Abstract
The double-stranded RNA (dsRNA)-dependent protein kinase PKR is thought to mediate a conserved antiviral pathway by inhibiting viral protein synthesis via the phosphorylation of the alpha subunit of eukaryotic initiation factor 2 (eIF2α). However, little is known about the data related to the lower vertebrates, including fish. Recently, the identification of PKR-like, or PKZ, has addressed the question of whether there is an orthologous PKR in fish. Here, we identify the first fish PKR gene from the Japanese flounder Paralichthys olivaceus (PoPKR). PoPKR encodes a protein that shows a conserved structure that is characteristic of mammalian PKRs, having both the N-terminal region for dsRNA binding and the C-terminal region for the inhibition of protein translation. The catalytic activity of PoPKR is further evidence that it is required for protein translation inhibition in vitro. PoPKR is constitutively transcribed at low levels and is highly induced after virus infection. Strikingly, PoPKR overexpression increases eIF2α phosphorylation and inhibits the replication of Scophthalmus maximus rhabdovirus (SMRV) in flounder embryonic cells, whereas phosphorylation and antiviral effects are impaired in transfected cells expressing the catalytically inactive PKR-K421R variant, indicating that PoPKR inhibits virus replication by phosphorylating substrate eIF2α. The interaction between PoPKR and eIF2α is demonstrated by coimmunoprecipitation assays, and the transfection of PoPKR-specific short interfering RNA further reveals that the enhanced eIF2α phosphorylation is catalyzed by PoPKR during SMRV infection. The current data provide significant evidence for the existence of a PKR-mediated antiviral pathway in fish and reveal considerable conservation in the functional domains and the antiviral effect of PKR proteins between fish and mammals.
The double-stranded RNA (dsRNA)-activated protein kinase PKR is an interferon (IFN)-induced serine/threonine kinase that mediates antiviral and antiproliferative effects via the phosphorylation of the alpha subunit of eukaryotic initiation factor 2 (eIF2α) (17). Most cells constitutively express a low level of PKR in a latent state, but following virus infection, PKR can be upregulated by newly produced type I IFN and activated as an antiviral effector and essential component of the cellular antiviral response. PKR activity also is involved in the regulation of differentiation, cell growth, apoptosis, and oncogenic transformation, at least partially through the phosphorylation of eIF2α (26, 40).
The phosphorylation of eIF2α is one of the well-characterized translational control mechanisms, and it also is used by PKR to mediate IFN antiviral effects. For translation initiation in normal cells, eIF2, an oligomer composed of three subunits (α, β, and γ), interacts with GTP and the initiator methionyl-tRNA to form a ternary complex of eIF2-GTP-Met-tRNA that subsequently binds to the 40S ribosomal subunit of the ribosome (20, 30). Following GTP hydrolysis, the released of eIF2-GDP complex must be continuously recycled by the guanine nucleotide exchange factor eIF2B to replace GDP with GTP for another round of initiation. In virus-infected cells, however, virus infection activates PKR, which phosphorylates eIF2α on serine 51. Phosphorylated eIF2α binds tightly to eIF2B, reducing the levels of functional eIF2B and consequently blocking GTP/GDP exchange on the eIF2 complex (20, 36, 45). As a result, cellular protein syntheses are shut off, and virus replication also is inhibited. In mammals, the overexpression of PKR inhibits the replication of some viruses, such as encephalomyocarditis virus (32), vaccinia virus (27), and human immunodeficiency virus (1), and PKR-deficient cells show the impaired induction of type I IFN and a higher permissiveness to encephalomyocarditis virus infection (11). Mice lacking PKR are extremely susceptible to vesicular stomatitis virus and influenza virus invasion (2, 43). In response, many viruses have evolved mechanisms to express some virus RNA and proteins or induce cellular products to counter or neutralize PKR functions (17).
However, little is known about eIF2α kinases except from the reports about mammals, birds, and plants. In fish, virus infection results in an innate IFN response that is characterized by the upregulation of some IFN-stimulated genes, such as Mx, ISG15, IFI56, and IFI58 (51), but no cDNA encoding fish PKR protein is isolated. Interestingly, a novel member of eIF2α kinase, designated PKR-like or PKZ, has been discovered recently in fish (3, 22, 39). Structurally similar to mammalian PKRs, the C-terminal catalytic domain of the putative PKR-like or PKZ protein contains 11 conserved kinase subdomains (3, 22, 39), with an invariant lysine in subdomain II that is essential for kinase activity (23), a kinase insert (KI) between subdomains IV and V that is indispensable for the interaction of the kinase and eIF2α (10), and a highly conserved eIF2α kinase motif, LFIQMEFCD, in subdomain V that is required for PKR kinase activity (4). However, this protein shows a unique N-terminal structure, with two Z-DNA binding domains instead of two tandem dsRNA binding motifs (dsRBM1 and dsRBM2) (3, 22, 39).
The upregulation by virus infection (22) and the possible capability of preventing protein synthesis, as determined by a cotransfection assay (39), indicate that fish PKR-like or PKZ plays an essential role in virus infection. However, its ability to inhibit virus replication is unknown. Moreover, although the phosphorylation of eIF2α is observed in rainbow trout cells treated with infectious pancreatic necrosis virus or poly(I:C) (18), there is no direct evidence for the correlation between the phosphorylation of fish eIF2α and an eIF2α kinase during the fish antiviral response. Therefore, these findings address two basic issues: whether there are authentic fish PKR enzymes and whether PKR-mediated eIF2α phosphorylation is a conserved and essential antiviral mechanism.
In 2006, we identified the Japanese flounder (Paralichthys olivaceus) eIF2α kinase gene HRI from UV-inactivated grass carp hemorrhagic virus (GCHV)-infected flounder embryonic cells (FEC) (52). In this study, we further cloned the flounder PKR gene, termed PoPKR (for Paralichthys olivaceus PKR), from the same system. Its antiviral role was sequentially delineated. Not only the binding affinities of the N terminus for dsRNA but also the catalytic activity of the C terminus for the inhibition of protein translation were revealed. The interaction between PoPKR and eIF2α was confirmed to be responsible for the phosphorylation of eIF2α, thus inhibiting Scophthalmus maximus rhabdovirus (SMRV) infection. These results strengthen the hypothesis that besides PKR-like or PKZ, fish have a mammalian PKR orthologue and possess a PKR-mediated antiviral pathway through the phosphorylation of eIF2α.
MATERIALS AND METHODS
Plasmids.
The prokaryotic expression constructs D1 (amino acids 1 to 200, containing dsRBM1), D2 (amino acids 100 to 350, containing dsRBM2), and BD (amino acids 1 to 350, containing the two dsRBMs) were generated by the insertion of different PCR products of flounder PKR into EcoRI/XhoI sites of pET-32c(+) (Novagen). The plasmids used for transfection, wild-type (WT) PKR (amino acids 1 to 688) and ΔN PKR (amino acids 286 to 688), were generated with corresponding PCR products inserted into NheI/BamHI sites of pcDNA3.1/Myc-His(−)A (Invitrogen). The catalytically inactive mutants K421R and ΔN-K421R were obtained from plasmids WT PKR and ΔN PKR, respectively, using the QuikChange site-directed mutagenesis kit (Stratagene). In K421R and ΔN-K421R, lysine 421 was replaced with arginine. The generated plasmids were verified by sequencing analyses. All of the primers used for plasmid construction are listed in Table 1.
TABLE 1.
