Abstract
Molecular genotyping has shown Mycobacterium tuberculosis lineages to be geographically restricted and associated with distinct ethnic populations. Whether tuberculosis (TB) caused by some M. tuberculosis lineages can present with a differential clinical spectrum is controversial because of very limited clinical data. We recently reported on the discovery of RDRio M. tuberculosis, a Latin American-Mediterranean sublineage that is the predominant cause of TB in Rio de Janeiro, Brazil. To investigate the clinical attributes of TB caused by RDRio strains, we studied a cohort of TB cases from Belo Horizonte, Brazil, in which clinical information recorded on a standardized questionnaire was collected at the time of microbiological testing. These patients were referred for culture and drug susceptibility testing because of the clinical suspicion of “complicated” TB, as demonstrated by high rates of multidrug resistance (12%) and cavitary TB (80%). We performed spoligotyping and RDRio genotyping on the M. tuberculosis strains and analyzed the clinical data from these patients. RDRio M. tuberculosis accounted for 37% of the total TB burden. Multivariate analysis found a significant association between TB caused by RDRio strains and pulmonary cavitation and residence in Belo Horizonte. Since cavitary TB is associated with higher sputum bacillary load, our findings support the hypothesis that RDRio M. tuberculosis is associated with a more “severe” disease as a strategy to increase transmission. Future studies are needed to confirm these observations and to better define the contribution of RDRio M. tuberculosis to the global TB epidemic.
Mycobacterium tuberculosis, the etiologic agent of tuberculosis (TB), is estimated to have infected one-third of the world's population and annually causes ∼8 million new TB cases and >2 million deaths (16, 58). The challenges posed by TB have been further worsened by the emergence of multi-drug-resistant and extensively drug-resistant M. tuberculosis strains.
Molecular typing, based on genetic markers, permits the rapid detection and species level identification of mycobacteria within the M. tuberculosis complex (MTC), as well as provides useful tools for examining the transmission and evolution of these microorganisms (7, 17, 30, 53). Genome-wide single nucleotide polymorphism (SNP) and deletion analyses have been used to organize the global M. tuberculosis population structure into overlapping phylogenies with major lineages that show distinct geographic distribution and that may be associated with specific host adaptation (1, 21, 23, 24, 27, 28, 50). Similar results have also been obtained with spoligotyping and IS6110-RFLP fingerprint analysis (18). The W/Beijing lineage, for example, is the predominant family in major SNP cluster II, accounts for ∼10% of strains causing TB globally, and is localized mainly to Asia but has spread internationally. W/Beijing has been associated with outbreaks and multiple drug resistance (MDR) (4, 32) and also contributed significantly to the resurgence of TB in the early 1990s in New York City (3, 12). As a result, W/Beijing has remained by far the most intensely studied M. tuberculosis family. However, the Latin American-Mediterranean (LAM) family, a member of SNP cluster VI, appears to be the single most prevalent M. tuberculosis lineage globally (8), accounting for ∼15% of the global TB burden. Despite its larger contribution to TB, much less is known about the epidemiology, biological behavior, and clinical attributes of disease caused by strains of the LAM family.
In a prior report, we described a genotype of M. tuberculosis named RDRio, a member of the LAM family (33). Several genetic typing methods and phylogenetic analyses of RDRio M. tuberculosis support the interpretation that RDRio M. tuberculosis is clonal in origin and likely derived from a single ancestor with a LAM9 spoligotype signature. Importantly, the RDRio sublineage is the predominant cause of recent TB transmission in Rio de Janeiro, as indicated by the higher rate of clustering when analyzed by variable-number tandem repeats of mycobacterial interspersed repetitive units (MIRU-VNTR) patterns (33). Retrospective reviews of bacteriological and patient records showed trends in the data suggesting that RDRio strains may cause a form of TB with a distinct clinical presentation even though an association with MDR or specific risk factors for TB was lacking.
Recently, Gagneux et al. reported on the phylogenetic structure of M. tuberculosis lineages and provided data lending support to the hypothesis that certain lineages are preferentially adapted to particular human populations (23). Ethnic groups residing in San Francisco were more likely to be infected with a M. tuberculosis strain linked to their country of birth. However, social, cultural, and environmental confounders were not excluded, although these may also be drivers of patho-evolutionary strategies. Importantly TB cases born in the United States were more likely to harbor a strain from the so-called Euro-American lineage, which includes the LAM family (23). Of particular interest is the increasing evidence, through in vitro and mouse studies, that specific M. tuberculosis strains possess unique genetic traits and virulence phenotypes (34, 45, 47, 54). However, clinical correlates of virulence phenotypes demonstrated by laboratory studies are limited (51, 55, 56). The study of RDRio strains from Rio de Janeiro showed that more than 70% of LAM9 strains were of the RDRio genotype; the over-representation of RDRio LAM9 strains at the expense of “wild-type” non-RDRio (WT) LAM9 strains suggests that the RDRio strains may possess a specific biological advantage. The limited clinical and bacteriological record review that we reported associated TB caused by RDRio M. tuberculosis with higher sputum bacterial counts, as well as more frequent reports of hemoptysis (the coughing of blood) and weight loss. Given the fact that the clinical information was collected retrospectively and extracted from often incomplete clinical reports, we were guarded in the interpretation that RDRio M. tuberculosis may cause a more “severe form” of TB.
Efforts to further characterize TB caused by RDRio M. tuberculosis should be undertaken given that the LAM family, like W/Beijing, has been associated by others with outbreaks in prisons and drug resistance (9, 30, 49), as well as the clinical associations noted in TB caused by RDRio M. tuberculosis in the published cohort (33). The availability of an existing cohort, in which clinical information was collected prospectively at the time of the clinical sample submission for culture and susceptibility testing, provided a unique opportunity to evaluate whether RDRio M. tuberculosis causes TB outside of Rio de Janeiro and to uncover distinct epidemiological and clinical features.
MATERIALS AND METHODS
Setting.
Minas Gerais is one of the most populous states in Brazil, and Belo Horizonte is its capital. In 2006, Belo Horizonte had a population of 2,399,920 and is the fifth largest city by number of residents in Brazil. In 2004, Minas Gerais reported 6,191 TB cases or 29.1 cases per 105 persons (37). The rates of human immunodeficiency virus (HIV) coinfection and death related to TB were 16.2 and 7.3%, respectively.
Patients and clinical isolates.
