Abstract
Kaposi's sarcoma-associated herpesvirus (KSHV)-encoded glycoprotein B (gB) is an important determinant of viral infectivity and virion egress. A small interfering RNA (siRNA)-based strategy was devised to inhibit KSHV gB gene expression. Transient cotransfection of plasmids constitutively expressing gB and anti-gB siRNAs in 293 cells substantially inhibited gB mRNA levels and protein production. Similarly, transient expression of siRNAs into the primary effusion lymphoma cell line BCBL-1 caused a substantial reduction of gB transcripts and protein synthesis. TaqMan real-time PCR assays against the lytic KSHV gene ORF59 and infectivity assays on 293 cells were employed to assess the effect of inhibiting gB synthesis on virion egress from BCBL-1 cells and infectivity on 293 cells, respectively. These experiments showed that gB was essential for virion egress and infectivity. Transfection of a codon-optimized gB gene with the first 540 nucleotides altered, and therefore not recognized by anti-gB siRNAs that target the native but not the codon-optimized sequence, efficiently rescued virion egress and infectivity in BCBL-1 cells in the presence of siRNAs inhibiting wild-type gB expression. To assess the role of the cytoplasmic domain of gB in virion egress, mutant gB genes were generated specifying carboxyl terminal truncations of 25 and 58 amino acids disrupting two prominent predicted α-helical domains associated with virus-induced cell fusion. A third truncation removed the entire predicted cytoplasmic terminus of 84 amino acids, while a fourth truncation removed 110 amino acids, including the terminal most hydrophobic, intramembrane anchoring sequence. Virion egress experiments revealed that all truncated gBs facilitated virion egress from BCBL-1 cells, with the exception of the largest 110-amino-acid truncation, which removed the gB anchoring sequence. Importantly, the gB truncation that removed the entire predicted cytoplasmic domain increased virion egress, suggesting the presence of a egress regulation domain located proximal to the intramembrane sequence within the cytoplasmic domain of gB. All supernatant virions were infectious on 293 cells, indicating that the carboxyl terminus of gB is not essential for either virion egress or virus infectivity.
Kaposi's sarcoma-associated herpesvirus (KSHV), also referred to as human herpesvirus 8, is a member of the gamma-2-herpesvirus family (genus Rhadinovirus) (46, 59). KSHV is etiologically associated with Kaposi's sarcoma, primary effusion or body cavity-based lymphoma, and multicentric Castleman's disease (4, 21, 60). KSHV can infect a variety of human cell types, including B, T, endothelial, epithelial, fibroblastic, and keratinocyte cells, and nonhuman cell types, including owl monkey kidney and baby hamster kidney fibroblastic cells (13, 15, 19, 20, 22, 28, 36, 43, 44, 50, 56, 62, 67). All herpesviruses initiate infection by means of direct binding to various receptors on cell surfaces that is mediated by several viral glycoproteins embedded in viral envelopes. Viral glycoproteins play important roles in virus attachment to susceptible cells, fusion of the viral envelope with either cellular or endosomal membranes, and virion morphogenesis and egress (26, 42, 57). Herpesviruses enter into susceptible cells by direct fusion of viral envelopes with cellular membranes via pH-independent or pH-dependent processes, depending on the cell type (7, 16, 40, 47, 48), or via receptor-mediated endocytosis (61), as has been shown for KSHV entry into human foreskin fibroblastic cells and B cells (1, 3).
Herpesviruses assemble their capsids within the nuclei of infected cells and most likely egress by an envelopment-de-envelopment process in which they acquire a primary envelope from the inner lamellae of the nuclear membrane and subsequently de-envelop by fusion of their initial viral envelopes with the outer nuclear lamellae, releasing capsids into the cytoplasm. Final cytoplasmic envelopment is thought to occur in the cytoplasm by the budding of cytoplasmic capsids into intracellular membranes, most likely derived from the trans-Golgi network (37-39, 45). KSHV codes for a number of glycoproteins, some of which have significant homology to glycoproteins of other herpesviruses. These include glycoprotein B (gB) (open reading frame 8 [ORF8]) (49), gH (ORF22), gM (ORF39), gL (ORF47) (46, 59), and gN (ORF53) (14, 30, 59). KSHV also encodes additional glycoproteins which do not have homologs in other herpesviruses, including gpK8.1A, gpK8.1B, K1, K14, and K15, which are expressed during lytic replication (59).
gB is one of the most conserved herpesvirus glycoproteins. It is an essential virion component for members of the alpha- and betaherpesvirus subfamilies and thought to function in virion attachment and virus entry into susceptible cells (10, 17, 52). Epstein-Barr virus (EBV), a gamma-1-herpesvirus (genus Lymphocryptovirus) (25), and murine gammaherpesvirus 68, a member of the gamma-2-herpesvirus (genus Rhadinovirus) subfamily, do not incorporate detectable levels of gB into their virions (24). However, virions of bovine herpesvirus 4, a gamma-2-herpesvirus (9, 34), contain gB (34). KSHV virions incorporate gB in the viral envelope, which is important for attachment to cell surfaces and entry via an RGD-dependent binding to integrins. Initially, the α3β1 integrin was implicated (2), but more recently, it was shown that αVβ3 is the integrin involved in gB RGD-mediated virus entry (23). KSHV gB is a type 1 membrane glycoprotein of 845 amino acids in length (6, 53, 55). It contains a predicted signal sequence of 23 amino acids, an extracellular domain containing multiple glycosylation sites, and multiple hydrophobic regions, of which the most carboxyl-terminal region serves to anchor gB in membranes (6, 53-55). gB has been shown to be involved in the egress of herpesviruses, such as alphaherpesvirus, pseudorabies virus, and EBV (8, 29, 32, 51, 52). KSHV gB was also shown to be essential for virion egress in 293 cells, since a KSHV mutant virus carrying a deletion of the gB gene was unable to egress from 293T cells, while the defect was rescued by exogenously provided gB (31). In contrast to these examples, herpes simplex virus type 1 (HSV-1) gB is not required for virion egress (11). More recently, however, it was shown that that the carboxyl terminus of HSV-1 gB is ubiquitinated and it appears to regulate virion egress (12).
RNA interference (RNAi) is the process by which double-stranded RNA silences gene expression, either by inducing the sequence-specific degradation of complementary mRNA or by inhibiting translation (41). Chemically synthesized short double-stranded RNA molecules of 21 or 22 nucleotides known as small interfering RNAs (siRNAs) were shown to cause mRNA degradation via the RNAi mechanism while evading the interferon response (18). Furthermore, endogenous expression of siRNA in the form of short hairpin RNAs, which bear a fold-back stem-loop structure (8-11), induced target gene silencing in mammalian cells. RNAi and siRNA technologies have been instrumental as molecular biological tools for the silencing of specific gene expression under a variety of experimental settings (27). For KSHV, siRNA approaches have been used to investigate specific viral functions (64, 66).
We employed an siRNA approach to conditionally silence KSHV gB gene expression in 293 and BCBL-1 cells. In this system, rescue of gB synthesis was accomplished by providing a synthetic gB with an altered DNA sequence which could not be recognized by the anti-gB siRNAs. This siRNA-based conditional silencing system was utilized to investigate the role of the cytoplasmic terminus of KSHV gB in virion egress. We found that the carboxyl terminus of gB contains a domain that regulates virion egress, but overall, the cytoplasmic domain of gB is not essential either for virion egress from BCBL-1 cells or for virus infectivity.
