Abstract
Tuberculosis culture usually requires sputum decontamination and centrifugation to prevent cultures from being overgrown by contaminating bacteria and fungi. However, decontamination destroys many tuberculous bacilli, and centrifugation often is not possible in resource-poor settings. We therefore assessed the performance of Mycobacterium tuberculosis culture with unprocessed samples plated directly by using tuberculosis-selective media and compared this procedure to conventional culture using centrifuge decontamination. Quadruplicate aliquots of strain H37RV were cultured in 7H9 broth with and without selective antimicrobials and after centrifuge decontamination. The subsequent comparison was made with 715 sputum samples. Split paired sputum samples were cultured conventionally with centrifuge decontamination and by direct culture in tuberculosis-selective media containing antibiotics. Centrifuge decontamination reduced tuberculosis H37RV colonies by 78% (P < 0.001), whereas direct culture in tuberculosis-selective media had no inhibitory effect. Similarly, in sputum cultures that were not overgrown by contaminants, conventional culture yielded fewer tuberculosis colonies than direct culture (P < 0.001). However, the sensitivity of conventional culture was greater than that of direct culture, because samples were less affected by contamination. Thus, of the 340 sputum samples that were tuberculosis culture positive, conventional culture detected 97%, whereas direct culture detected 81% (P < 0.001). Conventional and direct cultures both took a median of 8.0 days to diagnose tuberculosis (P = 0.8). In those direct cultures that detected drug resistance or susceptibility, there was a 97% agreement with the results of conventional culture (Kappa agreement statistic, 0.84; P < 0.001). Direct culture is a simple, low-technology, and rapid technique for diagnosing tuberculosis and determining drug susceptibility. Compared to that of conventional culture, direct culture has reduced sensitivity because of bacterial overgrowth, but in basic laboratories this deficit may be outweighed by the ease of use.
Worldwide, the majority of tuberculosis patients are diagnosed by a direct smear examination of sputum (20). However, culture diagnosis is more sensitive and allows for drug susceptibility to be determined simultaneously (12). In resource-poor settings, the success of treatment is threatened by multidrug-resistant tuberculosis (MDRTB) and extensively drug-resistant tuberculosis (7), which highlights the need for a rapid, simple, and cost-effective method of culture and susceptibility testing that can be carried out as close as possible to the point of care (15).
Microscopic observation drug susceptibility (MODS) testing allows both rapid and low-cost tuberculosis diagnosis in liquid culture with the simultaneous determination of drug susceptibilities (3, 12, 13). However, MODS requires sodium hydroxide decontamination and centrifuge concentration prior to culture in order to prevent contamination by bacteria and fungi. Centrifugation is costly and must be performed only in facilities with adequate biosafety (4, 21), while decontamination with sodium hydroxide is time-consuming, uses expensive consumables, and kills many viable tuberculous bacilli (14, 16).
Direct tuberculosis culture with media made selective by the addition of antibiotics has been used to determine the early bactericidal activity of drugs (5, 6, 17-19) and to detect tuberculosis in sputum (16) and extrapulmonary specimens (9-11). In this study, the ability of direct culture to detect tuberculosis and simultaneously determine drug susceptibility was compared to that of conventional decontaminated culture using the MODS technique, first with strain H37RV samples and subsequently in sputum.
MATERIALS AND METHODS
Clinical samples.
In 2006, 715 sputum samples were collected from 485 patients presenting with tuberculosis symptoms to health posts in a Peruvian shantytown. Participants included the symptomatic contacts of tuberculosis patients and new tuberculosis patients who had been diagnosed clinically or by positive sputum smear. New tuberculosis patients had just begun or were about to begin treatment. Human immunodeficiency virus testing was not done, but nationally, 3% of tuberculosis patients and 0.2% of adults are human immunodeficiency virus positive (8).
Preliminary studies.
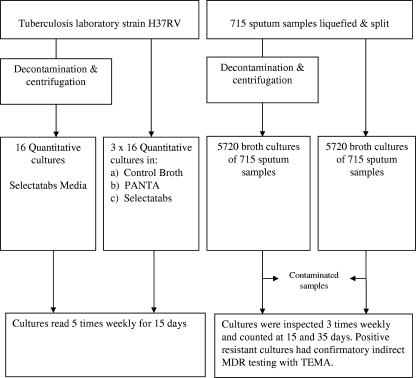
In initial experiments (Fig. 1), the effect of adding antimicrobials to the culture broth and the effect of centrifuge decontamination on growth in culture broth were determined using the H37RV laboratory strain of Mycobacterium tuberculosis.