From January to December of 2004, 240 patient samples were sent to the Minas Gerais State Reference Laboratory for Tuberculosis (Fundação Ezequiel Dias) to perform culture and drug susceptibility testing. This reference center serves Belo Horizonte and the surrounding communities/cities by providing culture and susceptibility testing for clinically suspected “complicated” TB, which may include suspected single drug resistance or MDR, advanced disease, and/or associated comorbidities. TB was diagnosed based on a positive culture on Löwenstein-Jensen medium and standard biochemical tests (41). Testing of susceptibility to anti-TB drugs was performed by the proportion method of Canetti and Grosset (11). Of the 124 culture-confirmed TB patients, 117 had sufficient demographic, clinical, and radiological information reported on a standardized questionnaire. The extracted DNA from 117 stored patient cultures were evaluated by spoligotyping in 2006 and later in 2007 for RDRio genotype and association with specific epidemiological and clinical features. Four patients were previously diagnosed and treated for TB in 2002 with the standard regimen (rifampin, isoniazid, and pyrazinamide for 2 months, followed by rifampin and isoniazid for 4 months) in accordance with Brazilian Ministry of Health Guidelines for the first episode of TB (36); at treatment's end, three of the four were cured. The fourth patient abandoned treatment prior to completion of the regimen. The patient was later retreated with four drugs over a more extended period and, after which time (in 2003), was considered cured. In 2004, however, the four patients again developed respiratory symptoms and were confirmed to have a recurrence of TB. Since the episodes were more than 1 year apart, they were considered to have a new episode of TB in this analysis. The present study was approved by the local Committee of Ethics in Research.
Data analysis.
The following data were extracted and entered into an electronic database in 2005 using information provided in a standardized questionnaire submitted with the clinical sample that included the following types of information: demographic (age, gender, community of residence), clinical (presence of cough, sputum production, fever, weight loss, HIV coinfection [yes or no]), radiological (cavitation on the chest radiography [yes or no]), and mycobacteriological (acid-fast bacillus [AFB] smear, susceptibility to tested anti-TB drugs).
Bacterial thermolysate and genotyping analysis.
A bacterial thermolysate was obtained as previously reported (33). A multiplex PCR was performed on these isolates to differentiate RDRio from WT strains as previously described (27). Briefly, two sets of primer pairs targeted either the IS1561′ locus (positive only in WT strains and corresponding to a band size of 530 bp) or the region flanking the RDRio locus (positive only in RDRio strains and corresponding to a band size of 1,175 bp). Mixed RDRio/WT strains are indicated by the presence of both bands, as previously validated (33). Spoligotyping was performed as described by Kamerbeek et al. (31), and the results were compared to SpolDB4 database of the Pasteur Institute of Guadeloupe (available at http://www.pasteurguadeloupe.fr:8081/SITVITDemo). Each strain isolated from the four patients with repeat episodes of TB was additionally genotyped by MIRU-VNTR as described by Supply et al. (53).
Statistical analysis.
Data were summarized by mean and standard deviation for continuous variables and by frequency and proportion for categorical variables. For univariate analysis and testing, we used nonparametric statistics: Wilcoxon test for continuous variables and the Fisher exact test for categorical variables. For multivariate analysis/testing, we used multiple logistic regression to estimate the adjusted odds ratio (OR), along with the 95% confidence interval (CI), controlling other variables. All analyses were performed by SAS 9.1 (SAS Systems, Cary, NC). All statistical testing and inferences were based on a two-sided hypothesis. To assess the discriminatory capacity for RDRio versus non-RDRio using multivariables included in the regression model, we computed area under the receiver-operator characteristic (ROC) curve (AUC).
A set of markers that perfectly discriminates between two classes has an AUC of 1.0 (a 100% true-positive rate), while an AUC of 0.5 means that the discriminatory capacity is no better than chance.
RESULTS
From the 117 isolates submitted to the multiplex PCR, a clear classification of either RDRio or WT was obtained in 105 isolates (The patterns of amplification are illustrated in Fig. 1). Twelve isolates and associated patient data were excluded from analysis. In 10 of the 12 isolates the amplification failed. Two other isolates had both “WT” and “RDRio” amplicons (two bands) by electrophoretic gel analysis, indicating a mixed RDRio and WT infection in which the available data did not permit the exclusion of laboratory cross-contamination since only one sample was available. However, the presence of infection with more than one strain of M. tuberculosis in the same episode is well described in the literature (19, 46, 60), and our previous report confirmed RDRio and WT strains in the same specimen (33). Notably, the proportion of almost 2% (2 of 107) is similar to the finding of the cohort in Rio de Janeiro and the rate of mixed infection in the literature (19, 33, 46).
FIG. 1.
Results of the multiplex RDRio PCR. Lane 1, 100-bp ladder; lane 2, amplification of the flanking RDRio region (found only in RDRio strains); lane 3, amplification of the IS1561′ region (found only in non-RDRio strains); lane 4, mixed RDRio and non-RDRio bands in the same isolate. Band sizes in base pairs are indicated.
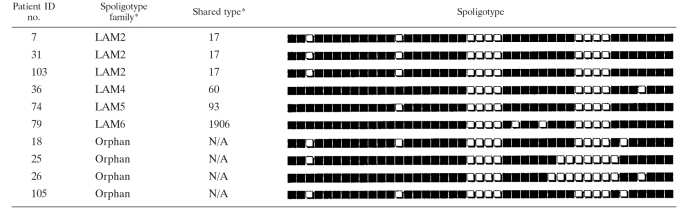
RDRio M. tuberculosis was the cause of 37% (39 of 105) of the TB cases from Belo Horizonte and surrounding cities in Minas Gerais State. This rate in Belo Horizonte for TB cases in 2004 is similar to the 30% reported for Rio de Janeiro for 18 months in 2002 thru 2003 (33). Of the total M. tuberculosis strains evaluated by spoligotyping by the present study, the LAM family as a whole was responsible for 56% of the TB burden in Minas Gerais. When segregated by WT and RDRio genotype, RDRio strains belonged exclusively to the LAM family and comprised of 66% of LAM strains. Of importance, 92% (23 of 25) of strains with a LAM9 spoligotype were of the RDRio sublineage (Table 1). In addition, all LAM1 and LAM2 spoligotypes were RDRio genotype, whereas the LAM3 spoligotype was solely of the WT group, results that are in accordance with our prior publication (33). In addition to LAM strains, the T, Haarlem, and X families were also found in the WT group (Table 1).
TABLE 1.
Spoligotyping patterns and genotype for each Belo Horizonte M. tuberculosis patient straina
*, according to the SpolDB4 and SITVIT webpages; WT, non-RDRio; #, primary isolate from the four patients with TB relapse.
