Abstract
Nearly a third of the human population is at risk of infection with the four serotypes of dengue viruses, and it is estimated that more than 100 million infections occur each year. A licensed vaccine for dengue viruses has become a global health priority. A major challenge to developing a dengue vaccine is the necessity to produce fairly uniform protective immune responses to all four dengue virus serotypes. We have developed two bivalent dengue virus vaccines, using a complex adenovirus vector, by incorporating the genes expressing premembrane (prM) and envelope (E) proteins of dengue virus types 1 and 2 (dengue-1 and -2, respectively) (CAdVax-Den12) or dengue-3 and -4 (CAdVax-Den34). Rhesus macaques were vaccinated by intramuscular inoculation of a tetravalent dengue vaccine formulated by combining the two bivalent vaccine constructs. Vaccinated animals produced high-titer antibodies that neutralized all four serotypes of dengue viruses in vitro. The ability of the vaccine to induce rapid, as well as sustained, protective immune responses was examined with two separate live-virus challenges administered at 4 and 24 weeks after the final vaccination. For both of these virus challenge studies, significant protection from viremia was demonstrated for all four dengue virus serotypes in vaccinated animals. Viremia from dengue-1 and dengue-3 challenges was completely blocked, whereas viremia from dengue-2 and dengue-4 was significantly reduced, as well as delayed, compared to that of control-vaccinated animals. These results demonstrate that the tetravalent dengue vaccine formulation provides significant protection in rhesus macaques against challenge with all four dengue virus serotypes.
Dengue viruses belong to the family Flaviviridae (3). Four antigenically distinct serotypes of dengue virus have similar clinical presentation, epidemiology, and distribution, especially in tropical and subtropical regions of the world, where 2.5 billion people are at risk of infection (9). Infection with any of the four dengue virus serotypes can cause diseases ranging from mild febrile illness and classic dengue fever to the severe and potentially fatal forms of dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) (29). Natural infection with any of the dengue virus serotypes provides only long-term homotypic immunity, and available epidemiologic data suggest an increased risk for DHF/DSS during secondary infections with a heterologous serotype (21, 22). Global expansion of dengue virus infections in recent decades has made the development of vaccines for dengue viruses a public health priority. Traditional vaccine approaches such as live attenuated viruses (LAV), inactivated viruses, and subunit vaccines, as well as novel approaches such as cloned, engineered viruses and chimeric viruses using yellow fever virus (YFV) backbone, are being pursued (1, 10, 26, 27); however, a licensed vaccine is not yet available.
To avoid the potential for increased risk of DHF/DSS due to postulated immune enhancement (13), a dengue virus vaccine should elicit protective immunity simultaneously to all four serotypes. Current approaches depend on developing four monovalent vaccine candidates and mixing them to produce a final tetravalent vaccine. This approach introduces considerable constraints regarding production and formulation. Vaccine approaches using live, replicating viruses have shown potential problems with mixed formulations, presumably stemming from serotype competition and/or dominance (7, 20, 30, 34). To address this potential problem, we are developing nonreplicating DNA vaccines and recombinant viral vector vaccines for dengue viruses. We have previously shown that a dengue virus type 1 (dengue-1) DNA vaccine expressing prM and full-length E genes induced neutralizing antibodies in nonhuman primates and provided partial protection from the corresponding live-virus challenge (28). It has been generally recognized that an inadequate uptake of naked DNA vaccine by host cells and the resulting poor expression of the antigen(s) are the leading cause of limited success with naked DNA vaccines. We therefore hypothesized that it may be beneficial to use replication-deficient recombinant viral vectors to enhance gene delivery and immunogenicity. Such vectored vaccines may be beneficial as stand-alone vaccine candidates or as components of heterologous prime-boost vaccination regimens. We have recently reported complete protection of cynomolgus monkeys vaccinated with a heterologous prime-boost regimen with monovalent dengue-1 vaccines, based on DNA and Venezuelan equine encephalitis (VEE) virus replicons (4).
Adenovirus vectors offer the advantages of being generally safe and easy to produce and store. In addition, there is substantial clinical experience with adenovirus vectors, albeit mostly in gene therapy. Second-generation complex adenovirus vectors with multiple deletions can harbor a larger foreign DNA load and are especially suited for developing multivalent vaccines such as tetravalent dengue vaccines. Vaccines based on this vector platform, expressing multiple antigens of Marburg virus (37) and Ebola virus (38), have already been described. Here we report results from a nonhuman primate study in which animals were vaccinated with a tetravalent dengue vaccine formulated by mixing two bivalent vaccine constructs, CAdVax-Den12 and CAdVax-Den34. Each bivalent construct was previously shown to elicit corresponding bivalent virus-neutralizing antibody in vaccinated mice (16). Results reported here demonstrate that the tetravalent dengue vaccine elicited a neutralizing antibody response to all four dengue virus serotypes and provided both short-term and long-term protection against challenges from each of the four serotypes.
MATERIALS AND METHODS
Vaccines.
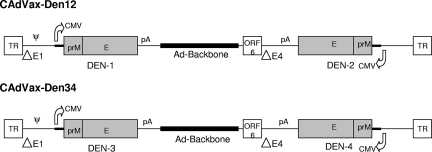
Construction and purification of CAdVax-Den12 and CAdVax-Den34 have been described previously (16). CAdVax-Den12 expressed the prM and E genes of dengue-1 and -2, and CAdVax-Den34 expressed the same genes of dengue-3 and -4. Genomic RNA of dengue-1 (strain Western Pacific 74) grown in Vero cells and low-passage clinical isolates (from the Philippines) of dengue-2, -3 and -4 were used as the source of prM and E genes. The dengue-3 prM and E gene sequences were optimized for human codon usage. Figure 1 shows a schematic diagram of the two bivalent vaccine constructs. Tetravalent dengue vaccine (CAdVax-DenTV) consisted of a mixture of equal proportions of CAdVax-Den12 and CAdVax-Den34. Control vaccine constructs expressed antigens from either the severe acute respiratory syndrome virus (CAdVax-C1) or the hepatitis C virus (CAdVax-C2).
