Abstract
Human immunodeficiency virus type 1 (HIV-1) infection has been implicated in impairing various aspects of NK cell function in viremic condition, and several viral factors contribute to these defects. Here, we evaluated the effect of HIV-1 Vpr on NK cell cytolytic function and cytokine (gamma interferon [IFN-γ]) production in the context of infection and exposure. Our data indicate that NK cells derived from a peripheral blood mononuclear cell culture infected in vitro with HIV-1 vpr(+) virus or exposed to recombinant Vpr protein exhibited reduced target cell killing in conjunction with diminished expression of CD107a and reduced IFN-γ production compared to their Vpr-negative counterparts. This Vpr-induced NK cell defect is in part through differential regulation of interleukin-12 and transforming growth factor β production by the infected target cells and concomitant activation of Smad3 signaling pathway. Collectively, these results illustrate the ability of Vpr to impair NK cell-mediated innate immune functions indirectly by dysregulating multiple cytokines in the infected target cells, thus increasing disease severity and affecting the final outcome in HIV-1 infection.
Natural killer (NK) cells are large granular lymphocytes that are critical components of both innate and adaptive immune responses (13). A role of NK cells in a variety of bacterial, fungal, and viral infections, including human immunodeficiency virus type 1 (HIV-1) infection, has been implicated (21, 44). NK cells were identified for their ability to lyse target cells by releasing cytokines, chemokines, and cytolytic granules containing perforins and granzymes in the absence of prior sensitization to antigens (53). HIV-1 infection compromises both innate and adaptive immune systems, and antiretroviral drug treatment fails to fully restore immune functions (5, 49). In vivo analyses indicate that NK cells from patients with HIV-1 viremia are defective in target cell lysis compared to those from aviremic or uninfected control subjects (15, 26). Disease severity in HIV-1 is associated with altered expression of different NK receptors and ligands, as shown by different studies (7, 9, 25, 29, 48). HIV-1 viral antigens have been shown to dysregulate NK cell function and its interaction with other accessory cells in the absence of direct infection through a multidirectional network of cytokines (26, 27, 39). Thus, the sequential deregulation of NK cell phenotypes together with compromised cytolytic function selectively contributes to the loss of natural immunity and immune suppression during HIV-1 infection (4, 6, 13).
The structural and accessory proteins expressed by HIV-1 are known to interact with components of both the innate and adaptive immune systems to evade host immune surveillance (16, 42). HIV-1 Nef is a potential regulator of NK cell cytotoxicity due to its involvement in major histocompatibility complex class I downregulation in infected CD4+ T cells (12). HIV-1 Tat has been shown to inhibit NK cell function through LFA-I-mediated Ca2+ influx (54). HIV-1 envelope protein also inhibits NK cell cytotoxicity and survival (1, 19, 47). From these studies it is evident that HIV-1 uses several of its gene products to disrupt the first line of defense against infection.
Recently Vpr has been shown to have a role in immune dysfunction, specifically the adaptive immune response, by impairing dendritic cell function as well as the antigen-specific T-cell response (23, 32). HIV-1 Vpr is a unique 14-kDa nonstructural protein associated with the virus particles and is available to host cells prior to de novo synthesis and productive infection (45), suggesting that Vpr might have a role in innate immunity and NK cell dysfunction. Therefore, here we evaluated the role of this viral protein in augmenting NK cell functions in the context of infection as well as exposure to free Vpr protein. Our results indicate that NK cells present in peripheral blood mononuclear cells (PBMC) infected with HIV-1 vpr(+) virus are defective in their ability to lyse NK-sensitive targets as well as produce gamma interferon (IFN-γ) and the degranulation marker CD107a. The observed Vpr-mediated NK cell dysfunction is, in part, through downregulation of interleukin-12 (IL-12) production and upregulation of transforming growth factor β (TGF-β) production by the infected target cells, as reconstitution of IL-12 and blocking of TGF-β resulted in partial recovery of these responses. Additionally, NK cells present in an HIV-1 vpr(+) virus-infected PBMC culture also exhibited increased phosphorylation of Smad3 along with elevated levels of SHIP. Together these results suggest a new role for HIV-1 Vpr in compromising NK cell function and innate immunity.
MATERIALS AND METHODS
Generation of HIV-1 vpr(+) and HIV-1 vpr(−) viruses and recombinant Vpr protein.
HIV-1 vpr(+) and HIV-1 vpr(−) viruses were generated using the proviral constructs pNL43R+E and pNL43R−E− from National Institutes of Health AIDS Research Reference Reagent Program (NIH AIDS RRRP). These constructs lack Nef expression, as murine heat-stable antigen CD24 was inserted into the open reading frame of the nef gene as described previously (14). HEK293T cells (2 × 106) were transfected with 7.5 μg of proviral constructs and 2.5 μg of HIV-1 envelope or vesicular stomatitis virus G-Env expression plasmid by the calcium phosphate precipitation method. The virus titer was measured by p24 enzyme-linked immunosorbent assay (ELISA), and infectivity was assessed by determination of multiplicity of infection using the HIV-1 reporter cell line TZM-bl (NIH AIDS RRRP) as described earlier (23). Recombinant Vpr and control glutathione S-transferase (GST) were produced in Escherichia coli following IPTG (isopropyl-β-d-thiogalactopyranoside) induction and purified according to the manufacturer's instructions (Novagen, San Diego, CA). Bacterial contaminants were removed by high-performance liquid chromatography purification, and the absence of endotoxin was confirmed using a reporter assay as described previously (24).
Isolation and infection of PBMC and macrophages with HIV-1 vpr(+) or HIV-1 vpr(−) virus.
Freshly isolated normal donor PBMC (5 × 106/ml) were stimulated with 1 μg/ml phytohemagglutinin (PHA)-P (Sigma, St. Louis, MO) and 200 U/ml human recombinant IL-2 (Chiron, Emeryville, CA) for 24 h, subsequently infected with HIV-1 vpr(+) or HIV-1 vpr(−) virus at an MOI of 0.1, and maintained in culture without removing the virus (unless mentioned otherwise). Similarly, CD14+ cell-depleted peripheral blood lymphocytes (PBL) were isolated and infected as described previously (46). Cells were washed and cultured in RPMI medium (Gibco, CA) containing 10% fetal bovine serum (HyClone, Logan, UT), 1% l-glutamine (Cambrex, MD), 1% penicillin-streptomycin (Gibco, CA), and IL-2 (200 U/ml, Chiron, Emoryville, CA). For virus replication studies, at 12 h postinfection cells were washed to remove the virus and maintained in culture. At 3 and 6 days postinfection, culture supernatants were assessed by p24 ELISA for virus titer and infected PBMC were assessed for expression of Gag (NIH AIDS RRRP) and Vpr (anti-Vpr antibody was a kind gift from John Kappes, University of Alabama) by immunoblot analysis using specific antibodies. The percentages of infected PBMC and NK cells were also calculated by detecting intracellular p24 (anti-p24-fluorescein isothiocyanate [FITC]; Coulter, Miami, FL) using flow cytometry as described previously (23).
Isolation and culture of CD3− CD56+ NK cells from normal and infected PBMC.
NK cells (CD3− CD56+) were isolated from total PBMC infected with HIV-1 vpr(−) or HIV-1 vpr(+) virus at 4 days postinfection using the negative selection method (Dynal Biotech, Brown Deer, WI). Similarly, NK cells were isolated from PBMC exposed to Vpr or control GST (100 ng/ml) at 3 days postexposure. A double-step purification was performed to obtain a higher purity (>95%).
NK cell-macrophage coculture.
Myeloid accessory cells (CD14+ monocytes) isolated using the positive selection method (Miltenyi Biotech, Auburn, CA) were further cultured as described previously (40). At day 3 cultured cells (1 × 106/ml) were infected with HIV-1 vpr(+) or HIV-1 vpr(−) virus for an additional 3 days as described above. Activated macrophages stimulated with lipopolysaccharide (LPS) for 24 h were cocultured with heterologous NK cells for 3 days, or, alternatively, 24-h macrophage culture supernatants following LPS stimulation were coincubated with NK cells (1 × 106/ml) at a 1:4 dilution in a total volume of 300 μl.
NK cell-mediated target cell lysis assay.
