Abstract
For influenza viruses to become infectious, the proteolytic cleavage of hemagglutinin (HA) is essential. This usually is mediated by trypsin-like proteases in the respiratory tract. The binding of plasminogen to influenza virus A/WSN/33 leads to the cleavage of HA, a feature determining its pathogenicity and neurotropism in mice. Here, we demonstrate that plasminogen also promotes the replication of other influenza virus strains. The inhibition of the conversion of plasminogen into plasmin blocked influenza virus replication. Evidence is provided that the activation of plasminogen is mediated by the host cellular protein annexin II, which is incorporated into the virus particles. Indeed, the inhibition of plasminogen binding to annexin II by using a competitive inhibitor inhibits plasminogen activation into plasmin. Collectively, these results indicate that the annexin II-mediated activation of plasminogen supports the replication of influenza viruses, which may contribute to their pathogenicity.
Influenza A viruses (IAV) can be discriminated based on their surface glycoproteins, hemagglutinin (HA; 16 subtypes) and neuraminidase (NA; 9 subtypes). All IAV subtypes are present in aquatic birds, but only a few subtypes, such as H1N1 and H3N2, circulate in the human population. IAV in humans are among the most common infectious pathogens and cause annual outbreaks of respiratory tract infections. For the infection of a target cell by IAV, the cleavage of the precursor HA molecule into the HA1 and HA2 subunits by trypsin-like proteases is required (15). After entry into the cell, the virus genome is released from the endosome following a low-pH-dependent fusion event mediated by HA. This fusion occurs only when HA is cleaved. Most of the strains have a single arginine at the HA cleavage site, and these viruses can replicate only in a limited number of tissues (17), mostly the upper respiratory tract, due to the presence of extracellular proteases at this site (4). The amino acid sequences encompassing the cleavage site correlate with the virulence of the virus (32). Indeed, the HA of highly pathogenic H5 and H7 pantropic avian virus subtypes contain multiple basic amino acids (R-X-R/K-R) at the junction of HA1 and HA2 that can be cleaved by an intracellular subtilisin-type enzyme, such as furin. Dissemination from the original site of infection is a virulence factor for highly pathogenic IAV, and it was reported that H5N1 human infection can lead to a fatal clinical outcome without signs of respiratory illness (1, 6, 35). Occasionally, viruses with a cleavage site consisting of a single arginine replicate outside the respiratory tract and cause mortality without signs of respiratory failure (34). In these cases, HA cleavage is essential for viral pathogenicity and replication outside the respiratory tract (15, 17, 31). IAV of the H3N2 subtype have been associated with neurologic complications of infection, although the underlying mechanism has not been elucidated (24). For the A/WSN/33 strain (H1N1), it has been demonstrated that plasminogen (PLG), which is abundantly present in plasma (14), can compensate for the absence of trypsin in vitro and in vivo in mice (12, 13). The conversion of PLG into plasmin, which possesses a trypsin-like protease activity, would provide an alternative mechanism for the cleavage of the HA molecule and, in mice, contribute to the dissemination of the virus and efficient replication in the brain (12, 13). For this particular strain, it was shown that the PLG binding activity was mediated through its NA and that a carboxy-terminal lysine and the lack of an oligosaccharide chain at position 146 of NA were essential for PLG binding (12, 13, 22). The aim of the present study was to investigate whether IAV strains other than A/WSN/33 have the ability to use PLG for the proteolytic activation of their HA. We report here that, in addition to influenza virus A/WSN/33, other human and avian IAV strains, including those of the H3 subtype, can use the conversion of PLG into plasmin for their replication. Since these other viruses do not have the specific carboxy-terminal lysine and do have an N-linked glycosylation site at position 146 on their NA, they cannot bind PLG through their NA and, thus, must have an alternative way to bind and activate PLG. We report that annexin II (A2), a host cellular protein that can bind and activate PLG, is associated with purified IAV particles. Furthermore, it was demonstrated that the presence of A2 in influenza virus particles can indeed facilitate the conversion of PLG into plasmin. Collectively, these results indicate that the conversion of PLG into plasmin provides the protease activity necessary for the cleavage of the precursor HA molecule and, thus, plays a role in the replication cycle and pathogenicity of IAV. Since the cellular host protein A2 that is incorporated into IAV particles can bind and activate PLG, this may be a common mechanism used by influenza viruses. The conversion of PLG into plasmin also may explain why some influenza viruses can replicate outside the respiratory tract in humans.
MATERIALS AND METHODS
Virus strains, cells, antibodies, and reagents.
The IAV A/WSN/33 (WSN/33; H1N1), A/PR/8/34 (PR/8/34; H1N1), A/Udorn/72 (Udorn/72; H3N2) (gifts from S. van der Werf, Pasteur Institute, Paris), and A/Turkey/Massachussets/65 (H6N2) (a gift from V. Jestin, AFSSA, Ploufragan, France) were used in the present study. The Madin-Darby canine kidney (MDCK; ATCC CCL 34) cell line was grown in minimum essential Eagle medium (EMEM; Cambrex, Belgium) supplemented with 5% fetal calf serum (FCS; Perbio, France), 2 mM l-glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin. For Western blot analyses, the following antibodies were used: anti-A2 (Santa Cruz Biotechnology, Heidelberg, Germany), anti-ERK (Cell Signaling Technology, Saint Quentin, France), anti-S100A10 (p11) (BD Biosciences, France), anti-NP (ATCC HB-65), anti-VP3 (a gift from N. Eterradossi, AFSSA, Ploufragan, France), anti-PLG (Kordia, The Netherlands), anti-NS1 (Santa Cruz Biotechnology, Heidelberg, Germany), and horseradish peroxidase-coupled antibodies directed against mouse, rabbit, or goat (P.A.R.I.S, Compiègne, France). Trypsin was purchased from Becton Dickinson (Sparks, MD), and PLG, 6-aminohexanoic acid (6-AHA), and lipoprotein(a) [Lp(a)] were from Sigma-Aldrich (Lyon, France). For immunogold labeling, a rabbit anti-A2 antibody (Biodesign, Saco, ME) was used.
Virus production.