Primers used for the cloning, expression, and transfection of PoPKR plasmids
| Name | Sequence (5′-3′) | Use |
|---|---|---|
| F1 | GTACATTCAGATGGAGCTGTG | PCR for the first cDNA fragments |
| R1 | AGGCTTCAGGTCTCTGTGGAT | |
| SMART-F | CAACGCAGAGTACGCGGG | RACE-PCR for the 5′ and 3′ cDNA of PoPKR |
| SMART-R | TCAACGCAGAGTACTTTTTTTTTTTTTTTT | |
| F2 | GTTCTGGCGCATTTGGTGAC | RACE-PCR for the 5′ and 3′ cDNA of PoPKR |
| R2 | ATCGTCGTCATTACTAGCAGT | |
| F3 | TGCGAGACTCAAAGAGAAGAGAAG | Real-time PCR for the expression of PoPKR |
| R3 | GGTTCCTTTGTACTCCGTTC | |
| Mx-F | GCCGTCATAGGAGACCAAA | Real-time PCR for the expression of PoMx |
| Mx-R | TTCCTCGTAGTCCCTGTAGC | |
| β-Actin-F | CACTGTGCCCATCTACGAG | Real-time PCR for the expression of β-actin as an internal control |
| β-Actin-R | CCATCTCCTGCTCGAAGTC | |
| F4 | TAGAATTCATGGCAACCATAAACTACGT | PCR for the prokaryotic expression plasmid bearing the complete cDNA of PoPKR |
| R4 | TACTCGAGGACAGAGTAACTTTCATGAG | |
| D1-F | TAGAATTCATGGCAACCATAAACTAC | PCR for the prokaryotic expression plasmid bearing dsRBM1 of PoPKR |
| D1-R | TACTCGAGACAGATTTCTATCACATCTT | |
| D2-F | TAGAATTCATGAGTAACAACAGTTATG | PCR for the prokaryotic expression plasmid bearing dsRBM2 of PoPKR |
| D2-R | TACTCGAGTGCTGCAAGTCTTATTTTG | |
| D1 + 2-F | TAGAATTCATGGCAACCATAAACTACGT | PCR for the prokaryotic expression plasmid bearing two dsRBMs |
| D1 + 2-R | TACTCGAGTTGTTTGGCTTCTTTCACAG | |
| K421R-F | CTTTGCAGCGGACGATCCTTACAGCACATAGCTTG | PCR for the mutant plasmid K421R |
| K421R-R | CAAGCTATGTGCTGTAAGGATCGTCCGCTGCAAAG | |
| WT-F | TAGCTAGCATGGCAACCATAAACTACGT | PCR for WT PoPKR plasmid |
| WT-R | TAGGATCCGACAGAGTAACTTTCATGAG | |
| ΔN-F | TAGCTAGCATGGACAACAAGGTAACTGT | PCR for ΔN PKR plasmid |
| WT-R | TAGGATCCGACAGAGTAACTTTCATGAG |
Cells, virus, and transfection.
FEC (7) were maintained in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal calf serum (FCS), and epithelioma papulosum cyprini (EPC) cells were grown in medium 199 with 10% FCS. SMRV was propagated, and its titers in EPC cells were determined as described previously (15).
Transient transfections were performed in FEC seeded in 6-well plates by using Genejuice reagent (Novagen) according to the manufacturer's instructions. When needed, Geneticin (400 μg/ml; Gibco) was used to select stable transformants of both FEC and EPC cells after they were transfected with K421R and empty vector pcDNA3.1 (1 μg/well).
For virus infection, cells were seeded in 6-well dishes (about 2 × 104 cells per well). Twenty-four hours later, the cells were washed three times and then treated with 1 ml FCS-free medium containing SMRV (1 × 104 50% tissue culture infectious doses [TCID50]/ml) and 1 ml FCS-free medium alone as a control. When needed, transfection was performed 24 h after cell seeding, and another 48 h later transfected cells were treated with SMRV as described above. Japanese flounders weighing 400 to 500 g were maintained in a tank containing 200 liters of seawater that was kept fully aerated at 17 to 18°C. After 1 week of not showing any clinical signs of disease, the fish were divided in two groups, each with three fish. One group of fish was intraperitoneally injected with 0.5 ml serum-free medium DMEM containing SMRV (1 × 109 TCID50/ml), and the other was intraperitoneally injected with serum-free medium (DMEM) alone as controls.
Identification of PoPKR.
The pair of primers F1 and R1 was designed according to the sequences of two conserved motifs, L(F/H)IQM and IHRDLKP, which are located at subdomains V and VI, respectively, within the C-terminal catalytic region of eIF2α kinases. The cDNA fragment homologous to known PKR genes was cloned from a SMART cDNA library made with mRNAs derived from UV-inactivated GCHV-infected FEC (9). Based on the cloned cDNA sequence, the full length of PoPKR cDNA was obtained by rapid amplification of cDNA ends (RACE)-PCR according to a previous report (52). Multiple-sequence alignments were generated by the Vector NTI program. The maximum-parsimony method was used with the ClustalW 1.8 program (48) to construct a phylogenetic tree based on the alignments of eIF2α kinase sequences containing full-length kinase domains but without KIs.
RNA, reverse transcription, and real-time PCR.
The total RNA from FEC and flounder tissues was extracted by the TRIzol method (Invitrogen) and reverse transcribed into cDNA with a random primer by using the RevertAid minus first-strand cDNA synthesis kit (Fermentas). Real-time PCR was performed with a DNA Engine Chromo 4 real-time system (MJ Research) with SYBR green I dye. PCR was performed to detect PoPKR mRNA in a 20-μl reaction volume that contained 1 μl cDNA, 0.2 μM of each primer, 1 U of Taq polymerase (Fermentas), 0.1 μM of each deoxynucleoside triphosphate, and 1× buffer for Taq polymerase. PCR conditions were as follows: 94°C for 4 min, then 94°C for 20 s, 56°C for 20 s, and 72°C for 20 s for 40 cycles, followed by 72°C for 10 min. β-Actin also was detected as a positive control. All samples were analyzed in triplicate, and the results were expressed as the relative change (n-fold) of the expression of β-actin with the 2−ΔΔCT method (29).
Poly(I:C) pull-down assay.
Poly(I:C)-agarose was made according to a previous report (46). Briefly, poly(C)-coated beads (Sigma) and poly(I) (2 mg/ml; Sigma) were resuspended in buffer (50 mM Tris [pH 7.0], 150 mM NaCl) and subsequently mixed at a volume ratio of 1:2. The mixture was rocked gently overnight at 4°C, centrifuged at 1,000 × g, washed once, resuspended in the same buffer as a 50% final slurry, and stored at 4°C for use.
For poly(I:C) pull-down assays, the prepared poly(I:C)-coated beads were equilibrated in binding buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1 mM EDTA, 1% NP-40) as a 10% slurry and then incubated with the purified fusion proteins containing dsRBM1 (D1), dsRBM2 (D2), and both dsRBMs (BD) in the presence of protease and phosphatase inhibitors. After gentle agitation for 1 h at 4°C, the beads were collected by centrifugation at 1,000 × g, rinsed three times with binding buffer, resuspended in three volumes of 1× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, and then analyzed by Western blotting.
Luciferase activity detection.
To detect luciferase activity, FEC were seeded in 24-well plates at a cell concentration of about 1 × 104 cells/well, and 24 h later they were cotransfected by using Genejuice reagent (Novagen) with 200 ng of reporter plasmid pGL3 promoter (Promega) and 200 ng of the respective pcDNA3.1 expression constructs. For each plasmid, triplicate transfections were performed. Cells were harvested 48 h after transfection and assayed for luciferase activity by using the luciferase assay system (Promega) after data were normalized for transfection efficiency by measuring the total amount of protein.
Polyclonal anti-PKR mouse serum preparation and Western blotting detection.
The complete protein of PoPKR was expressed by the prokaryotic vector pET-32c(+) (Novagen). The fusion protein was purified according to the protocol of the His·Bind purification kit (Novagen) and used to immunize mice for the preparation of polyclonal anti-PoPKR mouse serum according to a previous report (14).
For detection by Western blotting, protein extracts were separated by SDS-10% PAGE and electrotransferred onto nitrocellulose membranes. Membranes were incubated with anti-PKR polyclonal antibody at 1:1,000 in TBST buffer containing 1% milk (25 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% Tween 20), with final detection provided by alkaline phosphatase-conjugated horse anti-mouse antibody (SABC) and the 5-bromo-4-chloro-3-indolylphosphate-nitroblue tetrazolium method (Ameresco). To ensure equal protein loading, the same membranes were immunostained using polyclonal anti-human actin antibody (Santa Cruz) to detect the level of actin protein.
For the analysis of eIF2α phosphorylation, whole-cell extract lysates were prepared by washing cells twice with phosphate-buffered saline (PBS) and lysing them in lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% SDS, 1% NP-40, 1% Triton X-100, 10 mM glycerophosphate, 1 mM dithiothreitol, 1 mM EGTA, 10% glycerin) containing protease and phosphatase inhibitor cocktails (Sigma). Phosphorylated flounder eIF2α and total eIF2α were immunoblotted with rabbit polyclonal anti-phosphoserine 51 human eIF2α antibody (Cell Signaling Technology) and total human eIF2α antibody (Cell Signaling Technology), respectively.
Coimmunoprecipitation.
Protein samples for the coimmunoprecipitation assay were made by lysing cells or tissues in radioimmunoprecipitation assay buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, and a protease inhibitor cocktail). The prepared cell lysates were incubated with 1 to 10 μl of eIF2α antibody (0.2 to 2 μg; Santa Cruz) overnight at 4°C. The mixture was incubated with 20 μl of a resuspended volume of protein A/G agarose (Santa Cruz) overnight at 4°C. Immunocomplexes then were collected by centrifugation at 1,000 × g for 5 min at 4°C, washed four times with radioimmunoprecipitation assay buffer, and resuspended in 40 μl of 1× SDS-PAGE sample buffer. These samples were further analyzed by Western blotting.
Knockdown of PoPKR expression by siRNA.