PBMC (2 × 105 cells in a final volume of 200 μl) infected with HIV-1 vpr(−) or HIV-1 vpr(+) virus or exposed to Vpr and control protein as described above were used to assess NK cell-mediated cytolytic function using K562 cells as targets (kindly provided by Pawel Kalinski, University of Pittsburgh). Briefly, infected/exposed PBMC (12, 24, 48, 72, and 96 h and 6 days postinfection) were cocultured with K562 at an effector/target cell ratio of 10:1 in triplicate. Similarly, NK cells cocultured with macrophages or macrophage culture supernatants as described above were evaluated for target cell lysis. Cytotoxicity was assessed using a nonradioactive colorimetric assay measuring lactate dehydrogenase (LDH) released from lysed target cells following the manufacturer's instructions (Cytotox96 nonradioactive cytotoxicity assay; Promega, WI). Following 4 h of coculture at 37°C in a 96-well flat-bottom microtiter plate, 50-μl aliquots of cell-free supernatants were transferred to a new assay plate and incubated at room temperature with 50 μl reconstituted LDH substrate mix in the dark for 30 min. Target cells and effector cells incubated separately for the same period were used as target and effector spontaneous controls, respectively. Target cells treated with 0.8% Triton X lysis solution for 45 min prior to harvesting the supernatants served as the target maximum. Finally, the assay was terminated by adding 50 μl stop solution, and the release of LDH was measured at 490 nm using an ELISA plate reader. The percent cytotoxicity was calculated using the following formula: % cytotoxicity = (experimental value − effector spontaneous control value − target spontaneous control value)/(target maximum value − target spontaneous value) × 100.
NK cell degranulation assay.
To quantitate the cell surface expression of CD107a, infected or Vpr-exposed PBMC and PBL (total of 1 × 106/ml) were washed twice in phosphate-buffered saline and activated with K562 target cells (PBMC/K562 ratio = 1:1) in a total volume of 1 ml prewarmed culture medium following centrifugation for 5 min at 1,200 rpm to facilitate the contact of cells. PBMC stimulated for 1 hour with K562 were further incubated with 6 μl monensin (BD Bioscience, Mountain View, CA) at a final concentration of 6 μg/ml for an additional 3 hrs prior to surface staining for CD107a. NK cells were stained with anti-CD3-ECD and anti-CD56-PC5 (Beckman Coulter, Miami, FL) and anti-CD107a-FITC (BD Biosciences) antibodies for 1 hour in fluorescence-activated cell sorter (FACS) buffer (2% fetal bovine serum in phosphate-buffered saline) containing 5 mM EDTA on ice and were analyzed for surface expression of CD107a by flow cytometry as described previously (10).
Detection of intracellular IFN-γ.
Uninfected and infected PBMC and PBL (1 × 106 cells/ml) were further stimulated with K562 at an effector/target cell ratio of 10:1 for 6 h in the presence of 6 μl of monensin (BD Biosciences). Production of IFN-γ in fixed and permeabilized cells (cytoFix-cytoPerm kit; BD Biosciences, CA) was detected by intracellular staining using phycoerythrin-conjugated primary antibodies or isotype-matched controls (Caltag, Burlingame, CA) following surface staining with CD56-PC5 and CD3-ECD antibodies to distinguish CD3− CD56+ NK cells within the total PBMC. Briefly, cells were washed three times with FACS buffer, and surface markers were stained with anti-CD56-PC5 and anti-CD3-ECD for 1 hour. Intracellular IFN-γ staining was performed at room temperature for 60 min using 5 μl phycoerythrin-conjugated anti-IFN-γ antibodies (Coulter, FL), followed by two washes in Perm-Wash buffer. The cells were gated in ECD−, PC5+ channels to quantitate the expression of intracellular cytokine in specific NK cell subpopulations (CD3− CD56+ cells) and analyzed by flow cytometry as described previously (28, 35).
Detection of NK cell apoptosis.
At 4 and 6 days postinfection, PBMC and PBL (1 × 106 cells/ml) infected with HIV-1 vpr(+) or HIV-1 vpr(−) virus were washed twice with cold FACS buffer and surface staining of NK cells was carried out as described previously. To detect apoptosis, cells were resuspended in 100 μl sterile binding buffer containing 10 mM HEPES-NaOH (pH 7.4), 140 mM NaCl, and 2.5 mM CaCl2; incubated with annexin V-FITC (BD Biosciences) for 15 min at room temperature in the dark; and diluted four times with binding buffer before analysis by flow cytometry as described previously (24). The percentages of annexin V-positive and -negative populations among CD3− CD56+ NK cells were estimated.
Immunoblot analysis.
To detect p24 and Vpr in infected PBMC (5 × 107 cells), cells were lysed on day 3 postinfection and immunoblotted as described previously (23). NK cells (2 × 107) isolated from PBMC infected with HIV-1 vpr(+) or HIV-1 vpr(−) virus or treated with Vpr or control protein were lysed in radioimmunoprecipitation assay buffer as described previously (23). As a positive control, recombinant human TGF-β (10 ng/ml; R&D Systems, Minneapolis, MN) was used to treat the isolated normal and unexposed NK cells for the final 4 h. For NK cell signaling analysis, 20 micrograms of total cell lysates was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotted with anti-SHIP, anti-Smad2/3, anti-phopho-Smad2, or anti-phospho-Smad3 (all from Cell Signaling Technology, Beverly, CA) or anti-tubulin-α (as loading control; Neomarkers, Fremont, CA). Blots were developed using an ECL kit (Super Signal West Pico chemiluminescent substrate; Pierce, Richmond, IL) as described previously (23).
Quantitation of cytokines by ELISA.
Cytokines (IL-10, IL-12p70, IL-15, and TGF-β) released in the culture supernatants at different times postinfection or posttreatment were measured by ELISA using BD Opti-EIA kits as per the manufacturer's instructions (BD Biosciences).
Statistical analysis.
Results were analyzed using a paired Student t test. A P value of less than 0.05 was considered statistically significant.
RESULTS
HIV-1 vpr(+) virus infection of PBMC impairs NK-cell mediated cytolytic function, degranulation, and IFN-γ production.
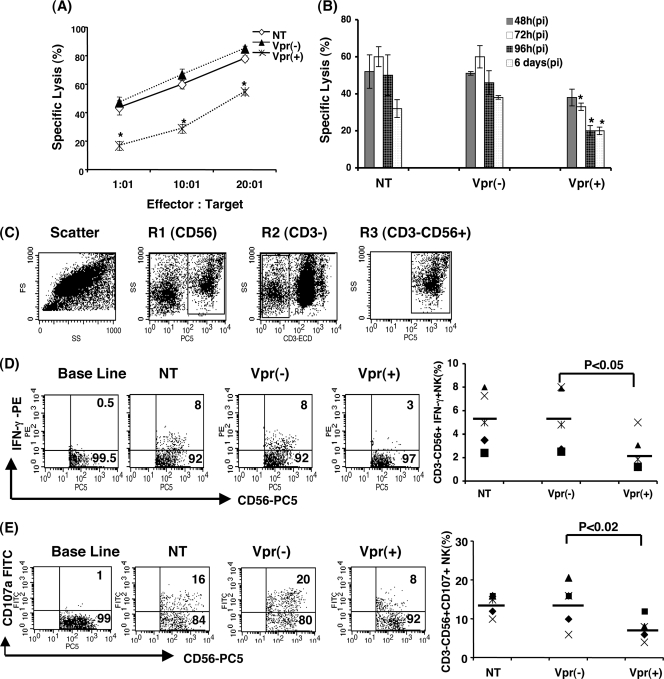
Previous studies have shown that HIV-1 Vpr dysregulates dendritic cells, T cells, and subsequently the adaptive immune response (23, 32, 46). However, the role of Vpr in NK cell dysfunction during HIV-1 infection is not well defined. We therefore first determined the effect of Vpr on NK cell function in the context of infection. As Nef is known to downregulate major histocompatibility complex class I molecules, here we compared the effect of Vpr on NK cell function in the absence of nef using viruses that lack Nef expression. PBMC were infected with HIV-1 vpr(+) or HIV-1 vpr(−) virus as described in Materials and Methods. NK cells present in the infected milieu were assessed for functional markers such as target killing, IFN-γ production, and CD107a expression. Infected and uninfected PBMC were cocultured with K562 targets at ratios of 1:1, 10:1, and 20:1, and target killing was measured. NK cells in PBMC infected with HIV-1 vpr(−) virus induced about 45% target cell lysis at a 1:1 ratio, whereas NK cells in PBMC infected with HIV-1 vpr(+) virus mediated 18% target lysis at the same ratio (Fig. 1A). A similar difference in specific lysis of targets was displayed by NK cells from HIV-1 vpr(+) virus-infected PBMC or HIV-1 vpr(−) virus-infected PBMC at 10:1 and 20:1 effector/target ratios. This suggests that NK cells in HIV-1 vpr(+) virus-infected culture is defective in killing the target cells. Next, to address how early this Vpr-mediated defect could be observed, PBMC infected for different time periods (12, 24, 48, 72, and 96 h and 6 days) were assessed for NK cell killing (Fig. 1B). The results indicate that inhibition of NK cell-mediated killing of target cells following infection of PBMC with HIV-1 vpr(+) virus was not detectable at 12 or 24 h postexposure (data not shown), whereas inhibition is evident as early as 48 h postinfection and was maintained up to 6 days (although at a much lower level), confirming a role for this protein during infection. The above-described reduction of NK cell-mediated target cell lysis by HIV-1 vpr(+)-infected PBMC was further confirmed using PKH-labeled K562 cells as targets in a similar setting of infection (data not shown).