MDCK cells were seeded at a density of 2 × 107 cells per 150-cm2 tissue culture flask and infected with IAV at a multiplicity of infection (MOI) of 10−3 in EMEM containing 1 μg/ml of trypsin. Two days postinfection, the supernatant was harvested and clarified by low-speed centrifugation (15 min at 3,600 × g and 4°C).
Virus purification.
Viruses were concentrated 100-fold by ultracentrifugation at 60,000 × g at 4°C for 105 min and resuspended in EMEM. Subsequently, they were purified by centrifugation in a 20 to 60% sucrose density gradient, a 20 to 45% sucrose velocity gradient, or a 14 to 60% iodixanol density gradient (Optiprep-Nycomed) for 2 h at 80,000 × g at 4°C. In some experiments, the three different techniques were used successively.
Virus composition analysis.
Purified viruses were loaded onto 4 to 12% NuPage Bis-Tris gels (Invitrogen). For protein identification by matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) analysis, gels were stained with Coomassie brilliant blue (CBB), proteins were excised from the gels and digested by trypsin, and proteins were identified. In parallel, proteins were analyzed by Western blot analysis as previously described (2, 27).
Kinetics of virus replication and inhibition by 6-AHA.
MDCK cells were infected with influenza viruses at an MOI of 10−3 in EMEM supplemented with 0.2 μM PLG. At various time points postinfection, supernatants were collected and virus titers were determined by plaque assay as previously described (28). The inhibition of virus replication by 6-AHA was analyzed after the infection of MDCK cells with IAV A/Turkey/Massachussets/65 or A/PR/8/34 at an MOI of 10−3 and A/Udorn/72 at an MOI of 10−5 in the presence of 10 μM PLG and various concentrations of 6-AHA. At various times postinfection, samples were collected and infectious virus titers were determined by plaque assay.
Inhibition of viral protein synthesis by 6-AHA using Western blot analysis.
MDCK cells were infected with IAV as described above in the presence of 5 μM PLG with or without 6-AHA (30 mg/ml). Forty-eight hours after infection, cells were lysed in lysis buffer (10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA, and 1% Triton X-100, vol/vol). Lysates were centrifuged at 14,000 × g for 40 min at 4°C, and proteins of the supernatants were analyzed by Western blotting using antibodies to NS1. The samples also were tested for the constitutive expression of tubulin, which was included as a control.
Detection of cell binding of PLG by flow cytometry.
MDCK cells were infected as described above at an MOI of 10−1. Eighteen hours postinfection, infected cells were incubated with human PLG in phosphate-buffered saline (PBS) (100 μg/ml) for 1 h at 4°C. Bound PLG was detected using goat anti-human PLG antibody (Kordia) and fluorescein isothiocyanate-labeled anti-goat antibody (Jackson ImmunoResearch) as previously described by flow cytometry analysis (29, 30).
NA sequence alignment.
The NA sequences of influenza A/Brevig Mission/1/18 (H1N1), A/WSN/1933 (H1N1), A/Puerto Rico/8/34 (H1N1), A/Udorn/1972 (H3N2), and A/turkey/Massachussets/3740/1965 (H6N2) viral strains (accession numbers AAF77036, AAA43397, NC_002018.1, AAA43419, and BAF48640, respectively) were retrieved from the NCBI nucleotide database. Sequences alignments were performed using the ClustalW server (http://www.ch.embnet.org/software/ClustalW.html). Since the amino acid at position 146 is subject to variability, the NA of the A/Puerto Rico/8/34 (H1N1) strain used in the present study was sequenced and determined to correspond to accession number NC_002018.1.
Immunogold labeling.
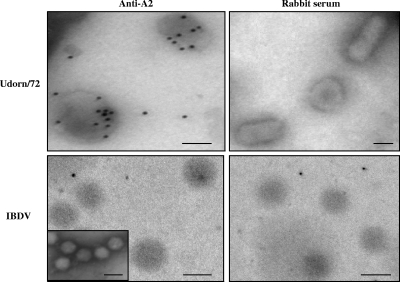
The immunogold labeling of A2 was performed with Optiprep gradient-purified virus particles of the A/Udorn/72 strain. Infectious bursal disease virus (IBDV) was used as a negative control. These virions were collected onto 300-mesh formwar-coated nickel grids and adsorbed on the grids for 30 min. After being extensively washed with PBS, virions were fixed for 5 min with a 1% paraformaldehyde solution. After being washed with PBS, immobilized virions were incubated for 2.5 h with the A2-specific polyclonal antibody (50 μg/ml in PBS-1% bovine serum albumin [BSA]) or control rabbit serum, washed two times for 3 min in PBS-1% BSA, and then incubated with goat anti-rabbit immunoglobulin G coupled to 10 nm colloidal gold particles (Tebu, France) for 30 min. After being washed, virus particles were fixed for 10 min with 2.5% glutaraldehyde in PBS and negatively stained using 1% water uranyl acetate for 1 min. Virions were examined with a Zeiss EM902 electron microscope operated at 80 kV (Carl Zeiss, France), and images were acquired with a charge-coupled device camera (Megaview III) and analyzed with ITEM software (MIMA2 platform; INRA-CRJ, Eloïse, France). Since immunogold labeling affects the structure of IBDV, electron microcopy was performed to confirm the identity of the structures as IBDV virions.
Conversion of PLG into plasmin by purified virus and inhibition experiments with 6-AHA or Lp(a).
Optiprep-purified IAV (106 infectious virions) were incubated for 3 h at 37°C with 1 μM PLG (A/PR/8/34 and A/Turkey/Massachussets/65) or 0.5 μM PLG (A/WSN/33 and A/Udorn/72) in the absence or presence of various concentrations of 6-AHA (2, 10, or 20 mg/ml) or Lp(a) (10 or 20 μM). Subsequently, PLG-to-plasmin conversion was tested by Western blot analysis using a specific antibody directed against all forms of PLG and its degradation products. As negative controls, purified IAV without PLG as well as purified IBDV (a nonenveloped virus) were used. 6-AHA is an inhibitor of PLG activation, whereas Lp(a) is a competitive blocker for PLG binding to A2 (11, 14).
RESULTS
Plasminogen supports replication of several IAV strains.