MATERIALS AND METHODS
Cells and virus propagation.
BCBL-1 cells harboring the rKSHV.152 genome constitutively expressing the green fluorescent protein (GFP) (gift from J. Vieira) were cultured in RPMI 1640 (Hyclone) medium with 10% heat-inactivated fetal bovine serum (Hyclone) with 2 mM l-glutamine, 1% penicillin-streptomycin (Gibco), and 250 mg of G418/ml (Gibco). 293A cells (ATCC) were cultured in Dulbecco's modified Eagle's medium with 10% fetal bovine serum and 1% penicillin-streptomycin. The KSHV lytic cycle was induced in the BCBL-1 cells by adding 25 ng/ml of 12-O-tetradecanoylphorbol-13-acetate (TPA; Sigma, St. Louis, MO). Virus from the supernatant was collected after 16 h and treated with DNase I before the extraction of viral DNA as previously described (35). For infectivity studies, the supernatants were collected 48 h after induction with TPA.
Antibodies.
Rabbit polyclonal antibodies generated in our laboratory against KSHV gB peptides were used to detect gB protein by Western blot analysis as previously described (6). Peptide antibodies specific to amino acids 167 to 191 of the gB protein were used in this study. Mouse anti-HuGAPDH (human glyceraldehyde-3-phosphate dehydrogenase) monoclonal antibodies were used to detect the GAPDH.
Vector construction.
SiRNAs were designed targeting the 5′ region of the KSHV gB expression sequence by use of Ambion's online siRNA target finder. Two siRNAs, si18 (sense strand, HindIII [5′ AGCTTCAAGTATGAACTCCCGAGATTCAAGAGATCTCGGGAGTTCATACTTGTTG 3′]; antisense strand, EcoRI [5′-AATTCAACAAGTATGAACTCCCGAGATCTCTTGAATCTCGGGAGTTCATACTTGA-3′]), located starting at nucleotide 403 after the ATG codon, and si22 (sense strand, HindIII [5′AGCTTGGTAAATGTCAACGGGGTATTCAAGAGATACCCCGTTGACATTTACCTTG 3′]; antisense strand, EcoRI [5′-AATTCAAGGTAAATGTCAACGGGGTATCTCTTGAATACCCCGTTGACATTTACCA-3′]), located starting at 484 bases from the ATG codon, were selected among 25 siRNAs against gB. The siRNAs contained HindIII and EcoRI overhangs at the 5′ and 3′ ends, respectively, for cloning purposes. Equimolar concentrations of the individual strands of siRNAs were mixed, denatured at 94°C for 5 min, and allowed to cool down slowly to room temperature to allow annealing of the two strands. The mixture was used for ligation into HindIII/EcoRI-digested p3xFLAG vector by use of T4 DNA ligase (NEB, Inc.). Ligation mix was transformed into one-shot TOP10 competent cells (Invitrogen) and selected on LB agar plus 100 μg/ml ampicillin. Recombinant clones were confirmed by restriction analysis and nucleic acid sequencing.
Codon optimization and construction of gB truncations.
The region consisting of the first 540 bases of KSHV gB from ATG to the HpaI restriction site, which carried the siRNA target sequences, was codon optimized using the DNAWorks website at http://helixweb.nih.gov/dnaworks. The wild-type KSHV gB was cloned in the expression vector p3xFLAG within the KpnI and BamHI sites. The codon-optimized fragment was used to replace the first 540-bp region between the KpnI and HpaI sites of the wild-type gB gene. The gB carboxyl-end truncations were generated by designing forward primers for the 5′ end of the codon-optimized gB and reverse primers carrying stop codons 75, 174, 252, and 330 bp from the 3′ end of gB to generate truncation gBp, gBg, gBc, and gBtrm gene constructs, respectively. The forward primers for codon-optimized gB and reverse primers carrying the opal mutations are indicated in Table 1. A 577-bp encephalomyocarditis virus internal ribosome entry site (IRES) sequence was inserted downstream of the siRNA in the p3xFLAG vector between the BglII and EcoRV restriction sites. The codon-optimized gB gene sequences coding for the gB truncations were cloned downstream of the IRES at the BamHI site. Plasmid vectors expressed siRNA individually or in combination with any one of the codon-optimized gBco, gBp, gBg, gBc, or gBtrm constructs.
TABLE 1.
List of primers
| Primer name (description) | Sequence (5′ → 3′) |
|---|---|
| gBco (gB optimized; forward) | ACAGCAAAGCTTATGACCCCCAGGAGCAGGCTG |
| gBp (25-aa gB truncation; reverse) | TTGTATGGATCCTCATGCGGTACGCTGAAACACCGA |
| gBg (58-aa gB truncation; reverse) | ATAGAAGGATCCTCAGATTTCCTCCCGTGTTGGGGC |
| gBc (84-aa gB truncation; reverse) | GGATCCTCTTCACTCACGCCTGGGCTA |
| gBtrm (110-aa gB truncation; reverse) | GGATCCTCACATGCCACCTAGGGGGTGTTT |
| gBUNI (gB universal; forward) | TCCAGACTACCCACGAGGAC |
| gBUNI (gB universal; reverse) | GTCAGGTTAATCGCGGACAT |
| gBwt (forward) | GACACCTTTCAGACGT |
| gBwt (reverse) | TCTCGGGAGTTCATACTTGT |
| gB (carboxy; forward) | ATCGTTATAGCAATCATC |
| gB (carboxy; reverse) | TCACTCCCCCGTTTCCGG |
| ORF59 (forward) | TCAGCTTCAGGAATACGTCCG |
| ORF59 (reverse) | GGCTATGCCAGCGTCGAGTA |
| ORF59 (probe) | FAMa-CGCGTGAGCTATTCGGTGCGAATA-TAMRA |
| GAPDH (forward) | GATTCCACCCATGGCAAATT |
| GAPDH (reverse) | AAGATGGTGATGGGATTTCCATT |
FAM, 6-carboxyfluorescein.
Transient transfection of 293 and BCBL-1 cells.
293 cells were seeded onto 12-well plates at 0.5 × 105 cells/well for 8 hours, medium was replaced with 500 μl of fresh medium, and cells were used for transfection experiments. BCBL-1 cells (0.5 × 105 cells/well) were suspended in fresh medium and used for each transfection. Transfections were carried out using SuperFect (Qiagen, Inc.) transfection agent according to the manufacturer's instructions.
Immunohistochemical studies.
To detect the expression of gB mutant genes, 293 cells were transiently transfected with plasmid vectors carrying gBco, gBp, gBg, gBc, and gBtrm as described earlier. Forty-eight hours posttransfection, the cells were fixed, reacted with anti-gB peptide antibody, and stained using the Vector NovaRED substrate kit (Vector Laboratories Inc., California), as per the manufacturer's instructions.
RT-PCR.