FIG. 1.
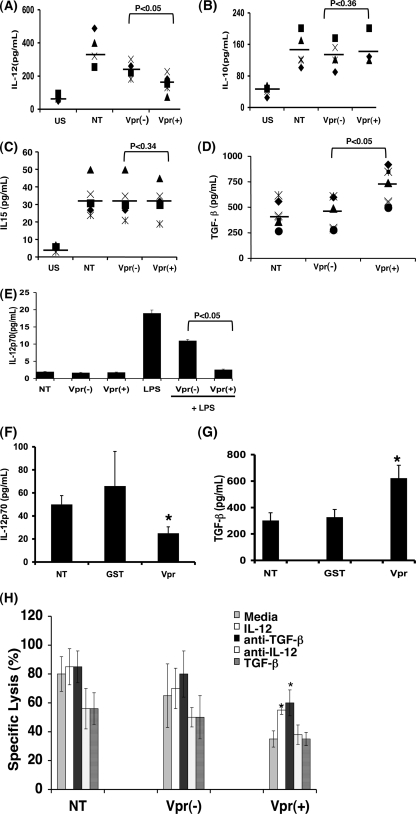
NK cells in PBMC infected with HIV1 vpr(+) virus are defective in cytolytic function, CD107a expression, and IFN-γ production. (A) PBMC infected with HIV-1 vpr(+) or HIV-1 vpr(−) virus were used to assess the NK cell cytolytic function. At 4 days postinfection, PBMC (effector) and K562 (target) cells were cocultured at ratios of 1:1, 10:1, and 20:1 for 4 h and specific lysis was measured. (B) PBMC were infected with HIV-1 vpr(−) or HIV-1 vpr(+) virus for 24, 48, 72, and 96 h and 6 days and assessed for NK cell lysis. The percent specific lysis was determined following the indicated postinfection (pi) time points. (C) Uninfected and infected PBMC were stimulated with K562 at a ratio of 10:1 in the presence of monensin for 4 h. NK cells were gated on the CD3-ECD−/CD56-PC5+ channel as shown. (D) Intracellular staining for IFN-γ was performed following surface staining with CD3-ECD and CD56-PC5 antibodies. Values in the upper right quadrants represent percent IFN-γ-producing CD3−CD56+ NK cells. The dot plot represents results from five independent experiments (right). (E) Expression of degranulation marker CD107a in infected PBMC was detected following 1 h of stimulation with K562 at a ratio of 10:1 followed by an additional 3 h incubation in the presence of monensin. Surface staining for CD107a was performed together with CD3-ECD and CD56-PC5. CD107a expression without K562 stimulation was considered baseline. Values in the upper right quadrants represent percent CD107a-expressing CD3− CD56+ NK cells. The dot plot represents results from five independent experiments (right panel). NT, uninfected PBMC; Vpr(+), PBMC infected with HIV-1 vpr(+) virus; Vpr(−), PBMC infected with HIV-1 vpr(−) virus. Data are representative of three independent experiments, each performed in triplicate. *, P < 0.05 for comparison of the Vpr(+) and Vpr(−) groups.
To further establish whether the reduced killing is correlated with functional defects, NK cells in the infected and uninfected cultures were assessed for IFN-γ production and expression of the NK cell degranulation marker CD107a within CD3− CD56+ cells. As shown in Fig. 1C, there was a substantial reduction in intracellular IFN-γ production (2.6-fold) and CD107a expression (2.5-fold) in CD3− CD56+ gated NK cells present in HIV-1 vpr(+) virus-infected PBMC compared to those present in HIV-1 vpr(−) virus-infected PBMC (Fig. 1D and E). Similar results were observed in multiple donors (n = 5). Together, these results indicate that NK cells present in infected PBMC have a decreased ability to lyse specific target cells and are functionally defective.
Vpr-induced defective NK cell function is independent of both direct infection and apoptosis of NK cells.
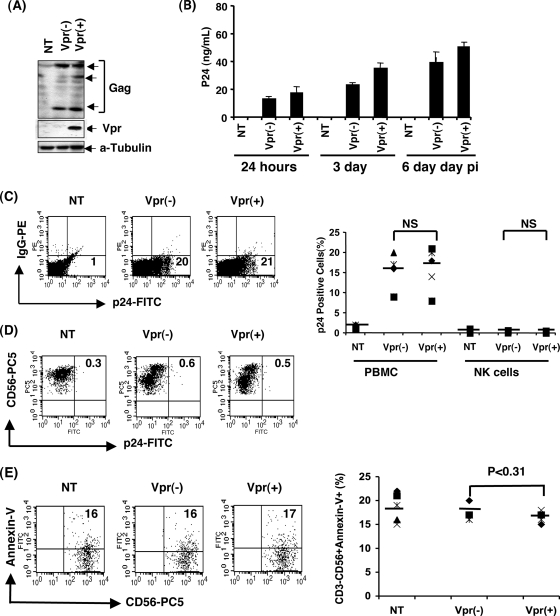
The results presented above indicate that NK cell function in the presence of Vpr is compromised. However, it is not known whether NK cells are infected by HIV-1 or whether NK cells undergo apoptosis during infection. To delineate this, PBMC infected with HIV-1 vpr(+) or HIV-1 vpr(−) virus were lysed on day 3 postinfection to detect viral proteins by immunoblot analysis. The results shown in Fig. 2A confirmed the presence of equal amounts of viral antigen (Gag) in both vpr(−)- and vpr(+)-infected PBMC and Vpr in the case of the HIV-1 vpr(+)-infected PBMC culture. Additionally, we also evaluated the viral replication by measuring the p24 in the culture supernatants (Fig. 2B). Our results indicate that there is no significant difference in virus replication between these two groups in this short-term, single-round infection.
FIG. 2.
Vpr-induced defective NK cell function is independent of direct infection of NK cells and apoptosis. (A) PBMC stimulated with PHA-P and recombinant human IL-2 were infected with HIV-1 vpr(−) or HIV-1 vpr(+) virus. At 3 days postinfection (pi), PBMC were lysed and assessed by immunoblotting to confirm the intracellular expression of Gag and Vpr. Tubulin was used as loading control. (B) Virus replication was monitored by measuring the p24 from the culture supernatant by ELISA on days 1, 3, and 6 postinfection. (C) Intracellular p24 was also detected in total PBMC by flow cytometry as a measure for determining percentage of p24 positive cells in HIV-1 vpr(+) or HIV-1 vpr(−) virus-infected PBMC. Numbers in the lower right quadrant represent percent p24 positive cells. (D) Detection of intracellular p24 in NK cells was tested within the same infected and uninfected PBMC populations by flow cytometry. PBMC gated on the CD3− CD56+ cell population were further assessed for infection by flow cytometry using intracellular p24 staining. Values in the upper right quadrant represent the percentage of cells positive for p24. The dot blot represents results from five different donors for infection in PBMC and NK cells. (E) Apoptosis in NK cells was determined using annexin V-FITC staining following surface staining with CD3-ECD and CD56-PC5 in the infected and uninfected cultures. The percentage of CD3− CD56+ NK cells positive for annexin V was evaluated on CD3− CD56+ gated NK cells. Values in the upper right quadrants and lower right quadrants represent percent apoptotic and nonapoptotic CD3− CD56+ NK cells, respectively. Results from five different donors are presented in the dot blot. NT, no treatment; Vpr(+), PBMC infected with HIV-1 vpr(+) virus; Vpr(−), PBMC infected with HIV-1 vpr(−) virus. A P value of <0.05 represents a significant difference in the HIV-1 vpr(+) virus-infected culture compared to HIV-1 vpr(−) virus-infected PBMC. NS, statistically not significant.