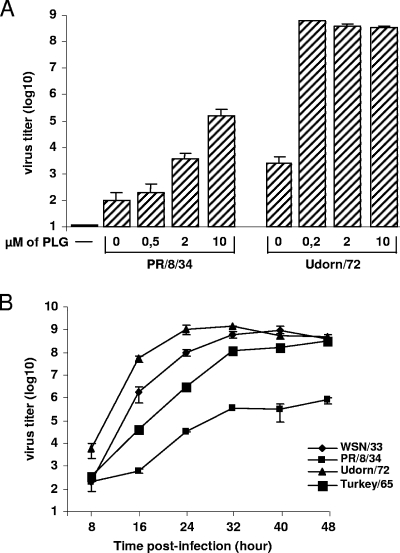
The aim of our study was to investigate whether IAV strains other than A/WSN/33 have the ability to use PLG for the proteolytic activation of their HA. For this purpose, MDCK cells were infected with IAV strains A/PR/8/34 and A/Udorn/72 at an MOI of 10−3 in the presence of different concentrations of PLG. After 24 h postinfection, infectious virus titers were determined by plaque assays. As shown in Fig. 1A, PLG facilitated A/PR/8/34 replication in a dose-dependent manner but at a lower level than that of the A/Udorn/72 strain. Thus, strains other than A/WSN/33 have the capacity to replicate in the presence of PLG.
FIG. 1.
PLG supports the replication of several IAV strains. (A) MDCK cells were left uninfected (−) or were infected with IAV A/PR/8/34 or A/Udorn/72 at an MOI of 10−3 in the absence or presence of various concentrations of PLG as indicated. After 24 h, infectious virus titers were determined by plaque assay. (B) MDCK cells were infected with A/WSN/33, A/PR/8/34, A/Udorn/72, and A/Turkey/Massachussets/65 at an MOI of 10−3 in the presence of 0.2 μM PLG. After the indicated times postinfection, the infectious virus titers in the culture supernatants were determined by plaque assay. Results show the mean values ± standard deviations from three independent experiments.
Since the average concentration of PLG in the serum is 2 μM (14), we performed growth kinetics experiments using 0.2 μM PLG. Thus, the levels of replication of IAV A/PR/8/34 (H1N1), A/Udorn/72 (H3N2), and A/Turkey/Massachussets/65 (H6N2) were compared to that of the A/WSN/33 strain in the absence or presence of 0.2 μM PLG. Infectious virus titers in the culture supernatants of infected MDCK cells were determined by plaque assay at various times postinfection. As shown in Fig. 1B, PLG supported the replication of all of the strains tested in a time course experiment, although the extent of PLG-dependent virus replication was strain dependent. Under our experimental conditions, the A/Udorn/72 (H3N2) strain replicated more efficiently than the others, including the A/WSN/33 strain.
Conversion of PLG into plasmin is necessary to support IAV replication.
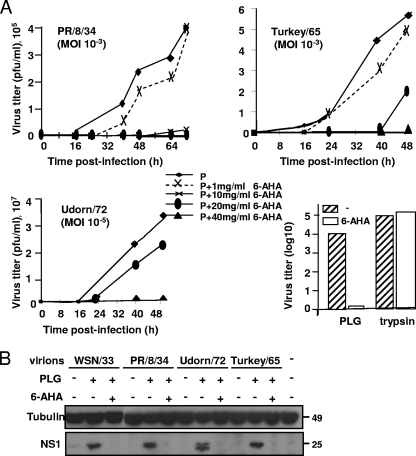
Plasmin is the enzymatically active form of its precursor, PLG. Thus, we next investigated whether the conversion of PLG into plasmin is required to support IAV replication. To this end, the effect of 6-AHA, an inhibitor of the PLG-to-plasmin conversion of IAV replication, was determined. As shown in Fig. 2A, the PLG-dependent replication of IAV A/PR/8/34, A/Udorn/72, and A/Turkey/Massachussets/65 was inhibited by 6-AHA in a dose-dependent fashion. The inhibition of PLG activation by 6-AHA was specific, since it did not affect the replication of IAV A/PR/8/34 in the presence of trypsin (Fig. 2A, lower right). Thus, our results show that plasmin generation from PLG is a replication-supporting factor used by several IAV strains.
FIG. 2.
Plasmin production is necessary for IAV replication. (A) MDCK cells were infected with IAV A/PR/8/34 at an MOI of 10−3, A/Turkey/Massachussets/65 at an MOI of 10−3, and A/Udorn/72 at an MOI of 10−5 in the presence of 10 μM PLG (P) and in the absence or presence of various concentrations of 6-AHA, as indicated. After the indicated times postinfection, the infectious virus titers in the culture supernatants were determined by plaque assay. The specificity of the 6-AHA inhibitor was determined on MDCK cells infected with IAV A/PR/8/34 (H1N1) at an MOI of 10−3. 6-AHA inhibits IAV replication in the presence of PLG (after 72 h) but had no effect when IAV were produced in the presence of 1 μg/ml trypsin (after 24 h). (B) Western blot analysis of lysates from MDCK cells left uninfected (−) or infected with the indicated IAV strains. Viral protein synthesis was assessed by Western blot analysis using the anti-NS1 antibodies and anti-tubulin as a control. The concentrations of PLG and 6-AHA used were 5 μM and 30 mg/ml, respectively. Numbers at the right of the figure refer to the molecular masses, in kilodaltons.
The nonstructural protein NS1 is not incorporated into virus particles but is synthesized intracellularly during influenza virus replication. To confirm that the conversion of PLG supports virus replication, the production of NS1 in PLG-treated MDCK cells infected with IAV A/WSN/33, A/PR/8/34, A/Udorn/72, and A/Turkey/Massachussets/65 in the presence or absence of 6-AHA also was tested by Western blot analysis. As shown in Fig. 2B, the nonstructural NS1 protein was detected in IAV-infected PLG-treated cells. The addition of 6-AHA to the culture fully blocked the synthesis of NS1 in cells infected with the respective viruses (Fig. 2B). The addition of 6-AHA did not affect the synthesis of the cellular protein tubulin, which was included as a control.
Collectively, we confirmed that the conversion of PLG into plasmin is needed to support IAV replication and viral protein synthesis.
PLG binding motif on NA in IAV strains.