FIG. 1.
Overview of methodology.
Logarithmic-phase cultures were diluted to a turbidity of 1 MacFarlane (∼3 × 108 CFU/ml). Quadruplet 50-μl aliquots were cultured in 450 μl culture medium under four different conditions: (i) with control medium, which was Middlebrook 7H9 and 12.5% OADC (oleic acid, albumin, dextrose, and catalase; Becton Dickinson); (ii) with control medium and PANTA (a selective medium designed for the inoculation of decontaminated samples, containing 40 U/ml polymyxin, 4 μg/ml amphotericin B, 16 μg/ml nalidixic acid, 4 μg/ml trimethoprim, and 4 μg/ml azlocilin; Becton Dickinson); (iii) with control medium and Selectatab antimicrobials (200 U/ml polymyxin, 0.1 mg/ml ticarcillin, 10 μg/ml amphotericin B, and 10 μg/ml trimethoprim; Mast Diagnostics, Bootle, United Kingdom); and (iv) after centrifuge decontamination in control medium with Selectatab antimicrobials. The samples were cultured undiluted and in three serial 1:20 dilutions. Colonies were counted on day 15.
Sample preparation.
Samples were transported from the health post to the laboratory at ambient temperature. On arrival at the laboratory, they were stored at 4°C until processed. Samples of less than 2 ml in volume were supplemented with phosphate-buffered saline at pH 6.8 (PBS) to 2 ml, and sputum in excess of 2 ml was discarded. All samples were liquefied with 500 μl of 0.5% N-acetyl cysteine and 2.9% sodium citrate and mixed for approximately 1 min. Two aliquots of 50 μl were removed for direct culture, and the remaining 2.4 ml underwent centrifuge decontamination.
Direct-culture technique.
For direct culture, one 50-μl aliquot was inoculated into a detection well of a 24-well tissue culture plate (Becton Dickinson) containing 450 μl of control medium (see above) supplemented with Selectatab antimicrobials. The other 50-μl aliquot was inoculated into an MDRTB testing well, which was identical to the detection well except that it contained 0.2 μg/ml rifampin and 1.0 μg/ml isoniazid in addition. The samples were cultured undiluted and in three serial 1:20 dilutions.
Conventional centrifuge decontamination technique.
The remainder of the sample was decontaminated by being mixed with an equal volume (2.4 ml) of 2% sodium hydroxide. After 20 min, 10 ml PBS was added. This was followed by centrifugation at 2,500 × g for 15 min at 17°C. The pellet was resuspended in PBS to the original volume (2.4 ml), and two aliquots of 50 μl were cultured (as described below).
Conventional culture technique.
The two 50-μl aliquots of decontaminated centrifuged sputum were inoculated into detection wells and MDRTB wells as described for direct culture, except that the broth was supplemented with PANTA instead of Selectatab antimicrobials. The samples were cultured undiluted and in three serial 1:20 dilutions.
Dilutions.
Three serial dilutions were made from both the detection and MDRTB wells for both direct and conventional cultures. After the contents of the inoculated well were mixed, 25 μl was removed and then inoculated into another well containing 475 μl of the same culture medium. Two further serial dilutions of 1:20 were made in the same manner. To minimize the risk of cross-contamination and occupational exposure, immediately after inoculation lids were applied to the culture plates, which were then sealed inside plastic ziplock bags. The bags were sealed for the duration of culture and were transparent, which allowed microscopy to be performed.
Culture-reading methods.
Cultures were incubated at 37°C and read with an inverted light microscope with ×40 magnification, as described previously (3). Positive M. tuberculosis cultures were identified by characteristic cord formation, and nontuberculous mycobacteria were identified by their lack of cording or, for Mycobacterium chelonae, by overgrowth before day 5. A positive sample was defined as one in which M. tuberculosis was visualized in any of the sample dilutions. Cultures were examined for M. tuberculosis growth three times per week from days 5 to 35, and the numbers of CFU were counted on days 15 and 35. Bacterial contamination was easily distinguished from the filamentous hyphae of fungal contamination on the basis of morphology. Contamination by bacteria and fungi could prevent the visualization of tuberculosis in the well. Partial contamination was defined as contamination of any kind that did not obscure the visualization of the well. A negative culture well was defined as one in which neither M. tuberculosis grew nor contamination was seen.
