Abstract
Generating and using regulatory T cells (Tregs) to modulate inflammatory disease represents a valuable approach to therapy but has not yet been applied as a means to control virus-induced immunopathological reactions. In this report, we developed a simplified technique that used unfractionated splenocytes as a precursor population and showed that stimulation under optimized conditions for 5 days with solid-phase anti-CD3 monoclonal antibody in the presence of transforming growth factor β (TGF-β) and interleukin-2 could induce up to 90% of CD4+ T cells to become Foxp3+ and able to mediate suppression in vitro. CD11c+ dendritic cells were intricately involved in the conversion process and, once modified in the presence of TGF-β, could convert Foxp3− CD4+ cells into Foxp3+ CD4+cells by producing TGF-β. The converted cells had undergone cell division, and the majority of them expressed activation markers along with surface molecules that would facilitate their migration into tissue sites. The primary reason for our study was to determine if such in vitro-converted Tregs could be used in vivo to influence the outcome of a virus-induced immunoinflammatory lesion in the eye caused by herpes simplex virus infection. We could show in three separate models of herpetic stromal keratitis that adoptive transfers of in vitro-converted Tregs effectively diminished lesion severity, especially when given in the initial phases of infection. The suppression effect in vivo appeared to be polyspecific. The protocol we have developed could provide a useful additional approach to control virus-induced inflammatory disease.
Numerous types of regulatory T cells (Tregs) were reported to control the immune responses to both self and foreign antigens (27). Impediments in Treg function can cause disease conditions (39). The best known example is the multiple-organ autoimmune disorders that occur in humans as well as in experimental animals when the gene for Foxp3 transcription factor is mutated (18, 24). Functional inactivation of Foxp3 gene alters the development and immunosuppressive activity of the natural regulatory CD4+ T-cell population. In recent years, naturally occurring Foxp3+ Tregs were shown to be the key cell type that maintains peripheral tolerance (16). Additionally, these cells also regulate pathogen- and allergen-induced inflammatory responses and boosting their functional activity may represent a valuable therapeutic approach to blunt transplant rejection. Previously, it was reported that depletion of natural Tregs (nTregs) prior to ocular infection with herpes simplex virus type 1 (HSV-1) increased the severity of immunoinflammatory lesions in the cornea (29). However, it would be valuable to know if Tregs can modulate virus-induced ongoing immunoinflammatory reactions and influence the disease progression.
In this report, we took advantage of the recent observations that Foxp3+ T cells with regulatory function can be generated from conventional T cells by appropriate in vitro activation conditions (4-6, 8, 34, 37). We modified the existing method to develop a simple in vitro technique to generate ovalbumin (OVA)-specific Foxp3+ regulatory CD4+ T cells from CD4+ Foxp3− cells. We then determined the ability of in vitro-generated Tregs to modulate the severity of an ocular immunoinflammatory reaction caused by infection with HSV in conventional and bystander models of HSV-1-induced stromal keratitis (SK). Our results showed that adoptive transfer of in vitro-generated OVA-specific Foxp3+ Tregs diminished lesion expression in three different models of herpetic SK in both an antigen-specific as well as nonspecific manner.
MATERIALS AND METHODS
Mice, virus, and cell lines.
Female 6- to 8-week-old BALB/c DO11.10 RAG2−/− mice were purchased from Taconic, Thy1.2+ BALB/c and CB.17 SCID mice were purchased from Charles River, and Thy1.1+ BALB/c mice were a kind gift from D. Woodland (Trudeau Institute, Saranac Lake, NY). All animals were housed in the animal facilities at the University of Tennessee. BALB/c DO11.10 RAG2−/− and CB.17 SCID mice were kept in our specific-pathogen-free facility, where food, water, bedding, and instruments were autoclaved and all manipulations were done in a laminar flow hood. All experimental procedures were in complete agreement with the Association for Research in Vision and Ophthalmology resolution on the use of animals in research. HSV-1 RE Hendricks and HSV-1 KOS were propagated and titrated on Vero cells (ATCC CCL81) using standard protocols. The virus was stored in aliquots at −80°C until use.
Corneal HSV-1 infection and clinical observations.
Corneal infections of unmanipulated BALB/c mice and DO11.10 RAG2−/− animals and adoptively transferred with induced Tregs (iTregs) were conducted under deep anesthesia. HSV RE and HSV KOS were used for induction of keratitis lesions in immunocompetent BALB/c and DO11.10 RAG2−/− animals, respectively. Sometimes Tregs were adoptively transferred in previously infected animals. Mice were scarified on their corneas with a 27-gauge needle, and a 3-μl drop containing the required viral dose was applied to the eye. The eyes were examined on different days postinfection (p.i.) with a slit-lamp biomicroscope (Kowa, Nagoya, Japan), and the clinical severity of keratitis and angiogenesis of individually scored mice were recorded. The scoring system was as follows: 0, normal eye; +1, mild corneal haze; +2, moderate corneal opacity or only the iris visible; +3, severe corneal opacity, iris invisible; +4, opaque cornea, ulcer formation; and +5, necrotizing SK. The angiogenesis was scored as described previously (15).
Antibodies and reagents.
CD4-allophycocyanin (APC) (RM4-5), Thy1.1-peridinin chlorophyll protein (OX-7), DO11.10-phycoerythrin (PE) (KJ1.26), CD25-fluorescein isothiocyanate (FITC) (7D4), glucocorticoid-induced tumor necrosis factor receptor (GITR)-FITC (DTA-1), folate receptor 4 (FR4)-FITC (eBio12A5), CD62L-FITC (MEL-14), CD103-FITC (M290), CD62L-APC (MEL-14) CD49d-PE (MFR4.B), ICAM-1-PE (3E2), ICOS-PE, PDL-1-PE (MIH5), PD-1-FITC (J43), Foxp3-PE (FJK-16S), Foxp3-FITC (FJK-16S), CD69-FITC (H1.2F3), CCR7-PE (4B12), CD11c-PE (HL3), anti-gamma interferon (IFN-γ)-FITC, anti-IL-10-FITC, and anti-IL-17-PE were purchased from BD PharMingen (San Diego, CA). Recombinant human transforming growth factor β1 (TGF-β1), anti-TGF-β1, -2, or -3 antibody (1D11), and anti-IL-10 antibodies (AB-417-NA) were obtained from R&D. Anti-CD3 (145.2C11) and anti-CD28 (37.51) were from BD Bioscience. Anti-PD1 antibody (J43) and anti-ICOS antibody (7E.17G9) were from ebioscience. Recombinant human IL-2 was obtained from Hemagen. OVA323-339 peptide was obtained from Genscript. Carboxyfluorescein succinimidyl ester (CFSE) was obtained from Molecular Probes and used at a final concentration of 0.5 μM for 15 min at 37°C in phosphate-buffered saline.
In vitro generation of CD4+ CD25+ Foxp3+ Tregs.
Splenocytes (SPCs) isolated from DO11.10 RAG2−/− mice were fractionated into CD4+ and CD4− T cells using a CD4+ T-cell isolation kit of T-cell-depleted SPCs (1 × 106), pulsed with various doses (2 μM, 1 μM, 0.62 μM, 0.31 μM, and 0.15 μM) of OVA peptide for 2 h at 37°C, and washed three times thereafter. OVA-pulsed cells were then cocultured in 1:1 ratio with purified CD4+ CD25− Foxp3− cells in the presence of 10 ng/ml of recombinant TGF-β (TGF-β) and 25 U of IL-2 for 5 days at 37°C, and thereafter the cells were characterized by flow cytometry. To discount the possibility of residual OVA peptide transfer along with transferred Tregs for subsequent in vivo experiments, OVA-specific Tregs were also generated with plate-bound anti-CD3 monoclonal antibody (MAb). To this end, 0.125 μg/ml of anti-CD3 MAb in a volume of 200 μl was coated overnight in 48-well flat-bottom plate in 0.1 M Na2HPO4 buffer (pH 9.0). Before establishing the culture of SPCs with the cocktail of various cytokines, plates were washed three times with medium, after which SPCs were cultured as described above but in the absence of OVA peptide. Total SPCs were isolated from DO11.10 RAG2−/− animals, and red blood cells (RBCs) were lysed using RBC lysing buffer. SPCs (2 × 106/ml) were cultured with various concentrations of recombinant human TGF-β1 (TGF-β1) (5, 10, or 15 ng), IL-2 (100, 50, or 25 U), and anti-CD3 antibody in a checkerboard fashion. The cultures were incubated for various periods ranging from 4 days to 7 days at 37°C in a humidified CO2 incubator to find the optimum dose of all constituents in the cocktail and the incubation periods that yielded a maximum percentage of iTregs. In certain experiments, SPCs were cultured in six-well culture plates to generate Tregs in bulk. The generated Tregs were phenotypically characterized by flow cytometry.
Purification of cells.