FIG. 1.
Schematic of the bivalent vaccine constructs CAdVax-Den12 and CAdVax-Den34. Salient features of dengue virus gene expression cassettes and adenovirus genome are shown. CMV, CMV promoter; pA, poly(A) site; TR, terminal repeats; ΔE1 and ΔE4 are deletions in the E1 and E4 regions, respectively; ψ, packaging sequence. Diagrams are not to scale. ORF, open reading frame.
Animals and study design.
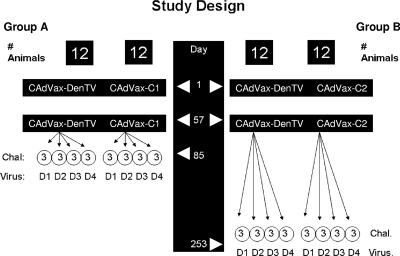
Rhesus macaques (Macaca mulatta) of either sex, 3 to 8 years old, were used in the study. Animals were prescreened for the presence of anti-dengue virus antibody by using the enzyme-linked immunosorbent assay (ELISA; flavivirus cross-reactive) and the plaque reduction neutralization test (PRNT), and those animals with evidence of previous exposure were excluded. Animals were not prescreened for antiadenovirus antibody. Forty-eight animals were divided into two groups, A and B, of 24 animals each (Fig. 2). Twelve animals in each group were vaccinated with CAdVax-DenTV. Twelve control animals in group A and in group B were vaccinated with CAdVax-C1 and CAdVax-C2, respectively. Vaccination consisted of two doses (on days 1 and 57) of 1 × 109 infectious units, delivered intramuscularly, divided equally between the two deltoid muscles. Four weeks after the final vaccination (day 85), the animals in group A were further divided into subgroups (n = 3) and challenged with live dengue-1, -2, -3, or -4. Animals in group B were similarly divided into subgroups and challenged at 24 weeks after the final vaccination (day 253). Prior to receiving the virus challenge, one of the vaccinated animals from group B died from causes unrelated to the study and was removed from data analyses. The experiments reported herein were conducted in compliance with the Animal Welfare Act and in accordance with the principles set forth in Guide for the Care and Use of Laboratory Animals (25a).
FIG. 2.
Study design. Twelve animals in group A and B each were vaccinated with CAdVax-DenTV vaccine or CAdVax-C1 or CAdVax-C2 on days 1 and 57. Group A and B animals were challenged (Chal.) with dengue-1, -2, -3, and -4 (D1 to D4) on days 85 and 253, respectively, as shown.
Immune responses.
Quantitative measurements of dengue virus-specific immunoglobulin G (IgG) in monkey sera were made by ELISA as previously described (4). Dengue virus-neutralizing antibody titers were determined by PRNT as previously described (23). Virus strains used in neutralization assays were the same as those used for virus challenge (see below). Serial twofold dilutions of individual monkey sera were used in the assay. A pool of the sera of all animals, collected before animals were primed, was used as the negative control for PRNT. Fifty percent neutralization titers (PRNT50) were determined by probit analysis using Minitab software. T-cell responses in vaccinated group B animals were measured by a gamma interferon enzyme-linked immunospot (IFN-γ ELISPOT) assay as previously described (4).
Antibodies to adenovirus were determined by ELISA. Immunoplates were coated with purified CAdVax vector (diluted 1:500 in phosphate-buffered saline) and allowed to dry overnight at 37°C. The next day, plates were washed, blocked, and incubated with serial dilutions of monkey sera (1:200 to 1:200,000). The remainder of the assay was carried out as previously described (27). Titers were determined by the inverse of the serum dilution that corresponded to an absorbance reading of two times the background absorbance for the assay.
Virus challenge and viremia.
Animals in groups A and B were challenged on days 85 and 253 after they received their primary vaccinations, respectively. Groups of three control and three vaccinated animals were challenged by subcutaneous inoculations of 5 × 105 PFU of dengue-1 (Western Pacific 74), dengue-2 (OBS8041), or dengue-3 (CH53489) or by 3.8 × 105 PFU of dengue-4 (341750). All challenge virus strains were unattenuated, virulent wild-type virus strains. The deduced amino acid sequence of the dengue-1 antigen in CAdVax-DenTV was identical to that of the challenge dengue-1, and the deduced amino acid sequences of the dengue-2, -3, and -4 antigens in CAdVax-DenTV were 97% or more homologous to those of the corresponding challenge virus strains.
Viremia was determined by using sera collected from daily bleeds following the challenge inoculation. Three hundred microliters of sera was diluted to 1 ml in Eagle's minimum essential medium (EMEM) supplemented with 2% fetal bovine serum, penicillin, and streptomycin. Two T-25 flasks of subconfluent monolayers of Vero cells were inoculated with 0.5 ml of diluted serum and incubated at room temperature for 1 h with gentle rocking. Four milliliters of EMEM containing 5% fetal bovine serum was added to each flask. Cells were incubated at 37°C in a 5% CO2 atmosphere for 14 days, with a medium change on day 7. The cells were then scraped off the flask, washed with phosphate-buffered saline, and spotted onto immunofluorescence slides. Cells were fixed using cold acetone and processed for indirect immunofluorescence using mouse monoclonal antibody 7E11 (specific for the nonstructural protein NS-1) and fluorescein isothiocyanate-conjugated anti-mouse Ig. Cells were examined with an Olympus fluorescence microscope for the presence or absence of dengue virus-specific antigen. With this method, a single PFU of virus could be detected by using serial dilutions of a virus stock of known titer, which resulted in a sensitivity of detection of about 7 PFU/ml. Serum samples that were found positive for the presence of virus by this method were further examined by direct plaque titration. Two-hundred-microliter volumes of 1:3 and 1:15 dilutions were used in duplicate for plaquing on Vero cell monolayers. The average titer was determined as PFU/ml.
