Abstract
Over 350 million people are chronically infected with hepatitis B virus (HBV), and a significant number of chronically infected individuals develop primary liver cancer. HBV encodes seven viral proteins, including the nonstructural X (HBx) protein. The results of studies with immortalized or transformed cells and with HBx-transgenic mice demonstrated that HBx can interact with mitochondria. However, no studies with normal hepatocytes have characterized the precise mitochondrial localization of HBx or the effect of HBx on mitochondrial physiology. We have used cultured primary rat hepatocytes as a model system to characterize the mitochondrial localization of HBx and the effect of HBx expression on mitochondrial physiology. We now show that a fraction of HBx colocalizes with density-gradient-purified mitochondria and associates with the outer mitochondrial membrane. We also demonstrate that HBx regulates mitochondrial membrane potential in hepatocytes and that this function of HBx varies depending on the status of NF-κB activity. In primary rat hepatocytes, HBx activation of NF-κB prevented mitochondrial membrane depolarization; however, when NF-κB activity was inhibited, HBx induced membrane depolarization through modulation of the mitochondrial permeability transition pore. Collectively, these results define potential pathways through which HBx may act in order to modulate mitochondrial physiology, thereby altering many cellular activities and ultimately contributing to the development of HBV-associated liver cancer.
Worldwide, an estimated 350 to 400 million people are chronically infected with human hepatitis B virus (HBV) (82, 97). HBV is the prototype member of the Hepadnaviridae, a family of hepatotropic viruses that can infect both birds and mammals (82). HBV has a partially double-stranded, circular DNA genome containing four overlapping, open reading frames that encode the viral envelope, capsid, polymerase, and X (HBx) proteins (82). Chronic infections with HBV are associated with the development of primary liver cancer, hepatocellular carcinoma (HCC) (3). The underlying molecular mechanisms that link chronic HBV infections to the development of HCC are incompletely understood, and both immune-mediated destruction of HBV-infected hepatocytes and concomitant liver regeneration, as well as activities of the HBx protein, are thought to be involved (82).
Numerous functions have been attributed to HBx, and how HBx influences HBV replication and HCC development is the subject of considerable debate. HBx expression facilitates development of HCC in HBx-transgenic mice, although whether HBx directly induces tumor formation or only sensitizes mice to carcinogens varies depending on the genetic background of the HBx-transgenic mice (43, 44, 56, 85, 101). Chronic infections with mammalian hepadnaviruses, all of which encode a X protein, cause HCC, while chronic infections with avian hepadnaviruses, which either do not encode a X protein or encode a highly divergent form of the protein, are not associated with the development of HCC (27). An X-like protein is encoded by duck HBV, but this protein is not required for duck HBV replication (15, 59). Studies from two groups demonstrated that woodchuck hepatitis virus (WHV) X protein (WHx) is required for WHV infection in vivo (16, 105). However, a different study suggested that WHV may replicate at low levels in the absence of WHx (103). In all three of these studies, woodchucks were injected with either wild-type or WHx-deficient WHV and monitored for evidence of WHV infection. In the first two studies, there was no evidence of WHV replication in the absence of WHx; however, in the third study, two of the four woodchucks injected with a WHx-deficient virus showed serological evidence of WHV infection. Unfortunately, the interpretation of the latter results are confounded by the observation that WHV DNA isolated from the two woodchucks injected with the WHx-deficient virus had reverted to the wild-type WHx sequence. Thus, these results could be interpreted to mean that WHx is not required for WHV replication or, because only wild-type WHx revertants were recovered, that WHx is required for WHV replication (103). Attempts to determine whether HBx is required for HBV replication have also produced various conclusions. Several studies have shown that HBx has an important role in HBV replication in HepG2 cells, a human hepatoblastoma cell line, but not in Huh7 cells, a human hepatoma cell line (8, 9, 53, 60, 91). A recent study in which a wild-type or HBx-deficient HBV cDNA was injected into mice demonstrated that approximately 75% of HBV replication in mouse liver is HBx dependent and that about 99% of the circulating viremia is HBx dependent (40). Collectively, these studies suggest that HBx and WHx are important contributors to HBV and WHV replication, but whether they are absolutely required is still unclear (reviewed in reference 10).
The link between chronic HBV infections and HCC, the impact of HBx expression on HBV replication, and the suspected role of HBx as a cofactor in HCC development have generated considerable interest in defining HBx activities. Several studies have shown that HBx can regulate cellular transcription and signal transduction pathways, the activity of proteasomes, cell cycle progression, and apoptosis (35; reviewed in reference 10). Although one dissenting result has been published (102), studies from four groups suggest that many reported activities of HBx may derive from HBx regulation of cytosolic calcium levels (9, 14, 63, 92). We recently showed that HBx regulation of cytosolic calcium levels involves modulation of the mitochondrial permeability transition pore (MPTP), a channel that spans through the outer mitochondrial membrane into the mitochondrial matrix and controls the migration of molecules into and out of mitochondria (51, 58, 70, 71). Exactly how HBx affects MPTP activity and mitochondrial physiology in normal hepatocytes is unknown.
HBx is localized to both the cytoplasm and the nuclei of cells (34). Immunofluorescence and cell fractionation studies with various cell types demonstrated that a fraction of cytosolic HBx colocalizes with mitochondria, both when expressed in the absence of other HBV proteins and when expressed in the context of HBV replication (25, 34, 36, 41, 58, 70, 84, 89, 90). HBx localizes to mitochondria in HBx-expressing COS cells, a monkey kidney cell line; in Huh7 cells, in HepG2.215 cells, a HepG2 cell line that stably expresses a replication-competent cDNA of HBV; and in HBx-transgenic mouse hepatocytes (17, 36, 70, 89). In one study, mitochondrially localized HBx expressed in Huh7 cells was sensitive to trypsin treatment and mitochondrially localized HBx expressed in COS cells was associated with a membrane fraction following alkaline treatment. Although these studies were conducted with different cell lines, they were collectively interpreted to mean that HBx localizes to the outer mitochondrial membrane (36). In support of this interpretation, results from a yeast two-hybrid screen showed that HBx can interact with voltage-dependent anion channel 3 (VDAC3). VDACs are channels that span the outer mitochondrial membrane and can function as a component of the MPTP (65, 71). Subsequent coimmunoprecipation studies in which HBx and VDAC3 expression vectors were cotransfected into COS cells provided additional evidence for this interaction (70). Finally, HBx expression induced mitochondrial membrane depolarization in Huh7 cells, although it had no direct impact on mitochondrial membrane potential in HepG2 cells (49, 70, 84, 89).
While the aforementioned studies demonstrated that HBx associates with a mitochondrially enriched fraction isolated from established cell lines and from HBx-transgenic mouse hepatocytes, no studies have characterized the precise mitochondrial localization of HBx expressed in the context of HBV replication or of HBx expressed in primary hepatocytes (17, 36, 70, 89). Furthermore, discrepant results regarding the impact of HBx expression on mitochondrial membrane potential have been published, and no studies have characterized the effect of HBx on mitochondrial physiology in normal hepatocytes (49, 70, 84, 89). We have used two model systems to characterize mitochondrially localized HBx and to study the impact of HBx expression on mitochondrial physiology. HepG2 cells are a model system for studying the role of HBx in HBV replication, and this cell line was used to initially characterize mitochondrially localized HBx when it was expressed in the absence of other HBV proteins and when it was expressed in the context of HBV replication. We then extended our studies to cultured primary rat hepatocytes and characterized the mitochondrial localization of HBx and the impact of HBx expression on mitochondrial membrane potential. Cultured primary rat hepatocytes provide a biologically relevant system for analyzing the effect of HBx in untransformed hepatocytes and have been used as a model system for understanding human liver diseases and normal hepatocyte physiology. Cholestasis, Wilson's disease, alcohol-induced liver pathologies, HBV replication and biology, and HCC have been studied in rat liver model systems, and the signaling pathways that are modulated during these processes have been analyzed in cultured primary rat hepatocytes (6, 19, 23, 37, 73). It is relatively easy to isolate large quantities of normal hepatocytes from a single rat, and protocols for maintaining and testing the differentiated status of cultured primary rat hepatocytes are well established (7, 74). Therefore, transfection of cultured rat hepatocytes with HBx-expressing and control plasmids facilitates analyzing HBx activities in hepatocytes derived from the same source.
In the studies described here, we demonstrate that a fraction of cytosolic HBx is associated with the outer mitochondrial membrane in HepG2 cells and cultured primary rat hepatocytes. We also show that HBx modulates mitochondrial membrane potential in cultured primary rat hepatocytes and that the effect of HBx on mitochondrial membrane potential depends on the status of NF-κB activity. We demonstrate that HBx activates NF-κB in cultured primary rat hepatocytes and that activation of NF-κB prevents mitochondrial membrane depolarization. Surprisingly, if the activity of NF-κB was inhibited, HBx induced mitochondrial membrane depolarization through modulation of the MPTP. These results suggest that HBx can modulate mitochondrial membrane potential through two different pathways; one pathway prevents mitochondrial depolarization, while the other induces depolarization. It is likely that the precise impact of these HBx activities on mitochondrial membrane potential will depend on the balance of these signals and will consequently affect cellular physiology, potentially contributing to HBV-associated liver diseases.
MATERIALS AND METHODS
Cells, transfections, and reagents.
HepG2 cells were obtained from the American Type Culture Collection and cultured in minimal essential medium supplemented with 10% fetal bovine serum, 1 mM nonessential amino acids, 1 mM sodium pyruvate, and 10 μg/ml gentamicin. All cells were maintained at 37°C in 5% CO2. HepG2 cells were transfected using FuGENE 6 (Roche) according to the manufacturer's protocol. Primary rat hepatocytes were transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. Tumor necrosis factor alpha (TNF-α) and carbonyl cyanide m-chlorophenylhydrazone (CCCP) were purchased from Calbiochem, and cycloheximide (CHX), cyclosporine (CsA), and FK506 (Tacrolimus) were purchased from Sigma.
Isolation and maintenance of primary rat hepatocytes in culture.
Hepatocytes were isolated from male Sprague-Dawley rats using a two-step profusion procedure as previously described (83). Briefly, the liver was first perfused with calcium-free liver perfusion buffer (Invitrogen) to disrupt hepatocyte desmosomes, followed by perfusion with a 3.5-mg/ml Liberase Blendzyme 3-containing buffer (Roche) to separate hepatocytes from the extracellular matrix. The liver was then removed and healthy hepatocytes isolated over a Percoll density gradient. On average, 1 × 108 cells were isolated per rat liver, and about 95% of cultured hepatocytes were viable. Animal surgery and hepatocyte isolation complied with all relevant federal and institutional policies. The hepatocytes were plated and cultured on collagen-coated tissue culture plates. Hepatocytes were maintained in Williams E medium supplemented with 2 mM l-glutamine, 1 mM sodium pyruvate, 4 μg/ml insulin-transferrin-selenium, 5 μg/ml hydrocortisone, 5 ng/ml epidermal growth factor, and 2% dimethyl sulfoxide (Fisher). For all studies with cultured primary rat hepatocytes, the hepatocytes were transfected at 24 h after plating and collected for analysis at 48 h posttransfection. Rat hepatocytes were monitored for retention of hepatocyte morphology throughout the time course of our studies and were also collected for reverse transcriptase PCR (RT-PCR) analysis of hepatocyte-specific mRNAs (see below).
RT-PCR analysis of hepatocyte mRNAs.