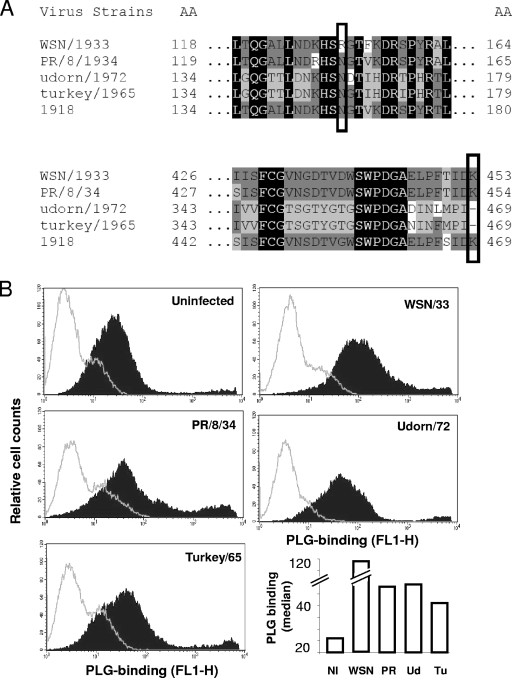
Previously it was demonstrated that PLG binds to the NA molecule of IAV A/WSN/33 and that a carboxy-terminal lysine and the lack of an oligosaccharide chain at position 146 determine this PLG binding capacity (12). We compared the relevant NA sequences of A/PR/8/34, A/Udorn/72, and A/Turkey/Massachussets/65 as well as all the other 7,311 NA sequences available to date in PubMed or Swissprot databases (not shown) to that of A/WSN/33 (Fig. 3A). Interestingly, only the NA sequence of influenza virus A/WSN/33 fulfilled the minimal requirement for binding PLG, which was previously characterized.
FIG. 3.
PLG binding to NA on the cell surface. (A) Partial sequence alignments of the NA of the indicated IAV strains. Boxes illustrate the amino acid at position 146 (top) and the carboxy-terminal amino acid residue (bottom). (B) MDCK cells were left uninfected (NI) or were infected with the indicated IAV strains. WSN, A/WSN/33; PR, A/PR/8/34; Ud, A/Udorn/72; and Tu, A/Turkey/Massachussets/65. Twelve hours after infection, cells were incubated with human PLG for 1 h, and flow cytometry analysis was performed to evaluate PLG binding to infected cells by using an anti-PLG antibody (closed histogram) or an isotype control (open histogram). The quantification of the median fluorescence intensity of PLG binding is shown in the bottom right panel.
Next, we tested PLG binding to IAV-infected cells, which was previously carried out by flow cytometry (12). First, our results show that PLG already binds to uninfected MDCK cells (Fig. 3B). MDCK cells infected with A/WSN/33 showed the strongest PLG binding activity in terms of median fluorescence intensity (Fig. 3B, lower right). This is in accordance with a previous report that showed that A/WSN/33 NA, but not the NA of other IAV, binds PLG on the cell surface of infected cells (12). However, MDCK cells infected with A/PR/8/34, A/Udorn/72, and A/Turkey/Massachussets/65 also showed an increase in PLG binding compared to that of uninfected cells. We concluded that A/WSN/33 displayed an increased binding of PLG through its NA in addition to the binding of PLG to infected cells by other means.
A2 is present in virus particles.
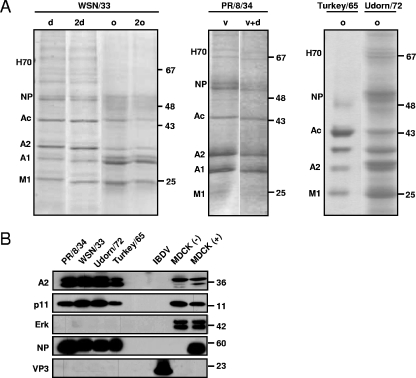
Since the NA of IAV A/PR/8/34, A/Udorn/72, and A/Turkey/Massachussets/65 are unlikely to bind PLG, we wished to investigate how these viruses can exploit PLG for their replication. Since it was shown that the host cellular PLG receptor A2 is associated with purified influenza virus particles (38), we hypothesized that A2 was involved in PLG-dependent virus replication. In order to test this hypothesis, IAV A/WSN/33, A/PR/8/34, A/Udorn/72, and A/Turkey/Massachussets/65 were purified from culture supernatants of infected MDCK cells. The resulting virions then were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and viral proteins were visualized by CBB staining (Fig. 4A) and identified by MALDI-TOF mass spectrometry analysis. In addition to the viral structural proteins NP and M1, we detected A2, host cell-derived heat shock protein, and actin in the purified virions. Thus, these results confirm that A2 is present in purified IAV particles (38).
FIG. 4.
Host cellular PLG receptor A2 is associated with purified influenza virus particles. (A) IAV A/WSN/33, A/PR/8/34, A/Turkey/Massachussets/65 (H6N2), and A/Udorn/72 were produced in MDCK cells and then purified by different techniques as described in Materials and Methods (d, sucrose density purification; 2d, two successive sucrose density purifications; o, Optiprep purification; 2o, two successive Optiprep purifications; v, sucrose velocity purification; and v+d, sucrose density purification followed by sucrose velocity purification). Virions then were subjected to a 4 to 12% SDS-PAGE, followed by CBB staining. The main bands were excised from the CBB-stained gel and then were subjected to MALDI-TOF analysis. The proteins identified, indicated at the left of the gel panel, are the following: heat shock protein 70 (H70), the viral NP protein (NP), actin (Ac), A2, annexin I (A1), and viral matrix protein 1 (M1). (B) Proteins of the indicated purified IAV and IBDV virions were separated in 4 to 12% SDS-PAGE, blotted onto nitrocellulose membranes, and probed with the anti-A2, anti-p11, anti-ERK, and anti-NP antibodies as well as with an anti-VP3 IBDV monoclonal antibody. Aliquots of total proteins from MDCK cells left uninfected (−) or infected (+) with the PR/8/34 IAV strain were used as controls. The numbers at the right of the figure refer to molecular masses, in kilodaltons.
