Abstract
TRIM5α is a potent barrier to cross-species retroviral transmission, and TRIM5αs from different species have divergent antiretroviral specificities. Multiple TRIM5 alleles circulate within rhesus macaque populations. Here we show that they too have different antiretroviral specificities, highlighting how TRIM5 genotypes contribute to protection in an individual or a population.
TRIM5α is an important mediator of antiretroviral innate immunity in mammals and represents a significant barrier to zoonotic transmission. It blocks retroviral infection in a species-specific manner; for example, human immunodeficiency virus type 1 (HIV-1) is restricted by Old World monkey TRIM5α but is not significantly restricted by human TRIM5α (12, 26, 31). TRIM5α consists of RING, B-box 2, and coiled-coil domains (RBCC), comprising a tripartite motif, as well as a C-terminal B30.2 domain, which determines antiviral specificity, and appears to interact directly with the incoming viral capsid (27). Recently, multiple TRIM5 alleles have been identified in an Old World monkey, the rhesus macaque (Macaca mulatta) (17). These alleles have surprisingly divergent B30.2 domains and are maintained at high frequencies in macaque populations. Because variation in the sequence of the B30.2 domain can have such profound effects on the antiretroviral specificity of TRIM5α, these divergent macaque B30.2 domains have likely been selected to interact with different viral capsids. Remarkably, one of the TRIM5 alleles, Mamu-7, encodes a TRIM5-cyclophilin A (CypA) fusion protein with a different spectrum of antiretroviral activity to TRIM5α (4, 13, 18, 29, 30). In Mamu-7/TRIMCyp, exon 6 is joined to a downstream CypA cDNA sequence, leaving a vestigial B30.2 domain in the genome (Fig. 1A). Here we demonstrate the differential restriction of HIV-2 by rhesus TRIM5 alleles and map the determinant of restriction to a polymorphism in the B30.2 V1 region. Furthermore, we show that the different TRIM5 alleles have dominant negative properties against each other when exogenously expressed.
FIG. 1.
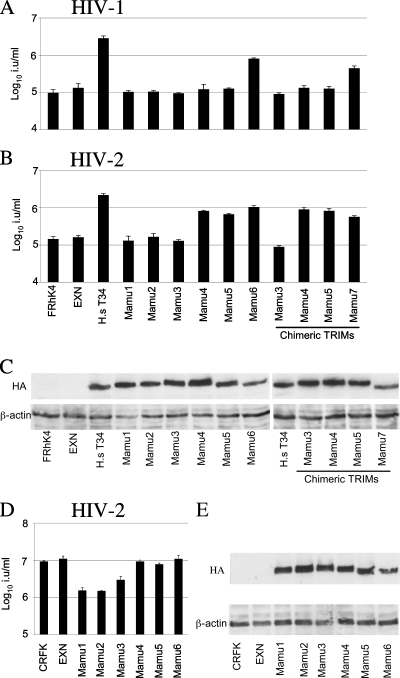
Sequence and antiretroviral specificities of the divergent B30.2 domains. (A) The exon structure of Macaca mulatta TRIM5 (solid line) and Mamu-7/TRIMCyp (dashed line). Numbers denote the exon number. (B) Predicted amino acid sequences of the polymorphic B30.2 domains. Nonsynonymous substitutions are uppercase and bold. Synonymous substitutions are lowercase and bold. The V1 region is also annotated. The titers of GFP-encoding VSV-G pseudotypes of HIV-1 (C), FIV (D), EIAV (E), HIV-2 (F), MLV N (G), SIVmac (H), and MLV B (I) were determined for feline CRFK cells expressing the Mamu-1 chimeras as shown, unmodified CRFK cells, or cells transduced with an empty vector (EXN). Titers are given in infectious units per ml (i.u/ml). Errors are standard deviations derived from triplicate infections and are representative of at least two experiments using independent viral stocks. HA-tagged TRIM5 expression levels were monitored by Western blotting (J) using anti-HA and anti-actin as a loading control.