To determine whether NK cells are infected with the virus, PBMC were stained for CD3 and CD56 cell surface molecules and intracellular p24 and assessed by flow cytometry. CD3− CD56+ NK cells did not show p24 staining, whereas total PBMC exhibited 20% p24 positive cells (Fig. 2C and D) within the same culture, suggesting that NK cells do not support virus replication in these in vitro cultures. Similar results were observed in multiple donors (n = 5), indicating that it is not a donor specific effect. Furthermore, assessment of NK cells for apoptosis by annexin V staining indicated that the percentages of apoptotic cells in both HIV-1 vpr(−) and HIV-1 vpr(+) virus-infected cultures at day 4 postinfection were similar (about 16%) (Fig. 2E). Together these results suggest that NK cell dysfunction caused by HIV-1 Vpr is not due to killing or loss of NK cells.
PBMC treated with recombinant Vpr display similar effects on NK cell function.
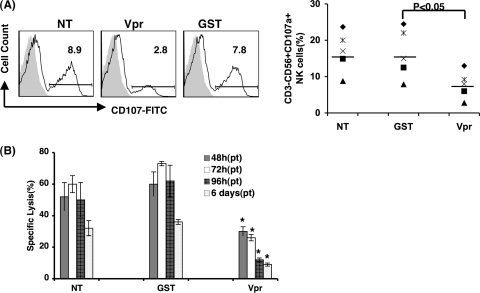
To test whether free Vpr protein in the absence of infection modulates NK cell function, total PBMC exposed to different concentrations of Vpr or GST (control) protein were assessed for their ability to express CD107a and to kill K562 targets. The results indicate that NK cells in PBMC culture treated with Vpr at as low a dose as 100 ng/ml exhibit maximum reduced expression of CD107a (2.7-fold) compared to the control GST treatment at 4 days posttreatment (Fig. 3A). Time course analysis indicated that Vpr-mediated inhibition of target cell lysis was detected as early as 48 h and maintained up to 6 days at an effector/target cell ratio of 10:1 (Fig. 3B). Next, to understand whether free Vpr could exert similar effects on NK cell function, we assessed the effect of Vpr on purified NK cells by measuring CD107a expression. NK cells exposed to Vpr did not show any significant changes compared to GST-treated or untreated control cells (data not shown), suggesting that unlike its effects on total PBMC, free Vpr failed to induce NK cell dysfunction as detected by CD107a expression.
FIG. 3.
Recombinant Vpr displays a similar ability to perturb NK cell function. (A) PBMC were treated with 100 ng/ml Vpr or control GST for 4 days, and expression of CD107a was determined as described for Fig. 1. Results from multiple donors (n = 5) are presented in a dot plot. (B) PBMC treated with Vpr or control protein for indicated post treatment (pt) time were further cocultured with K562 at an effector/target cell ratio of 10:1 in triplicate for 4 h, and the percent specific lysis was calculated as described in Materials and Methods. NT, no treatment; Vpr, PBMC treated with recombinant Vpr protein; GST, PBMC treated with recombinant GST protein. *, P < 0.05 for comparison of Vpr-treated PBMC and GST-treated PBMC. The data represent one of three similar experiments.
Vpr-induced dysregulation of NK cell function is due to the presence of infected/exposed target cells in PBMC.
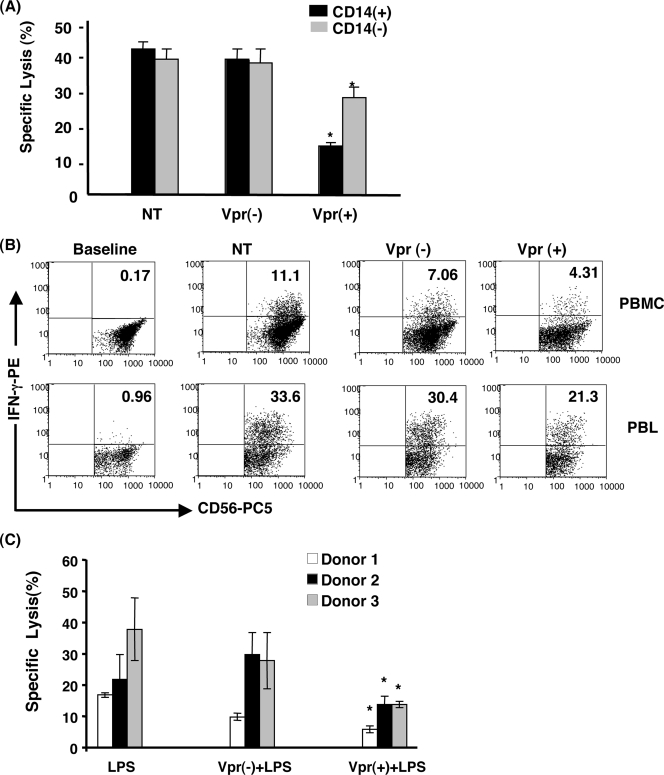
The results presented above indicate that the Vpr-induced NK cell dysregulation observed in total PBMC infected with or exposed to Vpr could be indirect, through its effects on infected target cells present in the culture. Within PBMC, both monocytes/macrophages and T cells are direct targets for HIV-1 infection and therefore have the potential to augment NK cell function. To address this, total PBMC and PBL were infected with HIV-1 vpr(+) or HIV-1 vpr(−) virus. At 4 days postinfection, PBMC and PBL were cocultured with K562 targets at an effector/target ratio of 10:1 and assessed for percent specific lysis. The data shown in Fig. 4A indicate that NK cells from HIV-1 vpr(+) virus-infected PBL have a reduced capacity to kill K562 targets compared to the NK cells isolated from HIV-1 vpr(+) virus-infected total PBMC (<1.5-fold versus > 2.5-fold), implying involvement of monocytes/macrophages. However, it is also important to note that the defect in Vpr-induced modulation of target cell lysis was not completely abrogated in PBL, suggesting that factors derived from nonmyeloid cells are also involved in the observed Vpr-induced inhibition of NK cell function. A similar modulation of intracellular IFN-γ production by NK cells following infection of PBMC and PBL with HIV-1 vpr(+) virus was detected (Fig. 4B). The observation that CD14 depletion leads to partial reversal of defects in NK cells upon infection of PBMC with HIV-1 vpr(+) virus further prompted us to elucidate the involvement of myeloid accessory cells such as macrophages in inducing NK cell-mediated inhibition of target cell lysis. As shown in Fig. 4C, coculture of NK cells with macrophage supernatants derived from HIV-1 vpr(+)-infected cells activated with LPS caused significant inhibition of target cell lysis compared to the uninfected or HIV-1 vpr(−)-infected control, indicating the inhibitory effect on NK cell function mediated by macrophage-derived factors in response to HIV-1 vpr(+) virus. Together these results suggest that Vpr-induced NK cell dysfunction is through factors produced by other HIV-1 target cells present in the infected milieu.
FIG. 4.
Vpr-induced dysregulation of NK cell function is indirectly through HIV-1-infected target cells in the milieu. PBMC isolated from normal donors were used for further purification of CD14+ cell-depleted PBL using CD14 isolation methods. (A) PBMC and PBL were infected with HIV-1 vpr(+) or HIV-1 vpr(−) virus for 4 days and cocultured with K562 at an effector/target cell ratio of 10:1. NK cell-mediated specific lysis was determined. Black bars indicate percent specific lysis of target cells by NK cells present in total PBMC. Gray bars denote percent target lysis by NK cells from PBL. (B) Evaluation of intracellular IFN-γ production by NK cells from PBMC and PBL cultures maintained under similar conditions as described in Materials and Methods. Baseline represents cells without K562 stimulation. NT, No treatment; Vpr(+), cells infected with HIV-1 vpr(+) virus; Vpr(−), cells infected with HIV-1 vpr(−) virus. Data are the representative of five independent experiments. (C) NK cell-mediated lysis of target cells following coculture of NK cells with macrophage supernatants. NK cells were coincubated with culture supernatants for 24 h. NK cell-specific lysis of K562 was measured by LDH release assay. Data are representative of three independent experiments. *, P < 0.05 in comparison with the corresponding HIV-1 vpr(−) virus-infected culture.