The majority of the TB patients were residents of Belo Horizonte compared to surrounding communities/cities—68 and 32%, respectively. Clinical data indicated a high rate of MDR and TB/HIV coinfections, 12 and 19.5%, respectively, compared to 0.9 and 12%, respectively, in the national Brazilian TB data (59, 59a). This cohort also had high rates for positive AFB smear and cavitary pulmonary disease: 88 and 84%, respectively. We next evaluated these epidemiological and clinical findings respective to whether TB was caused by RDRio or WT M. tuberculosis. Illustrated in Table 2 is a comparison of the sociodemographic, clinical, and bacteriological data of TB caused by RDRio and WT M. tuberculosis. The majority of patients were male (66%) while age, sex, and rate of HIV-1 infection were similar between TB caused by RDRio and WT M. tuberculosis (Table 2). The rate of AFB-positive smear, as well as single-drug resistance and MDR-TB caused by RDRio and WT M. tuberculosis, were similar. Multiple logistic regression analysis showed that RDRio M. tuberculosis was significantly associated with residence in the city of Belo Horizonte compared to outside communities (OR = 4.1 [95% CI = 1.4 to 11.6]; Table 3). The OR for RDRio infection leading to cavitary TB disease was >8-fold (95% CI = 1.5 to 53.0) greater than TB caused by WT strains. The multiple risk factors included in the logistic regression demonstrated an AUC of 0.743 in the capability to distinguish RDRio M. tuberculosis against WT (where 1.0 would represent perfect discrimination between two TB types).
TABLE 2.
Sociodemographic, clinical, and bacteriological data for pulmonary TB patients at the time of diagnosis
| Patient characteristic | Mean no. of patients (%)b
|
Pc | |
|---|---|---|---|
| RDRio (n = 39) | WT (n = 66) | ||
| Male gender | 25 (64) | 44 (67) | 0.83 |
| HIV positive | 6 (15) | 11 (17) | 1.00 |
| Presence of AFB in sputum | 30 (77) | 58 (88) | 0.17 |
| Resistance to at least one TB drug | 10 (26) | 15 (23) | 0.81 |
| MDR | 6 (15.4) | 7 (10.6) | 0.54 |
| City of residence | |||
| Belo Horizonte | 32 (82) | 39 (59) | 0.0018 |
| Neighboring cities | 7 (18) | 27 (41) | |
| Fever | 38 (97) | 64 (97) | 1.00 |
| Cough | 37 (95) | 64 (97) | 0.63 |
| Sputum production | 37 (95) | 63 (95) | 1.00 |
| wt loss | 38 (97) | 62 (94) | 0.65 |
| Cavity on CXRa | 37 (95) | 50 (76) | 0.015 |
CXR, chest radiography.
WT, non-RDRio. Values in parentheses indicate the percent frequency for the categorical variables. The mean ages (standard deviations) for the RDRio and WT groups were 42.6 years (14.5 years) and 45.0 years (17 years), respectively (P = 0.45). HIV serology testing was performed in 87 patients, of whom 17 were positive (19.5% of the patients tested).
The Wilcoxon test for continuous variables and the Fisher exact test for categorical variables were used for P value computation.
TABLE 3.
Demographic and clinical features associated with RDRio M. tuberculosis: results from multiple logistic regressiona
| Patient characteristic | OR (95% CI) | P |
|---|---|---|
| Age | 0.99 (0.96-1.01) | 0.32 |
| Male gender | 1.25 (0.49-3.19) | 0.64 |
| HIV positive | 0.56 (0.15-1.96) | 0.36 |
| Presence of AFB in sputum | 0.41 (0.13-1.33) | 0.14 |
| Resistance to at least one TB drug | 0.73 (0.26-2.03) | 0.54 |
| City of residence Belo Horizonte | 4.05 (1.4-11.6) | 0.009 |
| Cough | 1.24 (0.06-25.2) | 0.89 |
| Sputum production | 0.31 (0.01-7.0) | 0.46 |
| Cavity on CXR | 8.97 (1.52-53.0) | 0.016 |
AUC = 0.743. Fever and weight loss variables were omitted due to numerical instability of the model fit.
Four patients suffered TB recurrence. All of these patients had cavitary disease and were infected with LAM strains, three of which were RDRio genotype strains. In all four cases the spoligotyping and MIRU-VNTR patterns of the strains causing the second TB episode were identical to the strains causing the first episode of TB, suggesting that relapse rather than reinfection was the cause of the second episode (Tables 1 and 4). Of the three patients that completed treatment, the second episode strains remained susceptible to anti-TB drugs, as in the first episode strains. In the fourth case (caused by an RDRio strain), i.e., the patient who abandoned treatment, resistance to both rifampin and isoniazid developed in the subsequent TB episode.
TABLE 4.
Genotyping by spoligotyping, MIRU-VNTR, and RDRio/WT pattern in the first and subsequent isolates from the four patients with recurrent TBa
| Patient ID no. | Isolate ID no. | Genotyping based on:
|
||
|---|---|---|---|---|
| RDRio/WT | Spoligotype family | MIRU-VNTR | ||
| 46 | 46 | RDRio | LAM9 | 22325152321 |
| 111 | RDRio | LAM9 | 22325152321 | |
| 28 | 28 | WT | LAM3 | 12314152323 |
| 30 | WT | LAM3 | 12314152323 | |
| 20 | 20 | RDRio | LAM1 | 22326132321 |
| 100 | RDRio | LAM1 | 22326132321 | |
| 85 | 85 | RDRio | LAM9 | 22325152321 |
| 94 | RDRio | LAM9 | 22325152321 | |
Note that each pair of isolates from the same patient had a spoligotype pattern identical to that shown for the first isolate in Fig. 1, and here only the designated spoligotype family is indicated. WT, RDRio.
DISCUSSION
The LAM family is the leading contributor of TB worldwide by a single lineage, accounting for ∼15% of the submitted strains to the SpolDB4 database (8; Nalin Rastogi, unpublished data), while the T-spoligotype designation, the most frequently described spoligotype “family,” is a composite of more than one different lineage. The LAM family is prevalent in all of the Americas, the Caribbean, Europe, parts of Africa, and Oceania. The importance of the LAM family is reinforced by reports that it has been associated with outbreaks and MDR (9, 30, 49), including the recent description that a strain from the LAM4 subfamily is the leading cause of XDR-TB in South Africa (43). The association of W/Beijing family mainly with East-Asians and LAM predominantly with Euro-Americans has led to the speculation that M. tuberculosis adaptation to specific human ethnic hosts may be involved (23). Whether the selective association of a certain M. tuberculosis lineage with a distinct human ethnic group is due to genetic adaptation of the M. tuberculosis lineage or because of human social, cultural, epidemiological, and environmental forces are topics of current research. One line of reasoning is that an adapted M. tuberculosis lineage may be more virulent and/or transmissible in select human ethnic hosts.