To further characterize the degree of polymorphism in rhesus macaques, we sequenced TRIM5 exon 8 from DNA purified from 31 Indian and 38 Chinese Macaca mulatta monkeys from the Biomedical Primate Research Centre breeding colony in Rijswijk, The Netherlands (30). We identified the TRIM5 alleles Mamu-1, -3, -4, -5, and -7 in this cohort (17, 30). Predicted B30.2 domain amino acid sequences are shown in Fig. 1B. We also identified a mutation, G402D, in one animal. We are unsure whether this represents a mutation or a genuine polymorphism but have included it in our analyses. In order to explore the antiretroviral specificities of the various B30.2 domains, we generated a murine leukemia virus (MLV)-based vector (32) that expresses TRIM5α Mamu-1, driven by an internal cytomegalovirus promoter, with a silent SalI site at the V-301 and D-302 codons, facilitating the insertion of the entire exon 8 sequences from Mamu-1, -3, -4, -5, or -7 at this site. We then transduced CRFK cells with vectors encoding the different B30.2 domains appended to the hemagglutinin (HA)-tagged RBCC of Mamu-1, as described previously (32). We determined the infectious titers on these cells of vesicular stomatitis virus G glycoprotein (VSV-G) pseudotyped green fluorescent protein (GFP)-encoding retroviral vectors derived from HIV-1 (6, 33), HIV-2 (7), feline immunodeficiency virus (FIV) (21), simian immunodeficiency virus (SIVmac) (16), MLV (3), and equine infectious anemia virus (EIAV) (9) as described previously (2, 32). We used G418-selected pools of transduced cells and unmodified CRFK cells as a control.
We found that Mamu-1, -3, -4, and -5 B30.2 domains restricted HIV-1 infection (Fig. 1C), confirming previous results (17). In addition, all these chimeras restricted infection by FIV and EIAV (Fig. 1D and E), as has been described for Mamu-1 (8, 22). Interestingly, Mamu-1 and Mamu-3 chimeras restricted HIV-2 infection, whereas Mamu-4 and Mamu-5 chimeras did not (Fig. 1F). This indicates intraspecific variation in the target specificity of functional TRIM5 alleles. Importantly, the chimera including the vestigial Mamu-7 B30.2 domain did not restrict any of the viruses tested (Fig. 1C to I). This is most likely due to the frameshift and associated truncation of the B30.2 domain caused by the Δ1574-1575 polymorphism. Indeed, Mamu-1 engineered to contain this same polymorphism, Mamu-1 439Stop, is no longer able to restrict any of the viruses tested (Fig. 1C to I). We do not attribute these observations to TRIM5α expression levels, because all the proteins were expressed at similar levels, as assessed by Western blotting detecting the N-terminal HA tag (Fig. 1J). SIVmac, N-tropic MLV, and B-tropic MLV were not restricted by any of the rhesus alleles (Fig. 1G to I).
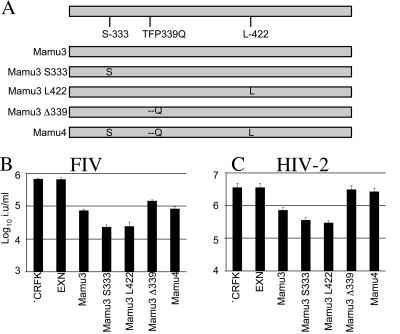
Only three polymorphic sites exist between the Mamu-3 B30.2 domain, which mediates the restriction of HIV-2, and the Mamu-4 B30.2 domain that does not (Fig. 1). To identify which polymorphism is responsible for the different antiviral specificities, we generated expression vectors encoding Mamu-3 B30.2 domains, with each polymorphism in isolation (Fig. 2A) appended to Mamu-1 RBCC. The mutant B30.2 chimeras were expressed in CRFK cells as described above. All mediated the restriction of FIV (Fig. 2B), indicating that they form functional restriction factors and were expressed appropriately. Interestingly, a single polymorphism, TFP339Q, is sufficient to ablate the ability of Mamu-3 to restrict HIV-2 (Fig. 2C).
FIG. 2.
The TFP339Q polymorphism ablates the restriction of HIV-2. (A) A schematic showing the nonsynonymous polymorphisms of the Mamu-3 and Mamu-4 B30.2 domains in addition to the Mamu-3 S333, Mamu-3 Δ339, and Mamu-3 L422 mutants is shown. FIV (B) and HIV-2 (C) were titrated onto CRFK cells expressing these molecules as described in the legend to Fig. 1. Titers are given in infectious units per ml (i.u/ml). Errors are standard deviations derived from triplicate infections and are representative of at least two experiments using independent viral stocks.