Cytokines produced by HIV-1 vpr(+) virus-infected or Vpr-exposed PBMC and/or macrophages are in part responsible for NK cell dysfunction.
The functional deficiency of NK cells in the absence of direct infection indicates that augmentation of factors (soluble and membrane bound) from infected/exposed targets might have a role in NK cell dysregulation. Therefore, we next delineated the involvement of cytokines that are known to affect NK cell survival/proliferation and functions (52). Supernatants collected at different time points from PBMC infected with HIV-1 vpr(+) or HIV-1 vpr(−) virus were assessed for IL-10, IL-12, IL-15, and TGF-β. As shown in Fig. 5A, there was a significant suppression of NK cell-activating proinflammatory cytokine IL-12p70 in PBMC infected with HIV-1 vpr(+) virus compared to the uninfected or HIV-1 vpr(−)-infected control in multiple donors. In contrast, production of IL-10 and soluble IL-15 was unaffected under similar condition (Fig. 5B and C). Analysis of anti-inflammatory cytokine TGF-β indicated that PBMC infected with HIV-1 vpr(+) virus produced a significantly higher (mean, 750 pg/ml) level of TGF-β than did HIV-1 vpr(−) virus-infected culture (mean, 400 pg/ml) (Fig. 5D). Time course analysis indicated that IL-12p70 expression was observed as early as 24 h postinfection and was maintained up to 96 h, whereas detectable levels of TGF-β were seen at 24 h and peaked at 48 h postinfection. This loss was maintained in the long-term cultures. The data presented in Fig. 5 represent the time point when the maximum amount of cytokine was produced. In order to evaluate the specific cell types involved in the observed Vpr-mediated dysregulation of IL-12 production, 7-day-old macrophages were infected with pseudotyped HIV-1 vpr(−) or HIV-1 vpr(+) virus for 72 h. Cells were washed and further stimulated with LPS for an additional 24 h. Production of soluble IL-12 in supernatants was measured (Fig. 5E). Macrophages infected with HIV-1 vpr(+) virus produced significantly less IL-12 than those infected with HIV-1 vpr(−) virus. Recombinant Vpr also exerted effects on IL-12 and TGF-β production similar to those in HIV-1 Vpr(+) virus-exposed PBMC culture (Fig. 5F and G). Collectively, these results indicate that both loss of IL-12 and gain of TGF-β in the HIV-1 vpr(+) virus-infected PBMC might play a role in NK cell dysfunction. Similar IL-12 downregulation by Vpr was reported previously (30).
FIG. 5.
Altered cytokines production by PBMC infected with HIV-1 vpr(+) virus promotes NK cell dysfunction. (A to D) PBMC infected with HIV-1 vpr(−) or HIV-1 vpr(+) virus were cultured as described in Materials and Methods. Supernatants were collected and assessed for IL-12 (A), IL-10 (B), IL-15 (C), and TGF-β (D) production by ELISA. Dot blots represent results from multiple donors (n = 5). US, unstimulated and uninfected PBMC; NT, stimulated and uninfected PBMC; Vpr(−), stimulated PBMC infected with HIV-1 vpr(−) virus; Vpr(+), stimulated PBMC infected with HIV-1 vpr(+) virus. *, P < 0.05, considered statistically significant for comparison to the HIV-1 vpr(−) virus groups. (E) Supernatant from PHA-stimulated PBMC treated with recombinant Vpr or control GST protein was assessed for IL-12. Production of IL-12 by macrophages infected with vpr(−) or vpr(+) virus was assessed. The supernatants derived from infected macrophages were used to measure IL-12p70 production. Data are representative of three similar experiments. (F and G) Production of IL-12 (F) and TGF-β (G) from PBMC treated with GST or Vpr, assessed by ELISA. *, P < 0.05 for comparison to GST control. NT, untreated; Vpr, recombinant Vpr treated; GST, control GST protein treated. Results represent one of three experiments. (H) Reversal of HIV-1 Vpr-induced dysregulation of NK cell lysis by recombinant IL-12 and anti-TGF-β. PBMC were pretreated with anti-TGF-β or anti-IL-12 antibody 2 h prior to infection or treated with IL-12 or TGF-β 12 h postinfection. NK cell function was assessed by measuring the percent specific lysis in different groups at an effector/target cell ratio of 10:1. NT, No treatment; Vpr(+), PBMC infected HIV-1 vpr(+) virus; Vpr(−), PBMC infected with HIV-1 vpr(−) virus. Data are the representative of one of three independent experiments. *, P < 0.05, considered statistically significant in comparison with medium-only group in HIV-1 vpr(+) virus-infected PBMC.
Next, we assessed whether Vpr-induced NK dysfunction could be reversed by reconstituting the loss of IL-12 with extracellular IL-12 protein or by blocking of overproduction of TGF-β with anti-TGF-β antibody. PBMC infected as described above were treated with recombinant IL-12 (100 ng/ml; Chemicon International, Temecula, CA) or anti-TGF-β (1 μg/ml; R&D Systems) at 12 h postinfection and maintained for an additional 3 days. As a positive control, cells were also treated with either recombinant TGF-β (5 ng/ml; R&D Systems) or anti-IL-12 (500 ng/ml; R&D Systems). NK cell-mediated cytotoxicity was measured in all the groups. The results indicate that treatment of HIV-1 vpr(+) virus-infected PBMC with recombinant IL-12 or anti-TGF-β increased NK cell-mediated lysis to 55% and 60%, respectively, compared to 35% lysis observed in medium-treated control (Fig. 5H). Conversely, single exposure to recombinant TGF-β or anti-IL-12 also inhibited NK cell-mediated killing of target cells in uninfected and HIV-1 vpr(−) virus-infected PBMC under similar conditions. However, in the case of HIV-1 vpr(+) virus-infected culture, no additional inhibition was observed. Similar results were observed in Vpr-treated PBMC (data not shown). Together, these results suggest that in addition to IL-12 and TGF-β, there may be other, unidentified soluble and membrane-associated factors that might also play a role in NK cell dysfunction.
HIV-1 Vpr-induced alteration of NK cell signaling involves an elevated level of SHIP and enhanced Smad3 phosphorylation.
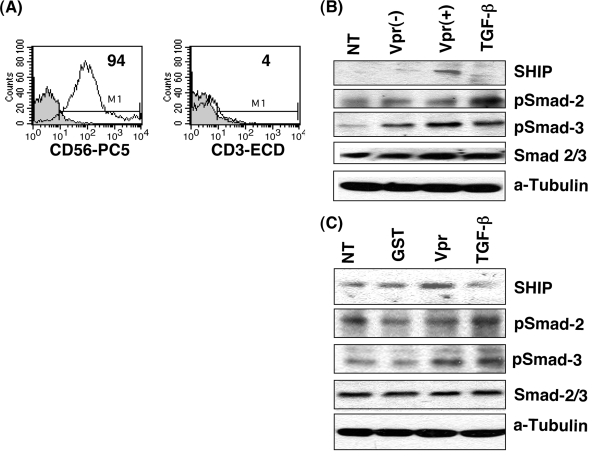
The results presented above indicate that NK cells in the presence of Vpr exhibit defective target cell killing and IFN-γ production. We further elucidated the impact of HIV-1 vpr(+) virus on phosphorylation of Smad2 and Smad3, which are known to have a role in IFN-γ production in NK cells (52). NK cells isolated from PBMC infected with HIV-1 vpr(+) or HIV-1 vpr(−) virus or treated with Vpr or GST were assessed for expression and phosphorylation of signaling molecules. The purity of the freshly isolated NK cells was about 94% as confirmed by flow cytometry (Fig. 6A). NK cells isolated from HIV-1 vpr(+)-infected PBMC exhibited elevated expression of inhibitory phosphatase SHIP and enhanced phosphorylation of Smad3, whereas the total Smad2/3 level remained unaltered. This activation is comparable with the level obtained when pure NK cells from the same PBMC culture were directly stimulated with TGF-β (10 ng/ml) for 4 h (Fig. 6B). Similar alterations of Smad2/3 and SHIP signaling were observed when NK cells were isolated from Vpr- or control protein-treated PBMC (Fig. 6C). Together, these results indicate that the diminished IFN-γ production in NK cells in the presence of HIV-1 vpr(+) virus could act in part through an increased amount of TGF-β production and activation of signaling pathway induced by TGF-β.