There is increasing evidence through in vitro and mouse studies that specific M. tuberculosis strains possess unique genetic traits and virulence phenotypes. These studies have noted that strains of certain lineages are associated with immunity-modifying capacities such as, NO resistance (C strain), poor granuloma formation (PGRS 004), heightened proinflammatory response (CDC1551), and dampened interleukin-6 (IL-6), IL-12, and tumor necrosis factor alpha mediated by a phenogylcolipid produced by the pks15/1 genes (W/Beijing) (22, 34, 44, 45, 52, 55). The data from the in vitro and mouse studies detailed above support that immunity-altering mechanisms provide biological advantages for M. tuberculosis. However, their ability to cause disease and enhance transmission in humans largely remains inferential. Several studies investigating specific strains in humans found that W/Beijing strains were associated with a lower frequency of patients manifesting with fever and cavitary disease at the time of diagnosis but more frequently developed fever during anti-TB treatment (56), while M. tuberculosis strain CDC1551 (Tennessee strain) was linked to a higher frequency of infection in close contacts (based on tuberculin skin testing conversion) but was not as virulent as other strains (22).
Specific large sequence polymorphisms (LSPs) can distinguish between M. tuberculosis lineages that have a predilection for specific global geographic regions and host ethnicity (7, 23, 38). Downsizing of the MTC genome has been shown as a potential mechanism for host adaptation that is underscored by the genome of Mycobacterium bovis, which has a broad host range and multiple gene regions of difference (26). Importantly, LSPs in certain M. tuberculosis strains have been linked to host immune modification. In particular, Central Asian (CAS) strains possess an LSP that has been associated with an increased capacity to promote host IL-10 production as an immune evasion strategy (39). Although RDRio strains have lost a phenoglycolipid involved in inhibiting proinflammatory cytokines due to a deletion within the pks15/1 gene, we speculate that RDRio M. tuberculosis may dampen immune recognition through the deletion of two of its surface antigens (PPE55 and PPE56), among other potential mechanisms. The PPE/PE_PGRS protein family is expressed on the surface of M. tuberculosis and is considered putative antigens in mycobacterial virulence and host immune response (6, 15, 44). Indeed, both PPE55 and PPE56 were shown to be expressed in vivo and upon entry into interferon-activated macrophages and are immunogenic in humans (54, 57). Lastly, LSPs involving either PPE55 and/or PPE56 have been noted in several clinical strains of M. tuberculosis and MTC species (26, 29, 40). We previously speculated that the loss of the two PPE genes in RDRio M. tuberculosis strains minimized host immune recognition, leading to enhanced M. tuberculosis virulence and transmissibility (33). Indeed, RDRio is significantly associated with more clusters (an indication of recent transmission) than WT strains, both in Brazilians (33) and in non-Brazilian populations (unpublished data), suggesting that RDRio strains may be a more significant source of recently transmitted TB.
Our recent publication associated RDRio TB with higher frequency of hemoptysis and weight loss than WT strains, despite a similar duration of illness prior to presentation, suggesting that RDRio strains could cause a more “severe” clinical disease (33). However, since these results were based on only 47% of the retrospective medical records available, we were cautious in that conclusion. The present study had the advantage that all laboratorial and clinical data were collected prospectively. RDRio strains were the cause of TB in >37% of this cohort of TB patients and >90% of the LAM9 strains. When analyzed by multiple logistic regression, residence in Belo Horizonte (a major metropolis compared to outer cities/communities) and cavitary lung disease were strongly associated with TB caused by RDRio. In fact, the OR for RDRio TB having cavitary disease was more than eight times greater than for WT TB. However, the corresponding CI is wide. A more accurate estimation (e.g., point and interval estimates) should be made, hopefully, by larger studies in the future. The robustness of the multivariate analysis was suggested by the area under the ROC curve of 0.743 (where 1.0 represents the 100% true rate). Although cavitary disease is not by itself a sign of more severe disease, it has been shown to be associated with a higher bacillary burden and/or increased transmissibility (13, 20, 48). The higher frequency of cavitary TB associated with RDRio strains by the present study is supported by our previous publication in which RDRio was associated with higher bacillary load (>200 colonies) compared to WT strains even though the qualitative AFB smear results between the two genotypes were similar (33). As was recently demonstrated, there is a clear association between cavitary disease and sputum bacillary load (42). These features of TB caused by RDRio genotype strains may in part explain why RDRio M. tuberculosis is prevalent in Brazil and elsewhere in the world, especially in locales with a high TB burden where so many other strains are competing (27). Additional inferential support for this supposition is that although the RDRio deletion seems to have derived from a LAM9 WT ancestor by phylogenetic analyses, 70% of the LAM9 strains in Rio de Janeiro were of the RDRio genotype. Importantly, in Belo Horizonte, more than 90% of the LAM9 strains were of the RDRio genotype. The higher percentage of RDRio strains circulating within the Belo Horizonte city compared to outlying communities might suggest an evolving outbreak, although potential study sampling bias, as well as differences in social/environmental factors cannot be excluded.
It should be emphasized that Minas Gerais State Reference Laboratory for TB is a reference laboratory serving the region for clinically “difficult” TB cases. Clinicians and health centers submit clinical samples because of severe clinical manifestation, the suspicion for drug resistance, and/or a second episode TB. This bias is reflected by the high percentage of positive AFB smears (88%), high rates of cavitary disease (84%), high rates of MDR strains (12%) and high rates of TB-HIV coinfection (19.5%). In this selection of TB cases enriched for clinical “difficult” patients, having cavitary disease was associated with a fourfold risk for TB caused by RDRio strains. The selection bias therefore enhanced our ability to make the current association between TB caused by RDRio strains and cavitary pulmonary disease. Although our preferred interpretation is that RDRio LAM strains cause more severe disease, as manifested by a higher rate of cavitary TB, we cannot exclude that these patients had more chronic infection with delayed medical care. However, in our retrospective cohort, patients with RDRio and WT strains presented for clinical care with a similar duration of illness (33). On the other hand, in a preliminary analysis of an ongoing prospective cohort in which the first 43 patient isolates were genotyped only by spoligotyping, we noted patients infected with LAM strains presented for clinical care with a significantly shorter duration of illness and with a higher frequency of fever, weight loss, and pulmonary cavitation than patients infected with other strain families (2). Given that there is an inherent bias in our study and a lack of data regarding duration of illness, future prospective studies are needed in which patients are evaluated that present with the full spectra of clinical TB disease states, have complete clinical assessments recorded, and are sufficient in sample size and power to detect differences in clinical disease severity.