Sputum drug susceptibility testing.
The MDR testing well was read as soon as M. tuberculosis growth was identified in the detection culture well, and MDRTB was defined by the presence of any M. tuberculosis growth in the isoniazid- and rifampin-containing culture. On day 15, all MDR testing wells were read regardless of the level of growth in the detection wells. A non-MDR sample was defined as one in which growth was observed in a detection culture but no growth was observed in the isoniazid- and rifampin-containing well. Confirmatory testing of drug susceptibility with the tetrazolium microplate assay (TEMA) (2) was performed on drug-resistant cultures. Any sample detected as resistant in MDRTB testing that had not recovered tuberculosis in any of the detection wells was considered a new positive sample in this phase of the analysis.
Analysis of combined cultures.
The combined result of all four dilutions was used to compare direct culture to conventional culture. A negative culture was defined as one in which there was no tuberculosis growth and no contamination in the most concentrated dilution, with no tuberculosis growth in more dilute wells. In order to not underestimate the effect of contamination (or overestimate the number of true-negative samples), a contaminated-negative culture was defined as one in which the most concentrated dilution was contaminated and there was no tuberculosis growth in the less concentrated dilution. Consequently, the contaminated-negative samples had at least one negative well, although the most concentrated dilution was contaminated. A positive culture was defined as one in which there was tuberculosis growth in any culture well.
Statistical analysis.
Results were analyzed using STATA 9 software. The data generated with pure laboratory tuberculosis strains were analyzed using an unpaired Student's t test. The median times to culture positivity were analyzed using the Wilcoxon signed rank test. The paired Student's t test was used to compare colony counts in the clinical study. The Z test of proportions was used to compare the differences in sensitivity. All P values were two sided, and P < 0.05 was considered statistically significant.
RESULTS
The 715 sputum samples were provided from 485 patients with a median age of 23 years and a male-to-female ratio of 1.2:1. Patients were treated for a median of 1 day prior to sample collection, and 276 samples were Ziehl-Neelson positive (Table 1).
TABLE 1.
Patient demographics
| Sample source | Age
|
No. (%) femaleb | No. of patients sampled | Mean no. of sputum samples per patient | Median no. of days of tuberculosis treatment prior to sample (IQR) | No. (%) positive by Ziehl-Neelsen sputum smearc | |
|---|---|---|---|---|---|---|---|
| Median, in yr (IQRa) | Range | ||||||
| New tuberculosis patients (n = 542) | 24 (12-36) | 1 mo-85 yr | 235 (43) | 328 | 1.7 | 31 (1-206) | 268 (49) |
| Suspected tuberculosis patients (n = 173) | 20.5 (11.5-30) | 1-77 yr | 91 (53) | 157 | 1.1 | 0 (0-0) | 8 (5) |
| Total or average (n = 715) | 23 (12-34) | 1 mo-85 yr | 326 (46) | 485 | 1.5 | 1 (0-125) | 276 (39) |
IQR, interquartile range.
Patient gender data were not available for seven samples.
Ziehl-Neelsen sputum smear data were unknown for 88 samples.
The effect of centrifuge decontamination on strain H37RV.
The conventional centrifuge decontamination of pure laboratory tuberculosis cultures caused a 78% reduction in the number of M. tuberculosis colonies recovered, as assessed by serial dilutions of strain H37RV (1,320 CFU/μl in control cultures and 320 CFU/μl after decontamination, which is a 0.56 log decrease; P < 0.001). Colony counts did not differ significantly between cultures in control broth with no antimicrobial additives and cultures in broth containing PANTA or Selectatab antimicrobial mixtures (control mixture, 1,060 CFU/μl; PANTA mixture, 2,520 CFU/μl [P = 0.1 compared to control results]; Selectatab mixture, 1,320 CFU/ml [P = 0.2 compared to control results]).
Mycobacterial growth in direct and decontaminated cultures.