FIG. 6.
HIV-1 Vpr treatment increases signaling molecules, SHIP, and phosphorylation of Smad2/3. NK cells were isolated from PBMC infected with HIV-1 vpr(+) or HIV-1 vpr(−) virus at 4 days postinfection by a negative selection method. (A) Purity of NK cells was determined by flow cytometry following CD3 and CD56 staining. (B) Expression of SHIP and Smad2/3 (total and phosphorylated) was assessed by immunoblot analysis. NT, No treatment; Vpr(+), PBMC infected with HIV-1 vpr(+) virus; Vpr(−), PBMC infected with HIV-1 vpr(−) virus. TGF-β, NK cells isolated from uninfected PBMC were treated with TGF-β (5 ng/ml) for 4 h prior to lysis as a positive control. (C) Expression of SHIP and Smad2/3 in NK cells isolated from PBMC treated with Vpr, GST, or TGF-β for 4 h was assessed. NT, untreated; GST, control protein-treated PBMC; Vpr, Vpr-treated PBMC; TGF-β, positive control. Results are representative of three similar experiments. Tubulin was used as loading control.
DISCUSSION
NK cells play a pivotal role in the induction of innate and adaptive immune responses. Defective NK cell function has been reported as a result of infection by a number of viruses, such as cytomegalovirus, hepatitis C virus, herpes simplex virus, human T-cell leukemia virus type 1, and HIV-1, as well as by other pathogens (8, 17, 22, 33, 50). NK cells from long-term nonprogressors and HIV-1-exposed seronegative individuals exhibited normal cytotoxic effector function (25, 31, 43), whereas rapid progression of diseases is associated with deregulation of NK cell phenotypes, leading to functional anergy and impaired lytic response (4, 5, 20). These findings show that HIV-1 infection induces a targeted deregulation of host immune surveillance involving NK cells.
One of the striking defects in NK cell subsets during HIV-1 pathogenesis is the inefficient cytotoxicity toward specific targets, and this impaired function is directly correlated with viral loads (2, 4), suggesting that the presence of viral antigens has a role in NK cell dysfunction. Although it is well accepted that NK cells are not direct targets of HIV-1, a number of HIV-1 viral proteins such as Env and Nef are known to have an impact on NK cell function (11, 18). Here, we present results indicating that Vpr modulates NK cell functions in the absence of direct effects on NK cell-mediated killing. Further, these results were in agreement with the in vivo analyses indicating that the defective NK cell function seen in HIV-1 patients is due not to loss of NK cells but rather to a shift in subsets (4, 26). Mechanistically, the most important question addressed in this study is how Vpr modulates the NK cell function. Based on the lack of p24 expression, it is clear that NK cells were not productively infected within the cultures. Thus, the observed defect in NK cell-mediated cytotoxicity could be largely due to the negative impact of Vpr on the functional modulation of other HIV-1 target cell types such as T cells and macrophage/monocytes. This was further supported by our observation that the extent of NK cell-mediated impaired target cell lysis, CD107a, expression, and intracellular IFN-γ production was less in the case of CD14+ monocyte-depleted PBMC similarly infected with HIV-1 vpr(+) virus. Our coculture experiments involving HIV-1 vpr(+)-infected macrophage supernatants and NK cells in conjunction with diminished IL-12 production by these macrophages further support this hypothesis. A similar impact of selective depletion of myeloid and plasmocytoid accessory cells in terms of NK cell activation was reported to occur in other infections (34, 41). The partial defect in NK cells in HIV-1 vpr(+)-infected CD14-depleted culture does not rule out the involvement of more than one accessory cell type and/or factor.
NK cells and their interaction with other cell types of the immune system through soluble and membrane-bound factors initiate a complex cross talk and signaling events (13). The observation that selective depletion of myeloid accessory cells leads to the partial abrogation of Vpr-mediated NK cell dysfunction in the setting of PBMC infection provides a further clue that augmentation of cytokines (soluble and membrane associated) from these cells could be an important modulating factor in NK cell defects. Interestingly, NK cells present in the infected PBMC culture exhibited the maximum functional defect compared to NK cells separated by transwells or exposed to supernatant from HIV-1 vpr(+)-virus infected PBMC (data not shown), suggesting a role for membrane-associated factors in target cells. Upregulation of proinflammatory cytokines such as IL-12 and IL-18 synergistically coregulates NK cell activation signals, leading to enhanced IFN-γ production as well as NK cell-mediated cytolytic responses (36). In contrast, overproduction of TGF-β, presumably by NK cells and Tregs, counterregulates at multiple levels of the signaling cascade involved in IFN-γ production (52). Our studies suggest that infection of PBMC with virus containing Vpr, in the absence of Nef, differentially modulates cytokine profiles, as evident by overproduction of TGF-β and suppression of IL-12. Similar dysregulation of cytokines reported by other studies using different forms of Vpr further supports this hypothesis (23, 30, 46). However, addition of recombinant IL-12 or anti-TGF-β antibody did not show complete reversal of the observed Vpr-mediated NK cell function, suggesting that other soluble and membrane-bound factors may be involved in NK cell dysfunction either independently or in concert. This observation is further enhanced by the fact that NK cells isolated directly from HIV-1 vpr(+)-infected PBMC concurrently expressed a relatively high level of SHIP, which has been shown previously to be elevated in functionally anergic NK cell subsets in HIV-1-infected viremic patients (3) and to be linked to TGF-β induced signaling.
Vpr is one of the virion-associated nonstructural proteins present in HIV-1 and is therefore accessible to immune effector cells during early infection. Our results further support that Vpr exposure as well as de novo-synthesized Vpr is cable of dysregulating cytokine production and subsequently NK cell function. Similar effects were indicated for virion-associated Vpr in the absence of productive infection (37, 38, 51). The present study illustrates the contribution of Vpr in perturbing the innate immune response through its ability to augment cell-to-cell interaction and disruption of the cytokine signaling network. Delineating the molecular mechanism(s) involving HIV-1 viral protein Vpr during infection will further improve our understanding of immune evasive strategies employed by Vpr to disarm innate and adaptive immune responses and will aid in the development of immunotherapeutic options for HIV-infected individuals.
Acknowledgments
The HIV-1 pNL43R+E−, pNL43R−E−, and pHIV-1 envelope expression plasmids were obtained from the NIH AIDS Research and Reference Reagents Program. We thank P. Kalinski of the University of Pittsburgh for providing the K562 cell line.
This work was supported by grant R56-AI50463 from NIAID, National Institute of Health, to V.A.
Footnotes
Published ahead of print on 16 April 2008.