In cells, A2 is found as a monomer (A2m) and as a heterotetramer (A2t) consisting of two A2 molecules and two molecules of the protein S100A10 (p11), which is a member of the S100 protein family (20). A2t is localized mainly in the endosomes and the plasma membrane, while A2m is mainly cytosolic (26). Thus, in order to investigate whether p11 also was incorporated into IAV, proteins incorporated into purified A/PR/8/34, A/WSN/33, A/Udorn/72, and A/Turkey/Massachussets/65 viruses were probed by Western blotting with anti-NP antibodies and antibodies to A2 and P11. The results confirmed the presence of A2 and p11 in all IAV strains tested in addition to the viral structural protein NP (Fig. 4B). In contrast, the cytoplasmic protein extracellular signal-regulated kinase (ERK) was not detected in the virions but was present in lysates of control MDCK and A/PR/8/34-infected MDCK cells, excluding a nonspecific incorporation of cellular proteins into the virions. Interestingly, A2 runs as a doublet in infected cells, which is not the case for uninfected cells, and we already noticed that different forms of A2 appeared after IAV infection (data not shown). The potential role of these different forms and/or posttranslational modifications in IAV pathogenicity is under investigation in our laboratory. Purified IBDV, a nonenveloped virus, was included as a negative control. The structural viral protein VP3 was readily detected, but A2 and p11 were not detected, indicating that IBDV particles did not incorporate these cellular proteins and that contaminants of cellular origin were not present. Collectively, these results demonstrate that A2 and p11 are incorporated into IAV particles, most likely as the heterotetramer A2t.
To further confirm that A2 is associated with IAV particles and is not a copurified contaminant of cellular origin, electron microscopic immunogold labeling was performed on purified virions by using an anti-A2 antibody. Immunogold staining showed that A2 is associated with IAV but not IBDV virions, which were used as a negative control (Fig. 5).
FIG. 5.
A2 is associated with purified influenza virus particles. Electron microscopic immunogold labeling was performed as described in Materials and Methods by using A2-specific antibodies or control rabbit serum as indicated. A purified IBDV preparation was used as a negative control to demonstrate the specific staining of A2 in the influenza virus particles. The insert in the bottom left shows an electron micrograph of the IBDV preparation to confirm the identity of virus particles seen after immunogold labeling. The bar is 60 nm.
A2 associated with IAV converts PLG into plasmin.
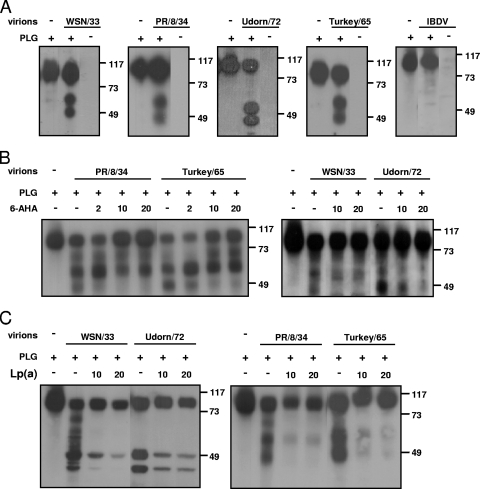
A2 is not only a receptor for PLG and plasmin but also is a mediator of the conversion of PLG into plasmin (14). Thus, in order to demonstrate that A2 incorporated into IAV can bind PLG and convert it into plasmin, purified virus preparations were incubated with PLG; the conversion into plasmin was monitored by Western blot analysis using an antibody that recognizes both PLG and plasmin. As shown in Fig. 6A, the incubation of PLG with all four purified influenza virus preparations tested resulted in the formation of two different forms of plasmin by purified virions (Fig. 6A). These two different forms of plasmin were previously found in other studies (21). The virus preparations did not contain PLG or plasmin. Furthermore, purified IBDV particles that do not contain A2 also failed to convert PLG into plasmin. The conversion of PLG into plasmin was inhibited by the addition of 6-AHA (Fig. 6B). With 6-AHA, only the formation of the low-molecular-weight protein was inhibited in a dose-dependent fashion. Stoichiometrically, this inhibition of plasmin formation coincided with the sustained presence of PLG after the reaction in the presence of 6-AHA (Fig. 6B).
FIG. 6.
Conversion of PLG into plasmin by purified IAV preparations. PLG was incubated (+) or left unincubated (−) at 37°C in presence or absence (−) of the indicated purified IAV strains. Proteins of the mixture then were detected by Western blot analysis using an antibody recognizing both PLG (∼100 kDa) and plasmin (∼55 kDa). (A) IBDV virions, used as a negative control, do not convert PLG into plasmin. The numbers at the right of the figure refer to the molecular masses, in kilodaltons. (B) Inhibition of PLG conversion by the 6-AHA inhibitor. (C) Inhibition of PLG conversion by the Lp(A) inhibitor.
We also used the inhibitor Lp(A), which binds to A2 and can compete with PLG for binding to A2. As shown in Fig. 6C, the addition of Lp(A) blocked the conversion of PLG into plasmin by all four viruses tested, which demonstrated that A2 incorporated into influenza virus particles is involved in the conversion of PLG into plasmin.
DISCUSSION
In the present paper, we have demonstrated that, in addition to the IAV strain A/WSN/33 (12), other influenza virus strains can use PLG for their replication. We also showed that the cellular protein A2 is incorporated into the virus particles. The presence of A2 in the virus particles provides the virus a means to convert PLG into plasmin, which could act as an alternative protease for the cleavage of the HA precursor molecule into the HA1 and HA2 subunits. The plasmin activity may not only contribute to enhancement of infectiousness of the virus but also increase the invasiveness of the virus in vivo, since plasmin also plays a role in the degradation of fibrin-rich extracellular matrices in the context of physiological cell migration (19). However, our results are laboratory observations, and further research is required to demonstrate that the mechanism described here plays a role in the invasiveness of IAV in vivo. The NA of A/WSN/33 definitely contributes to the replication of this strain in the presence of PLG, as described previously (12, 13), but whether it is indispensable or required for other influenza virus strains is a matter of debate. At present it is not clear whether the binding of PLG by A2 plays a role in the pathogenicity and tissue tropism of influenza viruses in vivo. Epithelial cells express A2 (3), and whether influenza viruses derived from these cells utilize the A2-PLG-dependent mechanism for their replication currently is under investigation in our laboratory.
