Abstract
The genus Aurantimonas, proposed in 2003, encompasses four species from environmental sources, including Aurantimonas altamirensis, isolated from a cave wall in Spain. Here, we report what we believe are the first cases of the recovery of A. altamirensis from human clinical materials.
The genera Aurantimonas and Fulvimarina are two members of the recently proposed family Aurantimonadaceae (8). The genus Aurantimonas currently consists of Aurantimonas coralicida, A. altamirensis, A. ureilytica, and A. frigidaquae, with the type species, A. coralicida, found previously to be the causative agent of the white plague type II on Caribbean scleractinian corals (7). A. altamirensis was isolated in the subterranean environment of the Altamira Cave (Cantabria, Spain) from a white microbial growth on the walls of the cave (9). A. ureilytica was isolated from an air sample collected from the Suwon region of South Korea (13), and A. frigidaquae was recovered from a water-cooling system in the Gwangyang region of South Korea (10).
During 2006 and 2007, strains of A. altamirensis were isolated from three different patients in two Canadian provinces. The first isolate was cultured from a contact lens and the lens cleansing solution from a patient with keratitis (case 1). The second isolate was recovered from a corneal culture from a patient who suffered a penetrating eye injury while sharpening a metal tool. Despite treatment with trifluridine, tobramycin-dexamethasone, and fluconazole eye drops, the infection progressed to form a dendritic corneal ulcer with perforation. The culture of corneal scrapings subsequently revealed Alternaria sp. and a gram-negative bacillus (case 2). The third isolate was recovered from the sputum of a cystic fibrosis patient who had recently received a 2-week course of tobramycin and piperacillin-tazobactam to treat the exacerbation of the respiratory condition by Staphylococcus aureus and Pseudomonas aeruginosa. Upon discharge, a regimen of ciprofloxacin for 3 weeks and daily inhaled colistin was instituted. Surveillance culture 3 weeks later yielded 2+ growth of S. aureus (as assessed using a scoring system in which 0 represents no growth, 1+ represents minimal growth, 2+ represents moderate growth, 3+ represents extensive growth, and 4+ represents maximal growth), as well as one colony of a gram-negative bacillus. The organism was left untreated, and the patient remained well (case 3).
The identification of patient strains at the primary-care level by using commercial identification kits (API 20NE and Vitek [both from bioMérieux, Inc.] and RapID NF [Remel]) gave rise to conflicting results. API 20NE strip identifications included Ochrobactrum anthropi and Sphingomonas paucimobilis, or the test could not provide adequate identification. The RapID NF strip (Remel) showed 99.9% agreement with the identification of Shewanella putrefaciens. The Vitek identification system (bioMérieux, Inc) could not provide adequate identification. Such ambiguities prompted clinicians to forward the isolates to a reference center for further characterization.
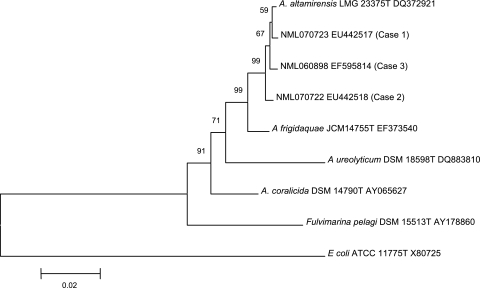
The three strains were identified by nearly full 16S rRNA gene sequencing in the Laboratoire de Santé Publique du Québec, Sainte-Anne-de-Bellevue, and the National Microbiology Laboratory, Winnipeg, Canada (1, 2). The strains were designated NML 070723 (the case 1 isolate), NML 070722 (the case 2 isolate), and NML 060898 (the case 3 isolate). Alignment was done using Clustal X (11), and phylogenetic relationships were inferred using the neighbor-joining method in MEGA 4.0 (12). The strains were found to have >99% identity to one another and 99.4% or greater identity to the A. altamirensis type strain (GenBank accession no. DQ372921) (Fig. 1). Pairwise analysis of 16S rRNA gene sequences from recently described species A. frigidaquae, which is closely related to, but discernible from, A. altamirensis (10), demonstrated 98.5% identity to the clinical isolates as well as the type strain of A. altamirensis (Fig. 1).
FIG. 1.