CD4+ and CD4− T cells were purified from naïve DO11.10 RAG2−/− mice using CD4+ T-cell isolation kits (Miltenyi Biotec). In addition, CD4+ and CD4− T cells were also purified from in vitro cultures for some in vivo experiments. CD11c+ DCs were isolated on magnetic bead columns (Miltenyi Biotec) after 48 h of in vitro culture from either those that contained the culture medium that resulted in Foxp3+ T-cell conversion or medium that lacked TGF-β. CD4+ CD25+ and CD4+ CD25− T cells were isolated from in vitro cultures using a CD4+ Treg isolation kit (Miltenyi Biotec). The depletion of T cells from splenocytes was achieved by using Thy1.2 microbeads (Miltenyi Biotec). All purification procedures were performed as per the manufacturers' instructions.
In vitro suppression assay.
In vitro suppression assays were performed to assess and compare the inhibitory activities of iTregs and splenic nTregs against anti-CD3-stimulated CD4+ CD25− T cells. To assess the suppressive activity of iTregs, CD4+ CD25+ T cells were purified from SPCs cultured for 5 days in the presence or absence of recombinant TGF-β1. CD4+ CD25− T cells were isolated from pooled spleens and lymph nodes (LNs) of DO11.10 RAG2−/− mice and labeled with 0.5 μM CFSE. T-cell-depleted SPCs were isolated from spleens of DO11.10 RAG2−/− mice using Thy1.2 microbeads and irradiated before use in the suppression assays. CFSE-labeled CD4+ CD25− T cells (5 × 104) were cultured in the presence of soluble anti-CD3 MAb (1.0 μg/ml), and irradiated SPCs (1 × 105) were cultured with sequentially diluted CD4+ CD25+ T cells from either culture in 96-well round-bottom plates. After 72 h, dilution of CFSE was analyzed by flow cytometry. The gate was applied on CD4+ CFSE+ T cells, and the intensity of CFSE staining was analyzed.
To compare the suppressive activity of iTregs with that of splenic nTregs, iTregs were isolated from the in vitro culture system and CD4+ CD25+ cells were isolated from the pooled spleens of BALB/c animals. CD4+ CD25− T cells were isolated from pooled spleens and LNs of Thy1.1 BALB/c animals and labeled with 0.5 μM CFSE. Cultures were set up as described above, and the dilution of CFSE in Thy1.1+ CD4+ gated cells was analyzed.
Measurement of in vivo activity of iTregs.
Three different systems were used to measure the in vivo activity of in vitro-generated Tregs. In the first series of experiments, cultured SPCs containing 5 × 105 of CD4+ CD25+ Foxp3+ T cells were adoptively transferred intravenously (i.v.) in DO11.10 RAG2−/− mice 1 day prior to or 6 days after ocular infection with 5 × 105 PFU of HSV-1 KOS, and the lesions were scored every alternate day beginning at day 4. At day 11, mice were sacrificed and cells recovered from cornea, draining LNs (DLNs), and spleen were analyzed by flow cytometry.
In the second set of experiments, 5 × 106 isolated CD4+ CD25− T cells from BALB/c animals were transferred with or without 1 × 106 nTregs or OVA Tregs into SCID animals, which were then infected with ocular HSV-1 (5 × 105 PFU). The SK lesion progression and angiogenesis were monitored for 12 days. To look for the proliferation of lesion-orchestrating CD4+ T cells, CFSE-labeled CD4+ CD25− T cells from BALB/c animals were transferred alone or with iTregs or nTregs. These animals were infected 24 h later with 5 × 105 PFU of HSV-1 RE. After 7 days of transfer, the proliferation of CD4+ CFSE+ T cells was analyzed by dilution of CFSE.
Finally, experiments were done to look for the disease-modulatory activity of Tregs in immunocompetent BALB/c animals. Five different doses (2 × 106, 1 × 106, 5 × 105, 2 × 105, and 5 × 104) of Foxp3+ T cells were adoptively transferred i.v. in BALB/c animals 1 day prior to the ocular HSV RE (5 × 105 PFU) infection. The disease severity was recorded for 15 days p.i. A minimum dose of Tregs which could inhibit the disease progression in these animals was used for subsequent studies in which cells were transferred 1 day before or 3 or 6 days p.i. Animals were sacrificed at different time intervals to collect and analyze lymphoid and nonlymphoid tissues.
Flow cytometric analysis. (i) Cell preparation.
Single-cell suspensions were prepared from the corneas, DLNs, and spleens of mice at different time points p.i. Corneas were excised, pooled groupwise, and digested with 60 U/ml Liberase (Roche Diagnostics) for 60 min at 37°C in a humidified atmosphere of 5% CO2 as described earlier (29). After incubation, the corneas were disrupted by grinding with a syringe plunger on a cell strainer and a single-cell suspension was made in complete RPMI 1640 medium.
(ii) Staining for flow cytometry.
The single-cell suspensions obtained from LNs, spleens, and corneal samples were stained for different cell surface molecules for fluorescence-activated cell sorting (FACS). All steps were performed at 4°C. Briefly, a total of 1 × 106 cells were first blocked with an unconjugated anti-CD32/CD16 MAb for 30 min in FACS buffer. After washing with FACS buffer, fluorochrome-labeled respective antibodies were added for 30 min. Finally, the cells were washed three times and resuspended in 1% paraformaldehyde.
To enumerate the number of IFN-γ-producing T cells, intracellular cytokine staining was performed as previously described (26). In brief, 106 freshly isolated SPCs and LN cells were cultured in U-bottom 96-well plates. Cells were left untreated, stimulated with 2 multiplicities of infection of UV-inactivated HSV-1, and incubated overnight at 37°C in 5% CO2. Brefeldin A (10 μg/ml) was added for the last 5 h of the culture period. After this period, cell surface staining was performed, followed by intracellular cytokine staining using a Cytofix/Cytoperm kit (BD PharMingen) in accordance with the manufacturer's recommendations. The antibodies used were anti-IFN-γ-FITC and anti-IL-17-PE. The fixed cells were resuspended in 1% paraformaldehyde and acquired with BD FACSCalibur. The data were analyzed using the CellQuestPro 3.1 (BD Biosciences) or Flowjo software.
BrdU incorporation assay.
Bromodeoxyuridine (BrdU) analysis was performed as described earlier (30). Briefly, mice were divided into four groups: naïve, naïve plus iTregs, infected, and infected plus iTregs. A total of 5 × 105 iTregs were transferred a day before ocular infection, and animals were then fed BrdU in drinking water at 1 mg/ml for 10 days after adoptive transfer of iTregs and ocular HSV-1 infection. After 10 days, host CD4+ Foxp3+ and CD4+ Foxp3− cells that incorporated BrdU were analyzed by staining with anti-BrdU antibody using an APC BrdU flow kit from BD Pharmingen as per the manufacturers' instructions.
Statistical analysis.
To calculate the statistical significance for disease severity between different groups, the unpaired two-tailed Student's t test was performed. All other analyses for statistically significant differences were performed with Student's t test. P ≤ 0.001, P ≤ 0.01, and P ≤ 0.05 were considered significant. Results are expressed as means ± standard deviation. For some experiments, as mentioned in the figure legends, one-way analysis of variance (ANOVA) was applied.
RESULTS
In vitro generation of OVA-specific Treg (iTregs).
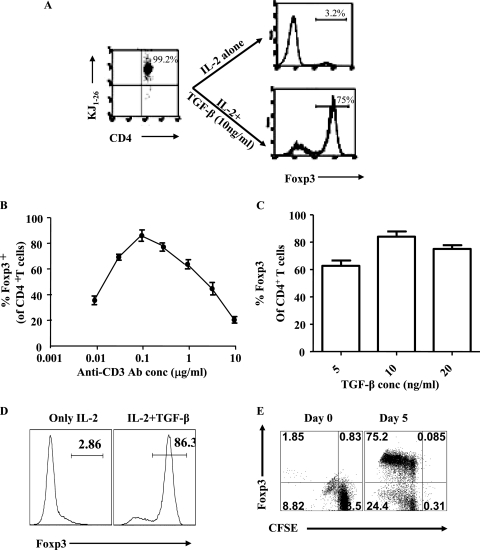
In initial experiments, splenic cells from naive DO11.10 RAG2−/− animals were fractionated on MACS columns into the T-cell fraction (greater than 95% KJ+ CD4+ and hence OVA specific) as well as the non-T-cell fraction. These non-T cells were pulsed with various concentrations of the OVA323-339 peptide and used to stimulate the T-cell fraction in a 1:1 ratio. The culture medium contained IL-2 (25 U/ml) as well as recombinant human TGF-β (10 ng/ml), conditions expected to be suitable to induce Foxp3+ T cells from conventional precursors (6, 8). Using these conditions, we observed that after a 5-day culture period, up to 70% (range 50 to 70%) of the surviving cells in the culture were Foxp3+. The dose of OVA peptide that gave maximum conversion efficiency was 0.31 μM.