The high degree of heterozygosity at the TRIM5 locus (17) led us to consider the impact of heterotrimerization on TRIM5α-mediated restriction (10). Short TRIM5 molecules, mutants, or splice variants that cannot restrict are known to have dominant negative properties against TRIM5α, and their overexpression rescues restricted viral infectivity (20, 25, 26). To investigate the possibility that the different alleles might have dominant negative effects against each other, we expressed the different TRIM5α chimeras used in Fig. 1 in the rhesus macaque cell line FRhK4, which is homozygous for Mamu-1 (30). We used an input equivalent to a multiplicity of infection of 10 on CRFK cells. For a positive control, we expressed human TRIM34, which has a strong dominant negative effect on rhesus TRIM5α-mediated restriction (30). As expected, the appended Mamu-7 B30.2 domain, which is unable to restrict HIV-1 or HIV-2 infection (Fig. 1), interfered with restriction mediated by endogenous TRIM5α Mamu-1 (Fig. 3A and B). In addition, Mamu-4 and Mamu-5 B30.2 domain chimeras, which do not mediate the restriction of HIV-2 (Fig. 1F), interfered with the restriction of HIV-2 by endogenous Mamu-1 (Fig. 3B). The restriction of HIV-1 remained unaffected, presumably because it was restricted by both endogenously and exogenously expressed TRIM5αs (Fig. 3A). Since multiple nonsynonymous single nucleotide polymorphisms exist between the rhesus TRIM5 alleles (17), we also examined whether the dominant negative effects were dependent on the Mamu-1 RBCC domains. To do this, we tested whether the expression of full-length Mamu-1 to Mamu-6 interfered with endogenous Mamu-1-mediated restriction in FRhK4 cells. Concordant with the data shown in Fig. 1, TRIM5 Mamu-1, Mamu-2, or Mamu-3 but not Mamu-4 or Mamu-5 was able to restrict HIV-2 when expressed in CRFK cells (Fig. 3D). HIV-1 was restricted by Mamu-1 to Mamu-5 as described previously (17; data not shown). Furthermore, full-length Mamu-4 and Mamu-5 interfered with HIV-2 restriction (Fig. 3B) but not HIV-1 restriction (Fig. 3A) by Mamu-1 in FRhK4 cells. In addition, expression of the apparently inactive Mamu-6 protein (17) rescued the restricted infection of both HIV-1 and HIV-2 under these conditions (Fig. 3A and B).
FIG. 3.
Dominant negative effects of Mamu-4, Mamu-5, and Mamu-7 B30.2 domains. FRhK4 cells were transduced with the TRIM5α expression vectors encoding full-length TRIM5α or Mamu-1 chimeras as shown. A human TRIM34 expression vector was used as a positive control. Forty-eight hours later, the titers of GFP-encoding VSV-G pseudotypes of HIV-1 (A) and HIV-2 (B) were determined for these cells. Titers are given in infectious units per ml (i.u/ml). Errors are standard deviations derived from triplicate infections and are representative of at least two experiments using independent viral stocks. (C) Protein expression in FRhK4 cells was monitored in parallel samples 48 h posttransduction using anti-HA and anti-actin as a loading control. Two gels were required, and human TRIM34 was loaded twice as an internal control. (D) The titers of GFP-encoding VSV-G pseudotypes of HIV-2 were determined for CRFK cells expressing full-length Mamu-1 to Mamu-6. (E) HA-tagged TRIM5 expression was monitored in CRFK cells by Western blotting using anti-HA and anti-actin as a loading control. H.s T34, Homo sapiens TRIM34.
The dominant negative activity of TRIM5 alleles that are functional but do not have the appropriate restriction specificity suggests that dominant negative activity results from incorporation into a heterotrimer and titration of the specific B30.2 domain. In other words, the incorporation of a TRIM5 molecule, unable to recognize a viral capsid, into the functional trimer reduces the avidity of the TRIM5α-capsid interaction. It seems paradoxical that the different TRIM5 alleles antagonize each other, as this would reduce, rather than increase, the protection in a heterozygote. We imagine that overexpression may exaggerate the dominant negative effects and that in vivo, TRIM5 alleles might be codominant in a heterozygote. Importantly, this is true for the mouse antiviral protein Fv1, which is codominant in mice and in heterozygous cell lines but dominant negative when overexpressed in a homozygous cell line in experiments similar to those shown in Fig. 3 (3). However, the fact that TRIM5 expression is strongly induced by type 1 interferon (1, 23) suggests that high levels of TRIM5 might occur in vivo during infection. In this case, high levels of both alleles may ensure minimal dominant negative activity.