Phylogenetic relationships inferred using the neighbor-joining method with 16S rRNA gene sequence data for species in the genus Aurantimonas and clinically derived isolates. The scale represents percentages of substitutions. Escherichia coli was used as a distant outlier, and Fulvimarina pegali was used as a family-level outlier.
Conventional phenotypic test methods were carried out at the National Microbiology Library (14), and the results were difficult to relate directly to those used previously to describe environmentally derived Aurantimonas spp. (7, 9, 10). The clinical isolates were strict aerobes, had a creamy yellowish pigment, grew well in air or 5% CO2 at 35°C and in air at 25°C, but showed no growth at 42°C. This observation differed from that for the nonclinical type strain of A. altamirensis, which grows best at 28°C (9). All strains were nonmotile, positive for catalase and oxidase, and metabolically oxidative, with triple sugar iron reaction results for the slant and butt being alkaline and neutral or neutral and neutral, respectively (9). All strains hydrolyzed urea but were not reactive with conventional esculin, citrate, cetrimide, indole, and gelatin. The deamination of phenylalanine to phenylpyruvic acid and growth on MacConkey agar were variable. Tests for nitrate reduction were negative except for NML 060898, which reduced nitrate, but not nitrite, as determined by conventional methods. In tests using oxidation-fermentation medium, glucose and xylose were oxidized. NML 070723 also oxidized mannitol, whereas in tests using oxidation-fermentation medium, lactose, sucrose, maltose, and fructose were not oxidized by any strain. The lack of motility, negativity for indole production, and the inability to hydrolyze esculin were consistent with the identification as A. altamirensis but not A. frigidaquae (10). API CH50 strip (bioMérieux, Inc) reactions differed slightly among the three isolates and were done for comparison with reactions used by Jurado et al. to characterize the type strain of A. altamirensis (9). The reaction results are summarized in Table 1.
TABLE 1.
API CH50 substrates which were reactive with clinically derived Aurantimonas strains and the type strain of A. altamirensisa
| Reaction result | Substrates tested with:
|
|||
|---|---|---|---|---|
| NML 070723 (case 1) | NML 070722 (case 2) | NML 060898 (case 3) | A. altamirensis LMG 23375T | |
| Positive | Galactose, glucose, d-fucose, l-fucose | Arabinose, arabinose, ribose, d-xylose, galactose, glucose, mannose, melibiose, d-fucose | Erythritol, l-arabinose, ribose, d-xylose, galactose, glucose, melibiose, d-fucose | Erythritol, dl-arabinose, ribose, d-xylose, galactose, glucose mannose, melibiose, gentiobiose, rhamnose, fucose |
| Negativeb | Erythritol, d- or l-arabinose, ribose, xylose, mannose, melibiose, gentiobiose, rhamnose | Erythritol, gentiobiose, rhamnose | d-Arabinose, mannose, gentiobiose, rhamnose | |
Data are from this study and reference 9.
The result was negative for the indicated strain, but the substrates listed reacted positively with at least one other strain.
Cellular fatty acid composition analyses were done as described previously (3) with the MIDI Sherlock system (Newark, DE), except that MIDI software version 4.5 was used. Library generation system software (MIDI) was used to compare profiles of the strains after growth on 5% sheep blood agar for 24 h in 5% CO2. Cellular fatty acid composition analyses of the three strains showed significant quantities (given as the average total volumes for the three strains, expressed as percentages rounded to the nearest integer) of C16:0 (8%), C17:1ω8c (2%), C17:1ω6c (2%), C17:0 (3%), C18:1ω7c (71%), C18:0 (2%), and C19:0 cycω8c (6%), with trace to small amounts of C15:0, C18:1ω9c, 2-OH C18:1, 3-OH C18:0, and C20:1ω7c and summed features 2, 3, and 5. These data were deemed to be highly consistent with those for members of the genus Aurantimonas (9, 10, 13).
Antimicrobial susceptibilities were determined by broth microdilution using Sensititre GN2F panels and cation-adjusted Mueller-Hinton broth (Nova Century Scientific Inc., Burlington, ON) according to the instructions of the panel manufacturer (Trek Diagnostics Inc.) and the CLSI guidelines for Pseudomonas aeruginosa and other non-Enterobacteriaceae (6). Antibiotic susceptibilities of the three strains are shown in Table 2.
TABLE 2.