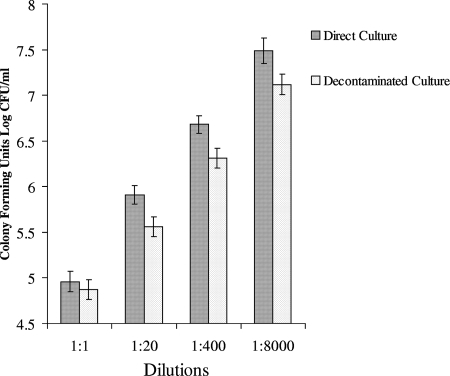
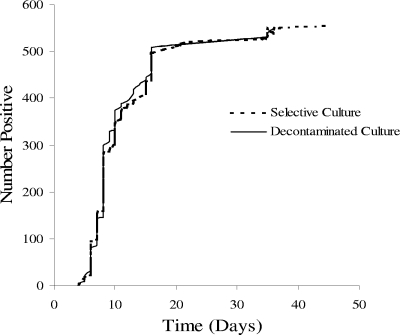
Considering only sputum cultures that were free from bacterial or fungal growth in both direct culture and decontaminated culture, direct cultures yielded higher M. tuberculosis colony counts than decontaminated cultures in all dilutions (P < 0.001 for combined dilutions) (Fig. 2), and direct culture yielded more positive tuberculosis cultures than conventional culture at a 1:8,000 dilution (P < 0.001). Both techniques diagnosed tuberculosis in a median of 8.0 days (P = 0.8) (Fig. 3).
FIG. 2.
Growth of Mycobacterium tuberculosis in direct and decontaminated culture. The comparison was made for those cultures not affected by contamination. The overall difference between the two groups are shown (P < 0.001). The individual P values for the dilutions are P = 0.14 (1:1), P < 0.001 (1:20), P < 0.001 (1:400), and P < 0.001 (1:8,000). Error bars indicate 95% confidence intervals. The numbers of CFU in both culture techniques appear to increase with increasing dilution, but this is simply an artifact caused by false-negative-contaminated cultures that occur more frequently at higher sample concentrations.
FIG. 3.
Number of days from sample processing until the detection of tuberculosis growth is shown for the samples that were positive in both direct culture and decontaminated culture. Five of 715 samples were read after the usual 35 days.
Sensitivity of direct culture and decontaminated culture.
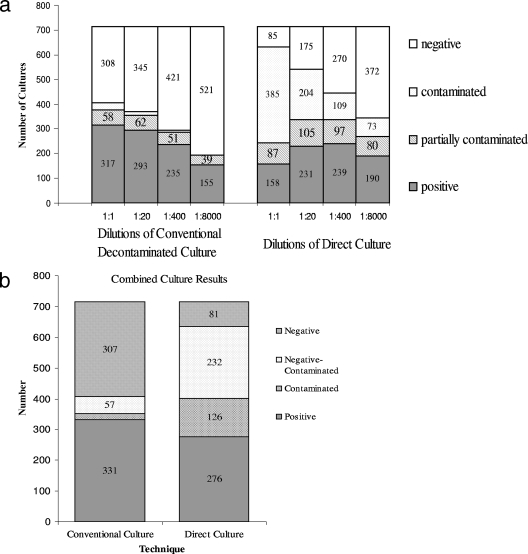
The greater sensitivity of direct culture compared to that of conventional culture for samples that yielded interpretable results by both techniques was outweighed by more frequent direct-culture failure because of bacterial and fungal overgrowth (Fig. 4a). Eighteen percent (126/715) of samples were contaminated (either partially or totally) in all direct-culture dilutions. Thirty-two percent (232/715) of samples were contaminated-negative. The corresponding contamination rates for decontaminated cultures were 3% (20/715) and 8% (57/715), both significantly less than that of direct culture (P < 0.001). Consequently, the diagnostic sensitivity of direct culture was less than that of decontaminated culture (81% versus 97% sensitivity in a comparison of the combined results of all dilutions; P < 0.001) (Fig. 4b and Table 2). In a comparison of the best-performing single culture wells, direct culture was most sensitive when sputum was diluted 1:400, which detected 70% (239/340) of positives; the sensitivity was 93% (316/340) for the best-performing, undiluted decontaminated culture (P < 0.001).
FIG. 4.