The authenticity of hepatocytes was confirmed by inspection of cell morphology and by detection of albumin, transferrin, and hepatocyte nuclear factor 4 (HNF4) mRNAs, indicators of differentiated hepatocytes (7, 74). RNA was isolated from hepatocytes using TRIzol (Invitrogen) according to the manufacturer's instructions. The iScript cDNA synthesis kit (Bio-Rad) was used for the reverse transcription step as directed by the manufacturer. The primers used for the PCRs were as follows: the forward oligonucleotide sequence for transferrin was 5′ GGCTCAGGAACACTTTGGC 3′ and the reverse oligonucleotide sequence was 5′ GTTGTTCCAGTTGATGCTGG 3′, the forward oligonucleotide sequence for HNF4 was 5′ CGGGCCACTGGCAAACAC 3′ and the reverse oligonucleotide sequence was 5′ GTAATCCTCCAGGCTCAC 3′, and the forward oligonucleotide sequence for albumin was 5′ GCCGAAAACTGTGACAAGTC 3′ and the reverse oligonucleotide sequence was 5′ TCTCGTAAAGCTCACAGTTAG 3′. The PCR protocol consisted of an initial 4-min denaturation at 94°C; followed by 30 cycles of denaturation at 94°C for 1 min, annealing at 55°C for 1 min, and extension at 72°C for 1 min; followed by a final extension at 72°C for 10 min. RT-PCR analysis of liver-specific mRNAs was conducted in hepatocytes immediately after isolation from perfused rat livers and on cultured rat hepatocytes at 24 and 48 h posttransfection.
Plasmids.
Full-length HBx was PCR amplified from HBV subtype ayw using oligonucleotides containing terminal XhoI or EcoRV restriction enzyme sites. Two constructs were made; the first, FL1-154 HBx, has an eight-amino-acid Flag epitope tag (IBI, Inc.) at the N terminus of HBx, and the second, 1-154 HBx, does not contain a Flag epitope tag. For the PCRs, the forward oligonucleotide sequence for FL1-154 HBx was 5′ GCCTCGAGATGGACTACAAGGACGACGATGATAAGATGGCTGCTAGGCTGTGC 3′ and the reverse oligonucleotide sequence was 5′ GCGATATGTCACTATTAGGCAGAGGTGAAAAAGTTGC 3′, and the forward oligonucleotide sequence for 1-154 HBx was 5′ GCCTCGAGATGGCTGCTAGGCTGTGCTGC 3′ and the reverse sequence was the same as for FL1-154 HBx. The PCR amplification protocol consisted of an initial 4-min denaturation at 94°C; followed by 25 cycles of denaturation at 94°C for 1 min, annealing at 60°C for 1 min, and extension at 72°C for 1 min; followed by a final extension at 72°C for 10 min. The HBx insert and pcDNA3.1(−) vector (Invitrogen) were digested with XhoI and EcoRV and ligated so that HBx is produced under the control of the cytomegalovirus promoter.
pGEMHBV (payw1.2) (79) contains a greater-than-unit-length cDNA of the HBV genome and has been previously described (79, 80). This construct expresses all of the HBV genes under the control of endogenous HBV enhancers and promoters. pGEM*7 (payw*7) (60) is identical to pGEMHBV except for a stop codon mutation in the seventh amino acid of the HBx sequence so that the HBx protein is not produced (60).
IκB-SR is cloned into the pLPCX vector and produces a mutant form of the IκB-α protein that has the serines at amino acid positions 32 and 36 mutated to alanines so that this mutant IκB cannot be phosphorylated and degraded (11, 13, 22, 94). The NF-κB-Luc reporter vector (pNF-κB-Luc) contains four NF-κB binding sites adjacent to a minimal promoter and the firefly luciferase coding region, as previously described (69). The pNF-κB-Luc-MUT reporter vector is identical to pNF-κB-Luc except that the NF-κB binding sites are mutated so that NF-κB cannot bind.
Antibodies.
Anti-Grp75, anti-hexokinase, anti-proliferating cell nuclear antigen, and anti-VDAC antibodies were purchased from Santa Cruz Biotechnology; anti-Flag-M2 antibody was purchased from Stratagene; anti-β-actin, anti-calnexin, anti-cathepsin L, and anti-peroxisomal membrane protein 70 (anti-PMP70) antibodies were purchased from Sigma; anti-cytochrome oxidase IV (anti-COXIV), anti-translocase of the outer membrane 20 (anti-TOM20), anti-mitogen-activated protein kinase (anti-MAPK), and anti-B-cell lymphoma 2 (anti-Bcl-2) antibodies were purchased from Cell Signaling; and anti-HBx antibody was purchased from Affinity BioReagents.
Isolation of mitochondria.
For all mitochondrial studies, hepatocytes were transfected at 24 h after plating and collected at 48 h posttransfection. Mitochondria were isolated using the mitochondrial isolation kit for mammalian cells (Pierce) according to the manufacturer's instructions, with some modifications. Briefly, following lysis of approximately 1 × 106 HepG2 cells or primary hepatocytes, cell debris and nuclei were pelleted at 300 × g, followed by centrifugation at 2,500 × g to pellet a mitochondrially enriched fraction. The mitochondrially enriched fraction was then either directly analyzed or separated through a 30% Percoll density gradient by ultracentrifugation using a Beckman Ti70.1 rotor at 95,000 × g for 15 min (this procedure was modified from that described in reference 32). Fractions were collected from the gradient, and proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 15% polyacrylamide gel. Proteins were transferred to a nitrocellulose membrane (Bio-Rad), followed by blocking for 2 h in 5% milk and incubation with primary antibody overnight. The membrane was then washed and incubated with a horseradish peroxidase-conjugated secondary antibody and visualized using an enhanced chemiluminescence (ECL) system (Bio-Rad). Western blot analysis was conducted using Grp75 as a mitochondrial marker (52), MAPK as a cytosolic marker (28), nucleolin as a nuclear marker (75), calnexin as an endoplasmic reticulum (ER) marker (47), cathepsin L as a lysosomal marker (64), and PMP70 as a peroxisomal marker (42), and an anti-Flag antibody was used to detect FL1-154 HBx.
Trypsin treatment of isolated mitochondria.
To identify proteins located peripherally on the outer mitochondrial membrane, mitochondria were isolated from approximately 6 × 106 HepG2 cells or primary rat hepatocytes (per treatment) using the Pierce mitochondrial isolation kit and subsequently treated with increasing concentrations of trypsin (Sigma) for 30 min on ice, followed by centrifugation at 6,700 × g for 15 min at 4°C (31, 36). The pellet fraction, containing proteins located within the mitochondria, was subsequently resolved on a 15% polyacrylamide gel. Western blot analysis was performed using hexokinase (62) and VDAC (17) as markers of a peripheral outer mitochondrial membrane protein and a protein located within the mitochondria, respectively. While cleavage of hexokinase was used as a control for a trypsin-sensitive mitochondrial protein, we included an additional control in the primary hepatocyte studies because mitochondrially associated HBx from primary rat hepatocytes was not sensitive to trypsin. Therefore, to show that the isolation procedure itself did not inhibit the ability of trypsin to cleave HBx in mitochondria isolated from cultured primary rat hepatocytes, these mitochondria were treated with both trypsin and 0.1% Triton X-100, which is an established technique used to disrupt mitochondria and render proteins contained within the mitochondria susceptible to trypsin treatment (46, 78).
Sodium carbonate treatment of isolated mitochondria.
To distinguish integral membrane proteins from peripheral membrane and soluble proteins, mitochondria isolated from approximately 6 × 106 HepG2 cells or primary rat hepatocytes using the Pierce mitochondrial isolation kit were treated with 0.1 M sodium carbonate (Na2CO3) (pH 11.5) for 30 min on ice, followed by centrifugation at 13,000 × g for 10 min at 4°C, as previously described (30). The pellet fraction, containing the inner and outer mitochondrial membranes, and the supernatant fraction, containing the peripheral membrane, intermembrane space and matrix proteins, were separated by SDS-PAGE on a 15% polyacrylamide gel. Western blot analysis was performed using hexokinase as a marker of a peripheral mitochondrial membrane protein and VDAC as a marker of an integral mitochondrial membrane protein.
Potassium chloride (KCl) treatment of isolated mitochondria.
To separate the outer and inner mitochondrial membranes, mitochondria isolated from approximately 6 × 106 primary rat hepatocytes using the Pierce mitochondrial isolation kit were treated with 10 mM KCl for 10 min on ice, as previously described (67). Following this incubation, samples were centrifuged at 2,500 × g to pellet the intact inner mitochondrial membrane and matrix, leaving the outer mitochondrial membrane and intermembrane space in the supernatant. The pellet fraction was washed and recentrifuged at 300 × g to remove any unlysed mitochondria. Western blot analysis was performed using Bcl-2 (24) and TOM20 (100) as markers of the outer mitochondrial membrane and COXIV (70) as a marker of the inner mitochondrial membrane.
Characterization of mitochondrial membrane potential.
The mitochondrial membrane potential was evaluated using the cationic dye JC-1 (5,5,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide; Invitrogen). As a positive control for membrane depolarization, cells were treated with various concentrations of CCCP (data not shown); maximum depolarization was observed by adding 10 μM CCCP 5 min prior to the addition of JC-1, which is consistent with other studies using primary rat hepatocytes (61). Cells were treated with TNF-α (5 ng/ml), CHX (25 μg/ml), CsA (5 μM), or FK506 (5 μM) for 18 h, where applicable. Primary hepatocytes were transfected at 24 h after plating, scraped into their growth medium at 48 h posttransfection, and incubated for 30 min in the dark with 2.5 μM JC-1 (77). Following incubation, cells were washed twice with phosphate-buffered saline and resuspended in 500 μl of phosphate-buffered saline for analysis on a Guava Technologies flow cytometer using the MitoPotential program according to the manufacturer's instructions.
Luciferase assays.
At 24 h after plating, primary hepatocytes were transfected with FL1-154 HBx, pcDNA3.1(−), IκB-SR, pNF-κB-Luc, or pNF-κB-Luc-MUT, where appropriate. Luciferase reporter assays were performed at 48 h posttransfection using the Promega luciferase assay system according to the manufacturer's instructions.
RESULTS
HBx expression and function.
Western blot analyses were conducted to confirm HBx expression following transfection of FL1-154 HBx or 1-154 HBx into HepG2 cells and primary rat hepatocytes (Fig. 1 and data not shown). HBx expression was detected in HBx-transfected HepG2 cells and primary hepatocytes and not in the pcDNA3.1(−)-transfected hepatocytes. Most experiments were conducted with FL1-154 HBx because less background was detected using the anti-Flag antibody than when the commercially available anti-HBx antibody was used. However, we compared both HBx expression constructs for the initial mitochondrial localization studies and saw similar results using both FL1-154 HBx and 1-154 HBx (data not shown).
FIG. 1.
HBx expression in HepG2 cells and primary rat hepatocytes. HepG2 cells or primary rat hepatocytes were transfected 24 h after plating with FL1-154 HBx or pcDNA3.1(−). At 48 h posttransfection, cells were collected and proteins were resolved by SDS-PAGE. Western blot analysis was performed using an anti-Flag antibody.
When HepG2 cells are transfected with an HBx-deficient HBV expression plasmid, pGEMHBV*7, HBV replication is severely diminished compared to HBV replication in HepG2 cells that are transfected with the greater-than-unit-length wild-type HBV DNA, pGEMHBV (60). We previously demonstrated that replication of the HBx-deficient HBV mutant can be rescued by cotransfection with FL1-154 HBx, demonstrating that this HBx expression vector produces functional HBx (58, 60). Additionally, the results of our previously published studies strongly suggest that activities associated with HBx expression from the FL1-154 vector are unlikely to be artifacts of overexpression (58). In these experiments, we assessed the ability of HBx to modulate cytosolic calcium levels when it was expressed from the FL1-154 plasmid or in the context of HBV replication; in both experimental conditions, similar increases in cytosolic calcium were observed and linked to regulation of the MPTP (58).
Confirmation of hepatocyte differentiation in culture.
