Abstract
Herpes simplex virus type 1 (HSV-1) glycoprotein C (gC) blocks complement activation, and glycoprotein E (gE) interferes with IgG Fc-mediated activities. While evaluating gC- and gE-mediated immune evasion in human immunodeficiency virus (HIV)-HSV-1-coinfected subjects, we noted that antibody alone was more effective at neutralizing a strain with mutations in gC and gE (gC/gE) than a wild-type (WT) virus. This result was unexpected since gC and gE are postulated to interfere with complement-mediated neutralization. We used pooled human immunoglobulin G (IgG) from HIV-negative donors to confirm the results and evaluated mechanisms of the enhanced antibody neutralization. We demonstrated that differences in antibody neutralization cannot be attributed to the concentrations of HSV-1 glycoproteins on the two viruses or to the absence of an IgG Fc receptor on the gC/gE mutant virus or to enhanced neutralization of the mutant virus by antibodies that target only gB, gD, or gH/gL, which are the glycoproteins involved in virus entry. Since sera from HIV-infected subjects and pooled human IgG contain antibodies against multiple glycoproteins, we determined whether differences in neutralization become apparent when antibodies to gB, gD, or gH/gL are used in combination. Neutralization of the gC/gE mutant was greatly increased compared that of WT virus when any two of the antibodies against gB, gD, or gH/gL were used in combination. These results suggest that gC and gE on WT virus provide a shield against neutralizing antibodies that interfere with gB-gD, gB-gH/gL, or gD-gH/gL interactions and that one function of virus neutralization is to prevent interactions between these glycoproteins.
Herpes simplex virus type 2 (HSV-2) infection is a significant risk factor for the acquisition of human immunodeficiency virus (HIV) (27, 39, 42). Individuals who are seropositive for HSV-2 have a twofold increased risk of acquiring HIV (39). Acquisition rates appear greatest following the initial HSV-2 infection, when HSV-2 reactivation is most frequent (3, 29, 40). Currently, 17% of adults in the United States are infected with HSV-2, with much greater prevalence rates in parts of South America and Africa (33, 44). While less is known about the epidemiological link between HSV-1 and HIV, studies suggest similar interactions (5, 6).
HSV-1 encodes glycoproteins involved in evading immunity, which is mediated by antibody or complement (22). Glycoprotein C (gC) binds complement component C3b, preventing the activation of the complement cascade (9, 11, 15, 19, 30, 31). Glycoproteins E and I (gE and gI) form a high-affinity receptor that binds the Fc region of immunoglobulin G (IgG), inhibiting complement activation and antibody-dependent cellular cytotoxicity (8, 10). HSV-1 strains that are defective in either IgG Fc or C3b binding or both due to targeted mutations in gC and gE are less virulent than the WT or marker-rescued viruses (23-26).
We were interested in determining whether antibody and complement levels are maintained at high enough concentrations in HIV-infected individuals to neutralize an HSV-1 strain with mutations in gC and gE (gC/gE) that is defective in immune evasion. If so, strategies aimed at blocking the immune evasion properties of gC and gE in HIV-infected subjects coinfected with HSV may represent a novel approach to preventing HSV recurrences (7, 20, 21).
We evaluated HIV-HSV-1-coinfected subjects and unexpectedly demonstrated that antibodies from -coinfected and HSV-1-monoinfected subjects had a greater neutralizing activity against an HSV-1 gC/gE mutant than against a WT virus. Since HSV-1-neutralizing antibodies primarily target the viral glycoproteins that are required for virus entry, we compared the neutralization of gC/gE mutant and WT viruses, using antibodies to gB, gD, or gH/gL, used either alone or in combination (16, 17, 34, 35, 38). We demonstrated that antibodies against each glycoprotein used alone showed neutralization activity that was comparable for both viruses; however, used in combination, antibodies were significantly more active against the gC/gE mutant than the WT virus. These results support a protective role for gC and gE on the WT virus in preventing antibodies from blocking interactions between gB, gD, and gH/gL.
MATERIALS AND METHODS
Cells and viruses.