To simplify matters, we also cultured unfractionated DO11.10 Rag2−/− naive SPCs in culture plates that had been preincubated with various concentrations of rat anti-mouse CD3 antibody. In preliminary experiments using 0.3 μg/ml to sensitize plates and the same TGF-β and IL-2 concentrations described above, up to 75% (range, 70 to 75%) of cells were observed to be Foxp3+ after 5 days of stimulation (Fig. 1A). When peptide (0.31 μM) was used for stimulation instead of anti-CD3, up to 45% (range, 30 to 45%) of CD4+ T cells became Foxp3 (data not shown). Subsequently, the system was optimized by using various concentrations of anti-CD3 and TGF-β, but using the same amount of IL-2. As shown in Fig. 1B and C, a concentration of 0.125 μg/ml of anti-CD3 and 10 ng/ml of TGF-β turned out to be optimal. With the optimal conditions, the frequency of CD4+ T cells that were Foxp3+ ranged from 80% to 90% of the CD4+ T cells in different experiments (Fig. 1D). These conditions were chosen for subsequent experiments in which in vitro-converted cells were used in vivo in an attempt to modulate HSV-induced ocular inflammatory lesions. During the conversion process, the Foxp3+ T cells underwent division (one to six times), although some converted cells must have died since the overall yield of Foxp3+ cells was usually around twofold the number of input Foxp3− CD4+ T cells (Fig. 1E).
FIG. 1.
In vitro generation of Foxp3+ CD4+ T cells from OVA-specific precursor Foxp3− CD4+ T cells. (A) Splenocytes from DO11.10 RAG2−/− mice were cultured in the presence of 0.3 μg/ml of anti-CD3 antibody, 25 U of recombinant IL-2, and the indicated concentrations of TGF-β. More than 99% of CD4+ T cells were KJ1.26 positive (gated on CD4+ T cells). After 5 days of culture, cells were analyzed for the expression of CD4 and Foxp3. Under these conditions, approximately 75% of CD4+ T cells became Foxp3+. (B) A dose-response curve for Foxp3 induction with various concentrations of anti-CD3 antibody, 10 ng/ml of TGF-β, and 25 U of IL-2 is shown. A dose of 0.125 μg/ml of anti-CD3 antibody (plate bound) was found to be optimal. (C) A dose-response histogram for Foxp3 induction with various concentrations of TGF-β, 25 U of IL-2, and 0.125 μg/ml of anti-CD3 is shown. At a dose of 10 ng/ml of TGF-β, 80 to 90% of CD4+ T cells converted to become Foxp3+. (D) Representative histograms for gated CD4+ T cells are shown to show the Foxp3 induction under optimal conditions (0.125 μg/ml of plate-bound anti-CD3 antibody, 25 U of IL-2, 10 ng/ml of TGF-β). (E) SPCs from DO11.10 RAG2−/− animals were CFSE labeled and cultured with plate-bound anti-CD3, IL-2, and TGF-β for 5 days. After 5 days, cells were stained with CD4 and Foxp3. CFSE dilution and Foxp3 expression were shown in gated CD4+ T cells. TGF-β induced CD4+ CD25+ Foxp3+ T cells to proliferate extensively.
Phenotypic characterization of iTregs.
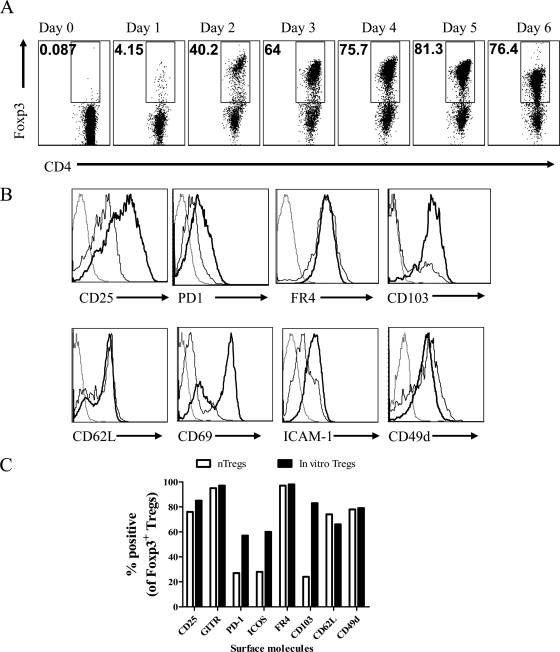
Using optimal Foxp3 conversion conditions, cultures were terminated at various times after initiation not only to establish the time when conversion was at its maximum, but also to measure the expression of additional phenotypes on the Foxp3+ T cells that could play a role in their migratory and functional properties in vivo. The results are expressed in Fig. 2A. Maximal frequencies of Foxp3 expression were evident after 5 days, and these frequencies sometimes exceeded 90% of the surviving CD4+ T cells.
FIG. 2.
Phenotypic characterization of in vitro-generated Foxp3+ T cells. (A) Representative FACS plots out of three similar experiments are shown for the kinetic analysis of Foxp3 induction in CD4+ CD25− Foxp3− T cells in in vitro cultures. Maximum conversion was observed at day 5 after initiation of culture (gated on CD4+ T cells). (B) Expression of surface markers on in vitro-converted Foxp3+ cells was compared with that of nTregs isolated from spleens of naïve immunocompetent animals. Representative histograms are shown. CD4+ Foxp3+ T cells were gated: dotted lines represent the isotype control, solid lines represent expression on nTregs, and solid boldface lines represent expression on iTregs. (C) A bar diagram of percentages of Foxp3+ nTregs and iTregs positive for various surface markers is shown.
As shown in Fig. 2B, most of the Foxp3+ T cells at the end of the culture period were CD103+, a molecule important for migration into tissue sites (1). This, as well as some other markers, was present on only a minor population of the CD4+ Foxp3+ nTreg population isolated from spleens of naïve immunocompetent mice (Fig. 2B). Additional phenotypic markers present on the majority of the in vitro-converted Foxp3+ T cells at the end of the culture period included CD25, GITR, FR4, PD1, ICOS, ICAM-1, CD62L, CD49d, CCR7, and CCR5. Their higher level (mean fluorescence intensity and percent positive) of expression on the in vitro-converted Tregs compared to nTregs indicated that the in vitro-converted cells were in a more activated state and more endowed with surface molecules that would permit tissue migration (Fig. 2B and C).
TGF-β-modified CD103+ CD11c+ DCs are involved in Foxp3 induction.
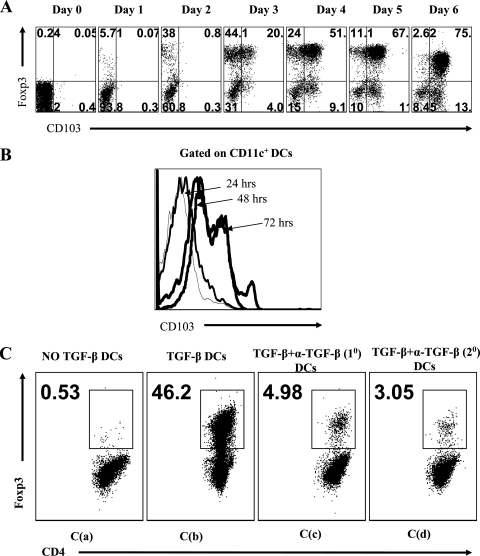
It was of interest to note that the expression of some phenotypic markers observed at the end of the culture period was not evident on those Foxp3+ cells that had been converted early during culture. The striking example of this was CD103, a molecule involved in tissue homing in vivo (1). Curiously, after 2 days of culture, the majority of CD11c+ DCs were already CD103+, but only a minor fraction (up to 2 to 5%) of CD4+ Foxp3+ cells were CD103+ (Fig. 3A and B). The DCs isolated from cultures lacking TGF-β, after 2 days, did not show this phenotype. We suspected that the CD103+ DCs could be the cells that were mainly responsible for causing the majority of CD4+ Foxp3+ conversion observed in our culture system. In support of this notion, we could demonstrate that the DCs isolated from cultures after 2 days could be used to drive Foxp3 expression in de novo cultures of purified CD4+ CD25− Foxp3− T cells that were T-cell receptor (TCR) stimulated in the presence of IL-2, but without additional TGF-β in the culture system. Accordingly, CD11c+ cells were isolated by positive selection from 2-day-stimulated SPCs with anti-CD3, IL-2, and TGF-β (conversion medium). In addition, CD11c+ cells were isolated from similar cultures that lacked TGF-β. The purity of CD11c+ DCs was between 85 and 90% in different experiments.
FIG. 3.