(a) Results for each of the eight culture wells are shown for the 715 sputum samples. (b) Results of all culture dilutions combined.
TABLE 2.
Performance of direct culture and decontaminated culture
| Culture type | Median no. of days to positive (P = 0.8) | % of agreement with MDRTB test resultsa (P < 0.001) | % Sensitivity of:
|
% Sensitivity (no. positive/total no.) of the most concentrated culture well if:
|
||
|---|---|---|---|---|---|---|
| All cultures (no. positive/total no.), combined dilutions (P < 0.001) | Most sensitive single dilution (P < 0.001) | All contamination excluded (P = 0.4) | Partial contamination included (P < 0.001) | |||
| Conventional decontaminated (n = 715) | 8.0 | 97 | 97 (331/340) | 93 (undiluted) | 98 (158/162) | 97 (180/185) |
| Direct (n = 715) | 8.0 | 97 | 81 (276/340) | 70 (1:400) | 96 (155/162) | 84 (156/185) |
For each culture type, Kappa is 0.84.
Drug susceptibility testing with direct and decontaminated cultures.
All 715 samples were tested for MDRTB (see Table SA1 in the supplemental material). The additional MDRTB culture wells yielded two positive (resistant) samples that were not detected in any of the detection wells. Four positive samples were detected in direct culture alone, and three positive samples were detected in conventional culture alone. Hence, the total number of samples that recovered M. tuberculosis (resistant or susceptible) by both tests was 342. Direct culture detected 280 (82%) of these samples, and conventional culture detected 334 (98%). For the 272 samples with interpretable MDRTB results (resistant or susceptible) from both direct and decontaminated cultures, there was a 97% agreement in MDRTB detection between the two methods (Kappa agreement statistic, 0.84; P < 0.001) (see Table SA1 in the supplemental material). Of the 86 samples that were determined to be MDRTB in decontaminated culture, 83 strains underwent confirmatory testing with TEMA; 76 (92%) yielded concordant results, 2 samples had TEMA results indicating monoresistance, and 5 samples had TEMA results indicating pansusceptibility. Of the 73 samples that were determined to be MDR by direct culture, 68 samples underwent confirmatory indirect drug susceptibility testing with TEMA, and 65 (96%) yielded concordant results, 2 samples had TEMA results indicating monoresistance, and 1 sample had TEMA results indicating pansusceptibility.
Contamination.
Partial contamination had a significant effect on diagnostic sensitivity (Table 2; also see Table SA2 in the supplemental material). Excluding all contaminated samples, the sensitivity of direct culture matched (96%) that of conventional culture (98%) (P = 0.4); however, when partial contamination alone was included, the sensitivity dropped (84% for direct culture and 97% for conventional culture; P < 0.001). Direct culture was more often contaminated with bacteria than fungi (84 and 21%, respectively, of all contaminated wells [P < 0.001]; 5% were contaminated with both). The ratio of bacterial-to-fungal contamination was similar for undiluted culture and sputum cultured at a 1:8,000 dilution (75 and 82%, respectively; P = 0.2). Diluting sputum samples increased the sensitivity of direct culture by reducing contamination and decreased the sensitivity of decontaminated culture.
Sample storage and transport.
The median time between sample collection and sample storage at 4°C was 2 days, and increased delay was not associated with higher contamination rates (49% for 0 to 3 days and 53% for 4 to 7 days of transport, respectively; P = 0.4). The median time between sample collection and sample processing was 7 days, and an increased delay to processing was not associated with higher contamination rates (53% for 0 to 5 days and 49% for 6 to 10 days, respectively; P = 0.4).
DISCUSSION
This is the first report that compares direct culture to conventional MODS culture, and our adaptation to the rapid and sensitive MODS technique also is novel. In both strain H37Rv and paired sputum cultures not affected by contamination, direct culture grew more colonies than conventional culture. In the most diluted cultures, direct culture also was more likely to detect tuberculosis than decontaminated culture. The serial dilution of sputum samples improved the sensitivity of direct culture and required only minimal equipment. However, contamination was too frequent to be operationally acceptable in most settings. Increased bacterial resistance to antibiotics as a result of widespread medical, veterinary, and farming use may explain the higher rate of contamination in our study compared to those of earlier reports (10, 11, 16). Furthermore, previous studies used agar, which has lower sensitivity for tuberculosis culture than broth but is less affected by bacterial and fungal contamination.