Results of antimicrobial susceptibility testing for A. altamirensisa
| Drug | MICb (μg/ml) (classification) for:
|
||
|---|---|---|---|
| Strain NML 070723 (case 1) | Strain NML 070722 (case 2) | Strain NML 060898 (case 3) | |
| Ampicillin | ≤4 (S) | ≤4 (S) | ≤4 (S) |
| Ampicillin-sulbactam | ≤4/2 (S) | ≤4/2 (S) | ≤4/2 (S) |
| Aztreonam | ≤8 (S) | ≤8 (S) | ≤8 (S) |
| Cefazolin | ≤4 (S) | ≤4 (S) | ≤4 (S) |
| Cefotetan | ≤8 (S) | ≤8 (S) | ≤8 (S) |
| Cefpodoxime | ≤2 (S) | ≤2 (S) | ≤2 (S) |
| Cefoxitin | ≤4 (S) | ≤4 (S) | ≤4 (S) |
| Cefuroxime | ≤4 (S) | ≤4 (S) | ≤4 (S) |
| Ceftriaxone | ≤1 (S) | ≤1 (S) | ≤1 (S) |
| Ceftazidime | ≤1 (S) | ≤1 (S) | ≤1 (S) |
| Cefepime | ≤4 (S) | ≤4 (S) | ≤4 (S) |
| Ticarcillin-clavulanic acid | ≤16/2 (S) | ≤16/2 (S) | ≤16/2 (S) |
| Piperacillin | ≤16 (S) | ≤16 (S) | ≤16 (S) |
| Piperacillin-tazobactam | ≤16/4 (S) | ≤16/4 (S) | ≤16/4 (S) |
| Imipenem | ≤2 (S) | ≤2 (S) | ≤2 (S) |
| Meropenem | ≤1 (S) | ≤1 (S) | ≤1 (S) |
| Amikacin | ≤8 (S) | ≤8 (S) | ≤8 (S) |
| Gentamicin | ≤2 (S) | ≤2 (S) | ≤2 (S) |
| Tobramycin | ≤4 (S) | ≤4 (S) | ≤4 (S) |
| Gatifloxacin | >8 (R) | >8 (R) | 4 (I) |
| Ciprofloxacin | >4 (R) | ≤0.5 (S) | 1 (S) |
| Nitrofurantoin | >128 (R) | >128 (R) | >128 (R) |
| Trimethoprim/sulfamethoxazole | >4/76 (R) | >4/76 (R) | 1/19 (S) |
Testing was done using broth microdilution (6).
S, susceptible; R, resistant; I, intermediate. When two values are given, they correspond respectively to the components in the drug combination.
To our knowledge, this is the first description of strains of the genus Aurantimonas isolated from clinical specimens and possibly associated with human infection. Phenotypic, chemotaxonomic, and genetic data shown here are unambiguously consistent with those described previously for this genus.
Because the recovery of this organism from humans has not been described previously, the clinical contribution to disease appears to be variable. It is possible that the organism was simply a contaminant derived from environmental and/or water sources (e.g., lens cleansing solution, medicated ocular solutions, or nebulized aerosols and related equipment). Contact lens cases can harbor organisms, such as gram-negative bacilli, that are not the causative organism of keratitis (5). Aerosols and nebulizing equipment can also act as reservoirs for microorganisms (4). In case 1, the simultaneous isolation from two related sources, in the absence of other organisms, from a patient with clinical disease is suggestive of an etiological role. In case 2, the concomitant recovery of Alternaria (a known ocular pathogen) from the corneal scrapings obscures the clinical relevance of the isolated Aurantimonas. Case 3 supports the possibility that the organism has no or low-level pathogenicity in certain clinical settings. These three cases illustrate the spectrum of clinical significance of A. altamirensis and provide guidance to clinical microbiologists on its accurate identification. Further studies are required to elucidate its clinical relevance.
This report also highlights the difficulty of correctly identifying this organism by phenotypically based laboratory methods alone. This agent gave rise to ambiguous identifications depending on which rapid identification method was used, and in such situations, microbiologists and clinicians should consider further characterization by 16S rRNA sequencing.
Nucleotide sequence accession numbers.
Sequences obtained for NML 070723 (the case 1 isolate), NML 070722 (the case 2 isolate), and NML 060898 (the case 3 isolate) have been deposited in GenBank under accession no. EU442517, EU442518, and EF595814, respectively.