Transiently transfected 293 cells were harvested 48 h after transfection and total RNA was extracted. Specifically, medium was aspirated and cells were scraped and resuspended evenly in 200 μl of ice-cold phosphate-buffered saline. Seven hundred microliters of Tri-Reagent (Molecular Research Center, Inc.) was added to the cells and mixed thoroughly to lyse the cells. After 15 min, 200 μl of chloroform was added and mixed thoroughly. The tubes were placed on ice for 15 min and centrifuged at 13 × 103 rpm for 15 min at 4°C. The supernatant was collected, precipitated in an equal volume of isopropanol, and left on ice for 30 min. The mix was centrifuged at 4°C for 20 min at 13 × 103 rpm. The resultant pellet was washed in 70% ethanol made with nuclease-free water, dried, and resuspended in 40 μl of nuclease-free water. The RNA was treated with Turbo DNase I (Ambion, Inc.) and quantified. First-strand cDNA was made using the high-capacity cDNA reverse transcription (RT) kit (Applied Biosystems, Inc.). Equal quantities of first-strand cDNA were used for PCR using the universal gB primers and the primers for GAPDH. The PCR products were resolved by electrophoresis on a 1% agarose gel.
For RT-PCR quantification of RNA derived from BCBL-1 cells, lytic replication of KSHV was induced by adding 25 ng/ml TPA to transiently transfected BCBL-1 cells 24 h after transfection. Induction was carried out in serum-free medium and the supernatant and cells were collected 16 h postinduction. Harvested cells were resuspended in 500 μl of ice-cold phosphate-buffered saline and split into two parts for RNA extraction (200 μl) and Western blot analysis (300 μl). Total RNA was extracted and first-strand cDNA was prepared as described earlier. Equal amounts of first-strand cDNA were used for PCR using the universal gB primers and the primers for GAPDH (Table 1) in the case of 293 cells. RT-PCR of RNA derived from BCBL-1 cells was carried out using primers specific to full-length wild-type gB and the carboxyl-terminal region of gB (Table 1) in addition to primers for universal gB and GAPDH. The PCR products were resolved by electrophoresis on a 1% agarose gel.
Western blot analysis.
The 293 or BCBL-1 cells were pelleted and resuspended in 100 μl of radioimmunoprecipitation assay lysis buffer and sonicated at 15% strength for 15 seconds. Cell debris was pelleted at 14,000 rpm for 10 min at 4°C and the total soluble protein (TSP) was transferred to a fresh tube and quantified using the bicinchoninic acid kit (Pierce Biotechnology, Inc.). Fifteen micrograms of TSP from each sample was loaded on a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel and resolved by electrophoresis. The proteins were transferred onto a nitrocellulose membrane and used for Western blot analysis. Rabbit polyclonal anti-gB and mouse monoclonal anti-GAPDH antibodies were used as primary antibodies. Horseradish peroxidase-tagged anti-rabbit/anti-mouse antibodies were used as secondary antibodies. ECL substrate (Amersham Biosciences) was used for chemiluminescent detection of proteins.
TaqMan real-time PCR analysis.
Real-time PCR was carried out on viral DNA from supernatants of BCBL-1 cells essentially as described previously (35). Specifically, the primers and probe (6-carboxytetramethylrhodamine [TAMRA]) for the real-time PCR were designed to detect ORF59 (Table 1). Supernatants were collected 16 h after lytic cycle induction and 200 μl was used for the extraction of viral DNA. The supernatants were treated with Turbo DNase I (Ambion, Inc.) for 2 h at 37°C. Viral DNA was extracted using the DNeasy blood and tissue kit (Qiagen, Inc.) as per the manufacturer's instructions. Equal volumes of viral DNA were used for TaqMan PCR analysis. Purified KSHV bacterial artificial chromosome 36 (BAC 36) DNA was used to generate the standard curve.
Infectivity of BCBL-1 supernatants on 293 cells.
Supernatants of BCBL-1 cells containing infectious virions were collected after TPA induction of viral replication for 48 h. 293 cells seeded at 75% confluence in 12-well plates were infected with BCBL-1 supernatants by use of 2 μg/ml Polybrene. The number of infected cells was visualized by counting 293 cells expressing enhanced GFP (EGFP) by use of a fluorescence microscope at 48 h postinfection. Infection was quantified by counting the number of infected cells in three different 10× viewing fields and calculating the mean value. The process was repeated for all tissue culture wells. The experiment was repeated three times.
RESULTS
Construction and testing of a conditional silencing system for KSHV gB expression.
The siRNAs si18 and si22, which target gB mRNA sequences starting at nucleotides 403 and 484, respectively (Fig. 1A), were designed as described in Materials and Methods. These two siRNAs were selected from a list of more than 25 siRNAs based on their specificity for the KSHV gB mRNA after extensive homology searches using BLAST and CLUSTALX algorithms. To develop conditional inhibition of gB expression using the si18 and si22 siRNAs, the first 540 bp of the DNA coding sequence of gB was codon optimized as described in Materials and Methods. Codon optimization substantially altered the 5′-most DNA sequence of the gB gene, effectively destroying recognition of the codon-optimized gB mRNA by the si18 and si22 siRNAs, while the encoded amino acid sequence was unaltered (Fig. 1B). The wild-type KSHV gB (gBw) and the codon-optimized gB (gBco) were cloned into plasmid p3xFLAG (Sigma, Inc.) The siRNAs si18 and si22 were cloned either individually in the 3×FLAG plasmid (psi18, psi22) or together in tandem with the gBco gene separated by a 577-bp encephalomyocarditis virus IRES situated immediately downstream from the siRNA sequences (psi18/22gBco) (see Materials and Methods).
FIG. 1.
Schematic representation of gB- and siRNA-expressing gene cassettes. (A) Schematic representation of the KSHV gB showing the position of the siRNA target sequences on the wild-type gB and the codon-optimized gB portion. The codon-optimized fragment replacing 540 bp in the 5′ end between Kpn1 and Hpa1 is shown as a light-shaded area of the gB gene. (B) Schematic of the siRNA gene expression cassette containing the siRNA (dark-shaded box) and gB genes separated by an IRES. The approximate locations of the different gB truncations are shown. The three different amplimers used for diagnostic PCR are indicated by light-shaded areas bracketed by PCR primers (arrows). CMV, cytomegalovirus.
Conditional silencing of gB expression was tested in transient expression experiments with 293 cells. Cells were cotransfected with mixtures of plasmids containing gBw mixed with either psi18 or psi22 or with an equimolar mixture of plasmids containing gBco with either psi18 or psi22 plasmids. RT-PCR analysis using primers which are equally specific for gBw and gBco (universal gB primers; gBUNI) showed a significant reduction in the levels of gB transcripts in 293 cells cotransfected with wild-type gB and siRNAs 18 and 22, as also evidenced after densitometric analysis using levels of GAPDH transcripts for normalization purposes (Fig. 2). In contrast, cotransfection of gBco with either psi18 or psi22 did not reduce the levels of gB transcripts (Fig. 2). gB transcripts were not detected in RNA derived from untransfected 293 cells or cells transfected with the transfecting agent alone (Fig. 2). Both gBw and gBco appeared to produce similar amounts of gB transcripts in the presence of a nonspecific siRNA provided for control purposes (Fig. 2).
FIG. 2.