Studies to determine the character of the contaminants and to evaluate the combination of selective media with disinfectants are under way. If bacterial and fungal overgrowth could be efficiently prevented, direct culture would be expected to have a greater diagnostic sensitivity than culture after centrifuge decontamination. The significant effect of partial contamination on tuberculosis growth in liquid culture is important because partial contamination impaired tuberculosis growth and therefore may cause false-negative reporting of culture results. We recommend that negative samples with partial contamination be recultured with more thorough decontamination.
A limitation of the current research was the lack of comparison with the Petroff technique, in which sputum is cultured after decontamination but without centrifugation (22). In this long-established technique that is used in parts of the developing world, sodium hydroxide is added to sputum; after an interval, excess buffer is added and the resultant diluted sample is applied directly to acidified egg-based media. However, the Petroff method has previously been shown to yield fewer CFU than direct culture in selective media (1).
The processing of sputum directly in selective culture media without centrifugation or decontamination is designed to allow reliable drug susceptibility testing in understaffed or poorly equipped laboratories. This study demonstrated that direct culture can rapidly and reliably diagnose tuberculosis and identify MDRTB, and it has the potential for implementation in basic laboratories, with the cost of reduced sensitivity compared to that of conventional centrifuge-decontaminated culture. Ongoing work aims to reduce the contamination of directly cultured sputum by improving the selective media in order to increase the diagnostic sensitivity.
Acknowledgments
We thank the tuberculosis working group at University Cayetano Heredia, Lima, Peru, whose generous collaboration made this work possible, and the patients who kindly agreed to give informed consent to provide sputum samples. We also thank David Moore for his help and support throughout the study.
This research was funded in part by the charity Innovation for Health and Development (IFHAD), Wellcome Trust Clinical Tropical Medicine Research Fellowships, and a grant from the Sir Halley Stewart Trust. R.H.G. is supported by U.S. Agency for International Development awards HRN-5986-A-00-6006-00 and GHS-A-00-03-00019-00 and a Global Research Activity Cooperative Agreement of the NIH/NIAID (T35A107646).
The funding agencies had no involvement in the conduct or publication of this research. The opinions expressed are those of the authors and do not necessarily reflect the views of the sponsors.
Ethical approval for the study was obtained from Imperial College London and Universidad Peruana Cayetano Heredia, Lima, Peru.
Footnotes
Published ahead of print on 30 April 2008.
Supplemental material for this article may be found at http://jcm.asm.org/.
REFERENCES
- 1.Allen, B. W., and D. A. Mitchison. 1992. Counts of viable tubercle bacilli in sputum related to smear and culture gradings. Med. Lab. Sci. 4994-98. [PubMed] [Google Scholar]
- 2.Caviedes, L., J. Delgado, and R. H. Gilman. 2002. Tetrazolium microplate assay as a rapid and inexpensive colorimetric method for determination of antibiotic susceptibility of Mycobacterium tuberculosis. J. Clin. Microbiol. 401873-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caviedes, L., T. S. Lee, R. H. Gilman, P. Sheen, E. Spellman, E. H. Lee, D. E. Berg, S. Montenegro-James, et al. 2000. Rapid, efficient detection and drug susceptibility testing of Mycobacterium tuberculosis in sputum by microscopic observation of broth cultures. J. Clin. Microbiol. 381203-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 1999. Biosafety in microbiological and biomedical laboratories, 4th ed. Centers for Disease Control and Prevention, Atlanta, GA.