To confirm that the cultured primary rat hepatocytes remained differentiated when maintained in the growth conditions described in Materials and Methods, cell morphology was continually monitored and RT-PCR analysis was performed to detect the expression of the hepatocyte-specific markers transferrin, albumin, and HNF4 (7, 74). Expression of each of these hepatocyte-specific markers was observed in freshly isolated hepatocytes and in hepatocytes 24 (data not shown) and 48 h posttransfection, the time of cell collection (Fig. 2). Note that due to the substantial difference in fragment sizes, we were able to analyze albumin and transferrin expression in the same sample. Hepatocyte morphology was also maintained throughout the experiment, and no alterations in cell appearance were noted during the time course of our studies. These observations are consistent with the many previously published studies that have used similar isolation and culture techniques to maintain differentiated rat hepatocytes (6, 19, 23, 37, 73).
FIG. 2.
Confirmation of hepatocyte differentiation in culture. RNA was extracted either from primary rat hepatocytes freshly isolated from a rat liver or from cultured primary rat hepatocytes maintained in Williams E medium as described in Materials and Methods. RT-PCR analysis was performed to analyze expression of albumin (A), transferrin (T), and HNF4 (H) as markers of differentiated hepatocytes. Expected band sizes are 914 bp for albumin, 530 bp for transferrin, and 530 bp for HNF4. Note that the primers for albumin and transferrin were combined in the same sample due to the substantial difference in PCR product size. Lanes AT (-RT) and H(-RT) represent samples in which isolated RNA was analyzed directly by PCR to confirm the absence of contaminating DNA.
HBx is localized to mitochondria.
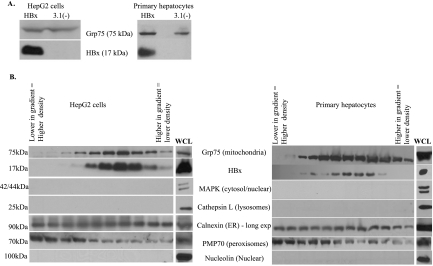
While one published report demonstrated that HBx was associated with a mitochondrially enriched fraction isolated from HBx-transgenic mouse hepatocytes, all other previous studies of the interaction between HBx and mitochondria were conducted with mitochondrially enriched fractions isolated from immortalized or transformed liver cell lines or cell lines that were not of hepatic origin (17, 34, 36, 41, 70, 84, 89). Our experiments determined whether HBx could be biochemically copurified with mitochondria isolated from HepG2 cells and from primary rat hepatocytes. HepG2 cells are a model system for studying functions of HBx that affect HBV replication, and cultured primary rat hepatocytes are an established model system of normal hepatocyte physiology (6, 19, 23, 37, 73). We first examined whether HBx was localized to a mitochondrially enriched fraction isolated from HepG2 cells and primary rat hepatocytes. Cells were transfected with FL1-154 HBx, and at 48 h posttransfection a mitochondrially enriched fraction was prepared. HBx localized to the mitochondrially enriched fraction in both HepG2 cells and primary hepatocytes (Fig. 3A). Similar to previously reported results in HepG2 cells (58), approximately 5% of cellular HBx localizes to the mitochondria in primary rat hepatocytes (data not shown). Comparable results were obtained using an HBx construct that did not contain a fused Flag epitope (data not shown). As a negative control, HepG2 cells were transfected with an arbitrary Flag-tagged protein (IκB-SR), and a mitochondrially enriched fraction was examined. As expected, this protein did not localize to mitochondria, negating the possibility that the Flag epitope was responsible for mitochondrial localization of HBx (data not shown).
FIG. 3.
Copurification of HBx with a mitochondrially enriched fraction isolated from HepG2 cells or primary rat hepatocytes. (A) Hepatocytes were transfected at 24 h after plating with FL1-154 HBx or pcDNA3.1(−), and a mitochondrially enriched fraction was isolated at 48 h posttransfection as described in Materials and Methods. Proteins were resolved by SDS-PAGE, and Western blot analysis was performed using an anti-Grp75 antibody and an anti-Flag antibody. (B) HepG2 cells or primary rat hepatocytes were transfected with FL1-154 HBx, followed by separation over a Percoll density gradient. Western blot analysis was performed using anti-Grp75, anti-Flag, anti-MAPK, anti-cathepsin L, anti-calnexin, anti-PMP70, and anti-nucleolin antibodies. WCL, whole-cell lysate.
While it is generally considered as an excellent method for isolating mitochondria, the technique used to prepare the mitochondrially enriched preparation may result in some contamination with other cytosolic organelles. To further purify mitochondria away from potential organellar contamination and confirm HBx localization to mitochondria, the mitochondrially enriched fraction was layered over a 30% Percoll density gradient and mitochondria were separated from other organelles by centrifugation. Fractions from the gradient were examined for the distribution of cytosolic organelles and for HBx. Western blot analyses were conducted to detect Grp75 as a mitochondrial marker, MAPK as a cytosolic marker, nucleolin as a nuclear marker, calnexin as an ER marker, cathepsin L as a lysosomal marker, and PMP70 as a peroxisomal marker. The anti-Flag antibody was used to detect Flag-tagged HBx. HBx colocalized with density-gradient-purified mitochondria in both HepG2 cells and primary hepatocytes. These fractions were free from lysosomal, cytosolic, and nuclear contamination (Fig. 3B). Attempts to eliminate all ER contamination were unsuccessful; prolonged overexposure of the Western blots showed a low level of ER in all fractions. However, this low level of ER contamination was detected equally in all fractions rather than showing the colocalization pattern of HBx and mitochondria. This low level of ER was likely a result of the large number of cells that were required for this experiment due to the low expression level of HBx. For reasons that are not entirely clear, the degree of peroxisomal contamination varied in different experiments, and in some experiments, no peroxisomal contamination was observed (compare Fig. 3B and 4B). However, even when peroxisomal contamination was noted in some mitochondrial fractions, the majority of peroxisomes peaked at a higher density than the peak for HBx and mitochondria (Fig. 3B).
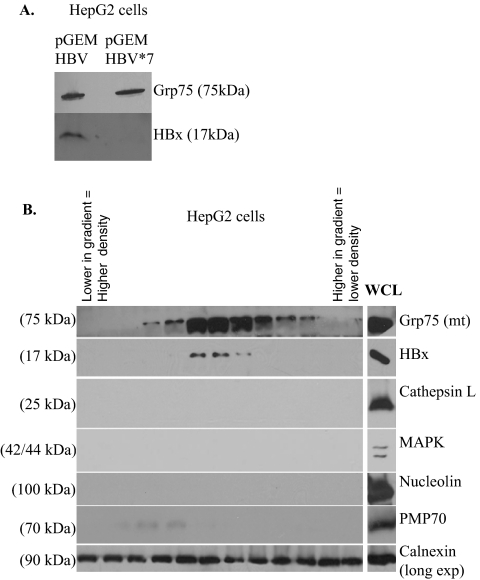
FIG. 4.
Copurification of mitochondria with HBx expressed in the context of HBV replication in HepG2 cells. (A) HepG2 cells were transfected with pGEMHBV or pGEMHBV*7, and at 48 h posttransfection a mitochondrially enriched fraction was isolated. Proteins were resolved by SDS-PAGE and Western blot analysis was performed using an anti-Grp75 antibody and an anti-HBx antibody. (B) HepG2 cells were transfected with pGEMHBV, and at 48 h posttransfection isolated mitochondria were separated over a Percoll density gradient. Western blot analysis was performed using anti-Grp75, anti-Flag, anti-cathepsin L, anti-calnexin, anti-MAPK, anti-nucleolin, and anti-PMP70 antibodies. WCL, whole-cell lysate.
To confirm that HBx localized to purified mitochondria in the context of HBV replication, Percoll density gradient purification of mitochondria was also performed in HepG2 cells transfected with pGEMHBV. Importantly, HBx localized to purified mitochondria when expressed in the context of HBV replication (Fig. 4). This is consistent with our results using HBx expressed in the absence of other HBV proteins and confirms that our results are not an artifact of HBx overexpression. Because HBx is expressed at a low level from the HBV genome, a large number of pGEMHBV-transfected HepG2 cells (approximately 7.5 × 107 cells) were processed to detect HBx localization to mitochondria. Primary rat hepatocytes have a decreased transfection efficiency compared to HepG2 cells (approximately two- to threefold lower) and therefore require substantially more cells for a similar analysis, thus making this study not feasible with cultured primary rat hepatocytes.
HBx is localized to the outer mitochondrial membrane.
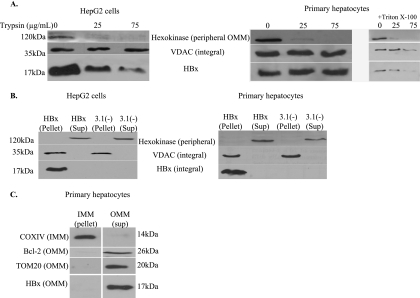
After establishing that a fraction of HBx localizes to mitochondria in hepatocytes, we addressed the localization of HBx within mitochondria. Isolated mitochondria were treated with increasing concentrations of trypsin, and Western blot analysis was performed using hexokinase as a marker of a peripheral outer mitochondrial membrane protein and VDAC as a marker of an integral mitochondrial membrane protein. This procedure distinguishes between proteins contained within mitochondria (i.e., integral membrane proteins and soluble proteins located in the intermembrane space or matrix) and proteins entirely or partially exposed on the surface of the outer mitochondrial membrane. Therefore, trypsin treatment will cleave hexokinase from the mitochondrial surface but will not cleave VDAC. Interestingly, we saw different results for HBx in HepG2 cells and primary rat hepatocytes. In HepG2 cells, mitochondrially associated HBx was susceptible to trypsin treatment (Fig. 5A), which is consistent with similar studies using Huh7 cells (36). However, HBx was not cleaved from the surface of mitochondria isolated from primary rat hepatocytes (Fig. 5A). The reason for these differences is currently unknown. As a control to show that the procedure used to isolate mitochondria from primary rat hepatocytes does not render mitochondrially associated HBx insensitive to trypsin, we demonstrated that VDAC and HBx become susceptible to trypsin in the presence of 0.1% Triton X-100 (Fig. 5A).
FIG. 5.
Localization of HBx to the outer mitochondrial membrane. (A) HepG2 cells or primary rat hepatocytes were transfected with FL1-154 HBx, and at 48 h posttransfection a mitochondrially enriched fraction was isolated. Trypsin treatment of isolated mitochondria was performed using increasing concentrations of trypsin. Western blot analysis was performed using anti-hexokinase, anti-VDAC, and anti-Flag antibodies. Treatment of mitochondria isolated from HBx-transfected primary hepatocytes with trypsin and Triton X-100 rendered HBx and VDAC susceptible to trypsin treatment. (B) HepG2 cells and primary rat hepatocytes were transfected with FL1-154 HBx, and at 48 h posttransfection a mitochondrially enriched fraction was isolated. Alkaline treatment of isolated mitochondria was performed, and proteins were resolved by SDS-PAGE. Western blot analysis was performed using anti-hexokinase, anti-VDAC, and anti-Flag antibodies. (C) Primary rat hepatocytes were transfected with FL1-154, followed by mitochondrial isolation and KCl treatment. Following differential centrifugation, proteins were resolved by SDS-PAGE, and Western blot analysis was performed using COXIV as an inner mitochondrial membrane (IMM) marker and Bcl-2 and TOM20 as markers of the outer mitochondrial membrane (OMM).