Of note, more than 80% of the patients in the present study had cavitary TB. All four patients who relapsed after completing 6 months of treatment also had cavitary disease on the chest X-ray examinations. As noted previously, the presence of cavitary disease appears to increase the risk of relapse and the development of drug resistance (10; rifapentine [Priftin] package insert [Hoechst Marion Roussel, Kansas City, MO]). For this reason, a joint statement of the American Thoracic Society, the Centers for Disease Control and Prevention, and the Infectious Disease Society of America recommends that patients with cavitation on chest X-ray examinations and whose second-month sputum culture remains positive receive a minimum of 9 months of treatment (5). Even though the present study was not designed to monitor patients for recurrence, our finding that all four cases of relapse had cavitary TB could be interpreted as supportive of the previous observations and the recommended longer duration of therapy. At least one study has reported a disproportionately high rate of relapse caused by LAM strains (25). Future studies will need to address whether the genotypic identification of RDRio infection alone or in association with cavitary TB is cause to extend treatment in affected patients.
We identified RDRio strains from every Brazilian region where samples were available, indicating that RDRio has spread throughout the whole country (data not shown). Moreover, our current data that the RDRio LAM sublineage is circulating internationally lends support that it may be more transmissible in certain more susceptible host ethnicities. These data include the following features. (i) RDRio strains were identified as a cause of TB in 11 of 20 countries tested from four of the world's continents (27). (ii) Comparative IS6110-RFLP phylogenetic analyses of selected M. tuberculosis from Rio de Janeiro against prototypic strains in a South African database suggests that RDRio strains are synonymous with the F9 and F13 IS6110-RFLP families (which are prevalent causes of ongoing TB in South Africa) (27, 51), as well as being similar to the SAF1 family recently described by Chihota et al. (14) as predominant strains causing TB in Zimbabwe (47.2%) and Zambia (65%). (iii) In Madrid, Spain, the most prevalent M. tuberculosis cluster strain is designated strain 5 (ST20 or LAM1) (35). Notably, strain 5 has remained a predominant clone in Madrid for the last 13 years, while other clone types have declined over this time period. Our group has recently described that strain 5 is a RDRio strain (27). Interestingly, its MIRU-VNTR has the pattern previously described to be informative of being a RDRio genotype (33). (iv) A genotype survey of TB in New York City covering the years 2001 to 2005 showed that ∼8% of all TB was caused by RDRio strains (unpublished data). (v) Finally, from the M. tuberculosis strains described in outbreaks from Russia (30, 49), both LAM9 and LAM1 were major contributors. Our previous and current studies found that these two LAM types were strongly associated with the RDRio genotype (33). These data provide support that the RDRio LAM sublineage may have some biological advantage and to be a significant global problem.
In summary, the available data suggest that RDRio M. tuberculosis strains are a major contributor to TB in Brazil and in several countries in the world. The current data suggest that RDRio LAM sublineage may cause more severe disease and/or may transmit more efficiently in certain ethnic populations. Larger prospective cohort studies are needed to provide more direct and conclusive evidence that RDRio LAM sublineage causes more severe forms of TB and/or transmits better than other lineages. Moreover, a study with a large sample size will minimize the width of the confidence interval that was seen in the present study with its smaller sample size. Such findings may impact on public health policies and assignment of limited resources for the control of TB in which one-third of the world's population is already a carrier and have a ∼10% life-time risk for reactivation TB.
Acknowledgments
Funding for this project was provided by National Institutes of Health grants R21 AI063147 and R21 AI063147 (to J.L.H.), National Institutes of Health Fogarty International Center grants (D43 TW00018 and U2R TW006885 [to J. R. Lapa e Silva, R. Chaisson, and J.L.H.] and U2R TW006901 [to Warren D. Johnson]), a grant from Faperj (process 110.288/2007) (A.L.K.), and a grant from the Laura Cook Hull Trust Fund (LCHTF; to Warren D. Johnson, Jr., principal investigator). L.C.O.L. was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico and was a Fogarty International Center trainee. S.M.S. was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes), Ministry of Education, Brazil. A.L.G. was supported in part by the LCHTF. S.W. was supported by training grant T32 AI 07613 from the National Institutes of Health, NIAID (to W. D. Johnson).
We thank Anna Cristina Carvalho (Institute of Infectious and Tropical Diseases, University of Brescia, Brescia, Italy) for preliminary data evaluation and comments and Warren D. Johnson for support and encouragement.
Footnotes
Published ahead of print on 7 May 2008.
REFERENCES
- 1.Baker, L., T. Brown, M. C. Maiden, and F. Drobniewski. 2004. Silent nucleotide. polymorphisms and a phylogeny for Mycobacterium tuberculosis. Emerg. Infect. Dis. 101568-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbosa, C., R. Noboa, L. Lazzarini, N. Boechat, J. Leung, A. Elias, P. Suffys, A. Kritski, J. R. Lapa e Silva, and J. L. Ho. 2007. Is the severity of tuberculosis different when patients are infected by strains of Mycobacterium tuberculosis belonging to diverse spoligotyping families? Am. J. Respir. Crit. Care Med. 175A417. [Google Scholar]