Data analyses.
All antibody data were log transformed for statistical analyses. Neutralizing antibody titers against four virus serotypes were evaluated by analysis of variance (ANOVA) and a t test. A repeated measure ANOVA was used to evaluate differences in IgG concentrations in response to four dengue virus serotypes between three points of observations. Viremia in the control and vaccinated animals challenged with dengue-1, -2, -3, or -4 was analyzed by logistic regression.
RESULTS
CAdVax-DenTV induces a tetravalent antibody response.
CAdVax-DenTV causes de novo synthesis of dengue virus antigens (prM and E) in infected cells (16). Antibodies to these dengue virus antigens are an important aspect of vaccine-induced immunity. The antibody response to each dengue virus serotype was measured by ELISA and PRNT. IgG response to all four dengue virus serotypes was detectable by ELISA in all vaccinated monkeys after their first vaccination (not shown). It should be noted that the ELISA format does not distinguish serotype cross-reactive antibodies. However, of the 23 vaccinated animals, only 5 had developed tetravalent virus-neutralizing antibody, while others had trivalent (7 of 23), bivalent (9 of 23), or monovalent (2 of 23) neutralizing antibody responses (not shown). When responses were measured 4 weeks after their second vaccination (day 85), all 23 vaccinated monkeys had tetravalent virus-neutralizing antibody responses (Table 1). Geometric mean PRNT50 titers for the four serotypes on day 85 ranged from 200 (dengue-2) to 937 (dengue-3). The dengue-2-neutralizing antibody response was significantly lower than that of the other serotypes (P < 0.05). Analysis of group B animals (n = 11) on day 253 showed that except for dengue-3, which showed a decline in the titers from day 85 to day 253, neutralizing antibody titers to the other serotypes were similar to those on day 85, indicating longevity of the virus-neutralizing antibody response. There were no significant differences among serotypes on day 253 (P = 0.1205).
TABLE 1.
Dengue virus neutralizing antibody response in vaccinated animalsa
| Group | Animal | Titer at day 85/253
|
|||
|---|---|---|---|---|---|
| Dengue-1 | Dengue-2 | Dengue-3 | Dengue-4 | ||
| A | RO4069 | 497 | 191 | 975 | 441 |
| RO4076 | 511 | 38 | 906 | 351 | |
| RO4018 | 981 | 381 | 1,135 | 773 | |
| RO4033 | 503 | 216 | 888 | 595 | |
| RO4020 | 681 | 49 | 1,325 | 387 | |
| RO4032 | 582 | 242 | 1,662 | 690 | |
| RO3031 | 537 | 240 | 981 | 605 | |
| RO3065 | 521 | 497 | 1,444 | 697 | |
| RO3026 | 773 | 500 | 1,405 | 708 | |
| RO4005 | 477 | 416 | 1,158 | 731 | |
| RO3053 | 937 | 578 | 1,451 | 849 | |
| RO4090 | 700 | 564 | 1,541 | 802 | |
| B | RO4055 | 869/565 | 90/106 | 148/1 | 230/109 |
| RO3040 | 943/548 | 161/236 | 397/20 | 282/369 | |
| RO3021 | 968/504 | 305/112 | 1,413/412 | 211/178 | |
| RO4086 | 778/952 | 369/302 | 1,051/738 | 477/517 | |
| RO4068 | 472/654 | 271/402 | 921/1,115 | 296/429 | |
| RO3057 | 412/385 | 41/101 | 620/248 | 300/178 | |
| RO3052 | 722/707 | 396/327 | 947/971 | 379/522 | |
| RO4034 | 460/438 | 147/226 | 448/164 | 219/301 | |
| RO3005 | 696/621 | 413/209 | 915/559 | 304/363 | |
| RO4026 | 620/597 | 502/189 | 1,233/1,249 | 310/527 | |
| RO3046 | 302/525 | 575/125 | 934/315 | 299/320 | |
| Geometric mean titerb | 621/575 | 200/191 | 937/220 | 431/312 | |
Neutralizing antibody titers were determined by PRNT and probit analysis. Values shown in bold are titers for the corresponding challenge virus type at the time of challenge. Where neutralizing antibody responses were not detectable, a titer of 1 was arbitrarily assigned for the purposes of determining the geometric mean titers. Animals were vaccinated on days 1 and 57. Group A animals were challenged on day 85, and group B animals were challenged on day 253.
Geometric mean titers were determined for days 85 and 253, with sample sizes of 23 and 11, respectively.
Long-lasting virus-neutralizing antibodies depend on a strong CD4+ helper T-cell response induced by the vaccine. However, the IFN-γ ELISPOT assay failed to detect T-cell responses in peripheral blood mononuclear cells (group B animals) collected at 4 weeks and 8 weeks after the second vaccination and stimulated in vitro with purified dengue-1 (not shown). All vaccinated animals showed moderate T-cell responses when measured at 4 weeks after the virus challenge (not shown).
Antivector immunity does not prevent response to revaccination.
