Abstract
In patients with Staphylococcus aureus bacteremia, intervention by infectious disease clinical pharmacists on the basis of the results of tests for mecA resulted in a 25.4-h reduction in the time to optimal antimicrobial therapy, from 64.7 ± 36.8 to 39.3 ± 15.5 h (P = 0.002), which may result in decreased mortality.
Although the mecA gene test is the most accurate method for the detection of methicillin-resistant Staphylococcus aureus (MRSA), it is not widely used at hospitals and many clinicians lack knowledge regarding the appropriate and timely application of mecA gene test results for the selection of optimal antimicrobial therapy (OAT) against S. aureus infections. The microbiology laboratory at the University of Michigan Health System routinely performs mecA gene testing by PCR for all staphylococci once daily, 6 days/week; results are generally available within 36 h after growth of the organism in culture. However, an additional 2 to 4 days elapse before the availability of final in vitro susceptibility testing results (i.e., oxacillin susceptibility with cefoxitin as the indicator drug).
We hypothesized that the use of an infectious disease (ID) clinical pharmacist to alert physicians and to provide clinical recommendations on specific antimicrobial therapy at the time of mecA gene test result availability would decrease the time to receipt of OAT. We compared the time to prescription of OAT following positive blood culture results with and without ID clinical pharmacist intervention to evaluate the impact of an ID clinical pharmacist-based mecA test result notification program on the percentage of patients receiving optimal antistaphylococcal therapy compared to the percentage of patients in a historical control group (who received the standard of care) receiving optimal antistaphylococcal therapy.
(This study was presented as a poster at the 45th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, 16 to 19 December 2005.)
The study consisted of two consecutive, prospective study phases. Phase I was a pilot study with concurrent quality assurance surveillance of the antimicrobial agents that patients with S. aureus bacteremia received without ID clinical pharmacist intervention. During phase II, the ID clinical pharmacist was alerted daily by the microbiology laboratory when the results of tests for the mecA gene were available. At that time, patient records were assessed to determine the target patients, defined as those who were not currently receiving OAT. The target patient's physician was contacted by telephone to explain the interpretation of the mecA gene test result and to offer recommendations for therapy. All target patients who met the selection criteria were enrolled in the study. During phase II (but not phase I), all patients received an intervention by an ID clinical pharmacist. Institutional review board approval was obtained for this study.
For the purposes of this investigation, “appropriate antimicrobial therapy” was defined as effective treatment with antimicrobials at the time of identification of microbiologically documented S. aureus bacteremia, and “OAT” was defined as any regimen containing vancomycin for MRSA bacteremia and any regimen containing nafcillin, β-lactam-β-lactamase inhibitor combinations, narrow-spectrum or expanded-spectrum cephalosporins, or vancomycin for methicillin-susceptible S. aureus (MSSA) infection. Vancomycin was not considered optimal therapy for MSSA bacteremia except in patients with true allergic reactions or severe adverse reactions (e.g., neutropenia) to β-lactam antibiotics. Alternatives to vancomycin (e.g., linezolid, quinupristin-dalfopristin, and daptomycin) were considered optimal if the patients had a true vancomycin allergy, were vancomycin intolerant, or had a documented treatment failure after the receipt of vancomycin. Additionally, only nafcillin was considered optimal therapy if the patient had concurrent meningitis or endocarditis due to MSSA.
The primary objective of this study was to evaluate the impact of interventions by ID clinical pharmacists on the adjustment of antimicrobial therapy on the basis of mecA gene test results, and the primary end point was the difference in time (in hours) to the receipt of optimal antistaphylococcal therapy for S. aureus bacteremia between phases I and II. Secondary end points included the time to the receipt of OAT and the rate of application of the mecA gene test results to adjustment of the antimicrobial regimen in the period prior to or with intervention by ID clinical pharmacists.
Statistical analysis was performed with the Statview program (version 5.0.1; SAS Institute Inc., Cary, NC). The t test (or the Mann-Whitney U test for nonparametric data) was used to compare the differences in time to optimal antibiotic therapy prior to and after implementation of pharmacy intervention. The Pearson chi-square test was applied to categorical variables of target groups between the two study phases with two-by-two tables. A P value of less than 0.05 was considered significant.
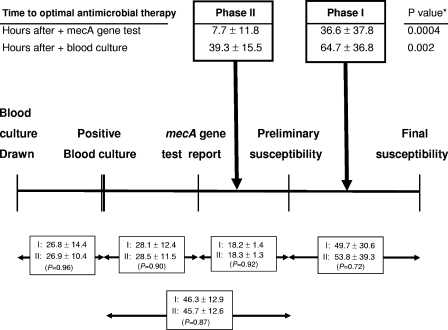
The times to the receipt of OAT after the time of collection of the blood sample for culture in which S. aureus bacteremia was detected were 64.7 ± 36.8 h in phase I and 39.3 ± 15.5 h in phase II (P = 0.002), a 25.4-h reduction in the time to OAT (Table 1; Fig. 1). The sample size of this study was insufficient for evaluation or comparison of infection-related outcomes or mortality, however, and a trend toward a decrease in the duration of S. aureus bacteremia was observed. An additional benefit was seen during the intervention phase after the discontinuation or deescalation of inappropriate therapy, resulting in decreased exposure to inappropriate antimicrobial agents and allowing optimal targeting of the causative pathogen.
TABLE 1.