REFERENCES
- 1.Ahmad, A., and J. Menezes. 1996. Defective killing activity against gp120/41-expressing human erythroleukaemic K562 cell line by monocytes and natural killer cells from HIV-infected individuals. AIDS 10143-149. [DOI] [PubMed] [Google Scholar]
- 2.Alter, G., J. M. Malenfant, R. M. Delabre, N. C. Burgett, X. G. Yu, M. Lichterfeld, J. Zaunders, and M. Altfeld. 2004. Increased natural killer cell activity in viremic HIV-1 infection. J. Immunol. 1735305-5311. [DOI] [PubMed] [Google Scholar]
- 3.Alter, G., T. J. Suscovich, M. Kleyman, N. Teigen, H. Streeck, M. T. Zaman, A. Meier, and M. Altfeld. 2006. Low perforin and elevated SHIP-1 expression is associated with functional anergy of natural killer cells in chronic HIV-1 infection. AIDS 201549-1551. [DOI] [PubMed] [Google Scholar]
- 4.Alter, G., N. Teigen, B. T. Davis, M. M. Addo, T. J. Suscovich, M. T. Waring, H. Streeck, M. N. Johnston, K. D. Staller, M. T. Zaman, X. G. Yu, M. Lichterfeld, N. Basgoz, E. S. Rosenberg, and M. Altfeld. 2005. Sequential deregulation of NK cell subset distribution and function starting in acute HIV-1 infection. Blood 1063366-3369. [DOI] [PubMed] [Google Scholar]
- 5.Azzoni, L., E. Papasavvas, J. Chehimi, J. R. Kostman, K. Mounzer, J. Ondercin, B. Perussia, and L. J. Montaner. 2002. Sustained impairment of IFN-gamma secretion in suppressed HIV-infected patients despite mature NK cell recovery: evidence for a defective reconstitution of innate immunity. J. Immunol. 1685764-5770. [DOI] [PubMed] [Google Scholar]
- 6.Azzoni, L., R. M. Rutstein, J. Chehimi, M. A. Farabaugh, A. Nowmos, and L. J. Montaner. 2005. Dendritic and natural killer cell subsets associated with stable or declining CD4+ cell counts in treated HIV-1-infected children. J. Infect. Dis. 1911451-1459. [DOI] [PubMed] [Google Scholar]
- 7.Ballan, W. M., B. A. Vu, B. R. Long, C. P. Loo, J. Michaelsson, J. D. Barbour, L. L. Lanier, A. A. Wiznia, J. Abadi, G. J. Fennelly, M. G. Rosenberg, and D. F. Nixon. 2007. Natural killer cells in perinatally HIV-1-infected children exhibit less degranulation compared to HIV-1-exposed uninfected children and their expression of KIR2DL3, NKG2C, and NKp46 correlates with disease severity. J. Immunol. 1793362-3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banerjee, P., G. Feuer, and E. Barker. 2007. Human T-cell leukemia virus type 1 (HTLV-1) p12I down-modulates ICAM-1 and -2 and reduces adherence of natural killer cells, thereby protecting HTLV-1-infected primary CD4+ T cells from autologous natural killer cell-mediated cytotoxicity despite the reduction of major histocompatibility complex class I molecules on infected cells. J. Virol. 819707-9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barbour, J. D., U. Sriram, S. J. Caillier, J. A. Levy, F. M. Hecht, and J. R. Oksenberg. 2007. Synergy or independence? Deciphering the interaction of HLA class I and NK cell KIR alleles in early HIV-1 disease progression. PLoS Pathog. 3e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bryceson, Y. T., M. E. March, D. F. Barber, H. G. Ljunggren, and E. O. Long. 2005. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. J. Exp. Med. 2021001-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cerboni, C., F. Neri, N. Casartelli, A. Zingoni, D. Cosman, P. Rossi, A. Santoni, and M. Doria. 2007. Human immunodeficiency virus 1 Nef protein downmodulates the ligands of the activating receptor NKG2D and inhibits natural killer cell-mediated cytotoxicity. J. Gen. Virol. 88242-250. [DOI] [PubMed] [Google Scholar]
- 12.Collins, K. L., and D. Baltimore. 1999. HIV's evasion of the cellular immune response. Immunol. Rev. 16865-74. [DOI] [PubMed] [Google Scholar]
- 13.Fauci, A. S., D. Mavilio, and S. Kottilil. 2005. NK cells in HIV infection: paradigm for protection or targets for ambush. Nat. Rev. Immunol. 5835-843. [DOI] [PubMed] [Google Scholar]
- 14.He, J., S. Choe, R. Walker, P. Di Marzio, D. O. Morgan, and N. R. Landau. 1995. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J. Virol. 696705-6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu, P. F., L. E. Hultin, P. Hultin, M. A. Hausner, K. Hirji, A. Jewett, B. Bonavida, R. Detels, and J. V. Giorgi. 1995. Natural killer cell immunodeficiency in HIV disease is manifest by profoundly decreased numbers of CD16+CD56+ cells and expansion of a population of CD16dimCD56− cells with low lytic activity. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 10331-340. [PubMed] [Google Scholar]
- 16.Iannello, A., O. Debbeche, E. Martin, L. H. Attalah, S. Samarani, and A. Ahmad. 2006. Viral strategies for evading antiviral cellular immune responses of the host. J. Leukoc. Biol. 7916-35. [DOI] [PubMed] [Google Scholar]
- 17.Kassim, S. H., N. K. Rajasagi, X. Zhao, R. Chervenak, and S. R. Jennings. 2006. In vivo ablation of CD11c-positive dendritic cells increases susceptibility to herpes simplex virus type 1 infection and diminishes NK and T-cell responses. J. Virol. 803985-3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kottilil, S., T. W. Chun, S. Moir, S. Liu, M. McLaughlin, C. W. Hallahan, F. Maldarelli, L. Corey, and A. S. Fauci. 2003. Innate immunity in human immunodeficiency virus infection: effect of viremia on natural killer cell function. J. Infect. Dis. 1871038-1045. [DOI] [PubMed] [Google Scholar]
- 19.Kottilil, S., K. Shin, J. O. Jackson, K. N. Reitano, M. A. O'Shea, J. Yang, C. W. Hallahan, R. Lempicki, J. Arthos, and A. S. Fauci. 2006. Innate immune dysfunction in HIV infection: effect of HIV envelope-NK cell interactions. J. Immunol. 1761107-1114. [DOI] [PubMed] [Google Scholar]
- 20.Kottilil, S., K. Shin, M. Planta, M. McLaughlin, C. W. Hallahan, M. Ghany, T. W. Chun, M. C. Sneller, and A. S. Fauci. 2004. Expression of chemokine and inhibitory receptors on natural killer cells: effect of immune activation and HIV viremia. J. Infect. Dis. 1891193-1198. [DOI] [PubMed] [Google Scholar]
- 21.Lanier, L. L. 2001. On guard—activating NK cell receptors. Nat. Immunol. 223-27. [DOI] [PubMed] [Google Scholar]
- 22.Lodoen, M. B., and L. L. Lanier. 2006. Natural killer cells as an initial defense against pathogens. Curr. Opin. Immunol. 18391-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Majumder, B., M. L. Janket, E. A. Schafer, K. Schaubert, X. L. Huang, J. Kan-Mitchell, C. R. Rinaldo, Jr., and V. Ayyavoo. 2005. Human immunodeficiency virus type 1 Vpr impairs dendritic cell maturation and T-cell activation: implications for viral immune escape. J. Virol. 797990-8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Majumder, B., N. J. Venkatachari, E. A. Schafer, M. L. Janket, and V. Ayyavoo. 2007. Dendritic cells infected with Vpr-positive human immunodeficiency virus type 1 induce CD8+ T-cell apoptosis via upregulation of tumor necrosis factor alpha. J. Virol. 817388-7399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mavilio, D., J. Benjamin, M. Daucher, G. Lombardo, S. Kottilil, M. A. Planta, E. Marcenaro, C. Bottino, L. Moretta, A. Moretta, and A. S. Fauci. 2003. Natural killer cells in HIV-1 infection: dichotomous effects of viremia on inhibitory and activating receptors and their functional correlates. Proc. Natl. Acad. Sci. USA 10015011-15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mavilio, D., G. Lombardo, J. Benjamin, D. Kim, D. Follman, E. Marcenaro, M. A. O'Shea, A. Kinter, C. Kovacs, A. Moretta, and A. S. Fauci. 2005. Characterization of CD56−/CD16+ natural killer (NK) cells: a highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc. Natl. Acad. Sci. USA 1022886-2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mavilio, D., G. Lombardo, A. Kinter, M. Fogli, A. La Sala, S. Ortolano, A. Farschi, D. Follmann, R. Gregg, C. Kovacs, E. Marcenaro, D. Pende, A. Moretta, and A. S. Fauci. 2006. Characterization of the defective interaction between a subset of natural killer cells and dendritic cells in HIV-1 infection. J. Exp. Med. 2032339-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meier, U. C., R. E. Owen, E. Taylor, A. Worth, N. Naoumov, C. Willberg, K. Tang, P. Newton, P. Pellegrino, I. Williams, P. Klenerman, and P. Borrow. 2005. Shared alterations in NK cell frequency, phenotype, and function in chronic human immunodeficiency virus and hepatitis C virus infections. J. Virol. 7912365-12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mela, C. M., C. T. Burton, N. Imami, M. Nelson, A. Steel, B. G. Gazzard, F. M. Gotch, and M. R. Goodier. 2005. Switch from inhibitory to activating NKG2 receptor expression in HIV-1 infection: lack of reversion with highly active antiretroviral therapy. AIDS 191761-1769. [DOI] [PubMed] [Google Scholar]