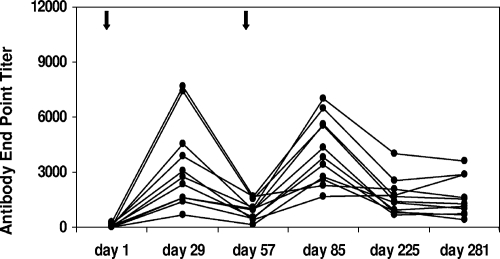
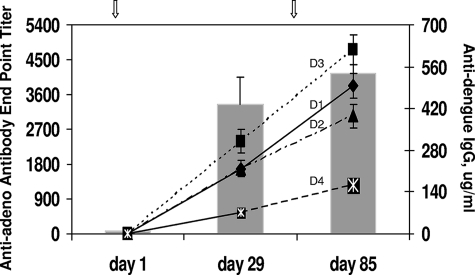
One of the concerns regarding virus vector-based vaccines, in general, is the effect of antivector immunity on the performance of vaccines. Since a significant percentage of the human population is preexposed to adenovirus, there is concern that vaccines based on adenovirus vectors may not be effective in the face of preexisting antiadenovirus antibodies. Although this study was not designed to address the effects of preexisting vector immunity, we have sought to determine the kinetics of antivector antibody elicited by an initial vaccination and its effect on the subsequent booster vaccine dose. To accomplish this, antiadenovirus antibody and anti-dengue virus IgG were measured at multiple time points for group B animals. Prior to receiving their first vaccination, all animals were either negative or weakly positive for antiadenovirus antibody, and the highest titer was <300. Animal RO3052 (Table 1), which had a prevaccination antiadenovirus antibody titer of 283, nevertheless produced anti-dengue virus antibodies at levels similar to those of animals who had lower or undetectable antiadenovirus antibody. Four weeks after receiving their first inoculation with CAdVax-DenTV, all animals had developed antivector antibody, with end point titers ranging from 652 to 7,680 (Fig. 3). These titers declined by day 57, when animals received a second dose of CAdVax-DenTV. Four weeks postbooster dose (day 85), antivector antibody titers had increased to levels similar to those seen at 4 weeks postpriming dose. Notably, anti-dengue virus antibody responses to each of the four serotypes also increased in response to the booster vaccination with CAdVax-DenTV (Fig. 4), indicating that antivector antibody levels on day 57 did not adversely affect the immunogenicity of the booster dose. By day 225, antivector antibody titers had once again declined to levels similar to those seen on day 57 (Fig. 3), indicating that it may be possible to administer repeat doses of a vaccine based on an adenovirus vector or to administer different vaccines based on this platform.
FIG. 3.
Antiadenovirus (vector) antibody responses in vaccinated animals. Antiadenovirus antibody in vaccinated animal sera (group B) was determined as described previously. End point ELISA titers are shown. Each line represents an individual animal in group B. Arrows indicate vaccinations.
FIG. 4.
Immunogenicity in the presence of antivector antibody. Averages of antiadenovirus antibody (bars) and anti-dengue virus antibody (lines) responses are shown for group B animals. Lines D1, D2, D3, and D4 represent dengue-1, -2, -3, and -4, respectively.
Protection from dengue virus challenge.
The efficacy of CAdVax-DenTV to protect vaccinated animals against a challenge from all four dengue viruses was determined by two separate virus challenges. The first challenge (group A) determined the short-term efficacy of vaccines (4 weeks after vaccination), whereas the second challenge (group B) determined the long-term efficacy (24 weeks after vaccination). Groups of three control and three vaccinated animals were each challenged with one of the four dengue virus serotypes. Animals were bled for 10 days following the virus challenge, and viremia was determined as described in Materials and Methods. Tables 2 and 3 show viremia for animals in group A (day 85 challenge) and group B (day 253 challenge), respectively. All four dengue virus types produced uniform viremia in control animals in both challenges, ranging from an average of 4.66 to 6.66 days and log titers of 0.77 to 2.89 PFU/ml. Four weeks after receiving their final vaccination (Table 2), all monkeys challenged with dengue-1 or dengue-3 were completely protected, showing 0 days of viremia compared to 5.66 days for the corresponding control groups (P = 0.0007 for both dengue-1 and -3). One of the three animals challenged with dengue-2 was viremic for a single day (group average, 0.33 days) compared to the control group average of 4.66 days (P = 0.0028). Similarly, each of the three animals challenged with dengue-4 became viremic for a single day (group average, 1 day) compared to the control group average of 6.66 days (P < 0.0001). Complete protection of vaccinated animals from the dengue-1 and dengue-3 challenges was also evident in group B animals, which were challenged at 24 weeks after vaccination (Table 3; P = 0.0007 and 0.0011, respectively). Significant protection against dengue-2 and dengue-4 challenges (P = 0.0028 and 0.0354, respectively) was also demonstrated in vaccinated group B animals. It is of interest to note that in group B animals (Table 3), although the duration of viremia with dengue-4 was significantly reduced in vaccinated animals compared to that in control animals, the virus titers appeared to be higher in the vaccinated animals than in the control animals.
TABLE 2.
Viremia in control and vaccinated monkeys challenged with dengue-1 to -4 at 4 weeks postvaccination
| Vaccine | Animal | Challenge virus serotype | No. of viremic days | Range of viremic daysa | Group average (days) | Range of virus titers (log10 PFU/ml) |
|---|---|---|---|---|---|---|
| Control (CAdVax-C1) | RO4073 | 1 | 6 | 1-7 | 5.66 | 0.77-2.64 |
| RO4070 | 1 | 5 | 2-6 | |||
| RO4066 | 1 | 6 | 2-7 | |||
| RO4061 | 2 | 4 | 1-4 | 4.66 | 0.77-2.89 | |
| RO3032 | 2 | 5 | 1-5 | |||
| RO4092 | 2 | 5 | 2-7 | |||
| RO3010 | 3 | 3 | 1-3 | 5.66 | 0.77-2.58 | |
| RO3125 | 3 | 6 | 1-6 | |||
| RO4012 | 3 | 8 | 2-9 | |||
| RO3021 | 4 | 5 | 1-7 | 6.66 | 1.25-2.34 | |
| RO4162 | 4 | 8 | 1-8 | |||
| RO4028 | 4 | 7 | 1-8 | |||
| CAdVax-DenTV | RO4069 | 1 | 0 | 0 | ||
| RO4076 | 1 | 0 | ||||
| RO4018 | 1 | 0 | ||||
| RO4033 | 2 | 1 | 7 | 0.33 | 2.65 | |
| RO4020F | 2 | 0 | ||||
| RO4032 | 2 | 0 | ||||
| RO3031 | 3 | 0 | 0 | |||
| RO3065 | 3 | 0 | ||||
| RO3026 | 3 | 0 | ||||
| RO4005 | 4 | 1 | 6 | 1 | 1.17-1.73 | |
| RO3053 | 4 | 1 | 6 | |||
| RO4090 | 4 | 1 | 4 |
The range is the period between the days on which viremia was first and last detected.