Splenic CD11c+ DCs are intricately involved in causing conversion of Foxp3− T cells into Foxp3+ CD4+ T cells. The kinetics of CD103 expression on CD4+ Foxp3+ T cells (A) and CD11c+ DCs (B) in an in vitro conversion culture is shown. The expression of CD103 was observed earlier on DCs than on CD4+ Foxp3+ T cells. (C) CD11c+ DCs were purified from 48-h SPC cultures in the presence of anti-CD3, IL-2, and either without TGF-β as used in panel C(a) or with TGF-β as used in panels C(b) and C(d) or with TGF-β plus anti-TGF-β as used in panel C(c). These DCs were then cocultured in 1:5 ratios with anti-CD3-stimulated purified CD4+ CD25− Foxp3− T cells isolated from naïve DO11.10 RAG2−/− animals in the presence of IL-2 alone or with anti-TGF-β antibody. Shown is a representative FACS plot illustrating Foxp3 induction when the DCs were isolated from primary culture with no TGF-β [C(a), primary culture in presence of TGF-β [C(b)], or primary cultures in presence of TGF-β and anti-TGF-β and when DCs were from primary culture with TGF-β but anti-TGF-β antibody was added in secondary cultures.
As shown in Fig. 3C, the addition of CD11c+ cells from cultures with the conversion medium caused up to 50% of CD4+ cells to become Foxp3+ after 4 days of further stimulation in the absence of TGF-β. In contrast, CD11c+ cells from the 2-day culture that lacked TGF-β failed to induce significant numbers of Foxp3+-converted cells in the secondary cultures. We interpret these experiments to mean that the CD11c+ cells were intricately involved in the in vitro conversion process, perhaps by being modified in their function during the initial culture period. This modification could include acting as a source of TGF-β. Thus, the addition of anti-TGF-β antibody in either primary or secondary cultures markedly inhibited the induction of Foxp3 in purified Foxp3− CD25− CD4+ cells (Fig. 3C). Furthermore, the DCs isolated after 2 days in the conversion system had higher TGF-β mRNA levels than did DCs stimulated under nonconverting conditions (data not shown).
Functional activity of Foxp3-converted cells.
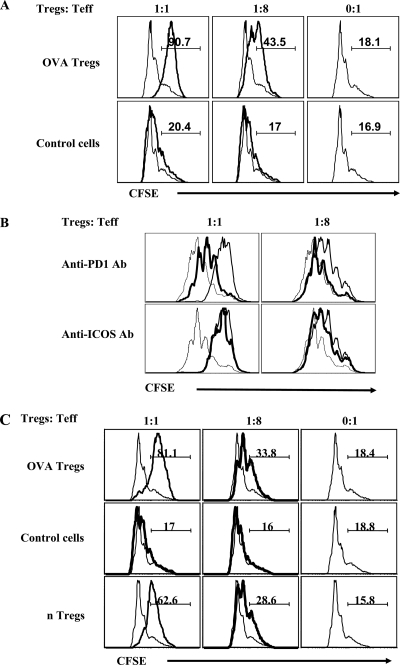
At the end of the 5-day culture period, CD25+ CD4+ T cells were isolated from both cultures stimulated with the conversion medium as well as cultures that were stimulated in the presence of IL-2 but without TGF-β. These cultures without TGF-β failed to generate significant numbers of Foxp3+ CD4+ T cells, as already stated. Both sets of CD25+ cells were added to anti-CD3-stimulated naïve CD4+ CD25− OVA-specific T cells isolated from pooled spleens and LNs of DO11.10 Rag2−/− mice and irradiated T-depleted splenocytes from the same strain of mice, to measure their antiproliferation activity. As shown in Fig. 4A, only the Foxp3+-containing population showed a dose-dependent suppressive activity. Experiments were also performed to determine possible mechanisms involved in the suppressive activity of iTregs. Neutralizing antibodies to either TGF-β, IL-10, ICOS, or PD-1 were added in suppression assays. The suppressive activity was only diminished significantly when PD-1 blocking antibody was added to the cultures (Fig. 4B). We interpret these experiments to mean that the suppression did not involve either TGF-β or IL-10 cytokines and that PD-1 engagement was likely involved in the suppression mechanism as previously observed by others (26).
FIG. 4.
In vitro-generated Tregs inhibit the proliferation of antigen-specific and polyspecific CD4+ CD25− T cells. CD25+ CD4+ T cells were isolated from both cultures stimulated with the conversion medium (Tregs) as well as cultures that were stimulated in the presence of IL-2 only (“control cells”). Additionally, CD4+ CD25+ T cells were also isolated from pooled spleens and LNs of BALB/c animals. These cells, in twofold serial dilutions, were used in suppression assays against the cultures of anti-CD3 antibody-stimulated CFSE-labeled CD4+ CD25− T cells (1 × 105) from naïve DO11.10 RAG2−/− mice (i.e., to measure antigen-specific effect) and Thy1.1 BALB/c animals (i.e., to measure polyspecific effect) with irradiated T-cell-depleted SPCs (2 × 105) from a homologous system as described in Materials and Methods. (A) The extent of CFSE dilution as an indication of suppressive activity of in vitro-generated CD4+ CD25+ regulatory T cells against anti-CD3-stimulated labeled CD4+ CD25− T cells from naïve DO.11.10 RAG2−/− animals is shown. Of the CD4+ CD25+ T cells, about 90% and 3% were Foxp3+ from cultures in the presence or absence of TGF-β, respectively. A first gate was applied on CD4+ T cells, and then the extent of CFSE dilution in CD4+ CFSE+ T cells was analyzed. (B) Blocking antibodies (Ab) to either TGF-β (10 μg/ml) or IL-10 (10 μg/ml) or PD-1 (10 μg/ml) or ICOS (10 μg/ml) were added to the suppression cultures, and the extent of division of CFSE in labeled cells was determined. Representative FACS plots at two dilutions of Tregs to T effectors are shown for PD-1- and ICOS-neutralized cultures. A dashed line represents dilution of CFSE when no Tregs were added, a solid line represents dilution of CFSE when Tregs were added, and a solid boldface line represents dilution of CFSE when, along with Tregs, either anti-PD1 or anti-ICOS antibodies were added. (C) Representative histograms indicating CFSE dilution in the Thy1.1 gated population is shown. CD4+ CD25+ T cells were isolated from the iTreg culture, control cells, and splenic nTregs from BALB/c animals and were used against anti-CD3-stimulated labeled CD4+ CD25− T cells from Thy1.1 animals. The markers show the percentages of cells that underwent less than two divisions.
In a second approach, CD25+ CD4+ T cells from in vitro conversion cultures and splenic CD25+ CD4+ nTregs isolated from immunocompetant BALB/c animals were compared for their ability to suppress the proliferation of CD25− CD4+ T cells. The latter were isolated from pooled spleens and LNs of naïve Thy1.1 BALB/c animals (hence polyspecific population) stimulated with anti-CD3 antibody and T-cell-depleted irradiated SPCs as a source of antigen-presenting cells. Proliferation of Thy1.1+ CD4+ T cells was analyzed by dilution of CFSE. In such experiments, the OVA-specific converted cells showed activity that was somewhat enhanced compared to that in the nTreg population (Fig. 4C).
In vivo activity of in vitro-converted cells in ocularly infected DO11.10 RAG2−/− mice.
The major objective of our investigation was to determine if in vitro-converted Foxp3+cells could influence the severity of ocular immunoinflammatory lesions induced in mice by HSV infection. The first model used TCR transgenic mice on a RAG−/− background, which were shown previously to develop SK upon ocular infection with HSV, even though their CD4+ T cells were almost all reactive with OVA323-339 peptide and not detectably cross-reactive with HSV antigens (11). The T cells in the ocular lesions of such animals were shown to react with the KJ1.26 MAb noted by others to react with the TCR of H-2d CD4+ T cells that recognize the OVA323-339 peptide (12). Since this KJ+ TCR had no demonstrable reactivity with HSV, we surmised that the activation of KJ+ CD4+ T cells was not TCR mediated but involved activation by one or more cytokines (11). We have referred to this as a bystander model of SK (11).
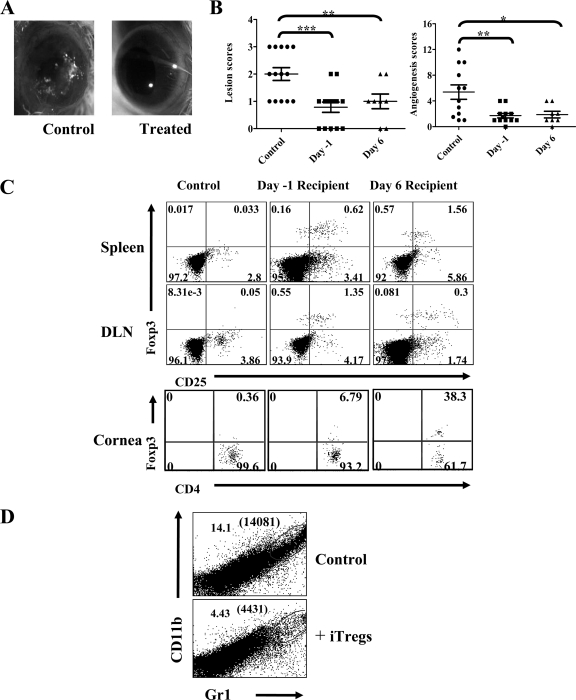
As already described, the Foxp3-converted cells in the in vitro system used in this communication were all KJ+ and hence OVA specific. Consequently, we speculated that such cells adoptively transferred into the infected DO11.10 RAG2−/− animals should modulate the severity of the ocular lesions. To test this, DO11.10 RAG2−/− mice were ocularly infected with 5 × 105 PFU of HSV KOS (which routinely induces SK in these animals) and some were given i.v. 5 × 105 Foxp3-converted cells either 24 h before or at 6 day p.i. The severity of lesions was then followed in control and adoptive transfer recipients over an 11- to 12-day observation period (animals began to die after day 11 from herpetic encephalitis).