- 5.Donald, P. R., F. A. Sirgel, F. J. Botha, H. I. Seifart, D. P. Parkin, M. L. Vandenplas, B. W. Van de Wal, J. S. Maritz, and D. A. Mitchison. 1997. The early bactericidal activity of isoniazid related to its dose size in pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 156895-900. [DOI] [PubMed] [Google Scholar]
- 6.Donald, P. R., F. A. Sirgel, A. Venter, E. Smit, D. P. Parkin, B. W. Van de Wal, and D. A. Mitchison. 2001. The early bactericidal activity of amikacin in pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 5533-538. [PubMed] [Google Scholar]
- 7.Gandhi, N. R., A. Moll, A. W. Sturm, R. Pawinski, T. Govender, U. Lalloo, K. Zeller, J. Andrews, and G. Friedland. 2006. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet 3681575-1580. [DOI] [PubMed] [Google Scholar]
- 8.Kawai, V., G. Soto, R. H. Gilman, C. T. Bautista, L. Caviedes, L. Huaroto, E. Ticona, J. Ortiz, M. Tovar, V. Chavez, R. Rodriguez, A. R. Escombe, and C. A. Evans. 2006. Tuberculosis mortality, drug resistance, and infectiousness in patients with and without HIV infection in peru. Am. J. Trop. Med. Hyg. 751027-1033. [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchison, D. A., B. W. Allen, L. Carrol, J. M. Dickinson, and V. R. Aber. 1972. A selective oleic acid albumin agar medium for tubercle bacilli. J. Med. Microbiol. 5165-175. [DOI] [PubMed] [Google Scholar]
- 10.Mitchison, D. A., B. W. Allen, and R. A. Lambert. 1973. Selective media in the isolation of tubercle bacilli from tissues. J. Clin. Pathol. 26250-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchison, D. A., B. W. Allen, and D. Manickavasagar. 1983. Selective Kirchner medium in the culture of specimens other than sputum for mycobacteria. J. Clin. Pathol. 361357-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore, D. A., C. A. Evans, R. H. Gilman, L. Caviedes, J. Coronel, A. Vivar, E. Sanchez, Y. Pinedo, J. C. Saravia, C. Salazar, R. Oberhelman, M. G. Hollm-Delgado, D. LaChira, A. R. Escombe, and J. S. Friedland. 2006. Microscopic-observation drug-susceptibility assay for the diagnosis of TB. N. Engl. J. Med. 3551539-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore, D. A., D. Mendoza, R. H. Gilman, C. A. Evans, M. G. Hollm Delgado, J. Guerra, L. Caviedes, D. Vargas, E. Ticona, J. Ortiz, G. Soto, and J. Serpa. 2004. Microscopic observation drug susceptibility assay, a rapid, reliable diagnostic test for multidrug-resistant tuberculosis suitable for use in resource-poor settings. J. Clin. Microbiol. 424432-4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray, S. J., A. Barrett, J. G. Magee, and R. Freeman. 2003. Optimisation of acid fast smears for the direct detection of mycobacteria in clinical samples. J. Clin. Pathol. 56613-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perkins, M. D., G. Roscigno, and A. Zumla. 2006. Progress towards improved tuberculosis diagnostics for developing countries. Lancet 367942-943. [DOI] [PubMed] [Google Scholar]
- 16.Rothlauf, M. V., G. L. Brown, and E. B. Blair. 1981. Isolation of mycobacteria from undecontaminated specimens with selective 7H10 medium. J. Clin. Microbiol. 1376-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sirgel, F. A., F. J. Botha, D. P. Parkin, B. W. Van De Wal, P. R. Donald, P. K. Clark, and D. A. Mitchison. 1993. The early bactericidal activity of rifabutin in patients with pulmonary tuberculosis measured by sputum viable counts: a new method of drug assessment. J. Antimicrob. Chemother. 32867-875. [DOI] [PubMed] [Google Scholar]
- 18.Sirgel, F. A., F. J. Botha, D. P. Parkin, B. W. Van de Wal, R. Schall, P. R. Donald, and D. A. Mitchison. 1997. The early bactericidal activity of ciprofloxacin in patients with pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 156901-905. [DOI] [PubMed] [Google Scholar]
- 19.Sirgel, F. A., P. R. Donald, J. Odhiambo, W. Githui, K. C. Umapathy, C. N. Paramasivan, C. M. Tam, K. M. Kam, C. W. Lam, K. M. Sole, and D. A. Mitchison. 2000. A multicentre study of the early bactericidal activity of anti-tuberculosis drugs. J. Antimicrob. Chemother. 45859-870. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. 2006. Global tuberculosis report: surveillance, planning, financing. WHO, Geneva, Switzerland.
- 21.World Health Organization. 2003. Laboratory biosafety manual, 2nd ed. (revised). WHO, Geneva, Switzerland.
- 22.World Health Organization. 1998. Laboratory services in tuberculosis control. Part III culture. WHO, Geneva, Switzerland.