameroon. J. Gen. Virol. 862047-2056. [DOI] [PubMed] [Google Scholar]
- 16.Lusida, M. I., M. Nagano-Fujii, C. A. Nidom, Soetjipto, R. Handajani, T. Fujita, K. Oka, and H. Hotta. 2001. Correlation between mutations in the interferon sensitivity-determining region of NS5A protein and viral load of hepatitis C virus subtypes 1b, 1c, and 2a. J. Clin. Microbiol. 393858-3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lusida, M. I., Surayah, H. Sakugawa, M. Nagano-Fujii, Soetjipto, Mulyanto, R. Handajani, Boediwarsono, P. B. Setiawan, C. A. Nidom, S. Ohgimoto, and H. Hotta. 2003. Genotype and subtype analyses of hepatitis B virus (HBV) and possible co-infection of HBV and hepatitis C virus (HCV) or hepatitis D virus (HDV) in blood donors, patients with chronic liver disease and patients on hemodialysis in Surabaya, Indonesia. Microbiol. Immunol. 47969-975. [DOI] [PubMed] [Google Scholar]
- 18.Magnius, L. O., and H. Norder. 1995. Subtypes, genotypes and molecular epidemiology of the hepatitis B virus as reflected by sequence variability of the S-gene. Intervirology 3824-34. [DOI] [PubMed] [Google Scholar]
- 19.Mulyanto, F. Tsuda, A. T. Karossi, S. Soewignjo, Roestamsjah, D. Sumarsidi, R. H. Trisnamurti, Sumardi, Surayah, L. Z. Udin, Melani-Wikanta, K. Kanai, and S. Mishiro. 1997. Distribution of the hepatitis B surface antigen subtypes in Indonesia: implications for ethnic heterogeneity and infection control measures. Arch. Virol. 1422121-2129. [DOI] [PubMed] [Google Scholar]
- 20.Nagasaki, F., H. Niitsuma, J. G. Cervantes, M. Chiba, S. Hong, T. Ojima, Y. Ueno, E. Bondoc, K. Kobayashi, M. Ishii, and T. Shimosegawa. 2006. Analysis of the entire nucleotide sequence of hepatitis B virus genotype B in the Philippines reveals a new subgenotype of genotype B. J. Gen. Virol. 871175-1180. [DOI] [PubMed] [Google Scholar]
- 21.Norder, H., A.-M. Couroucé, and L. O. Magnius. 1994. Complete genomes, phylogenetic relatedness, and structural proteins of six strains of the hepatitis B virus, four of which represent two new genotypes. Virology 198489-503. [DOI] [PubMed] [Google Scholar]
- 22.Norder, H., A.-M. Couroucé, P. Coursaget, J. M. Echevarria, S.-D. Lee, I. K. Mushahwar, B. H. Robertson, S. Locarnini, and L. O. Magnius. 2004. Genetic diversity of hepatitis B virus strains derived worldwide: genotypes, subgenotypes, and HBsAg subtypes. Intervirology 47289-309. [DOI] [PubMed] [Google Scholar]
- 23.Okamoto, H., F. Tsuda, H. Sakugawa, R. I. Sastrosoewignjo, M. Imai, Y. Miyakawa, and M. Mayumi. 1988. Typing hepatitis B virus by homology in nucleotide sequence: comparison of surface antigen subtypes. J. Gen. Virol. 692575-2583. [DOI] [PubMed] [Google Scholar]
- 24.Orito, E., M. Mizokami, H. Sakugawa, K. Michitaka, K. Ishikawa, T. Ichida, T. Okanoue, H. Yotsuyanagi, and S. Iino for the Japan HBV Genotype Research Group. 2001. A case-control study for clinical and molecular biological differences between hepatitis B viruses of genotypes B and C. Hepatology 33218-223. [DOI] [PubMed] [Google Scholar]
- 25.Orito, E., T. Ichida, H. Sakugawa, M. Sata, N. Horiike, K. Hino, K. Okita, T. Okanoue, S. Iino, E. Tanaka, K. Suzuki, H. Watanabe, S. Hige, and M. Mizokami. 2001. Geographic distribution of hepatitis B virus (HBV) genotype in patients with chronic HBV infection in Japan. Hepatology 34590-594. [DOI] [PubMed] [Google Scholar]
- 26.Sakamoto, T., Y. Tanaka, E. Orito, J. Co, J. Clavio, F. Sugauchi, K. Ito, A. Ozasa, A. Quino, R. Ueda, J. Sollano, and M. Mizokami. 2006. Novel subtypes (subgenotypes) of hepatitis B virus genotypes B and C among chronic liver disease patients in the Philippines. J. Gen. Virol. 871873-1882. [DOI] [PubMed] [Google Scholar]
- 27.Sastrosoewignjo, R. I., B. Sandjaja, and H. Okamoto. 1991. Molecular epidemiology of hepatitis B virus in Indonesia. J. Gastroenterol. Hepatol. 6491-498. [DOI] [PubMed] [Google Scholar]
- 28.Schaefer, S. 2007. Hepatitis B virus taxonomy and hepatitis B virus genotypes. World J. Gastroenterol. 1314-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stuyver, L., S. De Gendt, C. Van Geyt, F. Zoulim, M. Fried, R. F. Schinazi, and R. Rossau. 2000. A new genotype of hepatitis B virus: complete genome and phylogenetic relatedness. J. Gen. Virol. 8167-74. [DOI] [PubMed] [Google Scholar]
- 30.Sugauchi, F., M. Mizokami, E. Orito, T. Ohno, H. Kato, S. Suzuki, Y. Kimura, R. Ueda, L. A. Butterworth, and W. G. E. Cooksley. 2001. A novel variant genotype C of hepatitis B virus identified in isolates from Australian aborigines: complete genome sequence and phylogenetic relatedness. J. Gen. Virol. 82883-892. [DOI] [PubMed] [Google Scholar]
- 31.Sugauchi, F., E. Orito, T. Ichida, H. Kato, H. Sakugawa, S. Kakumu, T. Ishida, A. Chutaputti, C.-L. Lai, R. Ueda, Y. Miyakawa, and M. Mizokami. 2002. Hepatitis B virus of genotype B with or without recombination with genotype C over the precore region plus the core gene. J. Virol. 765985-5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takahashi, K., B. Brotman, S. Usuda, S. Mishiro, and A. M. Prince. 2000. Full-genome sequence analyses of hepatitis B virus (HBV) strains recovered from chimpanzees infected in the wild: implications for an origin of HBV. Virology 26758-64. [DOI] [PubMed] [Google Scholar]
- 33.Telenta, P. F. S., G. P. Poggio, J. L. Lopez, J. Gonzalez, A. Lemberg, and R. H. Campos. 1997. Increased prevalence of genotype F hepatitis B virus isolates in Buenos Aires, Argentina. J. Clin. Microbiol. 351873-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuasa, R., K. Takahashi, B. V. Dien, N. H. Binh, T. Morishita, K. Sato, N. Yamamoto, S. Isomura, K. Yoshioka, T. Ishikawa, S. Mishiro, and S. Kakumu. 2000. Properties of hepatitis B virus genome recovered from Vietnamese patients with fulminant hepatitis in comparison with those of acute hepatitis. J. Med. Virol. 6123-28. [PubMed] [Google Scholar]