RT-PCR analysis for detection of gB mRNA in transiently transfected 293 cells. 293 cells were either transfected with plasmid vectors expressing wild-type gB individually or cotransfected with vectors expressing siRNA and codon-optimized gB. RT-PCR was carried out using primers specific to gB (universal gB primers; 250 bp) and GAPDH primers, producing an 80-bp amplicon. cDNA from 293 cells and 293 cells transfected with SuperFect were used as controls. Additional controls included 293 cells cotransfected with vectors expressing either wild-type gB and nonspecific siRNA or codon-optimized gB (gBco) and nonspecific siRNA. The levels of gB transcripts obtained after densitometric analysis and normalization with GAPDH are shown aligned below for each sample.
Similar experiments were performed as outlined above, with the exception that cellular extracts were processed to detect gB expression in Western blots. gB expression was quantified by densitometry and normalized to GAPDH protein levels. Significant inhibition of gB expression was observed for 293 cells cotransfected with plasmids expressing gBw and either si18 or si22 in comparison to cells transfected with gBw and a nonspecific siRNA (Fig. 3). In contrast, the amount of gB detected in samples derived from cotransfections of 293 cells with gBco and either si18 or si22 was similar to that seen for gB from cells cotransfected with gBco and the nonspecific siRNA, indicating that the siRNAs did not inhibit gBco expression (Fig. 3).
FIG. 3.
Western blot analysis for detection of gB protein in transiently transfected 293 cells. (A) 293 cells either transfected with plasmid vectors expressing wild-type gB individually or cotransfected with vectors expressing siRNA and codon-optimized gB. TSP was extracted and used for Western blot analysis. Rabbit anti-gB antibodies were used to detect the 75-kDa gB protein species and mouse anti-HuGAPDH was used to detect GAPDH protein for normalization purposes. TSP from 293 cells and TSP from 293 cells transfected with SuperFect were used as controls. Additional controls included samples derived from 293 cells cotransfected with plasmid vectors expressing either wild-type gB and nonspecific siRNA or codon-optimized gB (gBco) and nonspecific siRNA. The levels of gB protein obtained after densitometric analysis of Western immunoblots and normalization with GAPDH protein are shown aligned below for each sample. α-, anti-.
Role of the carboxyl terminus of gB in virion egress from BCBL-1 cells.
To investigate the role of the carboxyl terminus of gB in virion egress, a panel of mutated gB genes was constructed, specifying different gB truncations by using the gBco gene as the template for mutagenesis. The carboxyl terminus of gB was truncated at specific sites to disrupt predicted α-helical structures (Fig. 4). The gB truncations include gBp, which has 25 amino acids deleted; gBg, with a deletion of 58 amino acids; gBc, with 84 amino acids deleted; and gBtrm, with 110 amino acids deleted. gBp disrupts the predicted α-helical domain α1, while the gBg truncation disrupts the predicted α-helical domain α2. gBc removed the entire predicted cytoplasmic domain of gB, while gBtrm removed the entire carboxyl terminus, including part of the predicted transmembrane domain (Fig. 4). Plasmids coding for the gB truncations were transfected into 293 cells and gBs were detected by immunohistochemistry. All gB constructs, including the gBco full-copy gB, were detected by anti-gB antibodies, except for gBtrm, which was not detectable (Fig. 5). Low levels of gBtrm were detected in supernatants of infected cells, suggesting that gBtrm was secreted into extracellular spaces (not shown).
FIG. 4.
Schematic diagram showing the predicted secondary structure of the KSHV gB carboxy-terminal cytoplasmic domain. The positions of the four truncations, namely, gBp, gBg, gBc, and gBtrm, are indicated.
FIG. 5.
Immunohistochemistry for detecting expression of gB mutants in 293 cells. Anti-gB antibodies were utilized to detect gB expression in transiently transfected cells at 48 h posttransfection as described in Materials and Methods.
To investigate the ability of si18 and si22 siRNAs to inhibit gB expression in BCBL-1 cells, similar experiments were performed as outlined earlier for the transient expression experiments with 293 cells by use of RT-PCR to detect gB mRNAs and GAPDH for normalization purposes. In these experiments, BCBL-1 cells were transfected and 24 h posttransfection cells were induced with TPA as detailed in Materials and Methods. Transfection of psi18 or psi22 in BCBL-1 cells significantly reduced endogenous gBwt mRNA levels detected by RT-PCR using primers that amplify a 338-bp gBwt-specific DNA fragment in comparison to what was seen for transfection with the nonspecific siRNA (Fig. 6A). In contrast, in BCBL-1 cells transfected with plasmids psi18gBco and psi22gBco, which coexpress the codon-optimized gB with si18 and si22, respectively, gB levels were approximately the same as those detected for BCBL-1 cells transfected with the nonspecific siRNA (Fig. 6B). RT-PCR products shown in the row labeled gBUNI were produced using gB primers, which amplify a 240-bp DNA fragment situated outside the codon-optimized region of gBco and are therefore predicted to amplify both gBwt and gBco sequences with equal efficiency.
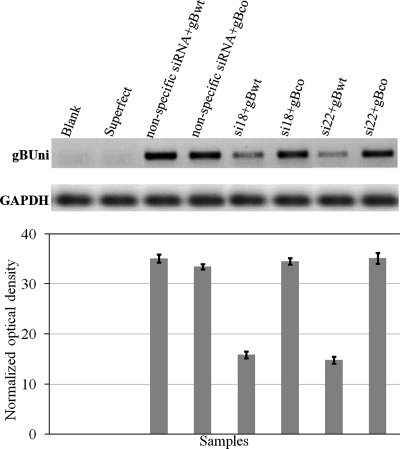
FIG. 6.
Detection of gB mRNA in BCBL-1 cells. Total RNA was extracted from transiently transfected BCBL-1 cells and first-strand cDNA was used for PCR using primers. Shown are wild-type gB (gBwt) (A), universal gB primers amplifying both wild-type and codon-optimized gB (gBUNI) (B), and gB carboxy primers amplifying the 3′ end of gB (gBCarb) (C) and GAPDH primers producing an 80-bp amplicon. The levels of gB transcripts obtained after densitometric analysis and normalization with GAPDH are shown aligned below for each sample.
Similar transfection experiments were performed with each of the gB constructs encoding carboxyl-terminal truncations of gB. Coexpression of gBp, gBg, gBc, and gBtrm with plasmids expressing either si18 or si22 elevated gB expression in BCBL-1 cells to levels similar to those observed for BCBL-1 cells transfected with the nonspecific siRNA (Fig. 6B). As expected, PCR primers which specifically amplify a 317-bp DNA fragment encoding the gB carboxyl terminus did not detect the elevated levels of the truncated gB transcripts observed with the universal gB primers, because the targeted gB gene sequence was absent from all gB mutant genes (Fig. 6C). The amount of gB transcripts detected is similar to that observed for si18- or si22-inhibitory conditions (Fig. 6C).