To further define the localization of HBx, mitochondria were isolated from HBx-transfected HepG2 cells or primary hepatocytes and were treated with sodium carbonate, pH 11.5. Alkaline treatment of mitochondria lyses the outer and inner mitochondrial membranes; this facilitates separation of integral membrane proteins from peripheral membrane proteins and soluble proteins in the intermembrane space or matrix of mitochondria (30). Hexokinase and VDAC were used as markers of peripheral and integral membrane proteins, respectively. Alkaline treatment of mitochondria isolated from HepG2 cells showed that at least a portion of HBx is an integral membrane protein (Fig. 5B). This is consistent with the results observed following alkaline treatment of mitochondria isolated from COS cells (36). Similarly, the results from alkaline treatment of mitochondria isolated from primary rat hepatocytes suggest that HBx is an integral membrane protein in these cells (Fig. 5B). The combined results of trypsin and alkaline treatment of mitochondria isolated from HepG2 cells suggest that HBx is partially inserted in the outer mitochondrial membrane and partially exposed on the surface of the outer mitochondrial membrane. For primary hepatocytes, these results suggest that HBx is an integral membrane protein.
Alkaline treatment of mitochondria isolated from HBx-transfected primary rat hepatocytes showed that HBx was localized to a membrane fraction but did not definitively localize it to either the inner or outer mitochondrial membrane. Further analyses were conducted with primary hepatocytes to distinguish between these two possibilities. Mitochondria were isolated from HBx-transfected primary hepatocytes and treated with 10 mM KCl as described in Materials and Methods. This treatment selectively lyses the outer mitochondrial membrane, while leaving the inner mitochondrial membrane intact. The outer membrane and intermembrane space are then separated from the inner mitochondrial membrane and matrix by differential centrifugation (67). Fractions were separated by SDS-PAGE, and Western blot analysis was performed to detect expression of Bcl-2 and TOM20 as markers of the outer mitochondrial membrane and of COXIV as a marker of the inner mitochondrial membrane. HBx was localized to the outer mitochondrial membrane fraction in primary rat hepatocytes (Fig. 5C). The results of the trypsin, alkaline, and potassium chloride treatment experiments collectively suggest that HBx is an integral outer mitochondrial membrane protein in primary rat hepatocytes.
Previous results from a yeast two-hybrid screen suggested that HBx interacts with VDAC3, and additional studies with COS cells suggested that HBx coimmunoprecipitates with exogenously expressed VDAC3 (70, 71). Attempts to coimmunoprecipitate HBx with endogenous VDAC in HepG2 cells or primary rat hepatocytes were unsuccessful (data not shown). However, this may be caused by the harsh lysis conditions required to extract mitochondrial membrane proteins; these may disrupt any interaction between HBx and endogenous mitochondrial proteins.
HBx regulates mitochondrial membrane potential.
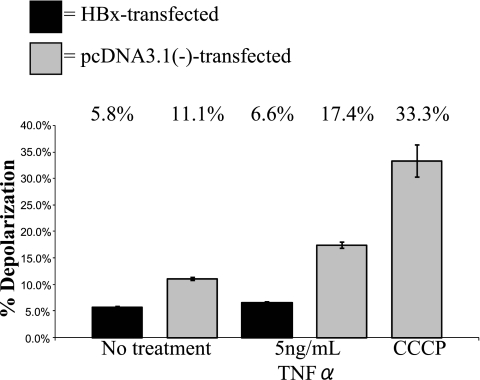
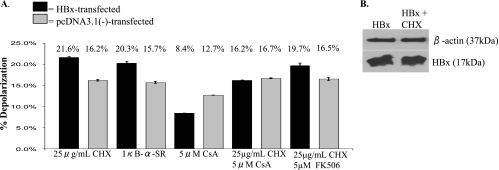
We next determined whether HBx affects mitochondrial physiology in primary rat hepatocytes. HBx has been reported to induce mitochondrial membrane depolarization in Huh7 cells (70, 84, 89) but to have no direct effect on mitochondrial membrane potential in HepG2 cells (50). The effect of HBx on mitochondrial membrane potential in primary hepatocytes is not known. HBx-transfected hepatocytes were incubated with the cationic dye JC-1 (72, 76), and mitochondrial membrane potential was evaluated by flow cytometry analysis. The extent of JC-1 accumulation in mitochondria depends on the mitochondrial membrane potential. JC-1 accumulates in mitochondria and forms red fluorescent J aggregates (∼590-nm emission) under conditions of mitochondrial polarization, but when the mitochondrial membrane depolarizes, JC-1 diffuses out of mitochondria, thereby decreasing aggregation and resulting in green fluorescence (∼525-nm emission). As a positive control to induce mitochondrial membrane depolarization, we used the uncoupler CCCP. CCCP functions by uncoupling electron transport from oxidative phosphorylation so that ATP synthesis can no longer take place (93). Various concentrations of CCCP were used (data not shown), and maximum depolarization was observed with 10 μM CCCP, which is consistent with other studies using CCCP in primary rat hepatocytes (61). On average, CCCP treatment caused detectable depolarization in about 30% of hepatocytes, which is comparable to previous reports in which CCCP was used to induce depolarization (54, 68). In contrast to previous studies performed with established cell lines, HBx expression in primary rat hepatocytes protected cells from mitochondrial membrane depolarization (5.8% depolarization in HBx-expressing cells, compared to 11.1% depolarization in negative control cells) (Fig. 6). In addition, the effect of TNF-α treatment on mitochondrial membrane depolarization was examined; TNF-α is a cytokine produced in the context of a chronic HBV infection and can be used to sensitize cells to mitochondrial membrane depolarization (29, 38). TNF-α treatment caused a slight amplification of depolarization in control cells, and HBx prevented this depolarization (6.6% depolarization in HBx-expressing cells, compared to 17.4% in negative control cells) (Fig. 6). Although the HBx-induced changes in the detectable percentage of hepatocytes with depolarized mitochondria are not large, they were consistently observed and are statistically significant (P ≤ 0.01); moreover, as stated above, the maximum detectable depolarization observed with CCCP treatment never exceeded 30% of hepatocytes. Finally, the transfection efficiency in cultured primary rat hepatocytes ranged from 30 to 40%, and the observed depolarization might be greater if all of the cultured hepatocytes expressed HBx.
FIG. 6.
HBx prevents mitochondrial membrane depolarization in primary rat hepatocytes. Primary rat hepatocytes were transfected with FL1-154 HBx. At 24 h posttransfection, hepatocytes were treated with TNF-α, as shown. Twenty-four hours later, cells were treated with CCCP for 5 min, where shown, and cells were then collected and stained with JC-1, followed by flow cytometry analysis. Statistical analysis, conducted using Student's t test, verified that these differences are statistically significant (P ≤ 0.01). Error bars represent the standard deviation.
CHX treatment alters HBx-dependent regulation of mitochondrial membrane potential.
Because our results were different from those of previous studies with established cell lines, we further examined the effect of HBx on mitochondrial membrane potential. Previous studies have shown that the effect of treatment on mitochondrial membrane potential can vary depending on the status of cellular protein synthesis and activation of cellular signal transduction pathways (38). To determine whether HBx-dependent mitochondrial membrane depolarization required protein synthesis and possible activation of signal transduction pathways, we first examined whether inhibition of protein synthesis influenced HBx-dependent modulation of mitochondrial membrane potential. In the presence of the translation inhibitor CHX, HBx induced mitochondrial membrane depolarization, suggesting an important role for protein production in HBx-dependent modulation of mitochondrial membrane potential (Fig. 7A). Surprisingly, CHX treatment of HBx-transfected hepatocytes did not substantially decrease HBx expression levels during the time course of these experiments (Fig. 7B). Addition of [35S]methionine confirmed that the levels of CHX treatment in our experiments completely inhibited protein synthesis (A. J. Clippinger and M. J. Bouchard, unpublished observations). Previous studies and our own analyses of [35S]methionine-pulse-labeled HBx have demonstrated that HBx has a short, bimodal half-life (4, 81; B. Yang and M. J. Bouchard, unpublished observations). Our results suggest that continued protein synthesis is required for the complete degradation of HBx; others have reported similar protein-stabilizing effects of CHX treatment (26, 99, 104).
FIG. 7.
Mitochondrial membrane potential in primary rat hepatocytes. (A) At 24 h after plating, primary rat hepatocytes were transfected with FL1-154 HBx, pcDNA3.1(−), or IκB-α mutant, as shown. At 24 h posttransfection, hepatocytes were treated with CHX, CsA, or FK506, where applicable. Cells were collected 24 h later, stained with JC-1, and analyzed by flow cytometry. Statistical analysis, conducted using Student's t test, verified that the differences between the HBx-transfected and negative control samples are statistically significant for CHX, IκB-SR, CsA, and CHX-FK506 treatment (P ≤ 0.05). Error bars represent the standard deviation. (B) Primary rat hepatocytes were collected at 48 h posttransfection as for panel A, and Western blot analysis was conducted to confirm that treatment with CHX did not substantially change the level of HBx expression.
HBx activates NF-κB in primary rat hepatocytes.
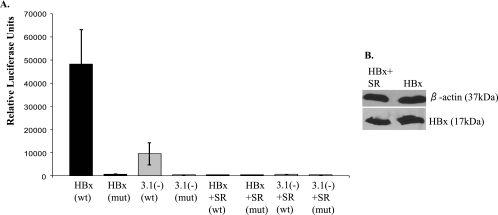
One consequence of CHX treatment is the inhibition of NF-κB-dependent expression of specific proteins. Therefore, one possible mechanism for HBx-induced protection of mitochondrial membrane depolarization could be through activation of NF-κB. Unlike primary hepatocytes, some established cell lines, including HepG2 cells, have low levels of NF-κB, and therefore it is not necessary to inhibit protein translation in order to induce mitochondrial membrane depolarization in response to various stimuli (38). As a first step in determining whether HBx activates NF-κB to modulate the mitochondrial membrane potential, we used an NF-κB-dependent luciferase reporter construct to assay HBx activation of NF-κB in primary rat hepatocytes. Hepatocytes were transfected with a plasmid containing the firefly luciferase gene under the control of a minimal promoter and four NF-κB binding sites (pNF-κB-Luc); the amount of luciferase produced correlates with NF-κB activity. As a control, cells were transfected with a plasmid identical to pNF-κB-Luc except all of the NF-κB binding sites were mutated so that NF-κB cannot activate expression of luciferase (pNF-κB-Luc-MUT). Primary hepatocytes were cotransfected with FL1-154 HBx or pcDNA3.1(−) and either wild-type pNF-κB-Luc or pNF-κB-Luc-MUT. Cells were collected at 48 h posttransfection and processed using the Promega luciferase assay system, and luciferase activity was measured using a luminometer. A fivefold induction in NF-κB activation was observed in HBx-expressing hepatocytes compared to negative control hepatocytes (Fig. 8A). This activation was not observed using pNF-κB-Luc-MUT, regardless of HBx expression. The activity of NF-κB was also examined in the presence of IκB-SR, a mutant form of the IκB-α protein which cannot be phosphorylated and degraded (11, 13, 22, 94). IκB-SR functions as a constitutive suppressor of NF-κB. IκB-SR decreased NF-κB activation, and cotransfection of HBx and IκB-SR abrogated the HBx-dependent activation of NF-κB, showing that IκB-SR is functional and further confirming that results from these luciferase assays represented changes in NF-κB activity (Fig. 8A). HBx expression was not substantially affected by IκB-SR (Fig. 8B).
FIG. 8.
NF-κB activation in primary rat hepatocytes. At 24 h after plating, primary rat hepatocytes were transfected with pNF-κB-Luc (wild type [wt]), pNF-κB-Luc-MUT (mut), FL1-154, pcDNA3.1(−), or IκB-SR, where applicable, and then collected at 48 h posttransfection. (A) Luciferase assays were performed as described in the Materials and Methods. This is a representative graph from three separate experiments, each performed in triplicate. Statistical analysis, conducted using Student's t test, verified that these differences are statistically significant (P ≤ 0.01). Error bars represent the standard deviation. (B) Hepatocytes were collected at 48 h posttransfection, and Western blot analysis was conducted to confirm that cotransfection of IκB-SR did not substantially change the level of HBx expression.