African green monkey kidney cells (Vero) were grown in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal bovine serum, 2 mM l-glutamine, 10 mM HEPES (pH 7.3), 20 μg/ml gentamicin, and 1 μg/ml amphotericin B (Fungizone; Life Technologies, Rockville, MD). Pools of purified virus were prepared by infecting Vero cells at a multiplicity of infection range of 2 to 5. Supernatant fluids 24 h postinfection were harvested for cell-free virus and centrifuged onto a 5% to 70% sucrose gradient (13). Virus-containing fractions were isolated and dialyzed against Dulbecco's phosphate-buffered saline (PBS) with Ca2+ and Mg2+, aliquoted, and stored at −70°C.
The WT strain, HSV-1 NS, is a low-passage clinical isolate obtained from an infected child (12). Mutant viruses derived from the NS strain, NS-gCΔC3 and NS-gE339, and the double mutant NS-gCΔC3,gE339 have been described previously (20, 24-26). The gC mutant virus NS-gCΔC3 has a deletion from amino acid 275 to 367, resulting in a loss of C3b binding (23). The gE mutant virus NS-gE339 has a four-amino-acid insertion at gE amino acid 339, resulting in the loss of IgG Fc binding (26). The gC/gE double-mutant virus NS-gCΔC3,gE339 contains both the gC and gE mutations in a single virus (20, 24).
Serum isolation.
Sera from HIV subjects at various stages of HIV disease (CD4 count, <200/μl, 200 to 500/μl, >500/μl) were obtained from the Clinical Core Laboratory of the University of Pennsylvania Center for AIDS Research. Sera were tested for antibodies to HSV-1 and HSV-2 by using a HerpeSelect 1 and 2 enzyme-linked immunosorbent assay (ELISA) IgG (Focus Technologies, Cypress, CA), which is a glycoprotein G-based assay that can detect type-specific IgG antibodies. Samples that were antibody positive to HSV-1 and antibody negative to HSV-2 (HSV 1+/2−) were selected for further evaluation. HIV-seronegative sera were obtained from healthy volunteers who participated in the GlaxoSmithKline HSV-2 glycoprotein D adjuvant (gD2) placebo-controlled vaccine trial (36). Sera selected were from HSV 1+/2− subjects who did not receive the gD2 vaccine. Subject consent was obtained prior to the study, and the University of Pennsylvania Human Subjects Committee approved the use of human sera for this research.
IgG preparations.
Patient IgG was purified from sera, using HiTrap protein G columns according to the manufacturer's instructions (Amersham Biosciences, Uppsala, Sweden). Fractions containing protein were pooled, dialyzed against PBS at 4°C, concentrated, and stored in aliquots at −20°C. IgG was purified from rabbits inoculated with the purified baculovirus proteins gB, gC, gD, gH/gL, or gI (2, 28, 32). The antibody to gI was produced in rabbits, using baculovirus-expressed gI amino acids 24 to 264 as the antigen (H. M. Friedman, unpublished). Pooled human IgG was purchased from the Michigan Department of Public Health, Lansing, that was prepared from the sera of thousands of HIV-negative donors (26).
Antibody neutralization assay.
Purified virus at a final concentration of 106 PFU/ml was incubated in 50 μl with an equal volume of 1% HSV 1+/2− serum treated with EDTA to inactivate the complement or with PBS for 1 h at 37°C (13, 14). Viral titers remaining were determined by plaque assay on Vero cells. Antibody neutralization was calculated as the difference between the titers of virus that was incubated with PBS and that of virus incubated with EDTA-treated serum to inactivate the complement. In some assays, pooled human IgG or rabbit antibodies to the purified baculovirus proteins gB, gC, gD, gH/gL, or gI were used (2, 24, 28, 32, 37).
Western blotting and densitometry analyses.
Purified WT and gC/gE mutant viruses, each at 2 × 105, 1 × 106, and 4 × 106 PFU, were run on 4 to 15% sodium dodecyl sulfate-polyacrylamide gels, transferred to Immobilon-P transfer membranes (Millipore Corp., Bedford, MA), and detected using polyclonal rabbit antibodies to gB, gC, gD, gE, gH/gL, gI, and VP5 (2, 21, 28, 32, 37, 41). Horseradish peroxidase-conjugated goat anti-rabbit IgG and enhanced chemiluminescence (Amersham Pharmacia, Piscataway, NJ) were used to visualize the primary antibodies. Densitometry analyses were performed using a ScanMaker i900 (Microtek Lab Inc., Carson, CA) unit to compare relative protein concentrations.