Similar experiments were performed with BCBL-1 cells as described above, with the exception that gB expression was detected using Western blot assays. Transfection of BCBL-1 cells with either psi18 or psi22 plasmids reduced the amount of gB detected using anti-gB specific antibody in comparison to gB levels in cells transfected with the nonspecific siRNA (Fig. 7A and B). As observed previously for the gB mRNA levels, coexpression of gBco, gBp, gBg, and gBc with plasmids expressing either si18 or si22 elevated gB expression in BCBL-1 cells to levels similar to those observed for BCBL-1 cells transfected with the nonspecific siRNA (Fig. 7A and B). Coexpression of gBtrm with either si18 or si22 did not elevate the overall amount of gB expression, as noted previously for the transient transfection experiments with 293 cells (Fig. 7A and B).
FIG. 7.
Western blot analysis for detection of gB in transiently transfected BCBL-1 cells. (A) Cells were transfected with plasmid vectors expressing si18 and various gB genes as indicated. (B) Cells were transfected with plasmid vectors expressing si22 and various gB genes as indicated. The levels of gB protein detected by Western immunoblot analysis and quantified by densitometric analysis and normalization with GAPDH protein are shown aligned below for each sample.
It has been previously shown that gB is essential for virion egress from 293 cells (31). Therefore, we investigated whether the inhibition of gB mRNA levels by the si18 and si22 siRNAs could reduce the amount of virion particles in the supernates of BCBL-1 cells. The number of virion particles in the supernates of BCBL-1 cells was quantified by real-time TaqMan PCR after DNase treatment of extracellular virions to specifically detect fully encapsidated viral DNA, as described above (see Materials and Methods) and previously (35). Transfection of BCBL-1 cells with either si18- or si22-expressing plasmids substantially reduced the amount of viral genomes detected in the supernates of BCBL-1 cells (Fig. 8). Coexpression of gBco with either si18 or si22 rescued virion egress to levels similar to those observed for BCBL-1 cells transfected with the nonspecific siRNA (Fig. 8). Coexpression of the gBp and gBg truncations with either si18 or si22 rescued virion egress to a level similar to that observed for the nonspecific siRNA control sample, with the exception of the gBtrm (Fig. 8). In contrast, coexpression of the gBc truncation with either si18 or si22 reproducibly increased virion egress by nearly 20% in comparison to what was seen for the nonspecific siRNA control sample, while coexpression of gBtrm with either si18 or si22 failed to rescue virion egress (Fig. 8).
FIG. 8.
Determination of virion egress by use of real-time PCR. Transiently transfected BCBL-1 cells were induced with 25 ng/ml TPA for 12 h in serum-free medium. Two hundred microliters of supernatant was collected and treated with Turbo DNase for 2 h. Viral DNA was extracted from equal volumes of supernatants by use of a DNeasy tissue and blood kit and used for real-time PCR with primers for ORF59 and the 6-carboxyfluorescein/TAMRA TaqMan probe (Table 1). Serial dilutions of BAC 36 plasmid containing the wild-type KSHV genome were used to generate the standard curve.
To determine the effect of the gB truncations on virion infectivity, supernatant virions were used to infected 293 cells. Infectivity was assayed by the expression of the EGFP gene, which is constitutively expressed by the rKSHV.152 genome (62). These results showed a correlation between the numbers of virion particles and infectious virions found in the supernatants of BCBL-1, revealing that all truncated gBs were able to increase production of infectious virions in the supernatants of BCBL-1 cells, with the exception of the gBtrm-transfected BCBL-1 cells (Fig. 9A and B).
FIG. 9.
Determination of infectious virions in BCBL-1 supernatants. (A) Supernatant virus from transiently transfected BCBL-1 cells was collected 48 h postinduction with TPA and used to infect 293 cells. Infected cells were visualized with a fluorescence microscope. (B) The average numbers of GFP-positive 293 cells in three different fields of visualization were determined.
DISCUSSION
The cloning of the KSHV genome as a BAC has enabled the rapid generation of KSHV mutant viruses (22, 67, 68). Virus mutants lacking specific gene functions have been generated and characterized in cell culture systems permissive for viral replication (31, 35, 65, 68). However, viral yields from cells transfected with KSHV mutant genomes are relatively low, hindering the elucidation of gene functions involved in virion infectivity, assembly, and egress from infected cells. In this study, we utilized anti-gB siRNAs to inhibit gB synthesis in BCBL-1 cells, which are known to produce large amounts of infectious virions after induction of the dormant KSHV genome to lytic replication. Transfection of BCBL-1 cells under anti-native gB siRNA conditions with a codon-optimized gB not susceptible to siRNA inhibition restored gB expression to near-native levels. We utilized this system to demonstrate that the cytoplasmic terminus of gB from amino acid residues 761 to 787 contains a domain that regulates virion egress; overall, however, this domain is not essential for either virion egress or infectivity.
Transient expression of either si18 or si22 siRNAs reduced gB mRNA levels and gB expression in both 293 and BCBL-1 cells by approximately 40 to 60%. Typically, more than 40% of 293 cells can be routinely transfected with plasmids by use of SuperFect (Qiagen, Inc.), as evidenced by transfection rates obtained with an EGFP-expressing plasmid (not shown). Therefore, the observed siRNA-mediated inhibition of mRNA levels and gB expression indicates that the majority of the 293 cells were cotransfected with both siRNA- and gBw-expressing plasmids. In BCBL-1 cells, approximately 20% of the cells can be routinely transfected with an indicator EGFP-expressing plasmid. Furthermore, approximately 15% of BCBL-1 cells can be reproducibly induced to KSHV lytic gene expression after induction with TPA (not shown). Therefore, if the transfection rate of BCBL-1 cells is independent of the rate of KSHV lytic gene expression, only 3% of BCBL-1 cells could be both transfected and induced to lytic gene expression. However, gB mRNA levels were reduced by 40 to 60%, and gB synthesis was reduced by more than 30%, indicating that a disproportionate number of BCBL-1 cells were both transfected and reactivated. Recently, it was shown that BCBL-1 cells in the S phase appeared to be more likely to undergo lytic reactivation than were those in G0/G1 phase, and these cells exhibited a smooth surface topology in comparison to the G0/G1-phase cells (63). Therefore, it is possible that BCBL-1 cells in the S phase may be more amenable to transfection using SuperFect (Qiagen, Inc.), accounting for the observed inhibition of gB mRNA levels and gB expression. Cotransfection with the codon-optimized gB, which was not susceptible to siRNA inhibition, effectively increased gB mRNA levels and gB expression. In these experiments, the siRNAs were cloned in tandem with the codon-optimized gB, which ensured their coexpression into the same transfected 293 and BCBL-1 cells. As discussed earlier, similar arguments can be made to explain the observed recovery of gB mRNA levels and gB synthesis encoded by the codon-optimized gB constructs.