HBx modulation of mitochondrial membrane potential is influenced by NF-κB activity.
To examine the possibility that HBx-dependent regulation of mitochondrial membrane potential is influenced by NF-κB activity, HBx- or pcDNA3.1(−)-transfected primary hepatocytes were cotransfected with IκB-SR. HBx induced mitochondrial membrane depolarization in primary rat hepatocytes in the presence of IκB-SR, similar to the result seen in the presence of CHX (Fig. 7A). These results show that HBx inhibition of mitochondrial membrane depolarization is NF-κB dependent in primary hepatocytes.
CsA abrogates HBx-dependent depolarization of mitochondrial membrane potential.
The effect of HBx on mitochondrial membrane potential was examined in the presence of CsA. CsA functions by inhibiting cyclophilin D, which is a component of the MPTP, and thereby inhibits opening of the MPTP (12). The addition of CsA, in the presence of CHX, abrogated the HBx-dependent depolarization of the mitochondrial membrane potential, suggesting that HBx can act through the MPTP as another means of regulating mitochondrial membrane potential (Fig. 7A). However, in the absence of CHX, HBx still protected CsA-treated hepatocytes from membrane depolarization compared to control-transfected cells. These results suggest that in cultured primary rat hepatocytes, HBx can cause mitochondrial membrane depolarization by affecting the function of the MPTP, that the HBx-dependent activation of NF-κB is independent of its regulation of the MPTP, and that HBx-dependent activation of NF-κB suppresses its ability to induce mitochondrial membrane depolarization. Addition of CsA caused a slight increase in the overall amount of membrane depolarization in both the HBx-transfected and negative control cells but blocked the ability of HBx to enhance this effect. This is consistent with data suggesting that CsA not only inhibits the MPTP but also can cause a slight increase in the amount of apoptosis in primary rat hepatocytes (33). CsA inhibits both calcineurin and cyclophilin D; calcineurin is a protein phosphatase that modulates the immune response through regulation of nuclear factor of activated T cells, and cyclophilin D is a component of the MPTP (12, 20). To determine if the effect of HBx on mitochondrial membrane potential in the presence of CsA was caused solely by the ability of CsA to inhibit cyclophilin D and not as a result of its effect on calcineurin, FK506 was used. FK506 functions as an immunosuppressant due to its ability to inhibit calcineurin; FK506 does not affect the MPTP (20). The effect of HBx on mitochondrial membrane potential in the presence of FK506 and CHX was comparable to the effect of HBx in the presence of CHX treatment alone, demonstrating that inhibition of calcineurin does not alter HBx modulation of mitochondrial membrane potential. Taken together, these results suggest that HBx activation of NF-κB prevents mitochondrial membrane depolarization in primary hepatocytes; however, under conditions where NF-κB is not active, HBx induces mitochondrial membrane depolarization by regulating the MPTP.
DISCUSSION
Chronic HBV infections are associated with the development of HCC (3). Although still debated, the nonstructural HBV X protein, HBx, has been implicated as a cofactor in the development of HBV-associated HCC (43, 44, 56, 85, 101). HBx is a multifunctional protein that can regulate cellular transcription and signal transduction pathways, proteasome activity, cell cycle progression, apoptosis, and HBV replication (35; reviewed in reference 10). Some of these HBx activities involve stimulation of signal transduction pathways by an HBx-induced elevation of cytosolic calcium levels (9, 14, 63, 92). It is likely that the subcellular distribution of HBx is important for its various functions, and several studies with established cell lines and HBx-transgenic mouse hepatocytes have suggested that a fraction of cytosolic HBx localizes to mitochondria (17, 25, 34, 36, 41, 58, 70, 84, 89, 90). Studies conducted with established cell lines suggest that HBx regulates cytosolic calcium levels through an MPTP-dependent pathway and may also modulate mitochondrial membrane potential (41, 58, 70, 84, 89). The localization of HBx within mitochondria and the effect of HBx on mitochondrial physiology in primary hepatocytes have not been previously addressed.
In this study, we initially characterized the mitochondrial localization of HBx in HepG2 cells, a cell line that serves as a model system for studying HBx-dependent HBV replication (60). We then extended our studies to cultured primary rat hepatocytes, a biologically relevant system that has been used to study various aspects of hepatocyte physiology (6, 19, 23, 37, 73). While similar studies could have been conducted with hepatocytes derived from HBx-transgenic mice, the necessity for control experiments with hepatocytes derived from a nontransgenic mouse and the consequential possibility that some subtle differences might not be related to HBx expression led us to focus our studies on HBx- and control-vector-transfected primary rat hepatocytes derived from the same source. Additionally, rats provided a more experimentally tractable system for isolating the large numbers of hepatocytes that were required for many of our experiments. For studies with cultured primary rat hepatocytes, we focused on the activities of HBx when it is expressed in the absence of other HBV proteins. The published reports on numerous tumors from HBV-associated HCC in which HBx is expressed in the absence of viral replication (21, 66, 98) suggest that it is important to understand the consequence of HBx expression for hepatocyte physiology both when expressed in the context of replication and when expressed in the absence of other viral proteins.
We first characterized the localization of HBx to a mitochondrially enriched fraction isolated from HepG2 cells; these studies were conducted with cells that were transfected with either an HBx expression vector or a replication-competent HBV DNA (Fig. 3A and 4). In both cases, HBx localized to mitochondria, confirming our previous immunofluorescence studies that showed colocalization of HBx and mitochondria and demonstrating that mitochondrially localized HBx was not an artifact caused by potential overexpression of HBx in the absence of HBV replication (58). We then showed that HBx localized to a mitochondrially enriched fraction isolated from HBx-transfected primary rat hepatocytes (Fig. 3A). Purification of mitochondria by density gradient fractionation further confirmed that HBx localized to mitochondria in HepG2 cells (Fig. 3B and 4B) and in primary rat hepatocytes (Fig. 3B).
We next used trypsin sensitivity assays, sodium carbonate treatment, and potassium chloride treatment to characterize the localization of HBx within mitochondria. The results of these studies showed that HBx was present in the outer mitochondrial membrane in both HepG2 cells and cultured primary rat hepatocytes (Fig. 5); these observations are consistent with similar studies using Huh7 and COS cells and with a possible VDAC3 interaction (36, 70, 71). The exact localizations of HBx in HepG2 cells and cultured primary rat hepatocytes were slightly different; a portion of HBx was sensitive to trypsin treatment in HepG2 cells, while mitochondrially localized HBx was entirely insensitive to trypsin when it was expressed in rat hepatocytes, unless these mitochondria were also exposed to Triton X-100. These results, combined with those from sodium carbonate and potassium chloride treatments, suggest that HBx is partially exposed on the surface of mitochondria and partially embedded in the outer mitochondrial membrane in HepG2 cells, whereas it is entirely embedded in the outer membrane of mitochondria in primary rat hepatocytes. The reasons for these differences are not known but may reflect variation in the way that HBx is inserted into the outer mitochondrial membrane or differences in an interaction between HBx and a mitochondrial protein. For example, it is possible that HBx interacts with VDAC3 and that it is the conformation of VDAC3 that varies between immortalized and transformed hepatocytes versus primary hepatocytes. There is 95% amino acid identity (98% similarity) between human and rat VDAC3, and this high degree of homology suggests that an interaction between HBx and VDAC3 would likely occur in both human and rat cells (2, 71). Additionally, because HBx regulates HBV replication in both HepG2 cells and primary rat hepatocytes, it is likely that HBx activities that affect HBV replication, such as regulation of calcium levels and of the MPTP, are similar in both HepG2 and primary rat hepatocytes (16, 58, 60, 86, 105). In ongoing studies, we are attempting to further characterize the differences in trypsin sensitivity of HepG2 and primary rat hepatocyte mitochondrially localized HBx and whether the VDAC conformation differs in HepG2 cells and rat hepatocytes.
HBx directly causes mitochondrial membrane depolarization in Huh7 cells but not in HepG2 cells (50, 70, 84, 89). While useful in defining possible activities of HBx, established cell lines may not completely mimic normal hepatocyte physiology and functions. Therefore, we characterized the effect of HBx on mitochondrial membrane potential in cultured primary rat hepatocytes and demonstrated that HBx inhibited mitochondrial membrane depolarization (Fig. 6). In the absence of any exogenous depolarization signal, mitochondrial membrane depolarization was observed in a small percentage of cultured primary rat hepatocytes. We consistently observed a small but statistically significant decrease in mitochondrial depolarization in HBx-expressing compared to control cells. The optimum transfection efficiency in primary rat hepatocytes was about 30 to 40%; therefore, it is possible that the percentage of hepatocytes with depolarized mitochondria would be even less if more hepatocytes in the assay were expressing HBx.
To determine whether HBx protects cells from mitochondrial membrane depolarization under conditions that might be present during an HBV infection of the liver, we also examined the effect of HBx on TNF-α modulation of mitochondrial membrane polarization. TNF-α is a cytokine that is present during immune-mediated responses to an HBV infection; prolonged treatment of primary rat hepatocytes with TNF-α caused mitochondrial membrane depolarization (29, 88) (Fig. 6). Because studies with other cell lines demonstrated that HBx induced mitochondrial depolarization, we expected that HBx would sensitize primary rat hepatocytes to TNF-α-induced mitochondrial membrane depolarization (50, 70, 84, 97). Surprisingly, HBx protected hepatocytes from TNF-α-induced depolarization; the increase in mitochondrial membrane depolarization that was observed in primary rat hepatocytes treated with TNF-α was inhibited by HBx expression (Fig. 6). These results suggest that in the context of an HBV infection, HBx expression may protect hepatocytes from cytokine-induced mitochondrial membrane depolarization.
Multiple cellular signal transduction pathways can affect mitochondrial membrane potential, and it is likely that compared to normal cells, many of these pathways are altered in established cell lines that have been derived from cancers (95). To begin to understand how HBx regulates mitochondrial membrane potential in cultured primary rat hepatocytes, we exposed HBx-expressing hepatocytes to CHX, an inhibitor of protein synthesis, and analyzed mitochondrial membrane potential. In the presence of CHX, HBx protection against depolarization was blocked, and HBx actually induced mitochondrial membrane depolarization (Fig. 7). These results support an important role for protein synthesis in HBx-dependent prevention of mitochondrial membrane depolarization and suggest that HBx modulation of mitochondrial membrane potential involves at least two activities. One activity prevents mitochondrial membrane depolarization and requires continual protein synthesis, while the other activity induces membrane depolarization. In hepatocytes in which protein synthesis is not blocked, the activity of HBx that prevents mitochondrial depolarization dominates HBx modulation of mitochondrial membrane potential.
CHX treatment is often used to inhibit cellular signal transduction pathways that are regulated by NF-κB, and HBx can activate NF-κB in various established cell lines (1, 18, 48, 55, 57, 87, 96). In the studies described here, we have demonstrated that HBx also stimulates NF-κB activity in primary rat hepatocytes (Fig. 8); this function of HBx was inhibited by IκB-SR, a mutant form of IκB-α that cannot be phosphorylated and degraded (11, 13, 22, 94). IκB-SR also blocked the ability of HBx to inhibit mitochondrial membrane depolarization, and HBx actually induced mitochondrial membrane depolarization in the presence of IκB-SR, similar to the results observed in the presence of CHX (Fig. 7). These results and those discussed above demonstrate that HBx expression has two different effects on mitochondrial membrane potential, depending on the status of NF-κB. It is possible that the variable, NF-κB-dependent effects of HBx on mitochondrial membrane potential might account for some of the discrepant results that have been published; the levels of NF-κB are decreased in some established liver cell lines compared to in primary hepatocytes (71, 84, 89). In addition, these results suggest that HBx modulation of mitochondrial membrane potential may change in hepatocytes if NF-κB levels are altered in response to different stimuli; signals that decrease NF-κB levels or activity in hepatocytes may cause HBx to change from an inhibitor of mitochondrial membrane depolarization to an activator of depolarization. The consequent effect of either mitochondrial membrane depolarization or protection against depolarization will likely affect hepatocyte physiology and contribute to HBV-associated disease. The factors that are stimulated by HBx-induced activation of NF-κB to prevent mitochondrial membrane depolarization are currently undefined.