The cumulative results of three similar experiments are shown in Fig. 5A and B. As is evident, the average severity of ocular lesions was significantly reduced in animals that received cells 24 h before infection. Similarly, lesion severity was also reduced significantly in the day 6 recipients. Samples were collected from both groups of animals to determine if the adoptively transferred Foxp3+ T cells could be demonstrated in the recipient tissues. It became possible to detect transferred cells since the DO11.10 RAG2−/− animals do not have detectable Foxp3+ cells even following infection with HSV. The results of such experiments revealed that both in day 1 and day 6 transfer recipients, appreciable numbers of Foxp3+ cells could be demonstrated by FACS analysis of collagenase-digested ocular samples as well as in the DLN and spleen (Fig. 5C). Reduced percentages as well as absolute numbers of polymorphonuclear leukocytes such as neutrophils (CD11b+ Gr1+) were found in the corneal tissues of transfer recipients as compared to control animals (Fig. 5D). These experiments clearly demonstrated that the in vitro Foxp3-converted cells may function in vivo to diminish herpetic lesions, although in the model studied, we could not establish how the inhibition was achieved or if this occurred by the action of Foxp3+ cells in the ocular tissue themselves or in the DLN in some way. It remains unclear how HSV infection causes SK in the DO11.10 RAG2−/− model, but we suspect the mechanism involves the activation of CD4+ T cells by inflammatory mediators generated by the infection. In support of this, CD4+ T cells collected from lymphoid tissues as well as cornea showed high frequencies of CD69+ cells, indicating they are activated. Transfer of iTregs into such animals significantly reduced the frequencies of activated cells (data not shown).
FIG. 5.
In vitro-generated OVA Tregs control SK severity in a bystander disease model. Foxp3+ CD4+ T cells (5 × 105) were adoptively transferred to DO11.10 RAG2−/− animals 24 h before or 6 days postocular HSV-1 infection. The disease severity and angiogenesis were recorded. (A) Gross eye pictures of control and transfer recipient animals from a representative experiment when 5 × 105 Foxp3+ cells were transferred 24 h before infection are shown. (B) Cumulative data on SK severity and angiogenesis from different experiments at 11 days p.i. are shown. Foxp3+ T cells (5 × 105) were transferred 24 h before or 6 days p.i. P values were calculated with one-way ANOVA using Dunnett's post-test settings. (C) Distribution of adoptively transferred Foxp3+ T cells in lymphoid organs (spleen and draining cervical LN) and ocular tissues at 11 days p.i. is shown. (D) Representative FACS plots demonstrating the infiltration of neutrophils (CD11b+ Gr1+) in collagenase-digested cornea from control and transfer recipient animals given 5 × 105 Foxp3+ cells transferred 24 h before infection are shown. Absolute numbers of neutrophils/cornea are shown in parentheses.
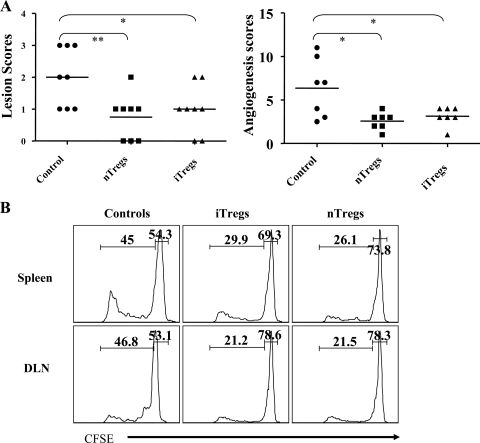
Inhibitory effects of Treg on SK in reconstituted SCID mice.
Whereas SCID animals ocularly infected with HSV fail to develop SK lesions, they do so if reconstituted with CD4+ CD25− T cells, even if such cells are taken from naïve animals (29). In a previous study, we demonstrated that SK severity in such animals was inhibited if CD4+ CD25+ T cells were cotransferred with the CD4+ CD25− population. As shown in Fig. 6A, this observation was repeated, but in addition we were able to show that OVA-specific iTregs were equally capable of modulating lesion severity. This occurred despite the fact that the iTreg population was KJ+ and hence OVA specific. Such experiments indicate that iTregs may act in a bystander-inhibitory fashion, but how such an effect was mediated requires further exploration. The effect appeared to involve inhibition of proliferation of effectors. This was shown in experiments wherein CFSE-labeled CD4+ CD25− cells (effectors) were transferred alone or with either iTregs or nTregs in SCID animals 24 h before ocular HSV-1 infection. As shown in Fig. 6B, the frequencies of proliferating cells, when measured at day 7 p.i., were reduced in recipients of both iTregs and nTregs as indicated by dilution of CFSE.
FIG. 6.
Cotransfer of in vitro-generated Tregs with polyspecific CD4+ CD25− T cells in SCID animals reduces the severity of SK. CD4+ CD25+ T cells (1 × 106) isolated from in vitro cultures and splenic nTregs (CD4+ CD25+ T cells) from naïve BALB/c animals were cotransferred with 5 × 106 CD4+ CD25− T cells 24 h before ocular HSV-1 infection (5 × 105 PFU). The disease severity and angiogenesis were recorded over a 12-day period. (A) The SK lesion scores and angiogenesis at 12 days p.i. are shown. (B) Proliferation of polyspecific CFSE-labeled CD4+ CD25− T cells in spleen and DLN in the presence of iTregs or splenic nTregs is shown.
Inhibition of SK by iTregs in immunocompetent BALB/c animals.
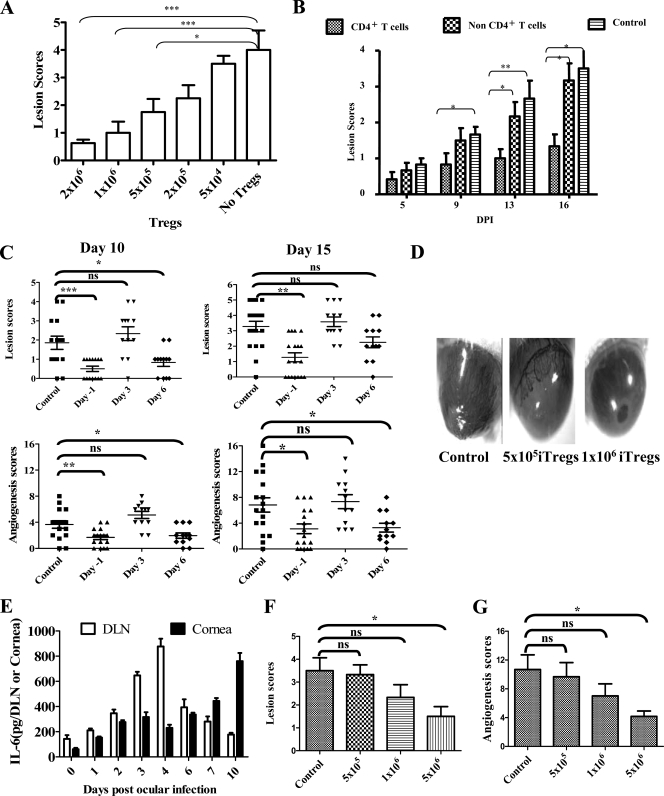
The final approach used to measure the efficacy of in vitro-converted Foxp3+ T cells was to use the immunocompetent ocularly infected BALB/c animals as the transfer recipients. In these experiments, animals were infected with 5 × 105 PFU of HSV RE, a dose which was expected to cause lesions in the majority of recipients, and the outcome was compared in control animals with those given different numbers of in vitro-converted cells day 1 prior to infection. The numbers of donor cells varied from 2 × 106 to 5 × 104 of Foxp3+ T cells which it must be emphasized were KJ+ and hence OVA specific. The results of a representative experiment are shown in Fig. 7A. As can be seen, lesions were markedly reduced in recipients given 2 × 106 donor cells but inhibition was also significant in those that received fivefold fewer cells. Using the 5 × 105 donor cell dose for transfer, we also compared the suppressive effects of donor cells that were fractionated into CD4+ and CD4− populations prior to transfer. As is evident in Fig. 7B, only the CD4+ fraction suppressed lesion severity in the BALB/c recipient animals.
FIG. 7.