PoPKR-specific short interfering RNA (siRNA), located 852 to 870 nucleotides downstream of the translation initiation codon, and nontargeting siRNA were designed and synthesized by GenePharma, with sequences of 5′-CGACUAUGACAACAAGGUATT-3′ and 5′-UUCUCCGAACGUGUCACGUTT-3′, respectively. FEC in 6-well culture plates were transfected with siRNA using Lipofectamine 2000 (Invitrogen) by following the manufacturer's protocols. siRNA solution was diluted to a final concentration of 100 nM. After 72 h, cells were infected with SMRV (1 × 104 TCID50/ml) and collected at the indicated times for detecting PKR protein levels and eIF2α phosphorylation during the SMRV infection course.
RESULTS
Cloning and characterization of the flounder PKR homologue.
In order to clone the flounder PKR gene, a pair of primers (F1 and R1) was designed against the sequences of two conserved motifs, L(F/H)IQM and IHRDLKP, within the C-terminal catalytic region of eIF2α kinases. A 190-bp cDNA homologous to known mammalian PKR genes was amplified from SMART cDNAs that were made with mRNAs from UV-inactivated GCHV-infected FEC. Subsequently, the full-length sequence of PoPKR was obtained by RACE-PCR.
The longest open reading frame of the PoPKR gene encodes a 688-amino-acid protein that is highly homologous to mammalian and avian PKRs (Table 2). The sequence analysis of the putative PoPKR protein displays two tandem dsRBMs within the N-terminal region that are significantly distinct from the identified fish PKR-like or PKZ genes that instead encode two Z-DNA binding domains in the corresponding sequences and 11 conserved catalytic subdomains of Ser/Thr protein kinases within the C-terminal region, which contains a KI between subdomains IV and V and a stretch (LYIQMELCD) within subdomain V that is similar to eIF2α kinase motif LFIQMEFCD (Fig. 1A and B). Similarly to mammalian PKRs, PoPKR contains two heparin binding domains (HBD) that were identified recently in human PKR (16), one present in residues 403 to 441 (HBD1) and the other in residues 537 to 609 (HBD2), and a series of conserved amino acids including Lys-421 and two Thr residues (Thr-577 and Thr-582) that are essential for kinase activity (13, 23, 50), although it is longer than any other known PKRs due to a relatively long linker between the two dsRBMs (Fig. 1B). A phylogenetic tree made with PoPKR and other known eIF2α kinases (Table 3) recovers four basic clades: PKR, HRI, GCN2, and PERK. PoPKR and fish PKR-like or PKZ proteins clearly form a subgroup that is clustered in the PKR subfamily with high support values, indicating that the cloned cDNA is a flounder PKR homologue (Fig. 1C).
TABLE 2.
Comparison of Paralichthys olivaceus PKR to those of other fish and other known PKR or PKR-like proteins
| Species | % Identity to Paralichthys olivaceus PKR | % Similarity to Paralichthys olivaceus PKR |
|---|---|---|
| Tetraodon nigroviridis PKR | 55.140 | 78.505 |
| Danio rerio PKZ | 34.967 | 65.256 |
| Carassius auratus PKR-like | 33.061 | 59.592 |
| Gallus gallus PKR | 34.615 | 59.532 |
| Mus musculus PKR | 34.435 | 59.478 |
| Sus scrofa PKR | 34.242 | 57.922 |
| Homo sapiens PKR | 34.224 | 61.603 |
| Bos taurus PKR | 34.024 | 59.067 |
| Cercopithecus aethiops PKR | 32.941 | 62.353 |
| Rattus norvegicus PKR | 32.927 | 61.324 |
| Oryctolagus cuniculus PKR | 31.315 | 61.765 |
FIG. 1.
Identification of flounder PKR. (A) Schematic diagram of the structure of the PoPKR domain. The dsRNA binding domain and catalytic domain are represented by the hatched boxes and shaded boxes, respectively. The solid black bars and roman numerals I to XI represent the catalytic subdomains, which are conserved in Ser/Thr kinases. The number indicates the amino acid positions. (B) Multiple alignments of PoPKR amino acid sequences with several typical PKRs from mammals and birds by the Vector NTI program. Amino acid numbering is shown to the right. Sequence gaps are represented by dashes. The conserved kinase subdomains are indicated by roman numerals (I to XI) and bars above the alignments. The dsRNA binding domain and the core sequences of the heparin binding domain are shown as open boxes. (C) Phylogenetic relationship of the eIF2α kinase family. Based on the multiple alignments of eIF2α kinase sequences containing full-length kinase domains but excluding the KI domains using the ClustalW 1.8 program, a rooted tree was constructed by the maximum-parsimony method. Human mitogen-activated protein kinase (MAPK) was used as the outgroup. Four eIF2α kinases, including PKR, PERK, HRI, and GCN2, are derived from flounder (Po), human (Hs), rabbit (Oc), rat (Rn), crucian carp (Ca), chicken (Gg), mouse (Mm), cow (Bt), pig (Ss), baker's yeast (Sc), fruit fly (Dm), nematode (Ce), and thale cress (At). PKR-like or PKZ proteins from crucian carp (Ca) and zebrafish (Dr) and a PKR deduced from a partial cDNA sequence of fugu (Tn) were included. The bootstrap confidence values shown at the nodes of the tree are validated by 1,000 repetitions.
TABLE 3.
List of all of the eIF2α kinases used in Fig. 1C
| Gene product | Species name (designation in Fig. 1C) | Common name | GenBank accession no.
|
GenBank accession no.
|
|---|---|---|---|---|
| Protein | Gene | |||
| PKR | Homo sapiens (Hs) | Human | NP_002750 | NM_002759 |
| PKR | Rattus norvegicus (Rn) | Norway rat | NP_062208 | NM_019335 |
| PKR | Mus musculus (Mm) | House mouse | NP_035293 | NM_011163 |
| PKR | Sus scrofa (Ss) | Pig | NP_999484 | NM_214319 |
| PKR | Bos taurus (Bt) | Cow | BAC66440 | AB104655 |
| PKR | Gallus gallus (Gg) | Chicken | NP_989818 | NM_204487 |
| PKR | Tetraodon nigroviridis (Tn) | Freshwater puffer fish | CAD67777 | AJ544919 |
| PKR | Paralichthys olivaceus (Po) | Bastard halibut | ABV21735 | EU118259 |
| PKZ | Danio rerio (Dr) | Zebrafish | NP_001013317 | NM_001013299 |
| PKR-like | Carassius auratus (Ca) | Goldfish | AAP49830 | AY293929 |
| PERK | Homo sapiens (Hs) | Human | AAF61199 | AF193339 |
| PERK | Rattus norvegicus (Rn) | Norway rat | NP_113787 | NM_031599 |
| PERK | Mus musculus (Mm) | House mouse | NP_034251 | NM_010121 |
| PERK | Drosophila melanogaster (Dm) | Fruit fly | AAF61200 | AF193340 |
| PERK | Caenorhabditis elegans (Ce) | Nematode | AAL30829 | AF435953 |
| HRI | Homo sapiens (Hs) | Human | AAF66736 | AF147094 |
| HRI | Rattus norvegicus (Rn) | Norway rat | AAA18255 | L27707 |
| HRI | Mus musculus (Mm) | House mouse | NP_038585 | NM_013557 |
| HRI | Oryctolagus cuniculus (Oc) | Rabbit | AAA31241 | M69035 |
| HRI | Gallus gallus (Gg) | Chicken | NP_989979 | NM_204648 |
| HRI | Tetraodon nigroviridis (Tn) | Freshwater puffer fish | CAG04442 | CAAE01014738 |
| HRI | Danio rerio (Dr) | Zebrafish | XM_688615 | XP_693707 |
| HRI | Paralichthys olivaceus (Po) | Bastard halibut | ABA39175 | DQ193596 |
| GCN2 | Homo sapiens (Hs) | Human | NP_001013725 | NM_001013703 |
| GCN2 | Mus musculus (Mm) | House mouse | NP_038747 | NM_013719 |
| GCN2 | Drosophila melanogaster (Dm) | Fruit fly | AAC47516 | U80223 |
| GCN2 | Saccharomyces cerevisiae (Sc) | Baker's yeast | AAA34636 | M27082 |
| GCN2 | Arabidopsis thaliana (At) | Thale cress | CAD30860 | AJ459823 |
Functional analyses of both N- and C-terminal domains of PoPKR.
In mammals, PKR is activated by the binding of its N-terminal dsRBMs to dsRNA (19, 41). Multiple alignments reveal that the core sequence of two PoPKR dsRBMs are approximately 65 residues, one residing in residues 6 to 71 and the other in residues 214 to 280 (Fig. 1A). Based on these sequences, three peptides, D1, D2, and BD (BD is residues 1 to 350 and contains dsRBM1 and dsRBM2), were expressed by a prokaryotic expression system as C-terminally His-tagged proteins (Fig. 2A) and purified by affinity chromatography (Fig. 2B).
FIG. 2.