TABLE 3.
Viremia in control and vaccinated monkeys challenged with dengue-1 to -4 at 24 weeks postvaccination
| Vaccine | Animal | Challenge virus serotype | No. of viremic days | Range of viremic daysa | Group average (days) | Range of virus titers (log10 PFU/ml) |
|---|---|---|---|---|---|---|
| Control (CAdVax-C2) | RO4011 | 1 | 7 | 1-8 | 5.66 | 0.77-2.85 |
| RO4196 | 1 | 5 | 1-5 | |||
| RO4044 | 1 | 5 | 1-5 | |||
| RO4031 | 2 | 4 | 1-4 | 4.66 | 1.07-2.15 | |
| RO4098 | 2 | 6 | 1-6 | |||
| RO4178 | 2 | 4 | 1-4 | |||
| RO4079 | 3 | 6 | 2-7 | 5.33 | 0.77-2.13 | |
| RO3277 | 3 | 7 | 1-7 | |||
| RO4080 | 3 | 3 | 1-7 | |||
| RO3269 | 4 | 5 | 1-6 | 5.00 | 0.77-1.77 | |
| RO3249 | 4 | 5 | 5-9 | |||
| RO4044 | 4 | 5 | 1-7 | |||
| CAdVax-DenTV | RO4055 | 1 | 0 | 0.00 | ||
| RO3040 | 1 | 0 | ||||
| RO3021 | 2 | 1 | 2 | 0.33 | —b | |
| RO4086 | 2 | 0 | ||||
| RO4068 | 2 | 0 | ||||
| RO3057 | 3 | 0 | 0.00 | |||
| RO3052 | 3 | 0 | ||||
| RO4034 | 3 | 0 | ||||
| RO3005 | 4 | 2 | 4-5 | 2.33 | 2.12-3.07 | |
| RO4026 | 4 | 2 | 4-5 | |||
| RO3046 | 4 | 3 | 3-5 |
The range is the period between the days on which viremia was first and last detected.
The day-2 serum sample from animal RO3021 was positive for virus by using cell infectivity assay, but virus presence was below the detection threshold by using the plaque assay.
Neutralizing antibody titers for all animals at the time of challenge are shown in Table 1. Examination of antibody titers and protection indicated that animals challenged with dengue-1 and -3 had antibody titers of >400 for dengue-1 and >150 for dengue-3 and were protected. Whether lower titers for dengue-1 and -3 are protective could not be determined by this study. For dengue-2, however, with the exception of animals RO4033 (1-day viremia) and RO4020 (0-day viremia), antibody titers of >100 appeared to be protective. The threshold antibody titer for protection against dengue-4 challenge appeared to be higher than for other dengue virus serotypes. Animals with titers in the 700 to 800 range (day-85 challenge) became viremic for 1 day, whereas those with titers in the 300 to 500 range (day-253 challenge) not only became viremic for 2 to 3 days but also exhibited higher virus titers (Table 3).
DISCUSSION
A major challenge to dengue vaccine development is the need to produce simultaneous protective immune responses to all four dengue virus serotypes. Candidate vaccines that use several different approaches (LAV, dengue-YFV chimeras, recombinant attenuated viruses, and DNA) are currently in various stages of development. All these approaches require four individual monovalent vaccines as components of the final tetravalent vaccine formulation. Mixed live vaccines such as those in the LAV approach have presented problems related to reactogenicity, serotype dominance, and competition (7, 20, 30, 34). Recombination is another concern when dealing with mixed formulations based on replicating live viruses. RNA viruses can recombine within and between species. A classic example is the emergence of Western equine encephalitis virus as a recombinant between a Sindbis-like virus and the Eastern equine encephalitis virus (12). Although homologous recombination among flaviviruses has been reported (8, 36), and Seligman and Gould (32) have argued that the possibility of untoward recombination events can never be dismissed, this issue has been controversial (6, 17, 25), and the possibility remains largely theoretical.
To address these issues and to reduce the complexity of dengue tetravalent vaccine, we have taken advantage of a complex adenovirus vector system. This is a nonreplicating adenovirus vector capable of harboring large foreign DNA, making it possible to create multivalent recombinant constructs. We have produced two bivalent constructs (CAdVax-Den12 and CAdVax-Den34), each expressing the prM and E proteins of two dengue virus serotypes. These bivalent vaccine constructs produced bivalent neutralizing antibody responses in vaccinated mice (16). We have now demonstrated that a tetravalent dengue vaccine (CAdVax-DenTV) formulated by mixing the two bivalent constructs is capable of eliciting high-titered neutralizing antibodies to all four dengue virus serotypes in rhesus macaques. Four weeks after they were vaccinated, all animals (23/23) produced neutralizing antibody responses to all four dengue virus serotypes. Antibody titers were fairly uniform across serotypes, except that titers for dengue-2 tended to be lower than those for the rest. These results demonstrate more uniform and consistent antibody responses than those in other published reports. For example, in a study of a tetravalent YFV-dengue virus chimera vaccine, only two of the four formulations tested produced neutralizing antibodies to all four dengue virus serotypes, and a wide range of titers was reported (11). Similarly, a recently published study of tetravalent LAV vaccine in rhesus monkeys reported only 70% seroconversion to dengue-4 (35), and although 100% seroconversion to all four dengue virus serotypes was reported in rhesus monkeys vaccinated with a single dose of tetravalent recombinant LAV vaccine, a second dose was necessary for protection against dengue-2 challenge (2).