In vitro-generated OVA-specific Tregs diminish the severity of SK in immunocompetent BALB/c transfer recipients in a dose-dependent manner. (A) Indicated doses of unfractionated Foxp3+ T cells were adoptively transferred in BALB/c animals 24 h before ocular HSV infection, and the disease severity was monitored until day 15. A bar diagram indicating the average SK lesion scores at different doses of transferred Foxp3+ cells at 15 days p.i. is shown. P values were calculated with one-way ANOVA using Dunnett's post-test settings, taking no Tregs as a control. (B) Fractionated CD4+ and non-CD4+ cells (5 × 105) isolated from in vitro conversion cultures were transferred in BALB/c animals. Average lesion scores are shown at 15 days p.i. Only CD4+ T cells could control the severity of SK. Student's t test (unpaired) was used to calculate the level of significance. (C) Foxp3+ cells (5 × 105) were transferred 24 h before or 3 days or 6 days p.i., and SK severity was recorded. Lesion scores and angiogenesis are shown at 10 days p.i. and 15 days p.i. No SK modulatory activity was shown by Tregs at 3 days p.i. transfer. P values were calculated with one-way ANOVA using Dunnett's post-test settings. (D) Eye pictures of control and iTreg transfer recipient animals from a representative experiment when 5 × 105 and 1 × 106 Foxp3+ cells were transferred 24 h before infection are shown. (E) The kinetics of the levels of proinflammatory cytokine IL-6 in cornea and DLN after ocular HSV-1 infection as determined by sandwich enzyme-linked immunosorbent assay is shown. (F and G) Indicated doses of iTregs were transferred at 3 days p.i. in BALB/c animals, and lesion severity (F) and angiogenesis (F) were recorded at day 15 and compared by one-way ANOVA using Dunnett's post-test settings.
In subsequent experiments, HSV-infected BALB/c animals were given adoptive transfers of Foxp3+-converted cells (5 × 105 Foxp3+ cells) either 1 day before infection or 3 or 6 days p.i. Animals were then scored for both the extent of angiogenesis and SK lesion severity scores over a 15-day observation period. The data in Fig. 7C and D show the cumulative data of individual animal scores of three separate experiments. As is evident, significant levels of inhibition occurred in the early transfer recipients. Transfers at day 6 provided suppression in some animals, but overall the results were not significant, especially at day 15 p.i., probably because of lower sample size and large variations. Interestingly, transfer of Tregs at day 3 invariably failed to produce suppression of SK or levels of angiogenesis. This might be explained by the fact that levels of proinflammatory cytokines such as IL-6 are high at this time in the DLN and cornea (Fig. 7E) Thus, cytokines such as IL-6 are known to blunt the function of Tregs (17). Inhibition could be achieved in the day 3 transfer model if 10-fold more cells were transferred on day 3 (Fig. 7F and G). Conceivably, the inhibition was evident because insufficient IL-6 was present to blunt the function of all of the transferred Tregs, but this issue needs to be formally explained.
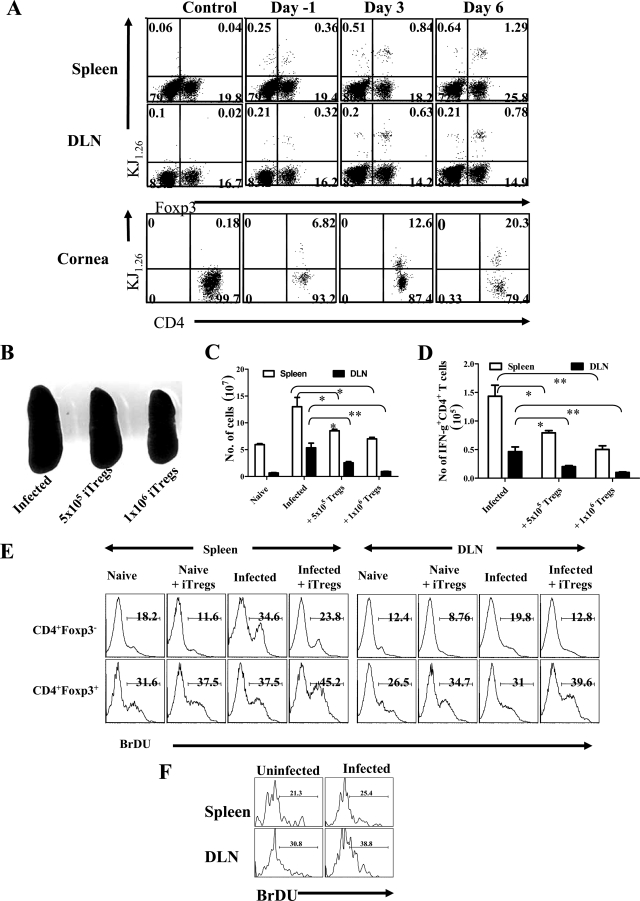
Our data demonstrate that with early transfer of Foxp3-converted OVA-specific T cells the cells are fully capable of inhibiting lesions caused by HSV, provided sufficient cells are transferred and transfer is performed early after infection. It is far from clear how the Tregs, which are OVA specific, act against an inflammatory lesion caused by HSV or in fact where the inhibitory effect is mediated. Experiments showed that adoptively transferred cells (based on determining the KJ+ markers) could be demonstrated to be present at least in appreciable numbers in the eye as well as lymphoid tissues at 16 days posttransfer (Fig. 8A). In long-term studies, transferred cells (2 to 3% of recovered CD4+ T cells) could be found in corneal tissues even after 3 months posttransfer but such cells were undetectable in lymphoid tissues at this time point (data not shown). It would be interesting to investigate if cornea-resident iTregs could prevent the recurrence of SKs.
FIG. 8.
Adoptively transferred Foxp3+ KJ1.26+ T cells were present in lymphoid and ocular tissues as well as diminished HSV-specific CD4+ T-cell immune response. BALB/c animals were given 5 × 105 iTregs before 24 h of ocular HSV infection or 3 or 6 days p.i., and 15 days p.i., DLNs, spleens, and ocular tissues were examined for the presence of CD+ Foxp3+ and KJ+ T cells. (A) Representative FACS plots are shown. (B to D) BALB/c animals were given the indicated numbers of iTregs before 24 h of ocular HSV infection, and 15 days p.i., immune parameters were studied. (B) Spleen size as an indication of generated immune response is shown in control and iTreg recipient animals. (C) Total cellularity in the spleen (blank bars) and DLN (filled bars) of controls and Treg recipient animals is shown. (D) Total numbers of IFN-γ+ CD4+ T cells in spleen and LN of control and Treg recipients are shown. (E and F) iTregs (5 × 105) were transferred into 12 animals that were then divided into four groups: naive, naïve plus iTregs, infected, and infected plus iTregs. Animals in the groups naïve plus iTregs and infected plus iTregs were given 5 × 105 iTregs. After 24 h, animals from the groups infected and infected plus iTregs were infected with HSV-1. All animals were given BrdU in drinking water for the next 10 days. After 10 days, spleens and cervical DLNs were analyzed for the frequencies of host CD4+ Foxp3+ or CD4+ Foxp3− and donor CD4+ KJ1.26+ Foxp3+ cells that incorporated BrdU. Representative FACS plots for host cells (E) and donor cells (F) are shown.
Although some adoptively transferred iTregs could be demonstrated in ocular tissues, their polyspecific suppressive activity could be mediated mainly in lymphoid tissues. Thus, one consequence of HSV infection is an increase in the spleen size as well as the DLN. Curiously, as shown in the Fig. 8B and C, the spleen size and total cellularity in iTreg recipients were reduced, approaching near those of normal animals, depending on the number of iTregs given. In addition, iTreg recipients showed lower numbers of HSV-specific IFN-γ-producing CD4+ T cells (Fig. 8D)—those principally responsible for mediating SK (22)—than was evident in control infected animals that did not receive iTregs. Accordingly, one outcome of the early iTreg transfer was suppression of the response of CD4+ T cells to HSV, although how this was achieved mechanistically remains to be explained. Regulatory cells transferred at day 6 had no effect on the magnitude of the anti-HSV immune response (data not shown).
We considered the possibility that iTreg adoptive transfers might serve to inhibit the division of host effectors while at the same time causing the expansion of the hosts' own nTregs, as was reported in a diabetes model by the Steinman group (32). To support such a possibility, we performed adoptive transfers with KJ+ Tregs and then compared the proliferative capacity of the host's CD4+ Foxp3+ and Foxp3− populations. Our results showed reduced proliferative responses of host CD4+ Foxp3− cells compared to infected controls not given iTregs but an increase in the proliferative response of the host CD4+ Foxp3+ population. Thus, the donor KJ+ Tregs appeared to mediate the suppression in at least two ways. These included inhibitory effects on the host effectors as well as an expansion of the host's own nTreg population. Curiously, the donor KJ+ cells themselves underwent proliferation in the host, which may also help explain how the minimal cell transfers were effective at modulating lesions.