While NF-κB activity was clearly implicated in protection of mitochondria from depolarization, we also wanted to determine how HBx could induce mitochondrial membrane depolarization. The results from our previous studies suggested a role for HBx in the regulation of the MPTP (9, 58). To test whether HBx modulated the MPTP as a means for inducing mitochondrial membrane depolarization, we treated HBx-transfected hepatocytes with CsA, an MPTP inhibitor. Combined treatment with CHX and CsA abrogated the HBx-induced depolarization of mitochondrial membrane potential. This result implies that in the absence of protein synthesis, HBx modulation of mitochondrial membrane potential is affected by HBx regulation of the MPTP (Fig. 7). FK506, a calcineurin inhibitor, did not affect HBx-induced mitochondrial membrane depolarization, suggesting that the result observed with CsA, which can inhibit both calineurin and MPTP activity, is due to inhibition of MPTP activity (Fig. 7). Overall, these studies show that HBx induction of mitochondrial membrane depolarization involves modulation of the MPTP. While it is likely that modulation of the MPTP by HBx requires its direct association with mitochondria and VDAC, attempts to identify a mutant HBx that does not localize to mitochondria have been confounded by significant differences in the stabilities of various truncated versions of HBx (A. J. Clippinger and M. J. Bouchard, unpublished observations). In addition, previous studies have suggested that there may be more than one mitochondrial localization signal in HBx. In these studies, mutant HBx proteins were used to map the region of HBx required for mitochondrial localization in Huh7 cells (36, 84, 89). One study showed that a putative mitochondrial localization signal for HBx was located between amino acids 54 and 70; however, another study showed that a mitochondrial localization signal was also located between amino acids 68 and 117 of HBx. Considering that HBx contains only 154 amino acids, these studies, and our observations of significant variations in the stability of truncated versions of HBx, may make it difficult to isolate a mutant HBx that is expressed at levels similar to wild-type HBx and is unable to localize to mitochondria. Cumulatively, our results demonstrate that HBx localizes to the outer mitochondrial membrane of mitochondria isolated from cultured primary rat hepatocytes and that in these hepatocytes, HBx prevents mitochondrial depolarization by activating NF-κB or, in the absence of NF-κB activity, modulates the MPTP to induce mitochondrial membrane depolarization.
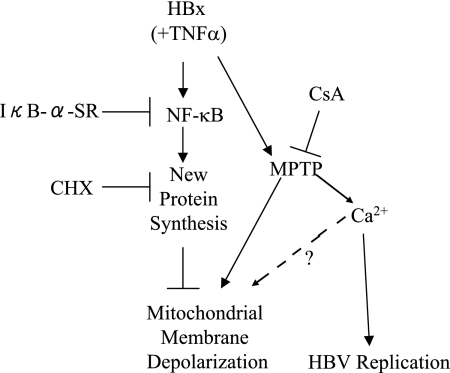
Although one dissenting result has been published (102), studies from four groups suggest that many HBx activities, including regulation of HBV replication, may result from HBx modulation of cytosolic calcium levels (9, 14, 63, 92). We recently reported that HBx expression elevates cytosolic calcium levels in HepG2 cells and that this activity could be blocked by CsA treatment (58). The MPTP is one component of mitochondria that is involved in the regulation of mitochondria and cytosolic calcium levels. Our previous studies suggest that HBx can modulate the MPTP even if protein synthesis is not inhibited and that HBx regulates cytosolic calcium levels through modulation of the MPTP. Those studies combined with the studies reported here allow us to generate a possible model for HBx regulation of cytosolic calcium levels, mitochondrial membrane potential, and HBV replication (Fig. 9). In this model, elevation of cytosolic calcium levels by HBx is an important HBx activity that regulates HBV replication, and this HBx activity involves modulation of the MPTP. Left unchecked, elevated cytosolic calcium levels can further activate the MPTP and induce mitochondrial membrane depolarization, which could negatively affect cellular metabolism and activate apoptotic pathways (5). To counter this effect, HBx also activates NF-κB, and this prevents mitochondrial membrane depolarization. It is likely that the combined effect of these HBx activities facilitates continued MPTP-regulated calcium signaling while preventing the detrimental effects that this might have for hepatocytes. In this regard, HBx may be functioning similarly to a cytokine such as TNF-α which can activate competing signals; the ultimate consequence of these signals will depend on the specific cell type stimulated. Our studies may also provide some clarification for discrepant results of HBx activities that have been reported and strengthen the argument that the precise consequence of HBx expression will depend on the status of signaling pathways and proteins within a cell. Importantly, we have demonstrated that in cultured primary rat hepatocytes, HBx modulates mitochondrial membrane potential by regulating both NF-κB activity and the MPTP. In the context of HBV replication in a hepatocyte, these activities may facilitate HBV replication while preventing mitochondrial membrane depolarization.
FIG. 9.
HBx regulation of mitochondrial membrane potential in primary rat hepatocytes. See Discussion for explanation.
In addition to facilitating HBV replication, HBx activation of NF-κB in hepatocytes and modulation of mitochondrial membrane potential may also have profound consequences for hepatocyte physiology. Various studies have implicated NF-κB activity in the development of liver cancer; activated NF-κB can inhibit apoptosis and enhance hepatocyte survival (reviewed in reference 39). In the context of a chronic HBV infection, HBx activation of NF-κB may provide a survival signal that eventually participates in the selection of transformed hepatocytes and the development of HCC. Mitochondrial dysfunction has also been correlated with chronic liver disease and carcinogenesis, and HBx regulation of mitochondrial membrane potential may contribute to activating pathways involved in cell transformation (45). One interesting possibility is that the status of NF-κB may change in the course of an HBV infection or in the process of hepatocyte transformation. If the activity of NF-κB is blocked in hepatocytes, HBx-induced depolarization of mitochondria could enhance activation of apoptotic pathways and eventually participate in the selection of hepatocytes that are resistant to proapoptotic signals. Therefore, both HBx activation of NF-κB and HBx modulation of the mitochondrial membrane potential could contribute to the development of HBV-associated HCC. Future studies will determine how these HBx activities influence hepatocyte signal transduction and survival pathways as well as HBV replication. It is quite likely that regulation of mitochondrial physiology by HBx will affect many cell functions, regulate HBV replication, and influence the development of HBV-associated HCC.
Acknowledgments
We thank Mauricio Reginato, Joseph Nickels, Maureen Murphy, and Timothy Block for discussions and advice.
This work was supported by NIH grants R01AI064844 to M.J.B. and F31AA016865 to A.J.C.
Footnotes
Published ahead of print on 30 April 2008.
REFERENCES
- 1.Andrisani, O., and S. Barnabas. 1999. The transcriptional function of the hepatitis B virus X protein and its role in hepatocarcinogenesis. Int. J. Oncol. 151-8. [DOI] [PubMed] [Google Scholar]
- 2.Anflous, K., O. Blondel, A. Bernart, M. Khrestchatisky, and R. Ventura-Clapier. 1998. Characterization of rat porin isoforms: cloning of a cardiac type-3 variant encoding an additional methionine at its putative N-terminal region. Biochim. Biophys. Acta 139947-50. [DOI] [PubMed] [Google Scholar]
- 3.Beasley, R., C.-C. Lin, L.-Y. Hwang, and C.-S. Chien. 1981. Hepatocellular carcinoma and hepatitis B virus: a prospective study of 22 707 men in Taiwan. Lancet ii1129-1133. [DOI] [PubMed] [Google Scholar]
- 4.Bergametti, F., D. Sitterlin, and C. Transy. 2002. Turnover of hepatitis B virus X protein is regulated by damaged DNA-binding complex. J. Virol. 766495-6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernardi, P. 1999. Mitochondrial transport and cations: channels, exchangers, and permeability transition. Physiol. Rev. 791127-1155. [DOI] [PubMed] [Google Scholar]
- 6.Bingham, M. J., T. J. Ong, K. H. Summer, R. B. Middleton, and H. J. McArdle. 1998. Physiologic function of the Wilson disease gene product, ATP7B. Am. J. Clin. Nutr. 67982S-987S. [DOI] [PubMed] [Google Scholar]
- 7.Block, G., J. Locker, W. Bowen, B. Petersen, S. Katyal, S. Strom, T. Riley, T. Howard, and G. Michalopoulos. 1996. Population expression, clonal growth, and specific differentiation patterns in primary cultures of hepatocytes induced by HGF/SF, EGF and TGFα in a chemically defined (HGM) medium. J. Cell Biol. 1321133-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blum, H., Z.-S. Zhang, E. Galun, F. von Weizsacker, B. Garner, T. Liang, and J. Wands. 1992. Hepatitis B virus X protein is not central to the viral life cycle in vitro. J. Virol. 661223-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouchard, M., L.-H. Wang, and R. Schneider. 2001. Calcium signaling by HBx protein in hepatitis B virus DNA replication. Science 2942376-2378. [DOI] [PubMed] [Google Scholar]
- 10.Bouchard, M. J., and R. J. Schneider. 2004. The enigmatic X gene of hepatitis B virus. J. Virol. 7812725-12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brockman, J., D. Scherer, T. McKinsey, S. Hall, X. Qi, W. Lee, and D. Ballard. 1995. Coupling of a signal response domain to IκB-alpha to multiple pathways for NF-κB activation. Mol. Cell. Biol. 152809-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Broekemeier, K., M. Dempsey, and D. Pfeiffer. 1989. Cyclosporin A is a potent inhibitor of the inner membrane permeability transition in liver mitochondria. J. Biol. Chem. 2647826-7830. [PubMed] [Google Scholar]
- 13.Brown, K., S. Gerstberger, L. Carlson, G. Franzoso, and U. Siebenlist. 1995. Control of IκB-α proteolysis by site-specific, signal-induced phosphorylation. Science 2671485-1488. [DOI] [PubMed] [Google Scholar]
- 14.Chami, M., D. Ferrari, P. Nicotera, P. Paterlini-Brechot, and R. Rizzuto. 2003. Caspase-dependent alterations of Ca2+ signaling in the induction of apoptosis by hepatitis B virus X protein. J. Biol. Chem. 27831745-31755. [DOI] [PubMed] [Google Scholar]
- 15.Chang, S. F., H. J. Netter, E. Hildt, R. Schuster, S. Schaefer, Y. C. Hsu, A. Rang, and H. Will. 2001. Duck hepatitis B virus expresses a regulatory HBx-like protein from a hidden open reading frame. J. Virol. 75161-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen, H., S. Kaneko, R. Girones, R. Anderson, W. Hornbuckle, B. Tennant, P. Cote, J. Gerin, R. Purcell, and R. Miller. 1993. The woodchuck hepatitis virus X gene is important for establishment of virus infection in woodchucks. J. Virol. 671218-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen, J., and A. Siddiqui. 2007. Hepatitis B virus X protein stimulates the mitochondrial translocation of Raf-1 via oxidative stress. J. Virol. 816757-6760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chirillo, P., M. Falco, P. L. Puri, M. Artini, C. Balsano, M. Levrero, and G. Natoli. 1996. Hepatitis B virus pX activates NF-κB-dependent transcription through a Raf-independent pathway. J. Virol. 70641-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chitturi, S., and G. C. Farrell. 2001. Etiopathogenesis of nonalcoholic steatohepatitis. Semin. Liver Dis. 2127-41. [DOI] [PubMed] [Google Scholar]
- 20.Crabtree, G., and E. Olson. 2002. NFAT signaling: choreographing the social lives of cells. Cell 109S67-S79. [DOI] [PubMed] [Google Scholar]
- 21.Dandri, M., P. Schirmacher, and C. Rogler. 1996. Woodchuck hepatitis virus X protein is present in chronically infected woodchuck liver and woodchuck hepatocellular carcinomas which are permissive for viral replication. J. Virol. 705246-5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DiDonato, J., F. Mercurio, C. Rosette, J. Wu-Li, H. Suyang, S. Ghosh, and M. Karin. 1996. Mapping of the inducible IκB phosphorylation sites that signal its ubiquitination and degradation. Mol. Cell. Biol. 161295-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diot, C., P. Gripon, M. Rissel, and C. Guguen-Guillouzo. 1992. Replication of hepatitis B virus in differentiated adult rat hepatocytes transfected with cloned viral DNA. J. Med. Virol. 3693-100. [DOI] [PubMed] [Google Scholar]
- 24.Dohi, T., E. Beltrami, N. Wall, J. Plescia, and D. Altieri. 2004. Mitochondrial survivin inhibits apoptosis and promotes tumorigenesis. J. Clin. Investig. 1141117-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doria, M., N. Klein, R. Lucito, and R. Schneider. 1995. The hepatitis B virus HBx protein is a dual specificity cytoplasmic activator of Ras and nuclear activator of transcription factors. EMBO J. 144747-4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernandez, E., and G. L. Craviso. 1999. Protein synthesis blockade differentially affects the degradation of constitutive and nicotinic receptor-induced tyrosine hydroxylase protein level in isolated bovine chromaffin cells. J. Neurochem. 73169-178. [DOI] [PubMed] [Google Scholar]
- 27.Ganem, D. 1996. Hepadnaviridae and their replication, p. 2703-2785. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, PA.