There are inherent technical limitations in multivalent vaccine studies with related antigens to conclusively demonstrate that each individual antigen expressed is eliciting a specific antibody response. Although dengue virus antibodies display cross-reactivity with assays such as ELISA and hemagglutination inhibition, they display the greatest serotype specificity with the neutralization test (19). Published reports from primate studies and human clinical samples suggest that a neutralizing antibody response elicited by a single dengue virus antigen is usually monotypic, and if there are minor cross-reactions, they disappear over several months (14, 15, 31). If the tetravalent neutralizing antibody responses demonstrated in this study were the result of cross-neutralization, one would have expected to see waning titers for one or more serotypes at 6 months postvaccination. The fact that stable antibody titers to all four dengue virus serotypes persisted for 6 months perhaps indicates that the CAdVax-DenTV vaccine is eliciting antibodies specific to all four dengue virus serotypes.
We were not able to demonstrate T-cell responses in vaccinated animals. Developing T-cell-based assays for dengue viruses in macaques has been generally difficult. Synthetic dengue virus E peptides have not worked well in in vitro stimulations of primate peripheral blood mononuclear cells (unpublished). Using purified dengue-1 to stimulate T cells in vitro, we were able to demonstrate modest T-cell responses in animals only after they had been challenged (data not shown), indicating that the vaccine did not elicit measurable T-cell response but the challenge inoculation did. Similar results were recently observed with cynomolgus monkeys vaccinated with a dengue-1 vaccine based on the VEE replicon system (4). One reason may be that the infecting virus presents certain epitopes in vivo that are not presented by antigens produced by the vaccine. Another possibility is that the wild-type virus elicits stronger T-cell responses and the sensitivity of the assay is not able to detect weaker responses elicited by the vaccine. This appears to be the case, since a high-titered and long-lasting dengue-neutralizing antibody response is indicative of a strong cognate CD4 T-cell response. The T-cell responses reported here are consistent with those reported for cynomolgus monkeys infected with dengue-3 (24).
Vaccinated rhesus monkeys were protected from viremia when challenged with any of the four dengue virus serotypes. Protection against viremia from dengue-1 and dengue-3 challenges was complete, and a significant reduction in the number of days of viremia from dengue-2 and dengue-4 was observed. Data further demonstrated that the immune responses elicited by the vaccine provided protection against all four serotypes when a challenge was mounted 1 month after vaccination and persisted through a 6-month period and was still capable of providing significant protection against all four serotypes. Complete protection against dengue-1 and -3 challenges was observed with animals challenged at 6 months after vaccination. There was more breakthrough viremia with the dengue-4 challenge at 6 months, however. It is possible that a higher threshold of neutralizing antibody is required for complete protection against dengue-4. Breakthrough viremia from dengue-4 challenge was also observed with the tetravalent YFV-dengue virus chimera vaccine (11), and complete protection against only dengue-2 was reported in the case of tetravalent LAV vaccine (35). It is of interest to note that vaccinated animals challenged with dengue-4 on day 253 had higher virus titers than control animals, although the duration of viremia in vaccinated animals was truncated. The possibility of antibody-mediated enhancement of infection and disease (13) is of particular concern for dengue vaccine candidates. The rhesus monkey model does not allow for disease monitoring, and the small number of animals used makes it difficult to draw definitive conclusions from these observations. Further in vivo and in vitro investigations are required for a clear understanding of this phenomenon.
It is interesting that for dengue-2 and dengue-4, two serotypes for which some breakthrough viremia was observed, the antigens are expressed from the E4 region of adenovirus, compared to dengue-1 and dengue-3 antigens, which are expressed from insertions in the E1 region (Fig. 1). Although all dengue virus antigen genes are under the control of human cytomegalovirus (hCMV) promoters, it is not known if there is any positional effect within the adenovirus genome. These considerations may be important in fine-tuning immune responses for maximal benefit in future versions of this dengue tetravalent vaccine.
Vector immunity has been a general concern with vaccines based on recombinant viral vectors, including adenovirus vectors (5). It is argued that in individuals previously exposed to adenoviruses, adenovirus vector-based vaccines may not be effective and that even in individuals not previously exposed, vector immunity due to first vaccination with an adenovirus vector-based vaccine will make subsequent vaccinations ineffective. However, Shiver et al. (33) have reported that in the case of an adenovirus vector-based human immunodeficiency virus (HIV) vaccine in nonhuman primates, preexisting immunity could be overcome by the use of a higher dose of vaccine. More recently, the use of higher doses of an adenovirus vector-based HIV vaccine has been shown to overcome preexisting adenovirus immunity in a phase I human clinical trial (5). However, the result from a recent phase II study of an HIV vaccine based on the adenovirus vector has been discouraging (18). There have been suggestions that high-level preexisting adenovirus antibody may have increased the risk for HIV infection. While caution is warranted, it is difficult to make comparisons; the HIV vaccine is a T-cell epitope-based vaccine against a chronic infectious virus, whereas dengue vaccines are based on antibody epitopes against an acute viral infection. Our data show that after the first dose of vaccine, appreciable levels of antiadenovirus antibody were detected. However, when animals were given a booster dose at day 57 (8 weeks) in the presence of these antiadenovirus antibodies, we were able to boost both antiadenovirus and anti-dengue virus antibody titers, indicating that the level of preexisting antiadenovirus antibody was not a major issue. By day 169 (week 24), antiadenovirus antibody levels had nearly declined to levels seen at 8 weeks. Thus, it is possible to administer a second booster dose at this time, if needed, or to vaccinate with a different vaccine built on the same platform. We believe that a proof-of-concept clinical trial for this tetravalent dengue vaccine and further development of improved vaccine constructs using this vector system are warranted.
Acknowledgments
We thank Meng Shi of the Walter Reed Army Institute of Research for help with statistical analysis.
Authors K.R., D.W., C.H., and K.P. are employees of the United States government or military service members. This work was prepared as part of their official duties.
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the U. S. government.
Footnotes
Published ahead of print on 14 May 2008.