Acknowledgments
The technical assistance of Cindy Munro and Matthew Walker is gratefully acknowledged.
Footnotes
Published ahead of print on 21 May 2008.
REFERENCES
- 1.Békal, S., C. Gaudreau, R. A. Laurence, E. Simoneau, and L. Raynal. 2006. Streptococcus pseudoporcinus sp. nov., a novel species isolated from the genitourinary tract of women. J. Clin. Microbiol. 442584-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernard, K., L. Shuttleworth, C. Munro, J. C. Forbes-Faulkner, D. Pitt, J. H. Norton, and A. D. Thomas. 2002. Propionibacterium australiense sp. nov., derived from granulomatous bovine lesions. Anaerobe 841-47. [Google Scholar]
- 3.Bernard, K. A., M. Bellefeuille, and E. P. Ewan. 1991. Cellular fatty acid composition as an adjunct to the identification of asporogenous, aerobic gram-positive rods. J. Clin. Microbiol. 2983-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blau, H., H. Mussaffi, M. Mei Zahav, D. Prais, M. Livne, B. M. Czitron, and H. A. Cohen. 2007. Microbial contamination of nebulizers in the home treatment of cystic fibrosis. Child Care Health Dev. 33491-495. [DOI] [PubMed] [Google Scholar]
- 5.Bourcier, T., F. Thomas, V. Borderie, C. Chaumeil, and L. Laroche. 2003. Bacterial keratitis: predisposing factors, clinical and microbiological review of 300 cases. Br. J. Ophthalmol. 87834-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. 2006. M100-S17, Table 2B-1, P. aeruginosa and other non-Enterobacteriaceae performance standards for antimicrobial susceptibility testing; 16th informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA.
- 7.Denner, E. B., G. W. Smith, H. J. Busse, P. Schumann, T. Narzt, S. W. Polson, W. Lubitz, and L. L. Richardson. 2003. Aurantimonas coralicida gen. nov., sp. nov., the causative agent of white plague type II on Caribbean scleractinian corals. Int. J. Syst. Evol. Microbiol. 531115-1122. [DOI] [PubMed] [Google Scholar]
- 8.Garrity, G. M. 2005. Taxonomic outline of the Archaea and Bacteria, p. 210. In G. M. Garrity et al. (ed.), Bergey's manual of systematic bacteriology, 2nd ed. Springer-Verlag, New York, NY.
- 9.Jurado, V., J. M. Gonzalez, L. Laiz, and C. Saiz-Jimenez. 2006. Aurantimonas altamirensis sp. nov., a member of the order Rhizobiales isolated from Altamira Cave. Int. J. Syst. Evol. Microbiol. 562583-2585. [DOI] [PubMed] [Google Scholar]
- 10.Kim, M. S., K. T. Hoa, K. S. Baik, S. C. Park, and C. N. Seong. 2008. Aurantimonas frigidaquae sp. nov., isolated from a water-cooling system. Int. J. Syst. Evol. Microbiol. 581142-1146. [DOI] [PubMed] [Google Scholar]
- 11.Larkin, M. A., G. Blackshields, N. P. Brown, R. Chenna, P. A. McGettigan, H. McWilliam, F. Valentin, I. M. Wallace, A. Wilm, R. Lopez, J. D. Thompson, T. J. Gibson, and D. G. Higgins. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 232947-2948. [DOI] [PubMed] [Google Scholar]
- 12.Tamura, K., J. Dudley, M. Nei, and S. Kumar. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 241596-1599. [DOI] [PubMed] [Google Scholar]
- 13.Weon, H. Y., B. Y. Kim, S. H. Yoo, J. H. Joa, K. H. Lee, Y. S. Zhang, S. W. Kwon, and B. S. Koo. 2007. Aurantimonas ureilytica sp. nov., isolated from an air sample. Int. J. Syst. Evol. Microbiol. 571717-1720. [DOI] [PubMed] [Google Scholar]
- 14.Weyant, R. S., C. W. Moss, R. E. Weaver, D. G. Hollis, J. G. Jordan, E. C. Cook, and M. I. Daneshvar. 1996. Identification of unusual pathogenic gram-negative aerobic and facultatively anaerobic bacteria. Williams & Wilkins, Baltimore, MD.