A2 is a cellular protein that promotes the formation of lipid microdomains, which are necessary for the budding of virus from infected host cells. The viral envelope of influenza viruses is derived from the host cell plasma membrane, and thus it is likely that enveloped viruses incorporate annexin, which is present in the plasma membrane of the host cell from which the viruses bud. We were able to detect A2, as well as actin, in highly purified influenza virus preparations, which is in concordance with results obtained by others (38). A2, which is a member of the annexin protein family of Ca2+-dependent membrane binding, also was associated with other enveloped viruses, like cytomegalovirus and human immunodeficiency virus type 1 (5, 39). This suggests that the acquisition of A2 from the host cell membrane is a property shared by many enveloped viruses. To further confirm that A2 is present in IAV virions, immunogold labeling of the virus preparations was performed. In purified IBDV preparations, neither A2 nor the biological activity associated with A2 (the conversion of PLG into plasmin) was detected, indicating that it is unlikely that cellular contaminants were responsible for the detection of A2, although we cannot rule out the presence of cellular contaminants completely, since immunogold labeling also was observed not to be associated with virus-like particles. As indicated above, A2 can bind PLG and convert it into plasmin (16). Thus, the incorporation of A2 into IAV particles provides the virus a way to use PLG as an alternative source of protease activity to cleave its HA precursor molecule, which is essential for the virus to become infectious. In addition, the capacity to use PLG could allow replication outside of the respiratory tract, as was demonstrated for the neurotropic influenza virus A/WSN/33 in mice. Indeed, this virus was able to replicate efficiently in vitro in the absence of trypsin but in the presence of PLG. The efficient use of PLG was attributed to unique features in the amino acid sequence of its NA (12, 13). It was shown that other viruses, which failed to share the properties of NA with influenza virus A/WSN/33, could not exploit PLG for their replication. Our data are in contrast to these earlier findings. We also demonstrated that influenza viruses that lack a carboxy-terminal lysine and that do have an N-linked glycosylation site at position 146 of the NA can replicate in a PLG-dependent fashion. The discrepancy with the earlier findings might be explained by the source of PLG that was used. Goto and Kawaoka used FCS as a source of PLG, whereas we used a commercially available well-defined purified PLG (11, 12). Other factors in the composition of serum, like protease inhibitors, may have influenced the outcome of the experiments. Furthermore, different batches of serum may contain different concentrations of PLG. It is important to note that a concentration of 0.2 μM PLG supported the replication of all influenza virus strains tested, which is well below the physiologic concentration of PLG (14). It is possible that IAV A/WSN/33 uses PLG more efficiently than, e.g., A/PR/8/34 and A/Udorn/72, which could explain its high virulence in mice and its neurotropism. However, the mouse model may not be the most appropriate model to study the pathogenesis of human influenza viruses (37, 43). The extent of PLG-dependent replication varied for different IAV strains. In particular, influenza virus A/Udorn/72 (H3N2) replicated well in the presence of PLG. It is of interest that viruses of this subtype have been associated more often with neurologic complications in humans than other subtypes (24). However, only influenza virus A/WSN/33 was shown to be neurovirulent in mice (13). Also, the recently isolated avian influenza viruses of the H5N1 subtype were shown to be neurovirulent in mice (23). Apparently, other host factors play a role in the pathogenesis and viral dissemination (36). For example, in mice with congenital or acquired abnormalities of mitochondrial beta oxidation, infection with the nonneurotropic H3N2 strain A/Aichi/2/68 leads to vascular dissemination, encephalitis, and severe brain edema (40-42). Other factors include the host immune response, since the fatal outcome of IAV infections has been associated with hypercytokinemia (7) and the dysregulation of the antiviral immune response (18). Atypical host factors such as congenital plasmin inhibitor deficiency (9) with elevated plasmin levels in the blood could influence the pathogenesis of IAV infections. Also, in other nonrespiratory diseases of IAV infections, like Reye's syndrome (25), encephalopathic syndrome (10), and encephalopathic necrosis (33), PLG-dependent replication may play a role.
It would be interesting to know whether there is a link between the pathogenesis of IAV infections and the synthesis of lipoprotein, which is present in human plasma in a wide range of concentrations and as numerous different isoforms (8). Both lipoprotein and PLG are synthesized in the liver. In Reye's syndrome, the replication of IAV is observed not only in the brain but also in the liver and is characterized by the accumulation of miniplasmin in the liver. More studies are needed to better understand this influenza-related disease, the role of PLG, and the potential beneficial effects of Lp(A) in its pathogenesis.
Collectively, the data presented in the present study indicate that certain influenza viruses can replicate in the presence of PLG despite the lack of the PLG binding motif in the NA described for IAV A/WSN/33 (12). The acquisition of the host cellular protein A2 during the budding process provides the virus an alternative way to bind PLG and to convert it into plasmin, which can support influenza virus replication. The differential capacity to use PLG for their replication may be the basis of differences in the virulence of IAV or their ability to disseminate from the initial site of infection to other tissues.
Acknowledgments
We thank S. van der Werf, N. Naffack, and V. Jestin for viruses.
Footnotes
Published ahead of print on 30 April 2008.