- 3.Bifani, P. J., B. B. Plikaytis, V. Kapur, K. Stockbauer, X. Pan, M. L. Lutfey, S. L. Moghazeh, W. Eisner, T. M. Daniel, M. H. Kaplan, J. T. Crawford, J. M. Musser, and B. N. Kreiswirth. 1996. Origin and interstate spread of a New York City multidrug-resistant Mycobacterium tuberculosis clone family. JAMA 275452-457. [PubMed] [Google Scholar]
- 4.Bifani, P. J., B. Mathema, N. E. Kurepina, and B. N. Kreiswirth. 2002. Global dissemination of the Mycobacterium tuberculosis W-Beijing family strains. Trends Microbiol. 1045-52. [DOI] [PubMed] [Google Scholar]
- 5.Blumberg, H. M., W. J. Burman, R. E. Chaisson, C. L. Daley, S. C. Etkind, L. N. Friedman, P. Fujiwara, M. Grzemska, P. C. Hopewell, M. D. Iseman, R. M. Jasmer, V. Koppaka, R. I. Menzies, R. J. O'Brien, R. R. Reves, L. B. Reichman, P. M. Simone, J. R. Starke, and A. A. Vernon. 2003. American Thoracic Society, Centers for Disease Control and Prevention, and the Infectious Diseases Society: treatment of tuberculosis. Am. J. Respir. Crit. Care Med. 167603-662. [DOI] [PubMed] [Google Scholar]
- 6.Brennan, M. J., and G. Delogu. 2002. The PE multigene family: a “molecular mantra” for mycobacteria. Trends Microbiol. 10246-249. [DOI] [PubMed] [Google Scholar]
- 7.Brosch, R., S. V. Gordon, M. Marmiesse, P. Brodin, C. Buchrieser, K. Eiglmeier, T. Garnier, C. Gutierrez, G. Hewinson, K. Kremer, L. M. Parsons, A. S. Pym, S. Samper, D. van Soolingen, and S. T. Cole. 2002. A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc. Natl. Acad. Sci. USA 993684-3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brudey, K., J. R. Driscoll, L. Rigouts, W. M. Prodinger, A. Gori, S. A. Al-Hajoj, C. Allix, L. Aristimuno, J. Arora, V. Baumanis, L. Binder, P. Cafrune, A. Cataldi, S. Cheong, R. Diel, C. Ellermeier, J. T. Evans, M. Fauville-Dufaux, S. Ferdinand, D. Garcia de Viedma, C. Garzelli, L. Gazzola, H. M. Gomes, M. C. Guttierez, P. M. Hawkey, P. D. van Helden, G. V. Kadival, B. N. Kreiswirth, K. Kremer, M. Kubin, S. P. Kulkarni, B. Liens, T. Lillebaek, M. L. Ho, C. Martin, C. Martin, I. Mokrousov, O. Narvskaia, Y. F. Ngeow, L. Naumann, S. Niemann, I. Parwati, Z. Rahim, V. Rasolofo-Razanamparany, T. Rasolonavalona, M. L. Rossetti, S. Rusch-Gerdes, A. Sajduda, S. Samper, I. G. Shemyakin, U. B. Singh, A. Somoskovi, R. A. Skuce, D. van Soolingen, E. M. Streicher, P. N. Suffys, E. Tortoli, T. Tracevska, V. Vincent, T. C. Victor, R. M. Warren, S. F. Yap, K. Zaman, F. Portaels, N. Rastogi, and C. Sola. 2006. Mycobacterium tuberculosis complex genetic diversity: mining the fourth international spoligotyping database (SpolDB4) for classification, population genetics and epidemiology. BMC Microbiol. 623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Candia, N., B. Lopez, T. Zozio, M. Carrivale, C. Diaz, G. Russomando, N. J. de Romero, J. C. Jara, L. Barrera, N. Rastogi, and V. Ritacco. 2007. First insight into Mycobacterium tuberculosis genetic diversity in Paraguay. BMC Microbiol. 775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canetti, G. 1965. The J. Burns Amberson Lecture: present aspects of bacterial resistance in tuberculosis. Am. Rev. Respir. Dis. 92687-703. [DOI] [PubMed] [Google Scholar]
- 11.Canetti, G., N. Rist, and J. Grosset. 1963. Measurement of sensitivity of the tuberculous bacillus to antibacillary drugs by the method of proportions: methodology, resistance criteria, results, and interpretation. Rev. Tuberc. Pneumol. 27217-272. [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. 1993. Outbreak of multidrug-resistant tuberculosis at a hospital-New York City, 1991. MMWR Morb. Mortal. Wkly. Rep. 42427-433. [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. 1995. Exposure of passengers and flight crew to Mycobacterium tuberculosis on commercial aircraft, 1992-1995. MMWR Morb. Mortal. Wkly. Rep. 44137-140. [PubMed] [Google Scholar]
- 14.Chihota, V., L. Apers, S. Mungofa, W. Kasongo, I. M. Nyoni, R. Tembwe, G. Mbulo, M. Tembo, E. M. Streicher, G. D. van der Spuy, T. C. Victor, P. van Helden, and R. M. Warren. 2007. Predominance of a single genotype of Mycobacterium tuberculosis in regions of Southern Africa. Int. J. Tuberc. Lung Dis. 11311-318. [PubMed] [Google Scholar]
- 15.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentiles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jegels, A. Krogh, J. McLean, S. Moule, L. Murphy, K. Oliver, J. Osborne, M. A. Quail, M. A. Rajandream, J. Rogers, S. Rutter, K. Seeger, J. Skelton, R. Squares, S. Squares, J. E. Sulston, K. Taylor, S. Whitehead, and B. G. Barrell. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393537-544. [DOI] [PubMed] [Google Scholar]
- 16.Corbett, E. L., C. J. Watt, N. Walker, D. Maher, B. G. Williams, M. C. Raviglione, and C. Dye. 2003. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch. Intern. Med. 1631009-1021. [DOI] [PubMed] [Google Scholar]
- 17.Crawford, J. T. 2003. Genotyping in contact investigations: a CDC perspective. Int. J. Tuberc. Lung Dis. 7S453-457. [PubMed] [Google Scholar]
- 18.Dale, J. W., G. H. Bothamley, F. Drobniewski, S. H. Gillespie, T. D. McHugh, and R. Pitman. 2005. Origins and properties of Mycobacterium tuberculosis isolates in London. J. Med. Microbiol. 54575-582. [DOI] [PubMed] [Google Scholar]
- 19.Das, S., S. Narayanan, L. Hari, N. S. Mohan, S. Somasundaram, N. Selvakumar, and P. R. Narayanan. 2004. Simultaneous infection with multiple strains of Mycobacterium tuberculosis identified by restriction fragment length polymorphism analysis. Int. J. Tuberc. Lung Dis. 8267-270. [PubMed] [Google Scholar]
- 20.Driver, C. R., M. Macaraig, P. D. McElroy, C. Clark, S. S. Munsiff, B. N. Kreiswirth, J. Driscoll, and B. Zhao. 2006. Which patients' factors predict the rate of growth of Mycobacterium tuberculosis clusters in an urban community? Am. J. Epidemiol. 16421-31. [DOI] [PubMed] [Google Scholar]