Earlier studies established that KSHV gB was essential for KSHV egress from 293 cells. In these studies, the KSHV gB gene was deleted via mutagenesis of the KSHV BAC pBAC36. Lack of gB led to inhibition of virion egress, while complementation of gB expression by transient expression of plasmids coding for gB recovered virion egress (31). Our results with BCBL-1 cells are in agreement with these published studies and suggest that egress pathways in 293 and BCBL-1 cells are similar with respect to gB functions in virion egress. Complementation of BCBL-1 cells with truncated gBs revealed that the cytoplasmic carboxyl terminus of gB was not essential for either virion egress or virus infectivity. Furthermore, it appeared that the cytoplasmic terminus contained a domain located proximal to the predicted intramembrane region that regulated virion egress, since deletion of the entire gB cytoplasmic domain significantly increased virion egress in comparison to what was seen for the shorter gB truncations. It is possible that the long carboxyl terminus of KSHV gB may bind to one or more other viral proteins and that this binding may affect the rate of cytoplasmic envelopment and virion egress. Apparently, gB functions in virion egress are not entirely conserved among different herpesviruses. For HSV-1, it was originally shown that gB is not essential for virion egress, since virions devoid of gB could egress out of cells but were not infectious (10). In contrast to these results, it was recently shown that the cytoplasmic terminus of gB may be required for efficient virion egress, since gB carboxyl-terminal truncations appeared to reduce virion egress by two- to threefold. This egress phenotype was attributed to the ubiquitination of the cytoplasmic terminus of HSV-1 gB (12). gB has also been shown to be essential for the egress of other herpesviruses, such as pseudorabies virus and EBV (8, 29, 32, 51, 52).
The process of final virion envelopment most likely occurs in the cytoplasm, and it is mediated by interactions between tegument proteins and the cytoplasmic termini of viral membrane proteins and glycoproteins (37, 38). All gB homologs have a rather large cytoplasmic domain ranging from 84 amino acids for KSHV to 109 amino acids for HSV-1. The KSHV gB was shown to interact with the ORF64 tegument protein, which could facilitate virion envelopment and infectious virus production (58). Potential ubiquitination of the carboxyl terminus of KSHV gB, as demonstrated for HSV-1 gB (12), or the presence of arginine-rich regions, as demonstrated for EBV (33), may affect intracellular transport and the localization of KSHV gB within intracellular membranes, indirectly affecting virion envelopment and egress.
All KSHV gB truncations with the exception of the largest (110-amino-acid) truncation were able to complement virion egress and virion infectivity under siRNA inhibitory conditions for the endogenous wild-type gB, indicating that the cytoplasmic domain of gB is not essential for either function. In contrast, partial deletion of the cytoplasmic terminus of HSV-1 gB increased virus-induced cell fusion, while larger deletions resulted in loss of viral infectivity (5). The cytoplasmic domains of KSHV and HSV-1 gB are predicted to be 84 and 109 amino acids, respectively. Despite their different amino acid lengths, two prominent predicted α-helical domains appear to be topologically conserved within their cytoplasmic domains. Furthermore, it was shown that a deletion of 25 amino acids from the KSHV gB terminus increased its ability to cause cell fusion obtained after cotransfection with gH- and gL-expressing plasmids (54), in agreement with the results obtained with a similar deletion in HSV-1 gB (5). Therefore, it appears that KSHV and HSV-1 gBs have both conserved as well as divergent functions in gB-mediated cell fusion, virion assembly, egress, and infectivity.
Overall, the results described herein suggest that KSHV gB domains other than the cytoplasmic domain are involved in virion egress and infectivity. It is likely that the absence of gB may lead to altered conformations of membrane-bound proteins and glycoproteins that are directly involved in cytoplasmic virion envelopment and egress. In this regard, it is possible that the intramembrane and extracytoplasmic domains of gB, either separately or together, are involved in direct or indirect interactions with membrane-bound proteins and glycoproteins involved in cytoplasmic virion envelopment and egress. Additional experiments are required to delineate these functional domains. The siRNA-mediated inhibition of KSHV gene synthesis described here provides an additional tool to investigate the structure and function of individual viral proteins in the KSHV virus life cycle, especially in BCBL-1 cells. The observed high transfection efficiency of BCBL-1 cells induced to lytic replication may enable the concurrent conditional silencing of multiple KSHV genes, enabling the dissection of potential cooperative functions in the KSHV life cycle and virus-induced tumorigenesis.
Acknowledgments
This work was supported by a subproject of grant NIH:NCRR P20 RR16456 to O.D., NIH:NIAID AI43000 to K.G.K., and Core Facilities of NIH NCRR P20 RR020159 to K.G.K.
We gratefully acknowledge Marc Boudreaux for proofreading the document and other Biommed staff for technical assistance.
Footnotes
Published ahead of print on 14 May 2008.
REFERENCES
- 1.Akula, S. M., P. P. Naranatt, N. S. Walia, F. Z. Wang, B. Fegley, and B. Chandran. 2003. Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) infection of human fibroblast cells occurs through endocytosis. J. Virol. 777978-7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2001. Human herpesvirus 8 envelope-associated glycoprotein B interacts with heparan sulfate-like moieties. Virology 284235-249. [DOI] [PubMed] [Google Scholar]
- 3.Akula, S. M., F. Z. Wang, J. Vieira, and B. Chandran. 2001. Human herpesvirus 8 interaction with target cells involves heparan sulfate. Virology 282245-255. [DOI] [PubMed] [Google Scholar]
- 4.Antman, K., and Y. Chang. 2000. Kaposi's sarcoma. N. Engl. J. Med. 3421027-1038. [DOI] [PubMed] [Google Scholar]
- 5.Baghian, A., L. Huang, S. Newman, S. Jayachandra, and K. G. Kousoulas. 1993. Truncation of the carboxy-terminal 28 amino acids of glycoprotein B specified by herpes simplex virus type 1 mutant amb1511-7 causes extensive cell fusion. J. Virol. 672396-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baghian, A., M. Luftig, J. B. Black, Y. X. Meng, C. P. Pau, T. Voss, P. E. Pellett, and K. G. Kousoulas. 2000. Glycoprotein B of human herpesvirus 8 is a component of the virion in a cleaved form composed of amino- and carboxyl-terminal fragments. Virology 26918-25. [DOI] [PubMed] [Google Scholar]
- 7.Bodaghi, B., M. E. Slobbe-van Drunen, A. Topilko, E. Perret, R. C. Vossen, M. C. van Dam-Mieras, D. Zipeto, J. L. Virelizier, P. LeHoang, C. A. Bruggeman, and S. Michelson. 1999. Entry of human cytomegalovirus into retinal pigment epithelial and endothelial cells by endocytosis. Investig. Ophthalmol. Vis. Sci. 402598-2607. [PubMed] [Google Scholar]