REFERENCES
- 1.Beigel, J. H., J. Farrar, A. M. Han, F. G. Hayden, R. Hyer, M. D. de Jong, S. Lochindarat, T. K. Nguyen, T. H. Nguyen, T. H. Tran, A. Nicoll, S. Touch, and K. Y. Yuen. 2005. Avian influenza A (H5N1) infection in humans. N. Engl. J. Med. 3531374-1385. [DOI] [PubMed] [Google Scholar]
- 2.Bernard, D., B. Riteau, J. D. Hansen, R. B. Phillips, F. Michel, P. Boudinot, and A. Benmansour. 2006. Costimulatory receptors in a teleost fish: typical CD28, elusive CTLA4. J. Immunol. 1764191-4200. [DOI] [PubMed] [Google Scholar]
- 3.Borthwick, L. A., A. Neal, L. Hobson, V. Gerke, L. Robson, and R. Muimo. 2008. The annexin 2-S100A10 complex and its association with TRPV6 is regulated by cAMP/PKA/CnA in airway and gut epithelia. Cell Calcium [Epub ahead of print.] [DOI] [PubMed]
- 4.Böttcher, E., T. Matrosovich, M. Beyerle, H. D. Klenk, W. Garten, and M. Matrosovich. 2006. Proteolytic activation of influenza viruses by serine proteases TMPRSS2 and HAT from human airway epithelium. J. Virol. 809896-9898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chertova, E., O. Chertov, L. V. Coren, J. D. Roser, C. M. Trubey, J. W. Bess, Jr., R. C. Sowder, Jr., E. Barsov, B. L. Hood, R. J. Fisher, K. Nagashima, T. P. Conrads, T. D. Veenstra, J. D. Lifson, and D. E. Ott. 2006. Proteomic and biochemical analysis of purified human immunodeficiency virus type 1 produced from infected monocyte-derived macrophages. J. Virol. 809039-9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Jong, M. D., V. C. Bach, T. Q. Phan, M. H. Vo, T. T. Tran, B. H. Nguyen, M. Beld, T. P. Le, H. K. Truong, V. V. Nguyen, T. H. Tran, Q. H. Do, and J. Farrar. 2005. Fatal avian influenza A (H5N1) in a child presenting with diarrhea followed by coma. N. Engl. J. Med. 352686-691. [DOI] [PubMed] [Google Scholar]
- 7.de Jong, M. D., C. P. Simmons, T. T. Thanh, V. M. Hien, G. J. Smith, T. N. Chau, D. M. Hoang, N. V. Chau, T. H. Khanh, V. C. Dong, P. T. Qui, B. V. Cam, Q. Ha do, Y. Guan, J. S. Peiris, N. T. Chinh, T. T. Hien, and J. Farrar. 2006. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat. Med. 121203-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de la Peña-Díaz, A., R. Izaguirre-Avila, and E. Angles-Cano. 2000. Lipoprotein Lp(a) and atherothrombotic disease. Arch. Med. Res. 31353-359. [DOI] [PubMed] [Google Scholar]
- 9.Favier, R., N. Aoki, and P. de Moerloose. 2001. Congenital alpha(2)-plasmin inhibitor deficiencies: a review. Br. J. Haematol. 1144-10. [DOI] [PubMed] [Google Scholar]
- 10.Gooskens, J., T. Kuiken, E. C. Claas, H. I. Harinck, J. C. Thijssen, H. J. Baelde, and A. C. Kroes. 2007. Severe influenza resembling hemorrhagic shock and encephalopathy syndrome. J. Clin. Virol. 39136-140. [DOI] [PubMed] [Google Scholar]
- 11.Goto, H., and Y. Kawaoka. 2000. Assays for functional binding of plasminogen to viral proteins. Methods 21159-163. [DOI] [PubMed] [Google Scholar]
- 12.Goto, H., and Y. Kawaoka. 1998. A novel mechanism for the acquisition of virulence by a human influenza A virus. Proc. Natl. Acad. Sci. USA 9510224-10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goto, H., K. Wells, A. Takada, and Y. Kawaoka. 2001. Plasminogen-binding activity of neuraminidase determines the pathogenicity of influenza A virus. J. Virol. 759297-9301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hajjar, K. A., and S. Krishnan. 1999. Annexin II: a mediator of the plasmin/plasminogen activator system. Trends Cardiovasc. Med. 9128-138. [DOI] [PubMed] [Google Scholar]
- 15.Horimoto, T., and Y. Kawaoka. 2005. Influenza: lessons from past pandemics, warnings from current incidents. Nat. Rev. Microbiol. 3591-600. [DOI] [PubMed] [Google Scholar]
- 16.Kassam, G., K. S. Choi, J. Ghuman, H. M. Kang, S. L. Fitzpatrick, T. Zackson, S. Zackson, M. Toba, A. Shinomiya, and D. M. Waisman. 1998. The role of annexin II tetramer in the activation of plasminogen. J. Biol. Chem. 2734790-4799. [DOI] [PubMed] [Google Scholar]
- 17.Klenk, H. D., and W. Garten. 1994. Host cell proteases controlling virus pathogenicity. Trends Microbiol. 239-43. [DOI] [PubMed] [Google Scholar]
- 18.Kobasa, D., S. M. Jones, K. Shinya, J. C. Kash, J. Copps, H. Ebihara, Y. Hatta, J. H. Kim, P. Halfmann, M. Hatta, F. Feldmann, J. B. Alimonti, L. Fernando, Y. Li, M. G. Katze, H. Feldmann, and Y. Kawaoka. 2007. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature 445319-323. [DOI] [PubMed] [Google Scholar]
- 19.Kramer, M. D., J. Reinartz, G. Brunner, and V. Schirrmacher. 1994. Plasmin in pericellular proteolysis and cellular invasion. Invasion Metastasis 14210-222. [PubMed] [Google Scholar]
- 20.Kwon, M., T. J. MacLeod, Y. Zhang, and D. M. Waisman. 2005. S100A10, annexin A2, and annexin a2 heterotetramer as candidate plasminogen receptors. Front. Biosci. 10300-325. [DOI] [PubMed] [Google Scholar]
- 21.Lee, E., D. E. Vaughan, S. H. Parikh, A. J. Grodzinsky, P. Libby, M. W. Lark, and R. T. Lee. 1996. Regulation of matrix metalloproteinases and plasminogen activator inhibitor-1 synthesis by plasminogen in cultured human vascular smooth muscle cells. Circ. Res. 7844-49. [DOI] [PubMed] [Google Scholar]