Long-term balancing selection can result in the maintenance of multiple alleles at high frequencies within a population. Such selection has occurred at the TRIM5 locus (17), and here we show that different rhesus macaque TRIM5 alleles have differing antiretroviral specificities. Importantly, they have different specificities against a virus (HIV-2) which comes from a lineage that is diverse and common in Old World monkeys, namely SIV from sooty mangabeys (14). This is concordant with the notion that these, or similar viruses, provided the selection that drove polymorphism. The relative youth of lentiviruses has been thought to preclude them from providing significant selection pressure on TRIM5 evolution. However, the recent identification of an endogenous lentiviral sequence in rabbits (11) increases the likely age of lentiviruses, and we expect that this will extend further as more endogenous lentiviruses are discovered. The TFP339Q polymorphism present in Mamu-4, -5, and -7 B30.2 domains alters the antiretroviral specificity of TRIM5α, presumably by preventing capsid-B30.2 domain interactions. TFP339Q is in the V1 region of the B30.2 domain that has been identified as evolving under positive selection (24).
Unsurprisingly, the truncated B30.2 domain of Mamu-7 has no apparent antiretroviral activity when fused to the Mamu-1 RBCC (Fig. 1). Whether Mamu-7 TRIM5 exon 8 is expressed in vivo remains unclear. Similar TRIMCyp-encoding alleles in Macaca nemestrina bear the same splicing mutant at the intron 6-exon 7 boundary that leads to exon skipping to the CypA cDNA (5). In M. nemestrina, at least, this mutation appears to prevent the expression of a functional TRIM5 from the TRIMCyp-encoding alleles. Instead, they encode a truncated TRIM5 (TRIM5θ) or a TRIM5 protein that lacks exon 7 (TRIM5η), and neither of these TRIM5s restricts HIV-1 (5). Interestingly, the exon 8 from these alleles does restrict HIV-1 when fused to human TRIM5 exons 2 to 7, indicating that it has the ability to interact with HIV-1 capsids if in the appropriate context (19). The fact that the mutation that is required to appropriately express TRIMCyp obviates the expression of full-length TRIM5α suggests that a single TRIM5 allele cannot encode antiviral TRIM5α and TRIMCyp, although this probably warrants further investigation. The apparently inactive Mamu-6 TRIM5α is dominant negative against Mamu-1 (Fig. 3). This suggests that Mamu-6, when expressed appropriately, is recruited into a TRIM5 trimer and that it cannot restrict due to a change in specificity resulting from polymorphisms in exon 6 and/or exon 8 (17).
The high degree of polymorphism at the TRIM5 locus is a vivid example of the antagonistic host-virus evolutionary relationship described by the Red Queen hypothesis (15, 28) and suggests that the protection provided by TRIM5 is powerful. Enhancing these natural innate antiviral pathways may eventually prove that they are more effective than traditional vaccine-based approaches. The key will be the translation of our molecular-level understanding into useful therapeutic intervention.
Acknowledgments
We thank Paul Bieniasz, Paul Clapham, Francois-Loic Cosset, Welkin Johnson, Andrew Lever, Kyriacos Mitrophanous, Claire Pardieu, Eric Poeschla, Adrian Thrasher, and Didier Trono for reagents and Imogen Lai for technical assistance.
This work was funded by Wellcome Trust fellowship 076608 and grant 073167 to G.J.T.
Footnotes
Published ahead of print on 14 May 2008.