- 21.Filliol, I., A. S. Motiwala, M. Cavatore, W. Qi, M. H. Hazbon, M. Bobadilla del Valle, J. Fyfe, L. Garcia-Garcia, N. Rastogi, C. Sola, T. Zozio, M. I. Guerrero, C. I. Leon, J. Crabtree, S. Angiuoli, K. D. Eisenach, R. Durmaz, M. L. Joloba, A. Rendon, J. Sifuentes-Osornio, A. Ponce de Leon, M. D. Cave, R. Fleischmann, T. S. Whittam, and D. Alland. 2006. Global phylogeny of Mycobacterium tuberculosis based on single nucleotide polymorphism (SNP) analysis: insights into tuberculosis evolution, phylogenetic accuracy of other DNA fingerprinting systems, and recommendations for a minimal standard SNP Set. J. Bacteriol. 188759-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedman, C. R., G. C. Quinn, B. N. Kreiswirth, D. C. Perlman, N. Salomon, N. Schluger, M. Lutfey, J. Berger, N. Poltoratskaia, and L. W. Riley. 1997. Widespread dissemination of a drug-susceptible strain of Mycobacterium tuberculosis. J. Infect. Dis. 176478-484. [DOI] [PubMed] [Google Scholar]
- 23.Gagneux, S., K. DeRiemer, T. Van, M. Kato-Maeda, B. C. Jong, S. Narayanan, M. Nicol, S. Niemann, K. Kremer, M. C. Gutierrez, M. Hilty, P. C. Hopewell, and P. M. Small. 2006. Variable host-pathogen compatibility in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 1032869-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gagneux, S., and P. M. Small. 2007. Global phylogeography of Mycobacterium tuberculosis and implications for tuberculosis product development. Lancet Infect. Dis. 7328-337. [DOI] [PubMed] [Google Scholar]
- 25.García de Viedma, D., M. Marín, S. Hernangómez, M. Díaz, M. J. Ruiz Serrano, L. Alcalá, and E. Bouza. 2002. Tuberculosis recurrences: reinfection plays a role in a population whose clinical/epidemiological characteristics do not favor reinfection. Arch. Intern. Med. 1621873-1879. [DOI] [PubMed] [Google Scholar]
- 26.Garnier, T., K. Eiglmeier, J. C. Camus, N. Medina, H. Mansoor, M. Pryor, S. Duthoy, S. Grondin, C. Lacroix, C. Monsempe, S. Simon, B. Harris, R. Atkin, J. Doggett, R. Mayes, L. Keating, P. R. Wheeler, J. Parkhill, B. G. Barrell, S. T. Cole, S. V. Gordon, and R. G. Hewinson. 2003. The complete genome sequence of Mycobacterium bovis. Proc. Natl. Acad. Sci. USA 1007877-7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gibson, A. L., R. C. Huard, N. C. Gey van Pittius, L. C. O. Lazzarini, J. Driscoll, N. Kurepina, T. Zozio, C. Sola, S. M. Spindola, A. L. Kritski, D. Fitzgerald, K. Kremer, H. Mardassi, P. Chitale, J. Brinkworth, D. G. de Viedma, B. Gicquel, J. W. Pape, D. van Soolingen, B. N. Kreiswirth, R. M. Warren, P. D. van Helden, N. Rastogi, P. N. Suffys, J. Lapa e Silva, and J. L. Ho. 2008. Application of sensitive and specific molecular methods to uncover the global dissemination of the major RDRio sublineage of the Latin American-Mediterranean Mycobacterium tuberculosis spoligotype family. J. Clin. Microbiol. 461259-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gutacker, M. M., B. Mathema, H. Soini, E. Shashkina, B. N. Kreiswirth, E. A. Graviss, and J. M. Musser. 2006. Single-nucleotide polymorphism-based population genetic analysis of Mycobacterium tuberculosis strains from four geographic sites. J. Infect. Dis. 193121-128. [DOI] [PubMed] [Google Scholar]
- 29.Huard, R. C., M. Fabre, P. de Haas, L. C. Lazzarini, D. van Soolingen, D. Cousins, and J. L. Ho. 2006. Novel genetic polymorphisms that further delineate the phylogeny of the Mycobacterium tuberculosis complex. J. Bacteriol. 1884271-4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ignatova, A., S. Dubiley, V. Stepanshina, and I. Shemyakin. 2006. Predominance of multidrug-resistant LAM and Beijing family strains among Mycobacterium tuberculosis isolates recovered from prison inmates in Tula Region, Russia. J. Med. Microbiol. 551413-1418. [DOI] [PubMed] [Google Scholar]
- 31.Kamerbeek, J., L. Schouls, A. Kolk, M. van Agterveld, D. van Soolingen, S. Kuijper, A. Bunschoten, H. Molhuizen, R. Shaw, M. Goyal, and J. van Embden. 1997. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 35907-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kruuner, A., S. E. Hoffner, H. Sillastu, M. Danilovits, K. Levina, S. B. Svenson, S. Ghebremichael, T. Koivula, and G. Källenius. 2001. Spread of drug-resistant pulmonary tuberculosis in Estonia. J. Clin. Microbiol. 393339-3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lazzarini, L. C., R. C. Huard, N. L. Boechat, H. M. Gomes, M. C. Oelemann, N. Kurepina, E. Shashkina, F. C. Mello, A. L. Gibson, M. J. Virginio, A. G. Marsico, W. R. Butler, B. N. Kreiswirth, P. N. Suffys, J. R. Lapa e Silva, and J. L. Ho. 2007. Discovery of a novel Mycobacterium tuberculosis lineage that is a major cause of tuberculosis in Rio de Janeiro, Brazil. J. Clin. Microbiol. 453891-3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manca, C., M. B. Reed, S. Freeman, B. Mathema, B. N. Kreiswirth, C. E. Barry III, and G. Kaplan. 2004. Differential monocyte activation underlies strain-specific Mycobacterium tuberculosis pathogenesis. Infect. Immun. 725511-5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martín, A., F. Chaves, J. Iñigo, M. Alonso, C. Sola, N. Rastogi, M. J. Ruiz Serrano, E. Palenque, E. Bouza, and D. Garcia de Viedma. 2007. Molecular, epidemiological, and infectivity characterization of a Mycobacterium tuberculosis strain prevalent in Madrid. Clin. Microbiol. Infect. 131210-1213. [DOI] [PubMed] [Google Scholar]
- 36.Ministério da Saúde. 2007. Guia de mesa da tuberculose. Ministério da Saúde, Rio de Janeiro, Brazil. http://portal.saude.gov.br/portal/svs/visualizar_texto.cfm?idtxt=21514.
- 37.Ministério da Saúde. 2006. Sistema nacional de vigilância em saúde: relatório de situação: Minas Gerais. Ministério da Saúde, Rio de Janeiro, Brazil. http://portal.saude.gov.br/portal/arquivos/pdf/relatorio_snvs_mg_2ed.pdf.