Poly(I:C) binding activity of dsRNA binding domains in the PoPKR N terminus. (A) Schematic structure of D1, D2, and BD used in this study that shows amino acid residue numbers. The dsRNA binding domains are indicated by the shaded box. (B) The expressed and purified His-tagged proteins D1, D2, and BD were separated in SDS-PAGE and stained with Coomassie brilliant blue. Lanes 1, protein marker; 2, lysate of mock-induced bacteria; 3, lysate of induced bacteria; and 4, purified fusion proteins. (C) A poly(I:C) pull-down assay was used to detect the dsRNA binding activity of the three purified fusion proteins, D1, D2, and BD. Proteins were incubated with poly(I:C)-linked agarose at 4°C overnight and then washed with binding buffer. The total purified fusion proteins and the poly(I:C)-linked agarose binding proteins were separated by SDS-PAGE and then detected by Western blotting (upper). The proportions of binding proteins were quantified and statistically analyzed (lower). T, total purified fusion proteins; B, the fusion protein binding to poly(I:C)-linked agarose; M, protein marker; C, poly(I:C) beads.
The three purified His-tagged proteins were challenged for binding to poly(I:C)-Sepharose. Following incubation with poly(I:C)-Sepharose and then elution with binding buffer, the total D1, D2, and BD fusion proteins and the proteins bound to poly(I:C)-Sepharose were separated and detected by SDS-PAGE and Western blotting. As shown in Fig. 2C, the proteins that bound to poly(I:C)-Sepharose were indeed the three expressed fusion peptides D1, D2, and BD (Fig. 2C, upper), and the binding ability of BD was higher than that of D1 and D2 (Fig. 2C, lower). In contrast, poly(I:C)-Sepharose was not able to pull down any fusion peptide (Fig. 2C, lane 1), and no related protein signal was detected in poly(I:C)-Sepharose alone (data not shown). Another His-tagged fusion protein, flounder HRI-N (52), could not be pulled down by poly(I:C)-Sepharose under the same conditions (Fig. 2C, lanes 8 and 9), excluding the possibility of a His tag binding to poly(I:C).
The catalytic activity of the WT PKR and three different mutants of PoPKR (ΔN PKR, K421R, and ΔN-K421R) (Fig. 3A) was analyzed by cotransfecting an expression construct encoding the luciferase reporter gene, which is a general approach to detecting mammalian PKR kinase activity (35). In mammalian studies, it is believed that the partial double-stranded mRNA that is transcribed from the cotransfected luciferase construct may bind to PKR, resulting in kinase activation and eIF2α phosphorylation, and, due to PKR activation, the expression of the luciferase reporter gene should be down-regulated at the translational level (24, 25). As shown in Fig. 3B, in comparison to the basal expression level of luciferase plasmid pGL3 with the empty vector pcDNA, cotransfection with WT PKR resulted in a significant reduction of luciferase activity. Similarly to human PKR (28, 34), cotransfection with the ΔN PKR mutant defective in N-terminal dsRBMs also yielded a low level of luciferase activity, indicating that the isolated kinase domain ΔN PKR also prevented luciferase translation. Interestingly, in contrast to WT PKR and ΔN PKR, two point mutants, K421R and ΔN-K421R, which were generated from the plasmids WT PKR and ΔN PKR by the replacement of lysine 421 with arginine, rescued luciferase synthesis. These data indicate that PoPKR is able to inhibit protein translation in vitro, and the residue lysine 421, the predicted ATP binding site, is required for the catalytic activity of PoPKR kinase.
FIG. 3.
Inhibition of luciferase synthesis by PoPKR. (A) Schematic representations of WT and mutant versions of PoPKR. The dsRNA binding domain and catalytic domain are indicated by the hatched boxes and shaded boxes, respectively. The numbering represents residue positions. Asterisks in the constructs of K421R and ΔN-K421R show the mutated amino acid positions (K421-R421) that abolish kinase activity. (B) FEC were cotransfected by the Genejuice procedure with 200 ng of the luciferase plasmid pGL3 promoter and 200 ng of each construct, including the empty pcDNA3.1 vector as a control and WT PKR, K421R, ΔN PKR, and ΔN-K421R. Cells were harvested 48 h after transfection and assayed for luciferase activity. The results of luciferase activity assays were normalized to the total protein concentration. Cotransfection with the pGL3 promoter and empty pcDNA3.1 vector was used as a control, and the detected luciferase activity was set at 100%. The relative luciferase activity in the experimental samples was obtained and compared to that of the control. Data represent means ± standard errors of triplicate experiments.
Increase of PoPKR expression and eIF2α phosphorylation by virus infection.
To further characterize the expression kinetics of PoPKR in response to virus infection, we prepared polyclonal anti-PoPKR antibody and detected PoPKR expression in SMRV-infected FEC. Compared to the low level of constitutive expression in mock-infected cells, a significant increase in PoPKR expression was observed after SMRV infection. For the mRNA level, the inductive PoPKR transcription was observed from 24 h and reached a peak at 96 h after SMRV infection (Fig. 4A). For the protein level, the PoPKR content was obviously increased at 72 h after SMRV infection (Fig. 4B). Under the same conditions, we also detected the expression status of PoMx, a known antiviral gene both in mammals and fish (49). As shown in Fig. 4C, the PoMx transcription was induced from 24 h, and a continually inductive expression was observed up to 96 h after SMRV infection.
FIG. 4.
Inductive expression of PoPKR and PoMx after SMRV infection. (A) Real-time PCR detection of PoPKR transcription in the SMRV-infected FEC. FEC were treated with FCS-free medium containing SMRV or FCS-free medium alone (control) and were sampled at the indicated times. The ratio of PoPKR mRNA to β-actin in control cells was set to 1, and all infected cells were normalized to this value. (B) Western blot detection of PoPKR protein in the SMRV-infected FEC. Western blotting was performed with a polyclonal anti-PoPKR serum made as described in Materials and Methods. (C) Real-time PCR detection of PoMx transcription in the SMRV-infected FEC under the same conditions.
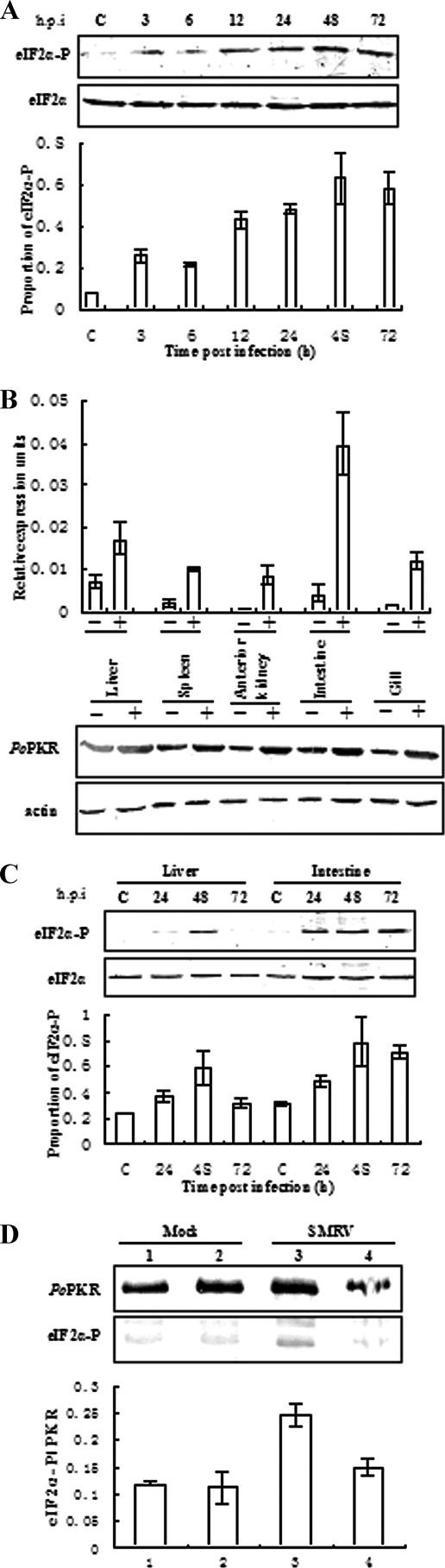
Subsequently, the phosphorylation status of eIF2α was examined in the SMRV-infected FEC. A pretest found that the polyclonal antibody of phosphorylated human eIF2α (Ser-51) was able to specifically recognize the phosphorylated eIF2α in flounder cells and tissues. As shown in Fig. 5A (upper), while the levels of total eIF2α were essentially equivalent, virus infection induced a clear and gradual upregulation for eIF2α phosphorylation along the increase of inducing time compared to that of control FEC. Quantitative and statistical analyses further confirmed that a consistent increase of eIF2α phosphorylation occurred as early as 3 h postinfection (p.i.) and reached a peak of about 7.3-fold upregulation at 48 to 72 h p.i. (Fig. 5A, lower panel).