- 28.Giodini, A., and P. Cresswell. 2008. Hsp90-mediated cytosolic refolding of exogenous proteins internalized by dendritic cells. EMBO J. 27201-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez-Amaro, R., C. Garcia-Monzon, L. Garcia-Buey, R. Moreno-Otero, J. Alonso, E. Yague, J. Pivel, M. Lopez-Cabrera, E. Fernandez-Ruiz, and F. Sanchez-Madrid. 1994. Induction of tumor necrosis factor α production by human hepatocytes in chronic viral hepatitis. J. Exp. Med. 179841-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goping, I., A. Gross, J. Lavoie, M. Nguyen, R. Jemmerson, K. Roth, S. Korsmeyer, and G. Shore. 1998. Regulated targeting of Bax to mitochondria. J. Cell Biol. 143207-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gotow, T., M. Shibata, S. Kanamori, O. Tokuno, Y. Ohsawa, N. Sato, K. Isahara, Y. Yayoi, T. Watanabe, J. F. Leterrier, M. Linden, E. Kominami, and Y. Uchiyama. 2000. Selective localization of Bcl-2 to the inner mitochondrial and smooth endoplasmic reticulum membranes in mammalian cells. Cell Death Differ. 7666-674. [DOI] [PubMed] [Google Scholar]
- 32.Graham, J. 1999. Isolation of mitochondria from tissues and cells by differential centrifugation, p. 3.3.1-3.3.15. In J. S. Bonifacino, M. Dasso, J. B. Harford, J. Lippincott-Schwartz, and K. M. Yamada (ed.), Current protocols in cell biology. John Wiley & Sons, Inc., New York, NY. [DOI] [PubMed]
- 33.Grub, S., E. Persohn, W. Trommer, and A. Wolf. 2000. Mechanisms of cyclosporine A-induced apoptosis in rat hepatocytes in primary cultures. Toxicol. Appl. Pharmacol. 163209-220. [DOI] [PubMed] [Google Scholar]
- 34.Henkler, F., J. Hoare, N. Waseem, R. Goldin, M. McGarvey, R. Koshy, and I. King. 2001. Intracellular localization of the hepatitis B virus HBx protein. J. Gen. Virol. 82871-882. [DOI] [PubMed] [Google Scholar]
- 35.Huang, J., J. Kwong, E. Sun, and T. Liang. 1996. Proteosome complex as a potential cellular target of hepatitis B virus X protein. J. Virol. 705582-5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huh, K., and A. Siddiqui. 2002. Characterization of the mitochondrial association of hepatitis B virus X protein, HBx. Mitochondrion 1349-359. [DOI] [PubMed] [Google Scholar]
- 37.James, S. J., I. P. Pogribny, M. Pogribna, B. J. Miller, S. Jernigan, and S. Melnyk. 2003. Mechanisms of DNA damage, DNA hypomethylation, and tumor progression in the folate/methyl-deficient rat model of hepatocarcinogenesis. J. Nutr. 1333740S-3747S. [DOI] [PubMed] [Google Scholar]
- 38.Johnson, B., E. Cepero, and L. Boise. 2000. Bcl-XL inhibits cytochrome c release but not mitochondrial depolarization during the activation of multiple death pathways by tumor necrosis factor. J. Biol. Chem. 27531546-31553. [DOI] [PubMed] [Google Scholar]
- 39.Karin, M. 2006. NF-κB and cancer: mechanisms and targets. Mol. Carcinogen. 45355-361. [DOI] [PubMed] [Google Scholar]
- 40.Keasler, V. V., A. J. Hodgson, C. R. Madden, and B. L. Slagle. 2007. Enhancement of hepatitis B virus replication by the regulatory X protein in vitro and in vivo. J. Virol. 812656-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim, S., H.-Y. Kim, S. Lee, S. Kim, S. Sohn, K. Kim, and H. Cho. 2007. Hepatitis B Virus X Protein induces perinuclear mitochondrial clustering in microtubule- and dynein-dependent manners. J. Virol. 811714-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koch, A., Y. Yoon, N. Bonekamp, M. McNiven, and M. Schrader. 2005. A role for Fis1 in both mitochondrial and peroxisomal fission in mammalian cells. Mol. Biol. Cell 165077-5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koike, K., K. Moriya, S. Iino, H. Yotsuyanagi, Y. Endo, T. Miyamura, and K. Kurokawa. 1994. High-level expression of hepatitis B virus HBx gene and hepatocellular carcinogenesis in transgenic mice. Hepatology 19810-819. [PubMed] [Google Scholar]
- 44.Koike, K., K. Moriya, H. Yotsuyanagi, Y. Shintani, H. Fujie, T. Tsutsumi, and S. Kimura. 1998. Compensatory apoptosis in preneoplastic liver of a transgenic mouse model for viral hepatocarcinogenesis. Cancer Lett. 134181-186. [DOI] [PubMed] [Google Scholar]
- 45.Krahenbuhl, S., J. Stucki, and J. Reichen. 1989. Mitochondrial function in carbon tetrachloride-induced cirrhosis in the rat-qualitative and quantitative defects. Biochem. Pharmacol. 381583-1588. [DOI] [PubMed] [Google Scholar]
- 46.Kuchler, K., G. Daum, and F. Paltauf. 1986. Subcellular and submitochondrial localization of phospholipid-synthesizing enzymes in Saccharomyces cerevisiae. J. Bacteriol. 165901-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Latha, K., W. Zhang, N. Cella, H. Y. Shi, and M. Zhang. 2005. Maspin mediates increased tumor cell apoptosis upon induction of the mitochondrial permeability transition. Mol. Cell. Biol. 251737-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lauer, U., L. Wei, M. Lipp, P. Hans Hofschneider, and A. Kekule. 1994. The hepatitis B virus preS2/St transactivator utilizes AP-1 and other transcription factors for transactivation. Hepatology 1923-31. [PubMed] [Google Scholar]
- 49.Lee, D. K., S. H. Park, Y. Yi, S. G. Choi, C. Lee, W. T. Parks, H. Cho, M. P. de Caestecker, Y. Shaul, A. B. Roberts, and S. J. Kim. 2001. The hepatitis B virus encoded oncoprotein pX amplifies TGF-beta family signaling through direct interaction with Smad4: potential mechanism of hepatitis B virus-induced liver fibrosis. Genes Dev. 15455-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee, Y. I., J. M. Hwang, J. H. Im, N. S. Kim, D. G. Kim, D. Y. Yu, H. B. Moon, and S. K. Park. 2004. Human hepatitis B virus-X protein alters mitochondrial function and physiology in human liver cells. J. Biol. Chem. 27915460-15471. [DOI] [PubMed] [Google Scholar]
- 51.Lemasters, J., and E. Holmuhamedov. 2006. Voltage-dependent anion channel (VDAC) as mitochondrial governator—thinking outside the box. Biochim. Biophys. Acta 1762181-190. [DOI] [PubMed] [Google Scholar]
- 52.Leu, J., P. Dumont, M. Hafey, M. Murphy, and D. George. 2004. Mitochondrial p53 activates Bak and causes disruption of a Bak-Mcl1 complex. Nat. Cell Biol. 6443-450. [DOI] [PubMed] [Google Scholar]
- 53.Leupin, O., S. Bontron, C. Schaeffer, and M. Strubin. 2005. Hepatitis B virus X protein stimulates viral genome replication via a DDB1-dependent pathway distinct from that leading to cell death. J. Virol. 794238-4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lim, M., P. Lim, R. Gupta, and U. Boelsterli. 2006. Critical role of free cytosolic calcium, but not uncoupling, in mitochondrial permeability transition and cell death induced by diclofenac oxidative metabolites in immortalized human hepatocytes. Toxicol. Appl. Pharmacol. 217322-331. [DOI] [PubMed] [Google Scholar]
- 55.Lucito, R., and R. Schneider. 1992. Hepatitis B virus X protein activates transcription factor NF-κB without a requirement for protein kinase C. J. Virol. 66983-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Madden, C., M. Finegold, and B. Slagle. 2001. Hepatitis B virus X protein acts as a tumor promoter in development of diethylnitrosamine-induced preneoplastic lesions. J. Virol. 753851-3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mahe, Y., N. Mukaida, K. Kuno, M. Akiyama, N. Ikeda, K. Matsushima, and S. Murakami. 1991. Hepatitis B virus X protein transactivates human interleukin-8 gene through acting on nuclear factor kB and CCAAT/enhancer-binding protein-like cis-elements. J. Biol. Chem. 26613759-13763. [PubMed] [Google Scholar]
- 58.McClain, S., A. Clippinger, R. Lizzano, and M. Bouchard. 2007. Hepatitis B virus replication is associated with an HBx-dependent mitochondrion-regulated increase in cytosolic calcium levels. J. Virol. 8112061-12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meier, P., C. A. Scougall, H. Will, C. J. Burrell, and A. R. Jilbert. 2003. A duck hepatitis B virus strain with a knockout mutation in the putative X ORF shows similar infectivity and in vivo growth characteristics to wild-type virus. Virology 317291-298. [DOI] [PubMed] [Google Scholar]
- 60.Melegari, M., P.-P. Scaglioni, and J. Wands. 1998. Cloning and characterization of a novel hepatitis B virus x binding protein that inhibits viral replication. J. Virol. 721737-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Misumi, Y., Y. Misumi, K. Miki, A. Takatsuki, G. Tamura, and Y. Ikehara. 1986. Novel blockade by brefeldin A of intracellular transport of secretory proteins in cultured rat hepatocytes. J. Biol. Chem. 26111398-11403. [PubMed] [Google Scholar]
- 62.Mulichak, A., J. Wilson, K. Padmanabhan, and R. Garavito. 1998. The structure of mammalian hexokinase-1. Nat. Struct. Biol. 5555-560. [DOI] [PubMed] [Google Scholar]
- 63.Oh, J. C., D. L. Jeong, I. K. Kim, and S. H. Oh. 2003. Activation of calcium signaling by hepatitis B virus-X protein in liver cells. Exp. Mol. Med. 35301-309. [DOI] [PubMed] [Google Scholar]
- 64.Oh, Y., C. Alpuche-Aranda, E. Berthiaume, T. Jinks, S. Miller, and J. Swanson. 1996. Rapid and complete fusion of macrophage lysosomes with phagosomes containing Salmonella typhimurium. Infect. Immun. 643877-3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Orrenius, S., B. Zhivotovsky, and P. Nicotera. 2003. Regulation of cell death: the calcium-apoptosis link. Nat. Rev. Mol. Cell Biol. 4552-565. [DOI] [PubMed] [Google Scholar]
- 66.Paterlini, P., K. Poussin, M. Kew, D. Franco, and C. Brechot. 1995. Selective accumulation of the X transcript of hepatitis B virus in patients negative for hepatitis B surface antigen with hepatocellular carcinoma. Hepatology 21313-321. [PubMed] [Google Scholar]