- 30.Mirani, M., I. Elenkov, S. Volpi, N. Hiroi, G. P. Chrousos, and T. Kino. 2002. HIV-1 protein Vpr suppresses IL-12 production from human monocytes by enhancing glucocorticoid action: potential implications of Vpr coactivator activity for the innate and cellular immunity deficits observed in HIV-1 infection. J. Immunol. 1696361-6368. [DOI] [PubMed] [Google Scholar]
- 31.Montoya, C. J., P. A. Velilla, C. Chougnet, A. L. Landay, and M. T. Rugeles. 2006. Increased IFN-gamma production by NK and CD3+/CD56+ cells in sexually HIV-1-exposed but uninfected individuals. Clin. Immunol. 120138-146. [DOI] [PubMed] [Google Scholar]
- 32.Muthumani, K., D. S. Hwang, A. Y. Choo, S. Mayilvahanan, N. S. Dayes, K. P. Thieu, and D. B. Weiner. 2005. HIV-1 Vpr inhibits the maturation and activation of macrophages and dendritic cells in vitro. Int. Immunol. 17103-116. [DOI] [PubMed] [Google Scholar]
- 33.Nattermann, J., G. Feldmann, G. Ahlenstiel, B. Langhans, T. Sauerbruch, and U. Spengler. 2006. Surface expression and cytolytic function of natural killer cell receptors is altered in chronic hepatitis C. Gut 55869-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newman, K. C., D. S. Korbel, J. C. Hafalla, and E. M. Riley. 2006. Cross-talk with myeloid accessory cells regulates human natural killer cell interferon-gamma responses to malaria. PLoS Pathog. 2e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nuvor, S. V., M. van der Sande, S. Rowland-Jones, H. Whittle, and A. Jaye. 2006. Natural killer cell function is well preserved in asymptomatic human immunodeficiency virus type 2 (HIV-2) infection but similar to that of HIV-1 infection when CD4 T-cell counts fall. J. Virol. 802529-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ortaldo, J. R., R. Winkler-Pickett, J. Wigginton, M. Horner, E. W. Bere, A. T. Mason, N. Bhat, J. Cherry, M. Sanford, D. L. Hodge, and H. A. Young. 2006. Regulation of ITAM-positive receptors: role of IL-12 and IL-18. Blood 1071468-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poon, B., M. A. Chang, and I. S. Chen. 2007. Vpr is required for efficient Nef expression from unintegrated human immunodeficiency virus type 1 DNA. J. Virol. 8110515-10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poon, B., K. Grovit-Ferbas, S. A. Stewart, and I. S. Chen. 1998. Cell cycle arrest by Vpr in HIV-1 virions and insensitivity to antiretroviral agents. Science 281266-269. [DOI] [PubMed] [Google Scholar]
- 39.Quaranta, M. G., A. Napolitano, M. Sanchez, L. Giordani, B. Mattioli, and M. Viora. 2007. HIV-1 Nef impairs the dynamic of DC/NK crosstalk: different outcome of CD56(dim) and CD56(bright) NK cell subsets. FASEB J. 212323-2334. [DOI] [PubMed] [Google Scholar]
- 40.Schafer, E. A., N. J. Venkatachari, and V. Ayyavoo. 2006. Antiviral effects of mifepristone on human immunodeficiency virus type-1 (HIV-1): targeting Vpr and its cellular partner, the glucocorticoid receptor (GR). Antiviral Res. 72224-232. [DOI] [PubMed] [Google Scholar]
- 41.Schleicher, U., J. Liese, I. Knippertz, C. Kurzmann, A. Hesse, A. Heit, J. A. Fischer, S. Weiss, U. Kalinke, S. Kunz, and C. Bogdan. 2007. NK cell activation in visceral leishmaniasis requires TLR9, myeloid DCs, and IL-12, but is independent of plasmacytoid DCs. J. Exp. Med. 204893-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schneider-Schaulies, S., and U. Dittmer. 2006. Silencing T cells or T-cell silencing: concepts in virus-induced immunosuppression. J. Gen. Virol. 871423-1438. [DOI] [PubMed] [Google Scholar]
- 43.Scott-Algara, D., L. X. Truong, P. Versmisse, A. David, T. T. Luong, N. V. Nguyen, I. Theodorou, F. Barre-Sinoussi, and G. Pancino. 2003. Increased NK cell activity in HIV-1-exposed but uninfected Vietnamese intravascular drug users. J. Immunol. 1715663-5667. [DOI] [PubMed] [Google Scholar]
- 44.Trinchieri, G. 1989. Biology of natural killer cells. Adv. Immunol. 47187-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tungaturthi, P. K., B. E. Sawaya, S. P. Singh, B. Tomkowicz, V. Ayyavoo, K. Khalili, R. G. Collman, S. Amini, and A. Srinivasan. 2003. Role of HIV-1 Vpr in AIDS pathogenesis: relevance and implications of intravirion, intracellular and free Vpr. Biomed. Pharmacother. 5720-24. [DOI] [PubMed] [Google Scholar]
- 46.Venkatachari, N. J., B. Majumder, and V. Ayyavoo. 2007. Human immunodeficiency virus (HIV) type 1 Vpr induces differential regulation of T cell costimulatory molecules: direct effect of Vpr on T cell activation and immune function. Virology 358347-356. [DOI] [PubMed] [Google Scholar]
- 47.Vieillard, V., J. L. Strominger, and P. Debre. 2005. NK cytotoxicity against CD4+ T cells during HIV-1 infection: a gp41 peptide induces the expression of an NKp44 ligand. Proc. Natl. Acad. Sci. USA 10210981-10986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ward, J., M. Bonaparte, J. Sacks, J. Guterman, M. Fogli, D. Mavilio, and E. Barker. 2007. HIV modulates the expression of ligands important in triggering natural killer cell cytotoxic responses on infected primary T-cell blasts. Blood 1101207-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weber, K., D. Meyer, V. Grosse, M. Stoll, R. E. Schmidt, and H. Heiken. 2000. Reconstitution of NK cell activity in HIV-1 infected individuals receiving antiretroviral therapy. Immunobiology 202172-178. [DOI] [PubMed] [Google Scholar]
- 50.Wills, M. R., O. Ashiru, M. B. Reeves, G. Okecha, J. Trowsdale, P. Tomasec, G. W. Wilkinson, J. Sinclair, and J. G. Sissons. 2005. Human cytomegalovirus encodes an MHC class I-like molecule (UL142) that functions to inhibit NK cell lysis. J. Immunol. 1757457-7465. [DOI] [PubMed] [Google Scholar]
- 51.Xiao, Y., G. Chen, J. Richard, N. Rougeau, H. Li, N. G. Seidah, and E. A. Cohen. 2008. Cell-surface processing of extracellular human immunodeficiency virus type 1 Vpr by proprotein convertases. Virology 372384-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu, J., M. Wei, B. Becknell, R. Trotta, S. Liu, Z. Boyd, M. S. Jaung, B. W. Blaser, J. Sun, D. M. Benson, Jr., H. Mao, A. Yokohama, D. Bhatt, L. Shen, R. Davuluri, M. Weinstein, G. Marcucci, and M. A. Caligiuri. 2006. Pro- and antiinflammatory cytokine signaling: reciprocal antagonism regulates interferon-gamma production by human natural killer cells. Immunity 24575-590. [DOI] [PubMed] [Google Scholar]
- 53.Zamai, L., A. R. Mariani, G. Zauli, L. Rodella, R. Rezzani, F. A. Manzoli, and M. Vitale. 1998. Kinetics of in vitro natural killer activity against K562 cells as detected by flow cytometry. Cytometry 32280-285. [DOI] [PubMed] [Google Scholar]
- 54.Zocchi, M. R., A. Rubartelli, P. Morgavi, and A. Poggi. 1998. HIV-1 Tat inhibits human natural killer cell function by blocking L-type calcium channels. J. Immunol. 1612938-2943. [PubMed] [Google Scholar]