FIG. 5.
Enhanced phosphorylation of eIF2α in response to SMRV infection. (A) Western blot detection (upper) and statistical analysis (lower) of the phosphorylated and total eIF2α proteins in the SMRV-infected FEC with eIF2α phosphospecific antibody (eIF2α-P) and eIF2α antibody (eIF2α) (Cell Signaling Technology). The extracts were prepared from the sampled cells at the indicated times. The relative eIF2α phosphorylation in the upper panel was imaged by BandScan software by counting the ratio of intensities between the phosphorylated bands and immunoblotting bands of eIF2α. C, control. (B) Real-time PCR (upper) and Western blot (lower) detection in different tissues of mock-infected flounder (−) and SMRV-infected flounder (+) for 72 h. The relative expression units of PoPKR mRNA in each tissue were represented by the ratio of PoPKR to β-actin. Tissue actin protein was detected by polyclonal anti-human actin (Santa Cruz). (C) Western blot detection (upper) and statistical analysis (lower) of eIF2α-P and total eIF2α in SMRV-infected flounder liver and intestine by eIF2α-P and eIF2α antibody at the indicated infection times. (D) FEC or flounder were mock infected and SMRV infected, cell lysates were prepared at 48 h p.i., and the lysates were subjected to immunoprecipitation with anti-human eIF2α antibody (Santa Cruz). After all immunocomplex samples were normalized and detected by Western blotting with anti-PoPKR antibody, the equalized samples were detected by the eIF2α phosphospecific antibody (eIF2α-P) (upper), and the proportions of the phosphorylated eIF2α and PoPKR were quantified and statistically analyzed (lower). Data represent means ± standard errors of triplicate experiments. Lanes 1 and 3, mock-infected and SMRV-infected FEC; Lanes 2 and 4, mock-infected and SMRV-infected flounder liver.
Furthermore, the tissue distribution and inductive expression of PoPKR was analyzed in healthy flounder and SMRV-infected flounder by real-time PCR and Western blotting. As shown in Fig. 5B, PoPKR was basally transcribed, and the inductive expression also was observed in all examined tissues, especially in liver and intestine. Due to the relatively high level of inductive expression, liver and intestine were selected to further study the phosphorylation of eIF2α upon virus infection. As shown in Fig. 5C (upper), consistently with the high expression of PoPKR, the enhanced phosphorylation of eIF2α was detected in both tissues, with a peak at 48 h p.i. (an approximately 2.5-fold increase by quantitative and statistical analyses [Fig. 5C, lower]). These results indicate that SMRV infection is able to induce PoPKR expression in vitro and in vivo and concomitantly enhances eIF2α phosphorylation.
Enhancement of eIF2α phosphorylation by PoPKR.
To evaluate whether the concomitantly enhanced phosphorylation of eIF2α was mediated by PoPKR in virus infection, we analyzed the interaction between PoPKR and eIF2α by using coimmunoprecipitation assays. As shown in Fig. 5D, when PoPKR data were normalized and remained constant in all of the coimmunoprecipitation samples, a significant increase in the level of the phosphorylated eIF2α was detected in SMRV-infected FEC and a weak one in the liver of SMRV-infected flounder compared to the basal level of phosphorylated eIF2α in mock-infected cells and liver. These results reveal that SMRV infection leads to a consistent increase in the phosphorylation of eIF2α that is bound to PoPKR in vitro and in vivo, indicating that this enhanced eIF2α phosphorylation is definitively attributed to PoPKR activation under virus infection.
Essential antiviral effect of PoPKR by phosphorylating eIF2α.
To test the effect of the PoPKR product on SMRV replication, FEC were transiently transfected by either WT PKR or mutant K421R, respectively. Following the infection of transiently transfected cells by SMRV, the viral yields were determined at various time points. As shown in Fig. 6A, in WT PKR-transfected FEC, viral titers reached 104.5 TCID50/ml at 24 h p.i. and peaked at 106.5 TCID50/ml at 48 to 72 h p.i., while in K421R-transfected cells, SMRV replication was substantially high, reaching titers of 108.4 TCID50/ml at 24 h p.i. and about 109 TCID50/ml at 48 h p.i., which was an approximately 275-fold increase over the titers of WT PKR-transfected cells. However, in control cells that were transfected with empty vector pcDNA, SMRV replication was moderate, with a titer of 105.5 TCID50/ml at 24 h p.i. and 107 TCID50/ml at 72 h p.i. These results indicated that the overexpression of WT PKR significantly inhibits the replication of SMRV, while the transfection of K421R increases the permissiveness to SMRV infection.
FIG. 6.
Inhibition of SMRV propagation by the overexpression of PoPKR through phosphorylating eIF2α. (A) FEC were transiently transfected with WT PKR, K421R, and pcDNA3.1 by the Genejuice procedure. At 48 h after transfection, the transfected cells were infected with SMRV and virus yields were measured in EPC cells at 3, 6, 12, 24, 48, and 72 h p.i. Data represent means ± standard errors of triplicate experiments. (B) Cell lysates were made from the infected cells at the indicated time points and were subjected to Western blot analysis with eIF2α-phosphospecific antibody (eIF2α-P) and eIF2α antibody (eIF2α) (Cell Signaling Technology) to detect phosphorylated eIF2α and total eIF2α, respectively. The actin detection with the antibody against actin was used as a loading control (lane C). (C) Quantitative and statistical analysis of the data shown in panel B by BandScan software. (D) The infected cell lysates from WT PKR transfection and K421R transfection at 48 h p.i. were subjected to immunoprecipitation with anti-human eIF2α antibody (Santa Cruz). The immunocomplex samples were subjected to Western blot analysis with polyclonal anti-PoPKR antibody. When the sample volumes were adjusted to make the PoPKR amounts equal, the phosphorlated eIF2α binding to PoPKR was detected by eIF2α-P (left), and the proportions of the phosphorylated eIF2α and PoPKR were quantified and statistically analyzed (right). Data represent means ± standard errors of triplicate experiments.
In accordance with the virus yields, a high level of eIF2α phosphorylation was observed in WT PKR-transfected cells and pcDNA-transfected cells (Fig. 6B). In comparison to their basal levels, at 48 h p.i. the level of eIF2α phosphorylation increased by up to 2.1-fold for WT PKR-transfected cells and 1.4-fold for pcDNA-transfected cells, whereas there was no significant increase in the level of eIF2α phosphorylation for K421R-transfected cells (Fig. 6C). The phosphorylation levels of eIF2α protein that bound to PoPKR were further examined by coimmunoprecipitation and Western blot analysis. As shown in Fig. 6D, PoPKR was coimmunoprecipitated by anti-human eIF2α antibody. When the level of immunoprecipitated PoPKR was equivalent between them, the level of phosphorylated eIF2α in the PoPKR-transfected FEC was about 5.9-fold higher than that in the K421R-transfected cells, further demonstrating that K421R had a dominant-negative effect, which was analogous to that of the catalytically inactive mutant K296R of human PKR (31). These results indicate that the overexpression of PoPKR is able to inhibit viral replication by regulating the phosphorylation of eIF2α.
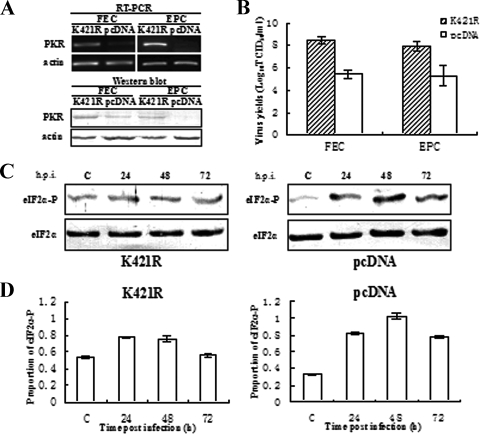
To better understand the antiviral effect of PoPKR as a translational regulator, stable transformants of FEC and EPC cells following transfection with either K421R or empty vector pcDNA were obtained and used to test the viral replication under the functional loss of PoPKR. RT-PCR and Western blot detection indicated that only a small quantity of endogenous PoPKR was observed in empty vector pcDNA transformants, but a high level of PoPKR was expressed in K421R transformants (Fig. 7A), indicating that the construct K421R was successfully integrated into the cellular genomes. As expected, a dramatic discrepancy in SMRV susceptibility was observed between the two transformants. At 48 h p.i., the viral titers were 108.5 and 107.9 TCID50/ml in K421R transformants of FEC and EPC cells, whereas they were only 105.4 and 105.3 TCID50/ml in the corresponding pcDNA transformants (Fig. 7B). These data meant that the loss of PoPKR function increased the susceptibility and level of infection of SMRV. Moreover, the kinetics of eIF2α phosphorylation in response to SMRV infection was analyzed in the FEC transformants. Along with the infection progress, an approximately 3.1-fold increase in phosphorylated eIF2α was detected in the control pcDNA transformant, whereas only a weak rise was seen in the level in the K421R transformant (Fig. 7C and D). The data suggested that the relative reduction of eIF2α phosphorylation should result from the overexpression of the catalytic inactivity of K421R. Therefore, the functional loss of PoPKR kinase reduces the level of eIF2α phosphorylation, and the essential antiviral effect of PoPKR is accomplished by phosphorylating eIF2α.