- 8.Brack, A. R., J. M. Dijkstra, H. Granzow, B. G. Klupp, and T. C. Mettenleiter. 1999. Inhibition of virion maturation by simultaneous deletion of glycoproteins E, I, and M of pseudorabies virus. J. Virol. 735364-5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bublot, M., P. Lomonte, A. S. Lequarre, J. C. Albrecht, J. Nicholas, B. Fleckenstein, P. P. Pastoret, and E. Thiry. 1992. Genetic relationships between bovine herpesvirus 4 and the gammaherpesviruses Epstein-Barr virus and herpesvirus saimiri. Virology 190654-665. [DOI] [PubMed] [Google Scholar]
- 10.Cai, W. H., B. Gu, and S. Person. 1988. Role of glycoprotein B of herpes simplex virus type 1 in viral entry and cell fusion. J. Virol. 622596-2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai, W. Z., S. Person, S. C. Warner, J. H. Zhou, and N. A. DeLuca. 1987. Linker-insertion nonsense and restriction-site deletion mutations of the gB glycoprotein gene of herpes simplex virus type 1. J. Virol. 61714-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calistri, A., P. Sette, C. Salata, E. Cancellotti, C. Forghieri, A. Comin, H. Gottlinger, G. Campadelli-Fiume, G. Palu, and C. Parolin. 2007. Intracellular trafficking and maturation of herpes simplex virus type 1 gB and virus egress require functional biogenesis of multivesicular bodies. J. Virol. 8111468-11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cerimele, F., F. Curreli, S. Ely, A. E. Friedman-Kien, E. Cesarman, and O. Flore. 2001. Kaposi's sarcoma-associated herpesvirus can productively infect primary human keratinocytes and alter their growth properties. J. Virol. 752435-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chandran, B., C. Bloomer, S. R. Chan, L. Zhu, E. Goldstein, and R. Horvat. 1998. Human herpesvirus-8 ORF K8.1 gene encodes immunogenic glycoproteins generated by spliced transcripts. Virology 249140-149. [DOI] [PubMed] [Google Scholar]
- 15.Ciufo, D. M., J. S. Cannon, L. J. Poole, F. Y. Wu, P. Murray, R. F. Ambinder, and G. S. Hayward. 2001. Spindle cell conversion by Kaposi's sarcoma-associated herpesvirus: formation of colonies and plaques with mixed lytic and latent gene expression in infected primary dermal microvascular endothelial cell cultures. J. Virol. 755614-5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Compton, T., R. R. Nepomuceno, and D. M. Nowlin. 1992. Human cytomegalovirus penetrates host cells by pH-independent fusion at the cell surface. Virology 191387-395. [DOI] [PubMed] [Google Scholar]
- 17.Cranage, M. P., T. Kouzarides, A. T. Bankier, S. Satchwell, K. Weston, P. Tomlinson, B. Barrell, H. Hart, S. E. Bell, A. C. Minson, et al. 1986. Identification of the human cytomegalovirus glycoprotein B gene and induction of neutralizing antibodies via its expression in recombinant vaccinia virus. EMBO J. 53057-3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elbashir, S. M., J. Harborth, W. Lendeckel, A. Yalcin, K. Weber, and T. Tuschl. 2001. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411494-498. [DOI] [PubMed] [Google Scholar]
- 19.Flore, O., S. Rafii, S. Ely, J. J. O'Leary, E. M. Hyjek, and E. Cesarman. 1998. Transformation of primary human endothelial cells by Kaposi's sarcoma-associated herpesvirus. Nature 394588-592. [DOI] [PubMed] [Google Scholar]
- 20.Foreman, K. E., J. Friborg, Jr., W. P. Kong, C. Woffendin, P. J. Polverini, B. J. Nickoloff, and G. J. Nabel. 1997. Propagation of a human herpesvirus from AIDS-associated Kaposi's sarcoma. N. Engl. J. Med. 336163-171. [DOI] [PubMed] [Google Scholar]
- 21.Ganem, D. 1998. Human herpesvirus 8 and its role in the genesis of Kaposi's sarcoma. Curr. Clin. Top. Infect. Dis. 18237-251. [PubMed] [Google Scholar]
- 22.Gao, S. J., J. H. Deng, and F. C. Zhou. 2003. Productive lytic replication of a recombinant Kaposi's sarcoma-associated herpesvirus in efficient primary infection of primary human endothelial cells. J. Virol. 779738-9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garrigues, H. J., Y. E. Rubinchikova, C. M. Dipersio, and T. M. Rose. 2008. Integrin αVβ3 binds to the RGD motif of glycoprotein B of Kaposi's sarcoma-associated herpesvirus and functions as an RGD-dependent entry receptor. J. Virol. 821570-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gong, M., and E. Kieff. 1990. Intracellular trafficking of two major Epstein-Barr virus glycoproteins, gp350/220 and gp110. J. Virol. 641507-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Honess, R. W. 1984. Herpes simplex and “the herpes complex”: diverse observations and a unifying hypothesis. The eighth Fleming lecture. J. Gen. Virol. 652077-2107. [DOI] [PubMed] [Google Scholar]
- 26.Kieff, E., and A. B. Rickinson. 2001. Epstein-Barr virus and its replication, p. 2511-2574. In D. M. Knipe and P. M. Howley (ed.), Fields virology, vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 27.Kim, D. H., and J. J. Rossi. 2007. Strategies for silencing human disease using RNA interference. Nat. Rev. Genet. 8173-184. [DOI] [PubMed] [Google Scholar]
- 28.Kliche, S., E. Kremmer, W. Hammerschmidt, U. Koszinowski, and J. Haas. 1998. Persistent infection of Epstein-Barr virus-positive B lymphocytes by human herpesvirus 8. J. Virol. 728143-8149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klupp, B. G., H. Granzow, E. Mundt, and T. C. Mettenleiter. 2001. Pseudorabies virus UL37 gene product is involved in secondary envelopment. J. Virol. 758927-8936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koyano, S., E. C. Mar, F. R. Stamey, and N. Inoue. 2003. Glycoproteins M and N of human herpesvirus 8 form a complex and inhibit cell fusion. J. Gen. Virol. 841485-1491. [DOI] [PubMed] [Google Scholar]
- 31.Krishnan, H. H., N. Sharma-Walia, L. Zeng, S. J. Gao, and B. Chandran. 2005. Envelope glycoprotein gB of Kaposi's sarcoma-associated herpesvirus is essential for egress from infected cells. J. Virol. 7910952-10967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lake, C. M., and L. M. Hutt-Fletcher. 2000. Epstein-Barr virus that lacks glycoprotein gN is impaired in assembly and infection. J. Virol. 7411162-11172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee, S. K. 1999. Four consecutive arginine residues at positions 836-839 of EBV gp110 determine intracellular localization of gp110. Virology 264350-358. [DOI] [PubMed] [Google Scholar]
- 34.Lomonte, P., P. Filee, J. R. Lyaku, M. Bublot, P. P. Pastoret, and E. Thiry. 1997. Glycoprotein B of bovine herpesvirus 4 is a major component of the virion, unlike that of two other gammaherpesviruses, Epstein-Barr virus and murine gammaherpesvirus 68. J. Virol. 713332-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luna, R. E., F. Zhou, A. Baghian, V. Chouljenko, B. Forghani, S. J. Gao, and K. G. Kousoulas. 2004. Kaposi's sarcoma-associated herpesvirus glycoprotein K8.1 is dispensable for virus entry. J. Virol. 786389-6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mesri, E. A., E. Cesarman, L. Arvanitakis, S. Rafii, M. A. Moore, D. N. Posnett, D. M. Knowles, and A. S. Asch. 1996. Human herpesvirus-8/Kaposi's sarcoma-associated herpesvirus is a new transmissible virus that infects B cells. J. Exp. Med. 1832385-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mettenleiter, T. C. 2004. Budding events in herpesvirus morphogenesis. Virus Res. 106167-180. [DOI] [PubMed] [Google Scholar]