REFERENCES
- 1.Blaney, J. E., Jr., C. T. Hanson, K. A. Hanley, B. R. Murphy, and S. S. Whitehead. 2004. Vaccine candidates derived from a novel infectious cDNA clone of an American genotype dengue virus type 2. BMC Infect. Dis. 439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaney, J. E., Jr., J. M. Matro, B. R. Murphy, and S. S. Whitehead. 2005. Recombinant, live-attenuated tetravalent dengue virus vaccine formulations induce a balanced, broad, and protective neutralizing antibody response against each of the four serotypes in rhesus monkeys. J. Virol. 795516-5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chambers, T. J., C. S. Hahn, R. Galler, and C. M. Rice. 1990. Flavivirus genome organization, expression, and replication. Annu. Rev. Microbiol. 44649-688. [DOI] [PubMed] [Google Scholar]
- 4.Chen, L., D. Ewing, H. Subramanian, K. Block, J. Rayner, K. D. Alterson, M. Sedegah, C. Hayes, K. Porter, and K. Raviprakash. 2007. A heterologous DNA prime-Venezuelan equine encephalitis virus replicon particle boost dengue vaccine regimen affords complete protection from virus challenge in cynomolgus macaques. J. Virol. 8111634-11639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen, P. 2006. Immunity's yin and yang. A successful vaccine must first avoid being eliminated by pre-existing immunity before it can promote a protective immune response. IAVI Rep. 101-5. [PubMed] [Google Scholar]
- 6.de Silva, A., and W. Messer. 2004. Arguments for live flavivirus vaccines. Lancet 364500. [DOI] [PubMed] [Google Scholar]
- 7.Edelman, R., S. S. Wasserman, S. A. Bodison, R. J. Putnak, K. H. Eckels, D. Tang, N. Kanesa-Thasan, D. W. Vaughn, B. L. Innis, and W. Sun. 2003. Phase I trial of 16 formulations of a tetravalent live-attenuated dengue vaccine. Am. J. Trop. Med. Hyg. 6948-60. [DOI] [PubMed] [Google Scholar]
- 8.Gould, E. A., S. R. Moss, and S. L. Turner. 2004. Evolution and dispersal of encephalitic flaviviruses. Arch. Virol. Suppl. 1865-84. [DOI] [PubMed] [Google Scholar]
- 9.Gubler, D. J. 1998. Dengue and dengue hemorrhagic fever. Clin. Microbiol. Rev. 11480-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guirakhoo, F., S. Kitchener, D. Morrison, R. Forrat, K. McCarthy, R. Nichols, S. Yoksan, X. Duan, T. H. Ermak, N. Kanesa-Thasan, P. Bedford, J. Lang, M. J. Quentin-Millet, and T. P. Monath. 2006. Live attenuated chimeric yellow fever dengue type 2 (ChimeriVax-DEN2) vaccine: phase I clinical trial for safety and immunogenicity: effect of yellow fever pre-immunity in induction of cross neutralizing antibody responses to all 4 dengue serotypes. Hum. Vaccin. 260-67. [DOI] [PubMed] [Google Scholar]
- 11.Guirakhoo, F., K. Pugachev, Z. Zhang, G. Myers, I. Levenbook, K. Draper, J. Lang, S. Ocran, F. Mitchell, M. Parsons, N. Brown, S. Brandler, C. Fournier, B. Barrere, F. Rizvi, A. Travassos, R. Nichols, D. Trent, and T. Monath. 2004. Safety and efficacy of chimeric yellow fever-dengue virus tetravalent vaccine formulations in nonhuman primates. J. Virol. 784761-4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hahn, C. S., S. Lustig, E. G. Strauss, and J. H. Strauss. 1988. Western equine encephalitis virus is a recombinant virus. Proc. Natl. Acad. Sci. USA 855997-6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halstead, S. B. 1988. Pathogenesis of dengue: challenges to molecular biology. Science 239476-481. [DOI] [PubMed] [Google Scholar]
- 14.Halstead, S. B., J. Casals, H. Shotwell, and N. Palumbo. 1973. Studies on the immunization of monkeys against dengue. I. Protection derived from single and sequential virus infections. Am. J. Trop. Med. Hyg. 22365-374. [DOI] [PubMed] [Google Scholar]
- 15.Halstead, S. B., and N. E. Palumbo. 1973. Studies on the immunization of monkeys against dengue. II. Protection following inoculation of combinations of viruses. Am. J. Trop. Med. Hyg. 22375-381. [DOI] [PubMed] [Google Scholar]
- 16.Holman, D. H., D. Wang, K. Raviprakash, N. U. Raja, M. Luo, J. Zhang, K. R. Porter, and J. Y. Dong. 2007. Two complex, adenovirus-based vaccines that together induce immune responses to all four dengue virus serotypes. Clin. Vaccine Immunol. 14182-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hombach, J., I. Kurane, and D. Wood. 2004. Arguments for live flavivirus vaccines. Lancet 364498-499. [DOI] [PubMed] [Google Scholar]
- 18.IAVI News. 5 February 2008, posting date. Further analysis of STEP AIDS vaccine trial reaffirms earlier trend. http://www.iavi.org/viewfile.cfm?fid=47758.
- 19.Innis, B. L. 1997. Antibody responses to dengue virus infection, p. 221-243. In D. J. Gubler and G. Kuno (ed.), Dengue and dengue hemorrhagic fever. CAB International, New York, NY.