Demographic characteristics, comorbidities, antimicrobial therapy, and species of staphylococci identified for 46 patients with Staphylococcus aureus bacteremia enrolled in the study
| Characteristic | Phase I (n = 30) | Phase II (n = 16) | P |
|---|---|---|---|
| Demographic characteristics | |||
| Age (yr) | |||
| Mean ± SD | 49.3 ± 20.4 | 40.2 ± 23.8 | 0.20 |
| Range | .25-85 | 0.17-82 | |
| No. (%) of male patients | 20 (66.6) | 8 (50.0) | 0.27 |
| No. (%) of patients with MSSA infection (mecA gene absent) | 24 (80.0) | 15 (93.8) | 0.22 |
| Duration of Staphylococcus aureus bacteremia (days) | 3.2 ± 4.0 | 1.8 ± 1.3 | 0.08 |
| No. (%) of patients seen by ID consult service | 13 (43.3) | 10 (62.5) | 0.24 |
| Empirical antimicrobial therapy (no. [%] of patients) | |||
| Vancomycin | 23 (76.7) | 15 (94.4) | 0.77 |
| β-Lactam-β-lactamase inhibitor | 3 (10.0) | 0 (0.0) | 0.19 |
| Expanded-spectrum cephalosporina | 0 (0.0) | 0 (0.0) | |
| Other | 4 (13.3) | 1 (6.3) | 0.50 |
| Specific antimicrobial therapy (no. [%] of patients) | |||
| Vancomycin | 6 (20.0) | 1 (6.3) | 0.18 |
| Nafcillin | 12 (40.0) | 7 (43.8) | 0.74 |
| β-Lactam-β-lactamase inhibitor | 4 (13.3) | 3 (18.8) | 0.66 |
| Expanded-spectrum cephalosporina | 6 (20.0) | 5 (31.3) | 0.39 |
| Other | 2 (6.7) | 0 (0.0) | 0.29 |
Narrow-spectrum or expanded-spectrum antistaphylococcal cephalosporin.
FIG. 1.
Time frame for the collection of blood samples for culture for the detection of S. aureus bacteremia, the reporting of mecA gene test and susceptibility test results for S. aureus, and the time to receipt of OAT during phases I and II. Data are reported in hours. *, P values for phase I versus phase II.
The timely use of appropriate empirical antimicrobial therapy and specific antimicrobial therapy-based susceptibility testing results are important determinants of clinical outcomes in patients with S. aureus bacteremia. In a retrospective cohort study of 64 patients infected with MSSA and 103 patients infected with MRSA, delayed treatment of hospital-acquired S. aureus bacteremia was associated with a higher rate of infection-related mortality and a longer length of hospital stay (P = 0.05). The breakpoint between “early” and “delayed” treatment was 44.75 h from the time that the first positive blood sample for culture in which S. aureus bacteremia was detected was drawn (2). The strongest clinical evidence to date supporting the concept that vancomycin is inferior to nafcillin for the treatment of MSSA bacteremia is from Chang et al. (1), who found that nafcillin was superior to vancomycin for the prevention of persistent bacteremia or relapse in patients with MSSA bacteremia. More recently, Stryjewski and colleagues (4) noted that hemodialysis-dependent patients treated with cefazolin (rather than vancomycin) for MSSA bacteremia experienced a lower risk of treatment failure.
The mecA PCR test provides a means for the more rapid identification of MRSA compared to the time to detection by traditional susceptibility testing methods and can be used to tailor OAT in a timely fashion. Novel methods for the detection and differentiation of MSSA and MRSA directly from blood culture bottles have recently become available (3). However, the clinician's utilization of this information is vital for tailoring the timely use of OAT. Despite the availability of the mecA test, prescribers failed to utilize the results without pharmacist intervention. Pharmacist intervention on the basis of the results of the mecA gene test resulted in a 25.4-h reduction in the time of receipt of OAT and a trend toward a decrease in the duration of S. aureus bacteremia. These results may result in decreased morbidity and mortality in patients with S. aureus bacteremia; further clinical study is needed in order to assess this possibility.
Acknowledgments
The authors have no funding or conflict of interest information to disclose with respect to this publication.
Footnotes
Published ahead of print on 7 May 2008.
REFERENCES
- 1.Chang, F. Y., B. B. MacDonald, J. E. Peacock, Jr., D. M. Musher, P. Triplett, J. M. Mylotte, A. O'Donnell, M. M. Wagener, and V. L. Yu. 2003. A prospective multicenter study of Staphylococcus aureus bacteremia: incidence of endocarditis, risk factors for mortality, and clinical impact of methicillin resistance. Medicine (Baltimore) 82322-332. [DOI] [PubMed] [Google Scholar]
- 2.Lodise, T. P., P. S. McKinnon, L. Swiderski, and M. J. Rybak. 2003. Outcomes analysis of delayed antibiotic treatment for hospital-acquired Staphylococcus aureus bacteremia. Clin. Infect. Dis. 361418-1423. [DOI] [PubMed] [Google Scholar]
- 3.Huletsky, A., P. Lebel, F. J. Picard, M. Bernier, M. Gagnon, N. Boucher, and M. G. Bergeron. 2005. Identification of methicillin-resistant Staphylococcus aureus carriage in less than 1 hour during a hospital surveillance program. Clin. Infect. Dis. 40976-981. [DOI] [PubMed] [Google Scholar]
- 4.Stryjewski, M. E., L. A. Szczech, D. K. Benjamin, Jr., J. K. Inrig, Z. A. Kanafani, J. J. Engemann, V. H. Chu, M. J. Joyce, L. B. Reller, G. R. Corey, and V. G. Fowler, Jr. 2007. Use of vancomycin or first-generation cephalosporins for the treatment of hemodialysis-dependent patients with methicillin-susceptible Staphylococcus aureus bacteremia. Clin. Infect. Dis. 44190-196. [DOI] [PubMed] [Google Scholar]