Statistical analysis.
Student's t test (Microsoft Excel software) was used to determine P values. Results were considered significant at a probability (P) value of <0.05.
RESULTS
Antibody neutralization of WT and gC/gE mutant viruses using serum from HIV-HSV-1-coinfected subjects.
To identify HIV subjects coinfected with HSV-1 (HSV 1+/2−), we tested serum for antibodies to HSV-1 and HSV-2 from the first 133 subjects enrolled in the Center for AIDS Research Clinical Core database, for whom both serum and CD4 T-cell counts were available. Overall, 39% had CD4 T-cell counts of >500/μl, 28% had 200 to 500/μl, and 33% had counts of <200/μl. Twenty-eight percent of subjects were seropositive for HSV-1 and seronegative for HSV-2 (HSV 1+/2−), 23% were HSV 1−/2+, 41% were doubly seropositive (HSV 1+/2+), and 8% were doubly seronegative (HSV 1−/2−). Sera from HSV 1+/2− subjects were selected for further analysis.
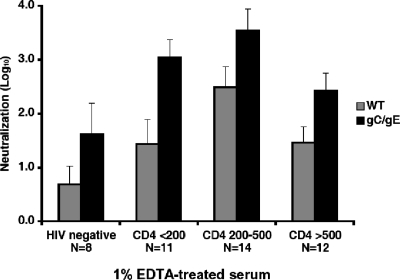
The neutralizing activity of the antibody was determined by using a 1:100 dilution of serum (1%) that was treated with EDTA to inactivate complement. Antibody neutralized both the WT virus and the gC/gE mutant virus at all stages of HIV disease; however, antibody neutralization of the gC/gE mutant virus was greater than that of the WT virus for each of the four groups tested (Fig. 1). We previously reported that gC and gE protect the virus from neutralization because these glycoproteins inhibit complement activation. Much higher concentrations of neutralizing antibodies were used in the current study than in our previous reports (20, 24). Nevertheless, we were surprised to find that antibody alone had a greater effect on the gC/gE mutant than on the WT virus.
FIG. 1.
Neutralization of the HSV-1 WT and gC/gE mutant viruses by antibody. The WT virus or the gC/gE mutant virus was incubated for 1 h at 37°C with 1% serum treated with EDTA to inactivate complement. Comparing antibody-mediated neutralization of the gC/gE mutant with that of the WT virus: HIV negative, P = 0.18; CD4 <200/μl, P < 0.01; CD4 200 to 500/μl, P < 0.06; and CD4 >500/μl, P < 0.04; all four groups combined, P < 0.0001.
Neutralization of the WT and gC/gE mutant viruses, using pooled human IgG.
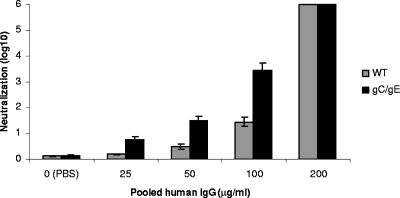
To further evaluate the enhanced neutralization of the gC/gE mutant virus by antibody, a dose-response experiment was performed using serial twofold dilutions of pooled human IgG. At 25, 50, and 100 μg/ml, the pooled human IgG neutralized more of the gC/gE mutant virus than the WT virus, demonstrating 0.6, 1.0, and 2.0 log10 differences, respectively, at increasing concentrations of IgG (Fig. 2). At 200 μg/ml, the IgG totally neutralized both viruses. Therefore, the gC/gE mutant virus was more readily neutralized than the WT virus by HSV antibodies in a dose-dependent fashion.
FIG. 2.
Neutralization of the WT and gC/gE mutant viruses by pooled human IgG. The WT and gC/gE mutant viruses were incubated with various concentrations of pooled human IgG and neutralization determined. Results shown here represent the means and standard errors of three determinations. Comparing the WT and gC/gE mutant viruses at 25, 50, and 100 μg/ml, P < 0.01.
The WT and gC/gE mutant viruses express similar levels of HSV glycoproteins on the virion.