DISCUSSION
In this report, we have confirmed the observations of others that Foxp3+ regulatory T cells can be generated in vitro from Foxp3− naïve CD4+ cells (6, 8). We developed a simplified technique that used unfractionated SPCs as a precursor population and showed that stimulation under optimized conditions for 5 days with solid-phase anti-CD3 MAb in the presence of TGF-β and IL-2 could induce up to 90% of CD4+ T cells to become Foxp3+ and able to mediate suppression in vitro. The converted cells had undergone cell division, and the majority of them expressed activation markers along with surface molecules that would facilitate their migration into tissue sites. The primary reason for our study was to determine if such in vitro-converted Tregs could be used in vivo to influence the outcome of a virus-induced immunoinflammatory lesion in the eye caused by HSV infection. We could show in three separate models of herpetic SK that adoptive transfers of in vitro-converted Tregs effectively diminished lesion severity, especially when given in the initial phases of infection. The protocol we have developed, which is novel for a virus-induced inflammatory disease, could provide a useful additional approach to control a chronic virus-induced lesion.
Previous studies on adoptive transfers with in vitro-converted Tregs have focused on the control of autoimmune lesions or facilitation of the acceptance of transplants (2, 9, 23, 31, 38). Moreover, the techniques used to produce the Treg populations were usually complex requiring purification of both responders and stimulators to achieve success. We have found that unfractionated SPCs can suffice as a responder population. In our case, these were transgenic T cells from naïve DO11.10 RAG2−/− mice which normally lack a population of Foxp3+ T cells (14). The T cells from such animals are predominantly CD4+ and express a TCR that recognizes the OVA peptide that can be conveniently identified with the KJ1.26 MAb. We could generate Foxp3+ KJ+ cells either by stimulating SPCs with OVA peptide in the presence of TGF-β and IL-2 or more effectively by stimulating them with solid-phase anti-CD3 MAb. It was not necessary to use additional costimulators such as anti-CD28, as is used in most other studies (6, 8, 19). The Foxp3 conversion process involved cell division and appeared to depend on the presence of antigen-presenting cells that responded initially to the TGF-β. Thus, we could show that 2-day stimulation of splenocytes in the conversion medium caused CD11c+ cells in the culture to become CD103+. Moreover, such cells could be used to drive Foxp3 expression in TCR-stimulated cultures of naive CD4+ Foxp3− T cells without the addition of TGF-β to cultures. We interpret these observations to mean that the CD103+ CD11c+ cells could be a source of TGF-β and possibly costimulation to the CD4+ cells that convert to become Foxp3+. Curiously, recent studies on Foxp3 conversion in the gut had demonstrated an essential role of CD103+ TGF-β-producing DCs (3, 7, 13, 21, 28).
The principal objective of our studies was to explore the value of adoptive transfers with Tregs as a means to influence the pathogenesis of a virus-induced immunoinflammatory lesion. In prior studies, we had shown that lesions caused by HSV infection in the eye were more severe in animals depleted of nTregs (29), so it was anticipated that adoptive transfers of Tregs might prove valuable to suppress the severity of SK lesions. However, as is well known when using Tregs therapeutically to control autoimmunity, the most potent Treg populations are those that are reactive with the same antigens as the effector cells that drive the lesions (9, 31, 32). Unfortunately, we have no simple means of generating HSV-specific Tregs in vitro, although this issue is under further investigation. There is, however, an SK model using TCR transgenic × Rag or SCID mice where the animals' T cells lack demonstrable cross-reactivity with HSV (11). Nonetheless, they develop stromal inflammatory lesions upon ocular infection with HSV. This has been referred to as the bystander model of SK (10). Using this model, we could show that the adoptive transfer of in vitro-generated Foxp3+ KJ+ T cells could inhibit the severity of SK in HSV-infected DO11.10 RAG2−/− mice, wherein lesions are orchestrated by CD4+ effectors that are also KJ+ (10). In this instance, therefore, the Treg control could be antigen specific.
Inhibition was most effective when the Tregs were given around the time of infection, but significant effects were also evident in animals in which therapy was delayed until day 6. We consider this latter observation particularly interesting, since it indicates that the adoptive transfer of Treg may represent a potential means of controlling ongoing viral inflammatory disease. We suspect that even greater efficacy in the day 6 therapeutic model could be observed if animals were kept alive longer than the usual time of their dying of herpetic encephalitis (around day 11). We are currently pursuing such experiments in animals treated with antivirals or protected by neutralizing antibody. In longer-living animals, multiple Treg administration will also be feasible, perhaps a necessary protocol to fully control lesions.
Unexpectedly, we were also able to show that in vitro-generated OVA-specific Foxp3+ Tregs could modulate lesions in HSV-infected BALB/c mice, where certainly the CD4+ T cells involved in orchestrating lesions were not KJ+ and OVA specific. Such observations could mean that adoptively transferred Tregs can act nonspecifically. This could be possible since the cells used for adoptive transfer were highly activated and in fact showed enhanced regulatory effects in vitro compared to nTregs isolated from normal animals. In fact, if the iTregs were transferred a week or more in animals prior to infection, control was not achieved (data not shown). During this time, we presume that cells may have lost their activation status, although this issue needs to be investigated.
Evidence for a nonspecific regulatory effect of the in vitro-converted Tregs was also seen in a second model of SK. In this model, SK can be induced in ocularly infected SCID mice as long as they are provided with adoptive transfers of normal or HSV-immune CD4+ T cells (29). We could demonstrate that cotransfer of the OVA-specific converted Tregs along with the polyspecific CD4+ CD25− T cells resulted in significantly reduced lesions. These results provide evidence of nonspecific bystander regulatory effects since the lesion-inducing effector cells would not be reactive to OVA peptide. Others too, have reported instances where Tregs could mediate bystander suppression (20, 35, 36). This is especially evident for regulatory cells that are abundant producers of cytokines such as IL-10 (23). However, we suspect that the bystander suppression effect in our system may not involve IL-10 because only a minor fraction of the cells could be shown to be IL-10 producers and the suppressive activity in vitro was found largely independent of cytokines. Nevertheless, this issue requires further investigation.
Although our results demonstrate that in vitro-converted Tregs could suppress the severity of SK lesions, the location and mechanism by which the inhibition occurs in vivo remain unclear. Thus, the cell population expressed several molecules that permitted the cells to access tissue sites as well as lymphoid tissue. In fact, adoptively transferred cells could be demonstrated at both the ocular inflammatory site as well as in lymphoid tissues. Some reports advocate that TGF-β-generated Foxp3+ cells have only a short life span in vivo (23, 25). This was not our experience, since some KJ+ Foxp3+ cells could still be recovered from the eyes of BALB/c mice 3 months after their administration. Finding cells in the eye does not mean that they exert their regulatory effect in that tissue. In fact, we strongly suspect that the suppressive effects of the early cell transfers were mediated mainly in lymphoid tissues where the effector T-cell response to the virus is being generated. Thus, recipients of such transfers had reduced HSV-specific effector CD4+ T-cell responses. Moreover, few if any effector T cells appear in the stroma until 6 or 7 days p.i. (33).
Another interesting observation was that the virus-induced immunopathology was suppressed by quite small numbers of adoptively transferred iTregs. Conceivably, the iTregs could in some way be causing the host's own nTregs to become activated and suppressive, as has been suggested to occur with similar studies in a diabetes model (32). In this model, control of pancreatic inflammation appeared to be mediated by the increased population of host-derived Tregs in the tissue. In fact, donor cells were not demonstrable in the recipient tissues. In our system, we could demonstrate the presence of transferred cells in the target tissues, but in addition we noted that host nTregs underwent increased proliferation in iTreg adoptive transfer recipients compared to controls. Such host nTregs could conceivably contribute to the suppression of lesions, as ongoing studies are attempting to demonstrate.
Finally, although the results were variable we could demonstrate that even in the BALB/c model transfer of Tregs at 6 days p.i., around the time of major access of effector T cells into the corneal stroma, could suppress lesion severity. Transfer of Tregs at this time period had no effect on the magnitude of the anti-HSV immune response, which is approaching its peak at this time. Conceivably the suppressive activity of such transfers could result from effects in the tissues themselves. It may be that multiple transfers would be a more effective way of controlling ongoing lesions, as we are currently investigating.
Acknowledgments
This work was supported by grants AI 106336501 from the National Institute of Allergy and Infectious Diseases and EY 05093 from the National Institutes of Health.
Footnotes
Published ahead of print on 14 May 2008.