FIG. 7.
Increase in permissiveness to SMRV replication by stable transfection with K421R. (A) RT-PCR (upper) and Western blot detection (lower) of PoPKR expression in stable transformants of FEC and EPC cells obtained from transfection with K421R and pcDNA3.1. (B) Stable K421R and pcDNA3.1 transformants of FEC and EPC cells were seeded in 6-well dishes and then infected with SMRV (1 × 104 TCID50/ml). Three days later, virus yields were measured in both cells. Data represent means ± standard errors of triplicate experiments. (C) K421R and pcDNA3.1 transformants of FEC were infected with SMRV (1 × 104 TCID50/ml) for 24, 48, and 72 h. The phosphorylated eIF2α and total eIF2α were detected with phosphospecific antibody (eIF2α-P) and eIF2α antibody (eIF2α) (Cell Signaling Technology), respectively. (D) Quantitative and statistical analysis of the data shown in panel C by BandScan software. Data represent means ± standard errors of triplicate experiments.
Inhibition of eIF2α phosphorylation in PoPKR knockdown FEC by siRNA.
To further confirm the phosphorylation of eIF2α by PoPKR, we used siRNA technology to analyze the PoPKR physiological function. When the PoPKR knockdown cells transfected with 100 nM of PoPKR-specific siRNA (si-PKR) and the nontargeting siRNA (si-NT)-transfected cells were infected with SMRV, the PoPKR-specific siRNA efficiently and significantly reduced the expression levels of PoPKR during the SMRV infection course, whereas the PoPKR expression in the nontargeting siRNA-transfected cells obviously increased at 72 h after SMRV infection (Fig. 8A). Moreover, although there was an obvious increase in eIF2α phosphorylation in the nontargeting siRNA-transfected cells, there was almost no enhancement of eIF2α phosphorylation in the PoPKR knockdown cells (Fig. 8B). The quantified analysis revealed that the phosphorylated eIF2α proportion in the control si-NT-transfected cells was about 1.9-fold as high as that in the PoPKR knockdown cells (Fig. 8C). The data indicated that PoPKR knockdown significantly inhibits eIF2α phosphorylation during SMRV infection, confirming that the enhanced eIF2α phosphorylation is catalyzed by PoPKR in virus infection.
FIG. 8.
Inhibition of eIF2α phosphorylation in the PoPKR knockdown FEC by siRNA. (A) Western blot detection of PoPKR expression in PoPKR knockdown FEC by siRNA (si-PKR) and nontargeting siRNA of mock-infected FEC (si-NT). FEC were transiently transfected with PKR-siRNA (si-PKR) and nontargeting siRNA (si-NT). Seventy-two hours later, the cells were infected with SMRV (1 × 104 TCID50/ml) for 24, 48, and 72 h, and their PKR levels were detected by polyclonal anti-PoPKR serum (upper). The actin protein (lower) was detected by polyclonal anti-human actin (Santa Cruz). (B) The phosphorylated eIF2α and total eIF2α was detected during the SMRV infection course with phosphospecific antibody (eIF2α-P) and eIF2α antibody (eIF2α) (Cell Signaling Technology), respectively. The actin protein (lower) was detected by polyclonal anti-human actin. (C) The proportions of the phosphorylated eIF2α and total eIF2α were quantified and statistically analyzed. Data represent means ± standard errors of triplicate experiments.
DISCUSSION
Mammalian PKR has been demonstrated to play a key role in host defense against viral infection through the phosphorylation of eIF2α (2, 43). Some studies also suggested that the PKR-mediated antiviral pathway is a conserved characteristic in vertebrates (18, 52). However, so far no reports exist to identify any authentic fish PKR gene and demonstrate an essential and nonredundant role in viral immunity. In this study, flounder PKR was cloned, and the putative protein showed a conserved structure that is characteristic of mammalian PKRs, with the identical properties including the N-terminal domain binding to poly(I:C) and the C-terminal domain inhibiting protein synthesis. Importantly, the overexpression of WT PKR and a catalytically inactive mutant K421R demonstrated that PoPKR was able to inhibit the proliferation of SMRV in the cultured cells by binding to and phosphorylating its substrate eIF2α, a mechanism similar to that in mammals (21).
The presented data revealed the constitutive expression of PoPKR in FEC and flounder tissues, and the expression was upregulated in response to SMRV infection. According to the studies of mammals (33), the upregulation of PoPKR seemed to be induced by IFN that was generated in SMRV-infected FEC. It has been confirmed that there was a complete IFN system in fish (51) and that IFN antiviral activity was induced from UV-inactivated GCHV-infected FEC (8). Therefore, we suggested that the constitutive PoPKR protein is present in a latent state, and its activation requires prior binding to dsRNA, a general by-product of virus replication. This hypothesis was demonstrated by a poly(I:C) pull-down assay in which not only the complete N terminus but also two dsRBMs of PoPKR separately displayed the capability to bind poly(I:C) in vitro. Subsequent experiments showed the interaction between PoPKR and eIF2α and the enhanced phosphorylation of eIF2α by PoPKR in SMRV-infected FEC and flounder tissues. Therefore, we believe that the latent PoPKR should be activated in the SMRV-infected cells, possibly through binding to dsRNA, and that both dsRBM1 and dsRBM2 in PoPKR, similarly to the corresponding dsRBMs in mammalian PKR (19, 41), have dsRNA binding ability.
The kinase activity of PoPKR also is established by cotransfecting WT PKR, ΔN PKR, K421R, and ΔN-K421R with the luciferase reporter gene construct (Fig. 3). By using the general method for evaluating PKR function in mammals (35), we confirm that PoPKR is able to inhibit protein translation in vitro. In addition to WT PKR, the isolated kinase domain ΔN PKR also prevents luciferase translation. Recently, a PACT binding motif that was located in the C-terminal catalytic domain of PKR was revealed to be responsible for the closed and inactive conformation of PKR (28). Based on the data, the removal of two dsRBMs might relieve the autoinhibition of PoPKR, which possibly contributes to the catalytic activity of ΔN PKR. However, mutating the predicated ATP binding site (K421R) resulted in the loss of catalytic activity for K421R and ΔN-K421R, validating the idea that residue lysine 421 is essential for the kinase activity and that the catalytic activity of the kinase is required for translation shutoff in vitro. Moreover, the physiological relevance of PoPKR in host defense against viral infection was demonstrated by two experiments. First, transient transfection of WT PoPKR reinforced the resistance of FEC to SMRV infection, since the reduction of viral yield was detected in transfected cells; however, a relief of viral replication was observed in the context of the transfection of catalytically inactive mutant K421R. Second, the increased viral titer was observed in K421R transformants of both EPC and FEC, indicating a general property of the dominant-negative effect of the mutant K421R and the ability of PoPKR to inhibit virus replication. These findings provide an insight into the structure-function relationship and confirm the conserved antiviral role of PoPKR.
Significantly, the cross-recognition of anti-human eIF2α antibody in flounder cells and tissues allowed us to easily detect the phosphorylation of endogenous flounder eIF2α. Interestingly, there was a constitutive phosphorylation of eIF2α in normal cultured cells and flounder tissues, indicating a constitutive activity of eIF2α kinases, possibly including PoPKR. Actually, a similar situation is observed in mammals (47). SMRV infection activated the upregulation of PoPKR, which was correlated with the fast phosphorylation of eIF2α. Moreover, eIF2α was verified to bind to PKR in normal or virus-infected cells and tissues, and the phosphorylation of eIF2α that was bound to PKR was promoted by the overexpression of WT PoPKR but impaired by the overexpression of K421R. Finally, the enhanced eIF2α phosphorylation during virus infection was further confirmed by the PoPKR-specific siRNA knockdown, which is similar to the technology used in mammalian PKR studies (5). Therefore, the enhanced phosphorylation of eIF2α by SMRV infection likely results from the catalytic activity of the bound PoPKR. In addition, viral replication was inhibited in the context of PoPKR overexpression, which is consistent with enhanced eIF2α phosphorylation. These results demonstrate that PoPKR functions as an antiviral effector through the regulation of eIF2α phosphorylation and, thus, blocks viral protein synthesis.