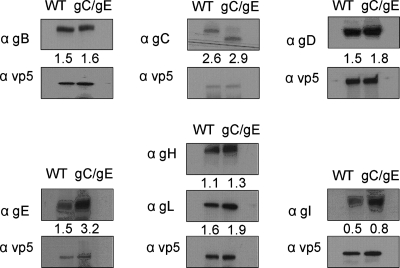
Additional studies were performed to evaluate the unexpected finding that antibody had a greater neutralizing effect on the gC/gE mutant virus than it did on the WT virus. One possible explanation for the greater susceptibility of the gC/gE mutant virus to antibody is that the mutant virus may incorporate fewer HSV-1 glycoproteins into the virion envelope. If this occurs, fewer antibody molecules would be required to neutralize the virus, since fewer target glycoproteins are present. We performed Western blotting and densitometry analysis using 2 × 105, 1 × 106, or 4 × 106 PFU purified WT or gC/gE mutant virus in alternating lanes of the gel to evaluate the relative concentrations of the glycoproteins, including those essential for virus entry, gB, gD, and gH/gL. Analysis of the HSV-1 capsid protein VP5 was included to ensure comparable loading of the WT and gC/gE mutant virus particles on the gel (18). We detected some differences in the relative concentrations of HSV-1 glycoproteins expressed on the WT and the gC/gE mutant viruses; however, concentrations of gB, gD, and gH/gL were slightly higher on the gC/gE mutant than on the WT virus, suggesting that the greater neutralizing activity of the mutant virus is not caused by lower concentrations of target glycoproteins (Fig. 3).
FIG. 3.
The gC/gE mutant and WT viruses express comparable concentrations of HSV-1 glycoproteins on the virion. Purified gC/gE mutant virus and WT virus were evaluated for VP5, gB, gC, gD, gE, gH, gL, and gI expression by Western blotting and densitometry analysis to compare relative glycoprotein concentrations. Each lane contains 1 × 106 PFU of the WT or gC/gE mutant virus. The number associated with each gel represents the ratio of the density of the glycoprotein band above the number to the density of the VP5 band below the number. The results are representative of two separate experiments that yielded similar findings.
Nonfunctional viral IgG Fc receptor does not explain the increased susceptibility of the gC/gE mutant virus to neutralizing antibody.
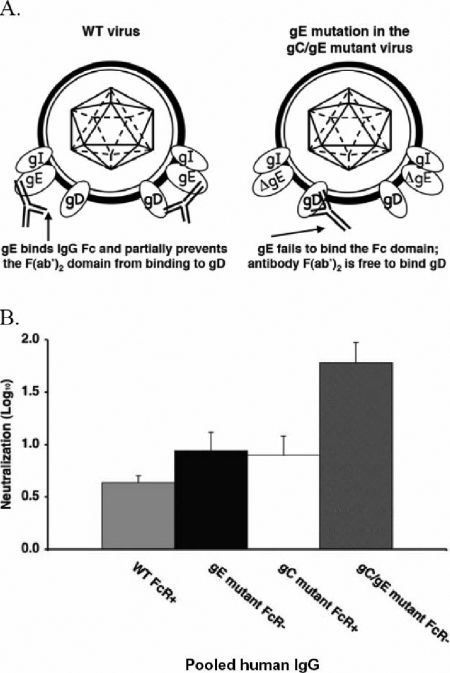
Another possible explanation for the increased antibody neutralization of the mutant virus is that the mutation in the HSV-1 IgG Fc receptor (FcγR) contributes to the enhanced neutralization. Figure 4A shows the possible mechanisms by which the HSV-1 FcγR may interfere with antibody neutralization. By binding the Fc domain of IgG, the HSV-1 FcγR on the WT virus may prevent the F(ab′)2 domain from interacting with its target antigen (Fig. 4A, at the left of the WT model). In contrast, the gE mutation in the gC/gE mutant virus eliminates FcγR activity, which may facilitate interactions between the F(ab′)2 domain and the antibody target, resulting in greater neutralization.
FIG. 4.