REFERENCES
- 1.Annacker, O., J. L. Coombes, V. Malmstrom, H. H. Uhlig, T. Bourne, B. Johansson-Lindbom, W. W. Agace, C. M. Parker, and F. Powrie. 2005. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J. Exp. Med. 2021051-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Battaglia, M., A. Stabilini, B. Migliavacca, J. Horejs-Hoeck, T. Kaupper, and M. G. Roncarolo. 2006. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J. Immunol. 1778338-8347. [DOI] [PubMed] [Google Scholar]
- 3.Benson, M. J., K. Pino-Lagos, M. Rosemblatt, and R. J. Noelle. 2007. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J. Exp. Med. 2041765-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bettelli, E., Y. Carrier, W. Gao, T. Korn, T. B. Strom, M. Oukka, H. L. Weiner, and V. K. Kuchroo. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441235-238. [DOI] [PubMed] [Google Scholar]
- 5.Cao, O., E. Dobrzynski, L. Wang, S. Nayak, B. Mingle, C. Terhorst, and R. W. Herzog. 2007. Induction and role of regulatory CD4+CD25+ T cells in tolerance to the transgene product following hepatic in vivo gene transfer. Blood 1101132-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, W., W. Jin, N. Hardegen, K. J. Lei, L. Li, N. Marinos, G. McGrady, and S. M. Wahl. 2003. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 1981875-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coombes, J. L., K. R. Siddiqui, C. V. Arancibia-Carcamo, J. Hall, C. M. Sun, Y. Belkaid, and F. Powrie. 2007. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 2041757-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davidson, T. S., R. J. DiPaolo, J. Andersson, and E. M. Shevach. 2007. Cutting edge: IL-2 is essential for TGF-beta-mediated induction of Foxp3+ T regulatory cells. J. Immunol. 1784022-4026. [DOI] [PubMed] [Google Scholar]
- 9.DiPaolo, R. J., C. Brinster, T. S. Davidson, J. Andersson, D. Glass, and E. M. Shevach. 2007. Autoantigen-specific TGFbeta-induced Foxp3+ regulatory T cells prevent autoimmunity by inhibiting dendritic cells from activating autoreactive T cells. J. Immunol. 1794685-4693. [DOI] [PubMed] [Google Scholar]
- 10.Gangappa, S., S. P. Deshpande, and B. T. Rouse. 1999. Bystander activation of CD4+ T cells can represent an exclusive means of immunopathology in a virus infection. Eur. J. Immunol. 293674-3682. [DOI] [PubMed] [Google Scholar]
- 11.Gangappa, S., S. P. Deshpande, and B. T. Rouse. 2000. Bystander activation of CD4+ T cells accounts for herpetic ocular lesions. Investig. Ophthalmol. Vis. Sci. 41453-459. [PubMed] [Google Scholar]
- 12.Haskins, K., R. Kubo, J. White, M. Pigeon, J. Kappler, and P. Marrack. 1983. The major histocompatibility complex-restricted antigen receptor on T cells. I. Isolation with a monoclonal antibody. J. Exp. Med. 1571149-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang, S., H. W. Lim, O. M. Andrisani, H. E. Broxmeyer, and H. K. Chang. 2007. Vitamin A metabolites induce gut-homing Foxp3+ regulatory T cells. J. Immunol. 1793724-3733. [DOI] [PubMed] [Google Scholar]
- 14.Kawahata, K., Y. Misaki, M. Yamauchi, S. Tsunekawa, K. Setoguchi, J. Miyazaki, and K. Yamamoto. 2002. Generation of CD4+CD25+ regulatory T cells from autoreactive T cells simultaneously with their negative selection in the thymus and from nonautoreactive T cells by endogenous TCR expression. J. Immunol. 1684399-4405. [DOI] [PubMed] [Google Scholar]
- 15.Kim, B., Q. Tang, P. S. Biswas, J. Xu, R. M. Schiffelers, F. Y. Xie, A. M. Ansari, P. V. Scaria, M. C. Woodle, P. Lu, and B. T. Rouse. 2004. Inhibition of ocular angiogenesis by siRNA targeting vascular endothelial growth factor pathway genes: therapeutic strategy for herpetic stromal keratitis. Am. J. Pathol. 1652177-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim, J. M., J. P. Rasmussen, and A. Y. Rudensky. 2007. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 8191-197. [DOI] [PubMed] [Google Scholar]
- 17.Korn, T., J. Reddy, W. Gao, E. Bettelli, A. Awasthi, T. R. Petersen, B. T. Backstrom, R. A. Sobel, K. W. Wucherpfennig, T. B. Strom, M. Oukka, and V. K. Kuchroo. 2007. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat. Med. 13423-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Bras, S., and R. S. Geha. 2006. IPEX and the role of Foxp3 in the development and function of human Tregs. J. Clin. Investig. 1161473-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu, Y., S. Amarnath, and W. Chen. 2006. Requirement of CD28 signaling in homeostasis/survival of TGF-beta converted CD4+CD25+ Tregs from thymic CD4+CD25− single positive T cells. Transplantation 82953-964. [DOI] [PubMed] [Google Scholar]
- 20.Martinic, M. M., and M. G. von Herrath. 2006. Control of graft-versus-host disease by regulatory T cells: which level of antigen specificity? Eur. J. Immunol. 362299-2303. [DOI] [PubMed] [Google Scholar]
- 21.Mucida, D., Y. Park, G. Kim, O. Turovskaya, I. Scott, M. Kronenberg, and H. Cheroutre. 2007. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 317256-260. [DOI] [PubMed] [Google Scholar]
- 22.Niemialtowski, M. G., and B. T. Rouse. 1992. Predominance of Th1 cells in ocular tissues during herpetic stromal keratitis. J. Immunol. 1493035-3039. [PubMed] [Google Scholar]
- 23.Roncarolo, M. G., and M. Battaglia. 2007. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat. Rev. Immunol. 7585-598. [DOI] [PubMed] [Google Scholar]
- 24.Rouse, B. T. 2007. Regulatory T cells in health and disease. J. Intern. Med. 26278-95. [DOI] [PubMed] [Google Scholar]
- 25.Selvaraj, R. K., and T. L. Geiger. 2007. A kinetic and dynamic analysis of Foxp3 induced in T cells by TGF-beta. J. Immunol. 1787667-7677. [DOI] [PubMed] [Google Scholar]
- 26.Sharma, M. D., B. Baban, P. Chandler, D. Y. Hou, N. Singh, H. Yagita, M. Azuma, B. R. Blazar, A. L. Mellor, and D. H. Munn. 2007. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J. Clin. Investig. 1172570-2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shevach, E. M. 2006. From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity 25195-201. [DOI] [PubMed] [Google Scholar]
- 28.Sun, C. M., J. A. Hall, R. B. Blank, N. Bouladoux, M. Oukka, J. R. Mora, and Y. Belkaid. 2007. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 2041775-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suvas, S., A. K. Azkur, B. S. Kim, U. Kumaraguru, and B. T. Rouse. 2004. CD4+CD25+ regulatory T cells control the severity of viral immunoinflammatory lesions. J. Immunol. 1724123-4132. [DOI] [PubMed] [Google Scholar]
- 30.Suvas, S., U. Kumaraguru, C. D. Pack, S. Lee, and B. T. Rouse. 2003. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J. Exp. Med. 198889-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang, Q., and J. A. Bluestone. 2006. Regulatory T-cell physiology and application to treat autoimmunity. Immunol. Rev. 212217-237. [DOI] [PubMed] [Google Scholar]
- 32.Tarbell, K. V., L. Petit, X. Zuo, P. Toy, X. Luo, A. Mqadmi, H. Yang, M. Suthanthiran, S. Mojsov, and R. M. Steinman. 2007. Dendritic cell-expanded, islet-specific CD4+ CD25+ CD62L+ regulatory T cells restore normoglycemia in diabetic NOD mice. J. Exp. Med. 204191-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas, J., and B. T. Rouse. 1998. Immunopathology of herpetic stromal keratitis: discordance in CD4+ T cell function between euthymic host and reconstituted SCID recipients. J. Immunol. 1603965-3970. [PubMed] [Google Scholar]
- 34.Thornton, A. M., E. E. Donovan, C. A. Piccirillo, and E. M. Shevach. 2004. Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. J. Immunol. 1726519-6523. [DOI] [PubMed] [Google Scholar]
- 35.Thornton, A. M., and E. M. Shevach. 1998. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 188287-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thornton, A. M., and E. M. Shevach. 2000. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J. Immunol. 164183-190. [DOI] [PubMed] [Google Scholar]
- 37.Yamagiwa, S., J. D. Gray, S. Hashimoto, and D. A. Horwitz. 2001. A role for TGF-beta in the generation and expansion of CD4+CD25+ regulatory T cells from human peripheral blood. J. Immunol. 1667282-7289. [DOI] [PubMed] [Google Scholar]
- 38.Yamazaki, S., A. J. Bonito, R. Spisek, M. Dhodapkar, K. Inaba, and R. M. Steinman. 2007. Dendritic cells are specialized accessory cells along with TGF-β for the differentiation of Foxp3+ CD4+ regulatory T cells from peripheral Foxp3 precursors. Blood 1104293-4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ziegler, S. F. 2006. FOXP3: of mice and men. Annu. Rev. Immunol. 24209-226. [DOI] [PubMed] [Google Scholar]