To our knowledge, this is the first report to reveal the biological functions of the fish PKR gene. In addition to PKR, there are three other well-characterized members of the eIF2α kinases, including HRI, GCN2, and PERK (6, 12, 42). These kinases possess an extensively conserved catalytic domain at their C terminus that ascribes them to the same eIF2α kinase family and a unique N-terminal regulatory region that confers on each kinase the ability to respond to a specific stress signal. Since 2004, a PKR-like or PKZ gene has been characterized in fish and thought to be an effector that is similar to mammalian PKRs (3, 22, 39). According to the phylogenetic analysis (Fig. 1C), PKR-like or PKZ proteins and flounder PKR cluster with mammalian PKRs, indicating that PKR-like or PKZ originates from PKR. Since fish PKR-like is significantly upregulated in virus-infected cells and tissues, it is likely that PKR-like also participates in the shutoff of protein synthesis in response to virus infection. In the current study, we also attempted, but failed to clone, the PKR-like gene from Paralichthys olivaceus, so the relative physiologic importance of fish PKR and PKZ and eIF2α phosphorylation was not clarified. However, we have successfully identified both PKR-like and PKR genes in Carassius auratus (unpublished data) and, more recently, in other fishes, including zebrafish (38), indicating the coexistence of both genes in the same fish species. Future work should be performed to clarify the role of fish PKR-like and the functional relationship with fish PKR during virus infection.
Collectively, the current study substantiates the hypothesis that fish have the orthologue of mammalian PKR and possess a PKR-mediated antiviral pathway through the phosphorylation of eIF2α. A marked structural difference between PoPKR and the identified mammalian and avian PKRs is the length of the linker between the two dsRBMs (the intramotif sequence [IMS]). The human PKR IMS is composed of about 24 amino acids (residues 78 to 100) and is responsible for the binding of differently sized RNAs and different binding efficiencies (19, 37). However, PoPKR has a long IMS that is composed of 142 amino acids. In addition, human PKR has three tyrosines that recently were identified to play a role in efficient kinase activation and eIF2α phosphorylation (44), but only one (corresponding to Tyr-110 of human PKR) is conserved in PoPKR. Finally, there is no direct evidence for PoPKR induction by IFN, although PoPKR could be induced by poly(I:C) (data not shown), a general inducer of IFN. Therefore, another key aspect of future work will be to clarify the unique function of the structure of PoPKR and a linker to the IFN antiviral response.
Acknowledgments
This work was supported by grants from the National Basic Research Program of China (2004CB117403), the National 863 High Technology Research Program (2007AA09Z429), the National Natural Science Foundation of China (30471333 and 30671617), and the Innovation Project of IHB, CAS (085A01-1-301).
Footnotes
Published ahead of print on 30 April 2008.
REFERENCES
- 1.Adelson, M. E., M. M. Camille, T. I. Kathryn, F. M. Nicholas, and J. S. Robert. 1999. Inhibition of human immunodeficiency virus (HIV-1) replication in SupT1 cells transduced with an HIV-1 LTR-driven PKR cDNA construct. Eur. J. Biochem. 264806-815. [DOI] [PubMed] [Google Scholar]
- 2.Balachandran, S., P. C. Roberts, L. E. Brown, H. Truong, A. K. Pattnaik, D. R. Archer, and G. N. Barber. 2000. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity 13129-141. [DOI] [PubMed] [Google Scholar]
- 3.Bergan, V., R. Jagus, S. Lauksund, Ø. Kileng, and B. Robertsen. 2008. The Atlantic salmon Z-DNA binding protein kinase phosphorylates translation initiation factor 2 alpha and constitutes a unique orthologue to the mammalian dsRNA-activated protein kinase R. FEBS J. 275184-197. [DOI] [PubMed] [Google Scholar]
- 4.Cai, R., and B. R. Williams. 1998. Mutations in the double-stranded RNA-activated protein kinase insert region that uncouple catalysis from eIF2α binding. J. Biol. Chem. 27311274-11280. [DOI] [PubMed] [Google Scholar]
- 5.Chang, K. S., Z. Cai, C. Zhang, G. C. Sen, B. R. G. Williams, and G. Luo. 2006. Replication of hepatitis C virus (HCV) RNA in mouse embryonic fibroblasts: protein kinase R (PKR)-dependent and PKR-independent mechanisms for controlling HCV RNA replication and mediating interferon activities. J. Virol. 807364-7374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, J. J., M. S. Throop, L. Gehrke, I. Kuo, J. K. Pal, M. Brodsky, and I. M. London. 1991. Cloning of the cDNA of the heme-regulated eukaryotic initiation factor 2α (eIF-2α) kinase of rabbit reticulocytes: homology to yeast GCN2 protein kinase and human double-stranded-RNA-dependent eIF-2α kinase. Proc. Natl. Acad. Sci. USA 887729-7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, S. L., G. C. Ren, Z. X. Sha, and C. Y. Shi. 2004. Establishment of a continuous embryonic cell line from Japanese flounder Paralichthys olivaceus for virus isolation. Dis. Aquat. Organ. 60241-246. [DOI] [PubMed] [Google Scholar]
- 8.Chen, Y. D., Y. B. Zhang, R. Zhu, J. Jiang, Q. Y. Zhang, and J. F. Gui. 2005. Construction of a subtractive cDNA library from the Paralichthys olivaceus embryonic cells induced by a double-stranded RNA virus. Virol. Sin. 20168-172. [Google Scholar]
- 9.Chen, Y. D., Y. B. Zhang, R. Zhu, F. T. Zhang, J. Jiang, Y. Shi, Q. Y. Zhang, S. L. Cheng, and J. F. Gui. 2005. Inductive expression and characterization analysis of Paralichthys olivaceus pigment epithelium-derived factor in a virally infected cell line. Biochem. Biophys. Res. Commun. 335799-809. [DOI] [PubMed] [Google Scholar]
- 10.Craig, A. W., G. P. Cosentino, O. Donzé, and N. Sonenberg. 1996. The kinase insert domain of interferon-induced protein kinase PKR is required for activity but not for interaction with the pseudosubstrate K3L. J. Biol. Chem. 27124526-24533. [DOI] [PubMed] [Google Scholar]
- 11.Der, S. D., and A. S. Lau. 1995. Involvement of the double-stranded-RNA-dependent kinase PKR in interferon expression and interferon-mediated antiviral activity. Proc. Natl. Acad. Sci. USA 928841-8845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dever, T. E., L. Feng, R. C. Wek, A. M. Cigan, T. F. Donahue, and A. G. Hinnebusch. 1992. Phosphorylation of initiation factor 2 alpha by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast. Cell 8585-596. [DOI] [PubMed] [Google Scholar]
- 13.Dey, M., C. Cao, A. C. Dar, T. Tamura, K. Ozato, F. Sicheri, and T. E. Dever. 2005. Mechanistic link between PKR dimerization, autophosphorylation, and eIF2α substrate recognition. Cell 122901-913. [DOI] [PubMed] [Google Scholar]
- 14.Dong, C. H., S. T. Yang, Z. A. Yang, L. Zhang, and J. F. Gui. 2004. A C-type lectin associated and translocated with cortical granules during oocyte maturation and egg fertilization in fish. Dev. Biol. 265341-354. [DOI] [PubMed] [Google Scholar]
- 15.Du, C. S., Q. Y. Zhang, C. L. Li, D. L. Miao, and J. F. Gui. 2004. Induction of apoptosis in a carp leucocyte cell line infected with turbot (Scophthalmus maximus L.) rhabdovirus. Virus Res. 101119-126. [DOI] [PubMed] [Google Scholar]
- 16.Fasciano, S., B. Hutchins, I. Handy, and R. C. Patel. 2005. Identification of the heparin-binding domains of the interferon-induced protein kinase, PKR. FEBS J. 2721425-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.García, M. A., J. Gil, I. Ventoso, S. Guerra, E. Domingo, C. Rivas, and M. Esteban. 2006. Impact of protein kinase PKR in cell biology: from antiviral to antiproliferative action. Microbiol. Mol. Biol. Rev. 701032-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garner, J. N., B. Joshi, and R. Jagus. 2003. Characterization of rainbow trout and zebrafish eukaryotic initiation factor 2alpha and its response to endoplasmic reticulum stress and IPNV infection. Dev. Comp. Immunol. 27217-231. [DOI] [PubMed] [Google Scholar]
- 19.Green, S. R., and M. B. Mathews. 1992. Two RNA-binding motifs in the double-stranded RNA-activated protein kinase DAI. Genes Dev. 62478-2490. [DOI] [PubMed] [Google Scholar]
- 20.Hershey, J. W. 1991. Translational control in mammalian cells. Annu. Rev. Biochem. 60717-755. [DOI] [PubMed] [Google Scholar]