- 38.Mostowy, S., D. Cousins, J. Brinkman, A. Aranaz, and M. A. Behr. 2002. Genomic deletions suggest a phylogeny for the Mycobacterium tuberculosis complex. J. Infect. Dis. 18674-80. [DOI] [PubMed] [Google Scholar]
- 39.Newton, S. M., R. J. Smith, K. A. Wilkinson, M. P. Nicol, N. J. Garton, K. J. Staples, G. R. Stewart, J. R. Wain, A. R. Martineau, S. Fandrich, T. Smallie, B. Foxwell, A. Al-Obaidi, J. Shafi, K. Rajakumar, B. Kampmann, P. W. Andrew, L. Ziegler-Heitbrock, M. R. Barer, and R. J. Wilkinson. 2006. A deletion defining a common Asian lineage of Mycobacterium tuberculosis associates with immune subversion. Proc. Natl. Acad. Sci. USA 10315594-15598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niobe-Eyangoh, S. N., C. Kuaban, P. Sorlin, J. Thonnon, V. Vincent, and M. C. Gutierrez. 2004. Molecular characteristics of strains of the Cameroon family, the major group of Mycobacterium tuberculosis in a country with a high prevalence of tuberculosis. J. Clin. Microbiol. 425029-5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nolte, F. S., and B. Metchock. 1995. Mycobacterium, p. 403-437. In P. R. Murray, F. J. Barron, M. A. Pfaller, F. C. Tenover, and R. I. Yolken (ed.), Manual of clinical microbiology, 6th ed. ASM Press, Washington, DC.
- 42.Palaci, M., R. Dietze, D. J. Hadad, F. K. Ribeiro, R. L. Peres, S. A. Vinhas, E. L. Maciel, V. do Valle Dettoni, L. Horter, W. H. Boom, J. L. Johnson, and K. D. Eisenach. 2007. Cavitary disease and quantitative sputum bacillary load in cases of pulmonary tuberculosis. J. Clin. Microbiol. 454064-4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pillay, M., and A. W. Sturm. 2007. Evolution of the extensively drug-resistant F15/LAM4/KZN strain of Mycobacterium tuberculosis in KwaZulu-Natal, South Africa. Clin. Infect. Dis. 451409-1414. [DOI] [PubMed] [Google Scholar]
- 44.Ramakrishnan, L., N. A. Federspiel, and S. Falkow. 2000. Granuloma-specific expression of Mycobacterium virulence proteins from the glycine-rich PE-PGRS family. Science 2881436-1439. [DOI] [PubMed] [Google Scholar]
- 45.Reed, M. B., P. Domenech, C. Manca, H. Su, A. K. Barczak, B. N. Kreiswirth, G. Kaplan, and C. E. Barry III. 2004. A glycolipid of hypervirulent tuberculosis strains that inhibits the innate immune response p84. Nature 43184-87. [DOI] [PubMed] [Google Scholar]
- 46.Richardson, M., N. M. Carroll, E. Engelke, G. D. van der Spuy, F. Salker, Z. Munch, R. P. Gie, R. M. Warren, N. Beyers, and P. D. van Helden. 2002. Multiple Mycobacterium tuberculosis strains in early cultures from patients in a high-incidence community setting. J. Clin. Microbiol. 402750-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riley, L. W. 2006. Of mice, men, and elephants: Mycobacterium tuberculosis cell envelope lipids and pathogenesis. J. Clin. Investig. 1161475-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shaw, J. B., and N. Wynn-Williams. 1954. Infectivity of pulmonary tuberculosis in relation to sputum status. Am. Rev. Tuberc. 69724-732. [DOI] [PubMed] [Google Scholar]
- 49.Shemyakin, I. G., V. N. Stepanshina, I. Y. Ivanov, M. Y. Lipin, V. A. Anisimova, A. G. Onasenko, O. V. Korobova, and T. M. Shinnick. 2004. Characterization of drug-resistant isolates of Mycobacterium tuberculosis derived from Russian inmates. Int. J. Tuberc. Lung Dis. 81194-1203. [PubMed] [Google Scholar]
- 50.Sreevatsan, S., X. Pan, K. E. Stockbauer, N. D. Connell, B. N. Kreiswirth, T. S. Whittam, and J. M. Musser. 1997. Restricted structural gene polymorphism in the Mycobacterium tuberculosis complex indicates evolutionarily recent global dissemination. Proc. Natl. Acad. Sci. USA 949869-9874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Streicher, E. M., T. C. Victor, G. van der Spuy, C. Sola, N. Rastogi, P. D. van Helden, and R. M. Warren. 2007. Spoligotype signatures in the Mycobacterium tuberculosis complex. J. Clin. Microbiol. 45237-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun, Y. J., T. K. Lim, A. K. Ong, B. C. Ho, G. T. Seah, and N. I. Paton. 2006. Tuberculosis associated with Mycobacterium tuberculosis Beijing and non-Beijing genotypes: a clinical and immunological comparison. BMC Infect. Dis. 6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Supply, P., S. Lesjean, E. Savine, K. Kremer, D. van Soolingen, and C. Locht. 2001. Automated high-throughput genotyping for study of global epidemiology of Mycobacterium tuberculosis based on mycobacterial interspersed repetitive units. J. Clin. Microbiol. 393563-3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Talaat, A. M., R. Lyons, S. T. Howard, and S. A. Johnston. 2004. The temporal expression profile of Mycobacterium tuberculosis infection in mice. Proc. Natl. Acad. Sci. USA 1014602-4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Valway, S. E., M. P. Sanchez, T. F. Shinnick, I. Orme, T. Agerton, D. Hoy, J. S. Jones, H. Westmoreland, and I. M. Onorato. 1998. An outbreak involving extensive transmission of a virulent strain of Mycobacterium tuberculosis. N. Engl. J. Med. 338633-639. [DOI] [PubMed] [Google Scholar]
- 56.van Crevel, R., R. H. Nelwan, W. de Lenne, Y. Veeraragu, A. G. van der Zanden, Z. Amin, J. W. van der Meer, and D. van Soolingen. 2001. Mycobacterium tuberculosis Beijing genotype strains associated with febrile response to treatment. Emerg. Infect. Dis. 7880-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Voskuil, M. I., D. Schnappinger, R. Rutherford, Y. Liu, and G. K. Schoolnik. 2004. Regulation of the Mycobacterium tuberculosis PE/PPE genes. Tuberculosis 84256-262. [DOI] [PubMed] [Google Scholar]
- 58.Reference deleted.
- 59.World Health Organization. 2007. Tuberculosis fact sheet no. 104. World Health Organization, Geneva, Switzerland. http://www.who.int/mediacentre/factsheets/fs104/en/index.html.
- 59a.World Health Organization. 2008. WHO global report WHO/HTM/TB/2008/393. World Health Organization, Geneva, Switzerland. http://www.who.int/tb/publications/global_report/2008/pdf/bra.pdf.
- 60.Yeh, R. W., P. C. Hopewell, and C. L. Daley. 1999. Simultaneous infection with two strains of Mycobacterium tuberculosis identified by restriction fragment length polymorphism analysis. Int. J. Tuberc. Lung Dis. 3537-539. [PubMed] [Google Scholar]