- 20.Kitchener, S., M. Nissen, P. Nasveld, R. Forrat, S. Yoksan, J. Lang, and J. F. Saluzzo. 2006. Immunogenicity and safety of two live-attenuated tetravalent dengue vaccine formulations in healthy Australian adults. Vaccine 241238-1241. [DOI] [PubMed] [Google Scholar]
- 21.Kliks, S. C., S. Nimmanitya, A. Nisalak, and D. S. Burke. 1988. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am. J. Trop. Med. Hyg. 38411-419. [DOI] [PubMed] [Google Scholar]
- 22.Kliks, S. C., A. Nisalak, W. E. Brandt, L. Wahl, and D. S. Burke. 1989. Antibody-dependent enhancement of dengue virus growth in human monocytes as a risk factor for dengue hemorrhagic fever. Am. J. Trop. Med. Hyg. 40444-451. [DOI] [PubMed] [Google Scholar]
- 23.Kochel, T. J., K. Raviprakash, C. G. Hayes, D. M. Watts, K. L. Russell, A. S. Gozalo, I. A. Phillips, D. F. Ewing, G. S. Murphy, and K. R. Porter. 2000. A dengue virus serotype-1 DNA vaccine induces virus neutralizing antibodies and provides protection from viral challenge in Aotus monkeys. Vaccine 183166-3173. [DOI] [PubMed] [Google Scholar]
- 24.Koraka, P., S. Benton, G. van Amerongen, K. J. Stittelaar, and A. D. Osterhaus. 2007. Efficacy of a live attenuated tetravalent candidate dengue vaccine in naive and previously infected cynomolgus macaques. Vaccine 255409-5416. [DOI] [PubMed] [Google Scholar]
- 25.Murphy, B. R., J. E. Blaney, Jr., and S. S. Whitehead. 2004. Arguments for live flavivirus vaccines. Lancet 364499-500. [DOI] [PubMed] [Google Scholar]
- 25a.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC.
- 26.Putnak, J. R., B. A. Coller, G. Voss, D. W. Vaughn, D. Clements, I. Peters, G. Bignami, H. S. Houng, R. C. Chen, D. A. Barvir, J. Seriwatana, S. Cayphas, N. Garcon, D. Gheysen, N. Kanesa-Thasan, M. McDonell, T. Humphreys, K. H. Eckels, J. P. Prieels, and B. L. Innis. 2005. An evaluation of dengue type-2 inactivated, recombinant subunit, and live-attenuated vaccine candidates in the rhesus macaque model. Vaccine 234442-4452. [DOI] [PubMed] [Google Scholar]
- 27.Putnak, R., D. A. Barvir, J. M. Burrous, D. R. Dubois, V. M. D'Andrea, C. H. Hoke, J. C. Sadoff, and K. H. Eckels. 1996. Development of a purified, inactivated, dengue-2 virus vaccine prototype in Vero cells: immunogenicity and protection in mice and rhesus monkeys. J. Infect. Dis. 1741176-1184. [DOI] [PubMed] [Google Scholar]
- 28.Raviprakash, K., K. R. Porter, T. J. Kochel, D. Ewing, M. Simmons, I. Phillips, G. S. Murphy, W. R. Weiss, and C. G. Hayes. 2000. Dengue virus type 1 DNA vaccine induces protective immune responses in rhesus macaques. J. Gen. Virol. 811659-1667. [DOI] [PubMed] [Google Scholar]
- 29.Rigau-Perez, J. G., G. G. Clark, D. J. Gubler, P. Reiter, E. J. Sanders, and A. V. Vorndam. 1998. Dengue and dengue haemorrhagic fever. Lancet 352971-977. [DOI] [PubMed] [Google Scholar]
- 30.Sabchareon, A., J. Lang, P. Chanthavanich, S. Yoksan, R. Forrat, P. Attanath, C. Sirivichayakul, K. Pengsaa, C. Pojjaroen-Anant, W. Chokejindachai, A. Jagsudee, J. F. Saluzzo, and N. Bhamarapravati. 2002. Safety and immunogenicity of tetravalent live-attenuated dengue vaccines in Thai adult volunteers: role of serotype concentration, ratio, and multiple doses. Am. J. Trop. Med. Hyg. 66264-272. [DOI] [PubMed] [Google Scholar]
- 31.Scott, R. M., S. Nimmannitya, W. H. Bancroft, and P. Mansuwan. 1976. Shock syndrome in primary dengue infections. Am. J. Trop. Med. Hyg. 25866-874. [DOI] [PubMed] [Google Scholar]
- 32.Seligman, S. J., and E. A. Gould. 2004. Live flavivirus vaccines: reasons for caution. Lancet 3632073-2075. [DOI] [PubMed] [Google Scholar]
- 33.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415331-335. [DOI] [PubMed] [Google Scholar]
- 34.Sun, W., R. Edelman, N. Kanesa-Thasan, K. H. Eckels, J. R. Putnak, A. D. King, H. S. Houng, D. Tang, J. M. Scherer, C. H. Hoke, Jr., and B. L. Innis. 2003. Vaccination of human volunteers with monovalent and tetravalent live-attenuated dengue vaccine candidates. Am. J. Trop. Med. Hyg. 6924-31. [DOI] [PubMed] [Google Scholar]
- 35.Sun, W., A. Nisalak, M. Gettayacamin, K. H. Eckels, J. R. Putnak, D. W. Vaughn, B. L. Innis, S. J. Thomas, and T. P. Endy. 2006. Protection of Rhesus monkeys against dengue virus challenge after tetravalent live attenuated dengue virus vaccination. J. Infect. Dis. 1931658-1665. [DOI] [PubMed] [Google Scholar]
- 36.Twiddy, S. S., and E. C. Holmes. 2003. The extent of homologous recombination in members of the genus Flavivirus. J. Gen. Virol. 84429-440. [DOI] [PubMed] [Google Scholar]
- 37.Wang, D., M. Hevey, L. Y. Juompan, C. M. Trubey, N. U. Raja, S. B. Deitz, J. Woraratanadharm, M. Luo, H. Yu, B. M. Swain, K. M. Moore, and J. Y. Dong. 2006. Complex adenovirus-vectored vaccine protects guinea pigs from three strains of Marburg virus challenges. Virology 353324-332. [DOI] [PubMed] [Google Scholar]
- 38.Wang, D., N. U. Raja, C. M. Trubey, L. Y. Juompan, M. Luo, J. Woraratanadharm, S. B. Deitz, H. Yu, B. M. Swain, K. M. Moore, W. D. Pratt, M. K. Hart, and J. Y. Dong. 2006. Development of a cAdVax-based bivalent Ebola virus vaccine that induces immune responses against both the Sudan and Zaire species of Ebola virus. J. Virol. 802738-2746. [DOI] [PMC free article] [PubMed] [Google Scholar]