- 38.Mettenleiter, T. C. 2002. Herpesvirus assembly and egress. J. Virol. 761537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mettenleiter, T. C. 2006. Intriguing interplay between viral proteins during herpesvirus assembly or: the herpesvirus assembly puzzle. Vet. Microbiol. 113163-169. [DOI] [PubMed] [Google Scholar]
- 40.Miller, N., and L. M. Hutt-Fletcher. 1992. Epstein-Barr virus enters B cells and epithelial cells by different routes. J. Virol. 663409-3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mittal, V. 2004. Improving the efficiency of RNA interference in mammals. Nat. Rev. Genet. 5355-365. [DOI] [PubMed] [Google Scholar]
- 42.Mocarski, E. S., and C. T. Courcelle. 2001. Cytomegaloviruses and their replication, p. 2629-2674. In D. M. Knipe and P. M. Howley (ed.), Fields virology, vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 43.Moore, P. S., S. J. Gao, G. Dominguez, E. Cesarman, O. Lungu, D. M. Knowles, R. Garber, P. E. Pellett, D. J. McGeoch, and Y. Chang. 1996. Primary characterization of a herpesvirus agent associated with Kaposi's sarcomae. J. Virol. 70549-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moses, A. V., K. N. Fish, R. Ruhl, P. P. Smith, J. G. Strussenberg, L. Zhu, B. Chandran, and J. A. Nelson. 1999. Long-term infection and transformation of dermal microvascular endothelial cells by human herpesvirus 8. J. Virol. 736892-6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagel, C. H., K. Dohner, M. Fathollahy, T. Strive, E. M. Borst, M. Messerle, and B. Sodeik. 2008. Nuclear egress and envelopment of herpes simplex virus capsids analyzed with dual-color fluorescence HSV1(17+). J. Virol. 823109-3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neipel, F., J. C. Albrecht, and B. Fleckenstein. 1997. Cell-homologous genes in the Kaposi's sarcoma-associated rhadinovirus human herpesvirus 8: determinants of its pathogenicity? J. Virol. 714187-4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nemerow, G. R., and N. R. Cooper. 1984. Early events in the infection of human B lymphocytes by Epstein-Barr virus: the internalization process. Virology 132186-198. [DOI] [PubMed] [Google Scholar]
- 48.Nicola, A. V., A. M. McEvoy, and S. E. Straus. 2003. Roles for endocytosis and low pH in herpes simplex virus entry into HeLa and Chinese hamster ovary cells. J. Virol. 775324-5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nixdorf, R., B. G. Klupp, A. Karger, and T. C. Mettenleiter. 2000. Effects of truncation of the carboxy terminus of pseudorabies virus glycoprotein B on infectivity. J. Virol. 747137-7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Panyutich, E. A., J. W. Said, and S. A. Miles. 1998. Infection of primary dermal microvascular endothelial cells by Kaposi's sarcoma-associated herpesvirus. AIDS 12467-472. [DOI] [PubMed] [Google Scholar]
- 51.Peeters, B., N. de Wind, M. Hooisma, F. Wagenaar, A. Gielkens, and R. Moormann. 1992. Pseudorabies virus envelope glycoproteins gp50 and gII are essential for virus penetration, but only gII is involved in membrane fusion. J. Virol. 66894-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pereira, L. 1994. Function of glycoprotein B homologues of the family herpesviridae. Infect. Agents Dis. 39-28. [PubMed] [Google Scholar]
- 53.Pertel, P. E. 2002. Human herpesvirus 8 glycoprotein B (gB), gH, and gL can mediate cell fusion. J. Virol. 764390-4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pertel, P. E., A. Fridberg, M. L. Parish, and P. G. Spear. 2001. Cell fusion induced by herpes simplex virus glycoproteins gB, gD, and gH-gL requires a gD receptor but not necessarily heparan sulfate. Virology 279313-324. [DOI] [PubMed] [Google Scholar]
- 55.Pertel, P. E., P. G. Spear, and R. Longnecker. 1998. Human herpesvirus-8 glycoprotein B interacts with Epstein-Barr virus (EBV) glycoprotein 110 but fails to complement the infectivity of EBV mutants. Virology 251402-413. [DOI] [PubMed] [Google Scholar]
- 56.Renne, R., D. Blackbourn, D. Whitby, J. Levy, and D. Ganem. 1998. Limited transmission of Kaposi's sarcoma-associated herpesvirus in cultured cells. J. Virol. 725182-5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roizman, B., and D. M. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2460. In D. M. Knipe and P. M. Howley (ed.), Fields virology, vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 58.Rozen, R., N. Sathish, Y. Li, and Y. Yuan. 2008. Virion-wide protein interactions of Kaposi's sarcoma-associated herpesvirus. J. Virol. 824742-4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Russo, J. J., R. A. Bohenzky, M. C. Chien, J. Chen, M. Yan, D. Maddalena, J. P. Parry, D. Peruzzi, I. S. Edelman, Y. Chang, and P. S. Moore. 1996. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc. Natl. Acad. Sci. USA 9314862-14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schulz, T. F., J. Sheldon, and J. Greensill. 2002. Kaposi's sarcoma associated herpesvirus (KSHV) or human herpesvirus 8 (HHV8). Virus Res. 82115-126. [DOI] [PubMed] [Google Scholar]
- 61.Spear, P. G., and R. Longnecker. 2003. Herpesvirus entry: an update. J. Virol. 7710179-10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vieira, J., P. O'Hearn, L. Kimball, B. Chandran, and L. Corey. 2001. Activation of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) lytic replication by human cytomegalovirus. J. Virol. 751378-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Whitman, A. G., O. F. Dyson, P. J. Lambert, T. L. Oxendine, P. W. Ford, and S. M. Akula. 2007. Changes occurring on the cell surface during KSHV reactivation. J. Electron Microsc. (Tokyo) 5627-36. [DOI] [PubMed] [Google Scholar]
- 64.Wies, E., Y. Mori, A. Hahn, E. Kremmer, M. Sturzl, B. Fleckenstein, and F. Neipel. 2008. The viral interferon-regulatory factor-3 is required for the survival of KSHV-infected primary effusion lymphoma cells. Blood 111320-327. [DOI] [PubMed] [Google Scholar]
- 65.Xu, Y., D. P. AuCoin, A. R. Huete, S. A. Cei, L. J. Hanson, and G. S. Pari. 2005. A Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 ORF50 deletion mutant is defective for reactivation of latent virus and DNA replication. J. Virol. 793479-3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao, J., V. Punj, H. Matta, L. Mazzacurati, S. Schamus, Y. Yang, T. Yang, Y. Hong, and P. M. Chaudhary. 2007. K13 blocks KSHV lytic replication and deregulates vIL6 and hIL6 expression: a model of lytic replication induced clonal selection in viral oncogenesis. PLoS ONE 2e1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou, F. C., Y. J. Zhang, J. H. Deng, X. P. Wang, H. Y. Pan, E. Hettler, and S. J. Gao. 2002. Efficient infection by a recombinant Kaposi's sarcoma-associated herpesvirus cloned in a bacterial artificial chromosome: application for genetic analysis. J. Virol. 766185-6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu, F. X., X. Li, F. Zhou, S. J. Gao, and Y. Yuan. 2006. Functional characterization of Kaposi's sarcoma-associated herpesvirus ORF45 by bacterial artificial chromosome-based mutagenesis. J. Virol. 8012187-12196. [DOI] [PMC free article] [PubMed] [Google Scholar]