REFERENCES
- 1.Asaoka, K., K. Ikeda, T. Hishinuma, K. Horie-Inoue, S. Takeda, and S. Inoue. 2005. A retrovirus restriction factor TRIM5alpha is transcriptionally regulated by interferons. Biochem. Biophys. Res. Commun. 3381950-1956. [DOI] [PubMed] [Google Scholar]
- 2.Besnier, C., Y. Takeuchi, and G. Towers. 2002. Restriction of lentivirus in monkeys. Proc. Natl. Acad. Sci. USA 9911920-11925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bock, M., K. N. Bishop, G. Towers, and J. P. Stoye. 2000. Use of a transient assay for studying the genetic determinants of Fv1 restriction. J. Virol. 747422-7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brennan, G., Y. Kozyrev, and S. L. Hu. 2008. TRIMCyp expression in Old World primates Macaca nemestrina and Macaca fascicularis. Proc. Natl. Acad. Sci. USA 1053569-3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brennan, G., Y. Kozyrev, T. Kodama, and S.-L. Hu. 2007. Novel TRIM5 isoforms expressed by Macaca nemestrina. J. Virol. 8112210-12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demaison, C., K. Parsley, G. Brouns, M. Scherr, K. Battmer, C. Kinnon, M. Grez, and A. J. Thrasher. 2002. High-level transduction and gene expression in hematopoietic repopulating cells using a human immunodeficiency [correction of imunodeficiency] virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter. Hum. Gene Ther. 13803-813. [DOI] [PubMed] [Google Scholar]
- 7.Griffin, S. D. C., J. F. Allen, and A. M. L. Lever. 2001. The major human immunodeficiency virus type 2 (HIV-2) packaging signal is present on all HIV-2 RNA species: cotranslational RNA encapsidation and limitation of Gag protein confer specificity. J. Virol. 7512058-12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hatziioannou, T., D. Perez-Caballero, A. Yang, S. Cowan, and P. D. Bieniasz. 2004. Retrovirus resistance factors Ref1 and Lv1 are species-specific variants of TRIM5alpha. Proc. Natl. Acad. Sci. USA 10110774-10779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ikeda, Y., M. K. Collins, P. A. Radcliffe, K. A. Mitrophanous, and Y. Takeuchi. 2002. Gene transduction efficiency in cells of different species by HIV and EIAV vectors. Gene Ther. 9932-938. [DOI] [PubMed] [Google Scholar]
- 10.Javanbakht, H., W. Yuan, D. F. Yeung, B. Song, F. Diaz-Griffero, Y. Li, X. Li, M. Stremlau, and J. Sodroski. 2006. Characterization of TRIM5alpha trimerization and its contribution to human immunodeficiency virus capsid binding. Virology 353234-246. [DOI] [PubMed] [Google Scholar]
- 11.Katzourakis, A., M. Tristem, O. G. Pybus, and R. J. Gifford. 2007. Discovery and analysis of the first endogenous lentivirus. Proc. Natl. Acad. Sci. USA 1046261-6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li, Y., X. Li, M. Stremlau, M. Lee, and J. Sodroski. 2006. Removal of arginine 332 allows human TRIM5α to bind human immunodeficiency virus capsids and to restrict infection. J. Virol. 806738-6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liao, C. H., Y. Q. Kuang, H. L. Liu, Y. T. Zheng, and B. Su. 2007. A novel fusion gene, TRIM5-cyclophilin A in the pig-tailed macaque determines its susceptibility to HIV-1 infection. AIDS 21(Suppl. 8)S19-S26. [DOI] [PubMed] [Google Scholar]
- 14.Ling, B., M. L. Santiago, S. Meleth, B. Gormus, H. M. McClure, C. Apetrei, B. H. Hahn, and P. A. Marx. 2003. Noninvasive detection of new simian immunodeficiency virus lineages in captive sooty mangabeys: ability to amplify virion RNA from fecal samples correlates with viral load in plasma. J. Virol. 772214-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lively, C. M., and M. F. Dybdahl. 2000. Parasite adaptation to locally common host genotypes. Nature 405679-681. [DOI] [PubMed] [Google Scholar]
- 16.Negre, D., P. E. Mangeot, G. Duisit, S. Blanchard, P. O. Vidalain, P. Leissner, A. J. Winter, C. Rabourdin-Combe, M. Mehtali, P. Moullier, J. L. Darlix, and F. L. Cosset. 2000. Characterization of novel safe lentiviral vectors derived from simian immunodeficiency virus (SIVmac251) that efficiently transduce mature human dendritic cells. Gene Ther. 71613-1623. [DOI] [PubMed] [Google Scholar]