- 67.Pedersen, P., J. Greenawalt, B. Reynafarje, J. Hullihen, G. Decker, J. Soper, and E. Eustamente. 1978. Preparation and characterization of mitochondria and submitochondrial particles of rat liver and liver-derived tissues. Methods Cell Biol. 20411-481. [DOI] [PubMed] [Google Scholar]
- 68.Poot, M., Y. Zhang, J. Kramer, K. Wells, L. Jones, D. Hanzel, A. Lugade, V. Singer, and R. Haugland. 1996. Analysis of mitochondrial morphology and function with novel fixable fluorescent stains. J. Histochem. Cytochem. 441363-1372. [DOI] [PubMed] [Google Scholar]
- 69.Puro, R., and R. Schneider. 2007. Tumor necrosis factor activates a conserved innate antiviral response to hepatitis B virus that destabilizes nucleocapsids and reduces nuclear viral DNA. J. Virol. 817351-7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rahmani, Z., K.-W. Huh, R. Lasher, and A. Siddiqui. 2000. Hepatitis B virus X protein colocalizes to mitochondria with human voltage-dependent anion channel, HVDAC3, and alters its transmembrane potential. J. Virol. 742840-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rahmani, Z., C. Maunoury, and A. Siddiqui. 1998. Isolation of a novel human voltage-dependent anion channel gene. Eur. J. Hum. Genet. 6337-340. [DOI] [PubMed] [Google Scholar]
- 72.Reers, M., S. Smiley, C. Mottola-Hartshorn, A. Chen, M. Lin, and L. Chen. 1995. Mitochondrial membrane potential monitored by JC-1 dye. Methods Enzymol. 260406-417. [DOI] [PubMed] [Google Scholar]
- 73.Rodriguez-Garay, E. A. 2003. Cholestasis: human disease and experimental animal models. Ann. Hepatol. 2150-158. [PubMed] [Google Scholar]
- 74.Runge, D., D. M. Runge, D. Jager, K. A. Lubecki, D. Beer Stolz, S. Karathanasis, T. Kietzmann, S. C. Strom, K. Jungermann, W. E. Fleig, and G. K. Michalopoulos. 2000. Serum-free, long-term cultures of human hepatocytes: maintenance of cell morphology, transcription factors, and liver-specific functions. Biochem. Biophys. Res. Commun. 26946-53. [DOI] [PubMed] [Google Scholar]
- 75.Sabherwal, N., K. Schneider, R. Blaschke, A. Marchini, and G. Rappold. 2004. Impairment of SHOX nuclear localization as a cause for Leri-Weill syndrome. J. Cell Sci. 1173041-3048. [DOI] [PubMed] [Google Scholar]
- 76.Salvioli, S., A. Ardizzoni, C. Franceschi, and A. Cossarizza. 1997. JC-1, but not DiOC6(3) or rhodamine 123, is a reliable fluorescent probe to assess delta psi changes in intact cells: implications for studies on mitochondrial functionality during apoptosis. FEBS Lett. 41177-82. [DOI] [PubMed] [Google Scholar]
- 77.Salvioli, S., J. Dobrucki, L. Moretti, L. Troiano, M. Garcia-Fernandez, M. Pinti, J. Pedrazzi, C. Franceschi, and A. Cossarizza. 2000. Mitochondrial heterogeneity during staurosporine-induced apoptosis in HL60 cells: analysis at the single cell and single organelle level. Cytometry 40189-197. [DOI] [PubMed] [Google Scholar]
- 78.Sardanelli, A. M., A. Signorile, R. Nuzzi, D. D. Rasmo, Z. Technikova-Dobrova, Z. Drahota, A. Occhiello, A. Pica, and S. Papa. 2006. Occurrence of A-kinase anchor protein and associated cAMP-dependent protein kinase in the inner compartment of mammalian mitochondria. FEBS Lett. 5805690-5696. [DOI] [PubMed] [Google Scholar]
- 79.Scaglioni, P., M. Melegari, and J. Wands. 1997. Biological properties of hepatitis B viral genomes with mutations in the precore promoter and precore open reading frame. Virology 233374-381. [DOI] [PubMed] [Google Scholar]
- 80.Scaglioni, P., M. Melegari, and J. Wands. 1997. Posttranscriptional regulation of hepatitis B virus replication by the precore protein. J. Virol. 71345-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schek, N., R. Bartenschlager, C. Kuhn, and H. Schaller. 1991. Phosphorylation and rapid turnover of hepatitis B virus X protein expressed in HepG2 cells from recombinant vaccinia virus. Oncogene 61735-1744. [PubMed] [Google Scholar]
- 82.Seeger, C., F. Zoulim, and W. Mason. 2007. Hepadnaviruses, p. 2977-3029. In D. M. Knipe and P. M. Howley (ed.), Field's virology, vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 83.Seglen, P. 1993. Isolation of hepatocytes by collagenase perfusion. Methods Toxicol. 1231-243. [Google Scholar]
- 84.Shirakata, Y., and K. Koike. 2003. Hepatitis B virus X protein induces cell death by causing loss of mitochondrial membrane potential. J. Biol. Chem. 27822071-22078. [DOI] [PubMed] [Google Scholar]
- 85.Slagle, B. L., T. H. Lee, D. Medina, M. J. Finegold, and J. S. Butel. 1996. Increased sensitivity to the hepatocarcinogen diethylnitrosamine in transgenic mice carrying the hepatitis B virus X gene. Mol. Carcinog. 15261-269. [DOI] [PubMed] [Google Scholar]
- 86.Sprinzl, M. F., H. Oberwinkler, H. Schaller, and U. Protzer. 2001. Transfer of hepatitis B virus genome by adenovirus vectors into cultured cells and mice: crossing the species barrier. J. Virol. 755108-5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Su, F., and R. Schneider. 1996. Hepatitis B virus HBx protein activates transcription factor NF-κB by acting on multiple cytoplasmic inhibitors of rel-related proteins. J. Virol. 704558-4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Su, F., and R. Schneider. 1997. Hepatitis B virus HBx protein sensitizes cells to apoptotic killing by tumor necrosis factor α. Proc. Natl. Acad. Sci. USA 948744-8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Takada, S., Y. Shirakata, N. Kaneniwa, and K. Koike. 1999. Association of hepatitis B virus X protein with mitochondria causes mitochondrial aggregation at the nuclear periphery, leading to cell death. Oncogene 186965-6973. [DOI] [PubMed] [Google Scholar]
- 90.Tanaka, Y., F. Kanai, T. Kawakami, K. Tateishi, H. Ijichi, T. Kawabe, Y. Aradawa, T. Kawakami, T. Nishimura, Y. Shirakata, K. Koike, and M. Omata. 2004. Interaction of the hepatitis B virus X protein (HBx) with heat shock protein 60 enhances HBx-mediated apoptosis. Biochem. Biophys. Res. Commun. 318461-469. [DOI] [PubMed] [Google Scholar]
- 91.Tang, H., L. Delgermaa, F. Huang, N. Oishi, L. Liu, F. He, L. Zhao, and S. Murakami. 2005. The transcriptional transactivation function of HBx protein is important for its augmentation role in hepatitis B virus replication. J. Virol. 795548-5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tarn, C., L. Zou, R. Hullinger, and O. Andrisani. 2002. Hepatitis B virus X protein activates the p38 mitogen-activated protein kinase pathway in dedifferentiated hepatocytes. J. Virol. 769763-9772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Terada, H. 1990. Uncouplers of oxidative phosphorylation. Environ. Health Perspect. 87213-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Traenckner, E., H. Pahl, T. Henkel, K. Schnidt, S. Wilk, and P. Baeuerie. 1995. Phosphorylation of human IκB-alpha on serines 32 and 36 controls IκB-alpha proteolysis and NF-κB activation in response to diverse stimuli. EMBO J. 141995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wallach, D., E. Varfolomeev, N. Malinin, Y. Gotsev, A. Kovalenko, and M. Boldin. 1999. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu. Rev. Immunol. 17331-367. [DOI] [PubMed] [Google Scholar]
- 96.Waris, G., K.-W. Huh, and A. Siddiqui. 2001. Mitochondrially associated hepatitis B virus X protein constitutively activates transcription factors STAT-3 and NF-κB via oxidative stress. Mol. Cell. Biol. 217721-7730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.WHO. 2000, posting date. Hepatitis B fact sheet. http://www.who.int/mediacentre/factsheets/fs204/en/print.html.
- 98.Wollersheim, M., U. Debelka, and P. Hofschneider. 1988. A transactivating function encoded in the hepatitis B virus X gene is conserved in the integrated state. Oncogene 3545-552. [PubMed] [Google Scholar]
- 99.Yang, M. X., and A. I. Cederbaum. 1997. Characterization of cytochrome P4502E1 turnover in transfected HepG2 cells expressing human CYP2E1. Arch. Biochem. Biophys. 34125-33. [DOI] [PubMed] [Google Scholar]
- 100.Yonashiro, R., S. Ishido, K. Shinkou, T. Fukuda, E. Goto, Y. Matsuki, M. Ohmura-Hoshino, K. Sada, H. Hotta, H. Yamamura, R. Inatome, and S. Yanagi. 2006. A novel mitochondrial ubiquitin ligase plays a critical role in mitochondrial dynamics. EMBO J. 253618-3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yu, D. Y., H. B. Moon, J. K. Son, S. Jeong, S. L. Yu, H. Yoon, Y. M. Han, C. S. Lee, J. S. Park, C. H. Lee, B. H. Hyun, S. Murakami, and K. K. Lee. 1999. Incidence of hepatocellular carcinoma in transgenic mice expressing the hepatitis B virus X-protein. J. Hepatol. 31123-132. [DOI] [PubMed] [Google Scholar]
- 102.Zhang, Z., U. Protzer, Z. Hu, J. Jacob, and T. J. Liang. 2004. Inhibition of cellular proteasome activities enhances hepadnavirus replication in an HBX-dependent manner. J. Virol. 784566-4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang, Z., N. Torii, Z. Hu, J. Jacob, and T. Liang. 2001. X-deficient woodchuck hepatitis virus mutants behave like attenuated viruses andinduce protective immunity in vivo. J. Clin. Investig. 1081523-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ziemiecki, A., R. R. Friis, and H. Bauer. 1982. Half-life of the Rous sarcoma virus transforming protein pp60src and its associated kinase activity. Mol. Cell. Biol. 2355-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zoulim, F., J. Saputelli, and C. Seeger. 1994. Woodchuck hepatitis virus X protein is required for viral infection in vivo. J. Virol. 682026-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]