- 22.Li, S., J. Schulman, S. Itamura, and P. Palese. 1993. Glycosylation of neuraminidase determines the neurovirulence of influenza A/WSN/33 virus. J. Virol. 676667-6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lipatov, A. S., S. Krauss, Y. Guan, M. Peiris, J. E. Rehg, D. R. Perez, and R. G. Webster. 2003. Neurovirulence in mice of H5N1 influenza virus genotypes isolated from Hong Kong poultry in 2001. J. Virol. 773816-3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maricich, S. M., J. L. Neul, T. E. Lotze, A. C. Cazacu, T. M. Uyeki, G. J. Demmler, and G. D. Clark. 2004. Neurologic complications associated with influenza A in children during the 2003-2004 influenza season in Houston, Texas. Pediatrics 114E626-E633. [DOI] [PubMed] [Google Scholar]
- 25.Mizuguchi, M. 2006. Influenza encephalopathy and Reye's syndrome. Nippon Naika Gakkai Zasshi 951263-1267. [DOI] [PubMed] [Google Scholar]
- 26.Rescher, U., and V. Gerke. 2004. Annexins—unique membrane binding proteins with diverse functions. J. Cell Sci. 1172631-2639. [DOI] [PubMed] [Google Scholar]
- 27.Riteau, B., D. F. Barber, and E. O. Long. 2003. Vav1 phosphorylation is induced by β2 integrin engagement on natural killer cells upstream of actin cytoskeleton and lipid raft reorganization. J. Exp. Med. 198469-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riteau, B., C. de Vaureix, and F. Lefevre. 2006. Trypsin increases pseudorabies virus production through activation of the ERK signalling pathway. J. Gen. Virol. 871109-1112. [DOI] [PubMed] [Google Scholar]
- 29.Riteau, B., P. Moreau, C. Menier, I. Khalil-Daher, K. Khosrotehrani, R. Bras-Goncalves, P. Paul, J. Dausset, N. Rouas-Freiss, and E. D. Carosella. 2001. Characterization of HLA-G1, -G2, -G3, and -G4 isoforms transfected in a human melanoma cell line. Transplant. Proc. 332360-2364. [DOI] [PubMed] [Google Scholar]
- 30.Riteau, B., N. Rouas-Freiss, C. Menier, P. Paul, J. Dausset, and E. D. Carosella. 2001. HLA-G2, -G3, and -G4 isoforms expressed as nonmature cell surface glycoproteins inhibit NK and antigen-specific CTL cytolysis. J. Immunol. 1665018-5026. [DOI] [PubMed] [Google Scholar]
- 31.Steinhauer, D. A. 1999. Role of hemagglutinin cleavage for the pathogenicity of influenza virus. Virology 2581-20. [DOI] [PubMed] [Google Scholar]
- 32.Stieneke-Grober, A., M. Vey, H. Angliker, E. Shaw, G. Thomas, C. Roberts, H. D. Klenk, and W. Garten. 1992. Influenza virus hemagglutinin with multibasic cleavage site is activated by furin, a subtilisin-like endoprotease. EMBO J. 112407-2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sugaya, N. 2002. Influenza-associated encephalopathy in Japan. Semin. Pediatr. Infect. Dis. 1379-84. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi, M., T. Yamada, Y. Nakashita, H. Saikusa, M. Deguchi, H. Kida, M. Tashiro, and T. Toyoda. 2000. Influenza virus-induced encephalopathy: clinicopathologic study of an autopsied case. Pediatr. Int. 42204-214. [DOI] [PubMed] [Google Scholar]
- 35.Tran, T. H., T. L. Nguyen, T. D. Nguyen, T. S. Luong, P. M. Pham, V. C. Nguyen, T. S. Pham, C. D. Vo, T. Q. Le, T. T. Ngo, B. K. Dao, P. P. Le, T. T. Nguyen, T. L. Hoang, V. T. Cao, T. G. Le, D. T. Nguyen, H. N. Le, K. T. Nguyen, H. S. Le, V. T. Le, D. Christiane, T. T. Tran, J. Menno de, C. Schultsz, P. Cheng, W. Lim, P. Horby, and J. Farrar. 2004. Avian influenza A (H5N1) in 10 patients in Vietnam. N. Engl. J. Med. 3501179-1188. [DOI] [PubMed] [Google Scholar]
- 36.Tumpey, T. M., T. R. Maines, N. Van Hoeven, L. Glaser, A. Solorzano, C. Pappas, N. J. Cox, D. E. Swayne, P. Palese, J. M. Katz, and A. Garcia-Sastre. 2007. A two-amino acid change in the hemagglutinin of the 1918 influenza virus abolishes transmission. Science 315655-659. [DOI] [PubMed] [Google Scholar]
- 37.van Riel, D., V. J. Munster, E. de Wit, G. F. Rimmelzwaan, R. A. Fouchier, A. D. Osterhaus, and T. Kuiken. 2007. Human and avian influenza viruses target different cells in the lower respiratory tract of humans and other mammals. Am. J. Pathol. 1711215-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watanabe, K., T. Fuse, I. Asano, F. Tsukahara, Y. Maru, K. Nagata, K. Kitazato, and N. Kobayashi. 2006. Identification of Hsc70 as an influenza virus matrix protein (M1) binding factor involved in the virus life cycle. FEBS Lett. 5805785-5790. [DOI] [PubMed] [Google Scholar]
- 39.Wright, J. F., A. Kurosky, E. L. Pryzdial, and S. Wasi. 1995. Host cellular annexin II is associated with cytomegalovirus particles isolated from cultured human fibroblasts. J. Virol. 694784-4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao, D., Y. Chen, M. Kuwajima, M. Shiota, and H. Kido. 2004. Accumulation of mini-plasmin in the cerebral capillaries causes vascular invasion of the murine brain by a pneumotropic influenza A virus: implications for influenza-associated encephalopathy. Biol. Chem. 385487-492. [DOI] [PubMed] [Google Scholar]
- 41.Yao, D., M. Kuwajima, Y. Chen, M. Shiota, Y. Okumura, H. Yamada, and H. Kido. 2007. Impaired long-chain fatty acid metabolism in mitochondria causes brain vascular invasion by a non-neurotropic epidemic influenza A virus in the newborn/suckling period: implications for influenza-associated encephalopathy. Mol. Cell Biochem. 29985-92. [DOI] [PubMed] [Google Scholar]
- 42.Yao, D., M. Kuwajima, and H. Kido. 2003. Pathologic mechanisms of influenza encephalitis with an abnormal expression of inflammatory cytokines and accumulation of mini-plasmin. J. Med. Investig. 501-8. [PubMed] [Google Scholar]
- 43.Zitzow, L. A., T. Rowe, T. Morken, W. J. Shieh, S. Zaki, and J. M. Katz. 2002. Pathogenesis of avian influenza A (H5N1) viruses in ferrets. J. Virol. 764420-4429. [DOI] [PMC free article] [PubMed] [Google Scholar]