- 17.Newman, R. M., L. Hall, M. Connole, G. L. Chen, S. Sato, E. Yuste, W. Diehl, E. Hunter, A. Kaur, G. M. Miller, and W. E. Johnson. 2006. Balancing selection and the evolution of functional polymorphism in Old World monkey TRIM5alpha. Proc. Natl. Acad. Sci. USA 10319134-19139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newman, R. M., L. Hall, A. Kirmaier, L. A. Pozzi, E. Pery, M. Farzan, S. P. O'Neil, and W. Johnson. 29 February 2008. Evolution of a TRIM5-CypA splice isoform in old world monkeys. PLoS Pathog. 4e1000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohkura, S., M. W. Yap, T. Sheldon, and J. P. Stoye. 2006. All three variable regions of the TRIM5α B30.2 domain can contribute to the specificity of retrovirus restriction. J. Virol. 808554-8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Passerini, L. D., Z. Keckesova, and G. J. Towers. 2006. Retroviral restriction factors Fv1 and TRIM5α act independently and can compete for incoming virus before reverse transcription. J. Virol. 802100-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poeschla, E. M., F. Wong-Staal, and D. J. Looney. 1998. Efficient transduction of nondividing human cells by feline immunodeficiency virus lentiviral vectors. Nat. Med. 4354-357. [DOI] [PubMed] [Google Scholar]
- 22.Saenz, D. T., W. Teo, J. C. Olsen, and E. M. Poeschla. 2005. Restriction of feline immunodeficiency virus by Ref1, Lv1, and primate TRIM5α proteins. J. Virol. 7915175-15188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakuma, R., A. A. Mael, and Y. Ikeda. 2007. Alpha interferon enhances TRIM5α-mediated antiviral activities in human and rhesus monkey cells. J. Virol. 8110201-10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sawyer, S. L., L. I. Wu, M. Emerman, and H. S. Malik. 2005. Positive selection of primate TRIM5alpha identifies a critical species-specific retroviral restriction domain. Proc. Natl. Acad. Sci. USA 1022832-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaller, T., S. Hué, and G. J. Towers. 2007. An active TRIM5 protein in rabbits indicates a common antiviral ancestor for mammalian TRIM5 proteins. J. Virol. 8111713-11721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stremlau, M., C. M. Owens, M. J. Perron, M. Kiessling, P. Autissier, and J. Sodroski. 2004. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature 427848-853. [DOI] [PubMed] [Google Scholar]
- 27.Stremlau, M., M. Perron, M. Lee, Y. Li, B. Song, H. Javanbakht, F. Diaz-Griffero, D. J. Anderson, W. I. Sundquist, and J. Sodroski. 2006. Specific recognition and accelerated uncoating of retroviral capsids by the TRIM5alpha restriction factor. Proc. Natl. Acad. Sci. USA 1035514-5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Valen, L. 1973. A new evolutionary law. Evol. Theory 11-30. [Google Scholar]
- 29.Virgen, C. A., Z. Kratovac, P. D. Bieniasz, and T. Hatziioannou. 2008. Independent genesis of chimeric TRIM5-cyclophilin proteins in two primate species. Proc. Natl. Acad. Sci. USA 1053563-3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson, S. J., B. L. J. Webb, L. M. J. Ylinen, E. Verschoor, J. L. Heeney, and G. J. Towers. Independent evolution of an antiviral TRIMCyp in rhesus macaques. Proc. Natl. Acad. Sci. USA 1053557-3562. [DOI] [PMC free article] [PubMed]
- 31.Yap, M. W., S. Nisole, C. Lynch, and J. P. Stoye. 2004. Trim5alpha protein restricts both HIV-1 and murine leukemia virus. Proc. Natl. Acad. Sci. USA 10110786-10791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang, F., T. Hatziioannou, D. Perez-Caballero, D. Derse, and P. D. Bieniasz. 2006. Antiretroviral potential of human tripartite motif-5 and related proteins. Virology 353396-409. [DOI] [PubMed] [Google Scholar]
- 33.Zufferey, R., D. Nagy, R. J. Mandel, L. Naldini, and D. Trono. 1997. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat. Biotechnol. 15871-875. [DOI] [PubMed] [Google Scholar]