- 21.Hovanessian, A. G. 2007. On the discovery of interferon-inducible, double-stranded RNA activated enzymes: the 2′-5′oligoadenylate synthetases and the protein kinase PKR. Cytokine Growth Factor Rev. 18351-361. [DOI] [PubMed] [Google Scholar]
- 22.Hu, C. Y., Y. B. Zhang, G. P. Huang, Q. Y. Zhang, and J. F. Gui. 2004. Molecular cloning and characterisation of a fish PKR-like gene from cultured CAB cells induced by UV-inactivated virus. Fish Shellfish Immunol. 17353-366. [DOI] [PubMed] [Google Scholar]
- 23.Katze, M. G., M. Wambach, M. L. Wong, M. Garfinkel, E. Meurs, K. Chong, B. R. Williams, A. G. Hovanessian, and G. N. Barber. 1991. Functional expression and RNA binding analysis of the interferon-induced, double-stranded RNA-activated, 68,000-Mr protein kinase in a cell-free system. Mol. Cell. Biol. 115497-5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaufman, R. J., M. V. Davies, V. K. Pathak, and J. W. B. Hershey. 1989. The phosphorylation state of eucaryotic initiation factor 2 alters translational efficiency of specific mRNAs. Mol. Cell. Biol. 9946-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaufman, R. J., and P. Murtha. 1987. Translational control mediated by eucaryotic initiation factor-2 is restricted to specific mRNAs in transfected cells. Mol. Cell. Biol. 71568-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koromilas, A. E., S. Roy, G. N. Barber, M. G. Katze, and N. Sonenberg. 1992. Malignant transformation by a mutant of the IFN-inducible dsRNA-dependent protein kinase. Science 2571685-1689. [DOI] [PubMed] [Google Scholar]
- 27.Lee, S. B., Z. Melkova, W. Yan, B. R. Williams, A. G. Hovanessian, and M. Esteban. 1993. The interferon-induced double-stranded RNA-activated human p68 protein kinase potently inhibits protein synthesis in cultured cells. Virology 192380-385. [DOI] [PubMed] [Google Scholar]
- 28.Li, S., G. A. Peters, K. Ding, X. Zhang, J. Qin, and G. C. Sen. 2006. Molecular basis for PKR activation by PACT or dsRNA. Proc. Natl. Acad. Sci. USA 10310005-10010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25402-408. [DOI] [PubMed] [Google Scholar]
- 30.Majumdar, R., and U. Maitra. 2005. Regulation of GTP hydrolysis prior to ribosomal AUG selection during eukaryotic translation initiation. EMBO J. 243737-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meurs, E. F., J. Galabru, G. N. Barber, M. G. Katze, and A. G. Hovanessian. 1993. Tumor suppressor function of the interferon-induced double-stranded RNA-activated protein kinase. Proc. Natl. Acad. Sci. USA 90232-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meurs, E. F., Y. Watanabe, S. Kadereit, G. N. Barber, M. G. Katze, K. Chong, B. R. Williams, and A. G. Hovanessian. 1992. Constitutive expression of human double-stranded RNA-activated p68 kinase in murine cells mediates phosphorylation of eukaryotic initiation factor 2 and partial resistance to encephalomyocarditis virus growth. J. Virol. 665805-5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meurs, E., K. Chong, J. Galaru, N. S. B. Thomas, I. M. Kerr, B. R. G. Willians, and A. G. Hovannessian. 1990. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell 62379-390. [DOI] [PubMed] [Google Scholar]
- 34.Nanduri, S., F. Rahman, B. R. Williams, and J. Qin. 2000. A dynamically tuned double-stranded RNA binding mechanism for the activation of antiviral kinase PKR. EMBO J. 195567-5574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel, C. R., P. Stanton, and C. G. Sen. 1996. Specific mutations near the amino terminus of double-stranded RNA-dependent protein kinase (PKR) differentially affect its double-stranded RNA binding and dimerization properties. J. Biol. Chem. 27125657-25663. [DOI] [PubMed] [Google Scholar]
- 36.Rhoads, R. E. 1993. Regulation of eukaryotic protein synthesis by initiation factors. J. Biol. Chem. 2683017-3020. [PubMed] [Google Scholar]
- 37.Robertson, H. D., and M. B. Mathews. 1996. The regulation of the protein kinase PKR by RNA. Biochimie 78909-914. [DOI] [PubMed] [Google Scholar]
- 38.Rothenburg, S., N. Deigendesch, M. Dey, T. E. Dever, and L. Tazi. 2008. Double-stranded RNA-activated protein kinase PKR of fishes and amphibians: varying the number of double-stranded RNA binding domains and lineage-specific duplications. BMC Biol. 612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rothenburg, S., N. Deigendesch, K. Dittmar, F. Koch-Nolte, F. Haag, K. Lowenhaupt, and A. Rich. 2005. A PKR-like eukaryotic initiation factor 2alpha kinase from zebrafish contains Z-DNA binding domains instead of dsRNA binding domains. Proc. Natl. Acad. Sci. USA 1021602-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scheuner, D., R. Patel, F. Wang, K. Lee, K. Kumar, J. Wu, A. Nilsson, M. Karin, and R. J. Kaufman. 2006. Double-stranded RNA-dependent protein kinase phosphorylation of the α-subunit of eukaryotic translation initiation factor 2 mediates apoptosis. J. Biol. Chem. 28121458-21468. [DOI] [PubMed] [Google Scholar]
- 41.Schmedt, C., S. R. Green, L. Manche, D. R. Taylor, Y. Ma, and M. B. Mathews. 1995. Functional characterization of the RNA-binding domain and motif of the double-stranded RNA-dependent protein kinase DAI (PKR). J. Mol. Biol. 24929-44. [DOI] [PubMed] [Google Scholar]
- 42.Shi, Y., K. M. Vattem, R. Sood, J. An, J. Liang, L. Stramm, and R. C. Wek. 1998. Identification and characterization of pancreatic eukaryotic initiation factor 2 α-subunit kinase, PEK, involved in translational control. Mol. Cell. Biol. 187499-7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stojdl, D. F., N. Abraham, S. Knowles, R. Marius, A. Brasey, B. D. Lichty, E. G. Brown, N. Sonenberg, and J. C. Bell. 2000. The murine double-stranded RNA-dependent protein kinase PKR is required for resistance to vesicular stomatitis virus. J. Virol. 749580-9585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Su, Q., S. Wang, D. Baltzis, L. K. Qu, A. H. Wong, and A. E. Koromilas. 2006. Tyrosine phosphorylation acts as a molecular switch to full-scale activation of the eIF2α RNA-dependent protein kinase. Proc. Natl. Acad. Sci. USA 10363-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sudhakar, A., A. Ramachandran, S. Ghosh, S. E. Hasnain, R. J. Kaufman, and K. V. Ramaiah. 2000. Phosphorylation of serine 51 in initiation factor 2 alpha (eIF2 alpha) promotes complex formation between eIF2 alpha(P) and eIF2B and causes inhibition in the guanine nucleotide exchange activity of eIF2B. Biochemistry 3912929-12938. [DOI] [PubMed] [Google Scholar]
- 46.Sumpter, R., Y. M. Loo, E. Foy, K. Li, M. Yoneyama, T. Fujita, S. M. Lemon, and M. Gale. 2005. Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J. Virol. 792689-2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tallóczy, Z., W. Jiang, H. W. Virgin IV, D. A. Leib, D. Scheuner, R. J. Kaufman, E. L. Eskelinen, and B. Levine. 2002. Regulation of starvation- and virus-induced autophagy by the eIF2α kinase signaling pathway. Proc. Natl. Acad. Sci. USA 99190-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 224673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang, Y. B., Q. Li, and J. F. Gui. 2004. Differential expression of two Carassius auratus Mx genes in cultured CAB cells induced by grass carp hemorrhage virus and interferon. Immunogenetics 5668-75. [DOI] [PubMed] [Google Scholar]
- 50.Zhang, F., P. R. Romano, T. Nagamura-Inoue, B. Tian, T. E. Dever, M. B. Mathews, K. Ozato, and A. G. Hinnebusch. 2001. Binding of double-stranded RNA to protein kinase PKR is required for dimerization and promotes critical autophosphorylation events in the activation loop. J. Biol. Chem. 27624946-24958. [DOI] [PubMed] [Google Scholar]
- 51.Zhang, Y. B., J. Jiang, Y. D. Chen, R. Zhu, Y. Shi, Q. Y. Zhang, and J. F. Gui. 2007. The innate immune response to grass carp hemorrhage virus (GCHV) in cultured Carassius auratus blastula (CAB) Cells. Dev. Comp. Immunol. 31232-243. [DOI] [PubMed] [Google Scholar]
- 52.Zhu, R., Y. B. Zhang, Y. D. Chen, C. W. Dong, F. T. Zhang, Q. Y. Zhang, and J. F. Gui. 2006. Molecular cloning and stress-induced expression of Paralichthys olivaceus heme-regulated initiation factor 2α kinase. Dev. Comp. Immunol. 301047-1059. [DOI] [PubMed] [Google Scholar]