The role of the HSV-1 FcγR in antibody neutralization. (A) Possible model to explain the greater susceptibility of the gC/gE mutant virus to neutralization by antibody alone. On the left side of the WT virus model, gE binds the Fc domain of IgG, preventing the F(ab′)2 from binding antigen (shown here as gD). On the right side of the WT virus model, antibody bipolar bridging is shown in which the Fab domain binds to gD and the Fc domain of the same IgG molecule binds to gE (10). If antibody binding occurs as shown on the left side but not the right side of the WT virus model, the HSV-1 FcγR (comprised by gE/gI) may prevent some F(ab′)2 domains from interacting with their target antigens. In the model of the gC/gE mutant virus, ΔgE fails to bind the IgG Fc domain, allowing the F(ab′)2 domain to bind antigen (shown as gD) and to neutralize the virus. (B) A nonfunctional viral FcγR does not explain the increased susceptibility of the gC/gE mutant virus to antibody neutralization. Viruses were incubated with 100 μg/ml of pooled human IgG, and the amount of neutralization was determined. Results shown represent mean titers ± standard errors (n = 8 to 17 for each virus). No significant differences were detected between the antibody neutralization of the FcγR-defective virus (NS-gE339 [gE mutant FcR−]) and that of the FcγR-intact virus (NS-gCΔC3 [gC mutant FcR+]); P = 0.47. The gC/gE mutant virus was neutralized significantly more than either the FcγR− virus or the FcγR+ virus; P < 0.05 and P < 0.02, respectively.
To evaluate this possibility, pooled human IgG was used to compare the neutralization of a gE mutant strain that is defective in FcγR activity with that of a gC mutant virus that has an intact FcγR. If the viral FcγR inhibits neutralization, we postulated that the mutant virus with an intact FcγR would be less neutralized than the mutant virus with a defective FcγR. Viruses were incubated with PBS or 100 μg/ml pooled human IgG as the source of HSV antibodies. Antibody was equally effective at neutralizing the FcγR-intact and the FcγR-defective viruses and was even more active against the gC/gE double-mutant virus (Fig. 4B). Therefore, a nonfunctional viral FcγR does not explain the increased susceptibility of the gC/gE mutant virus to neutralization mediated by antibody alone. In addition, since the gC/gE double-mutant virus was neutralized more readily than either of the single-mutant viruses from which it was derived, we conclude that the mutations in gC and gE both contribute to increasing the susceptibility of the gC/gE mutant virus to neutralizing antibody.
gC and gE immune evasion domains shield critical neutralizing epitopes on gB, gD, and gH/gL.
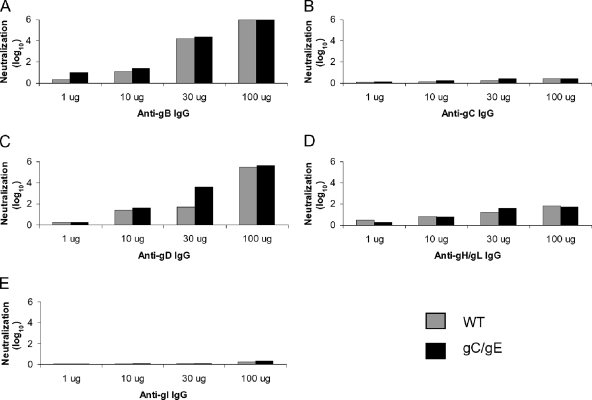
We next performed assays to compare neutralization of the WT and the gC/gE mutant viruses following incubation with rabbit antibodies that interact with gB, gD, or gH/gL, and as controls, antibodies to gC and gI that are dispensable glycoproteins for virus entry were included. Antibodies to gB and gD and, to a lesser extent, to gH/gL neutralized both viruses in a dose-dependent fashion, while antibodies to gC and gI had little neutralizing activity against either virus (Fig. 5). Surprisingly, we detected minimal differences between the neutralization of the WT virus and that of gC/gE mutant virus by antibodies to gB, gD, or gH/gL, except when anti-gD IgG was used at 30 μg/ml (Fig. 5C). The results shown were from experiments performed once at each IgG concentration; however, experiments were performed three additional times using anti-gD IgG at 30 μg/ml, and the differences between results for the gC/gE mutant and WT viruses were reproducible (1.5 log10 greater neutralization of the gC/gE mutant than of the WT virus, mean of four determinations; P < 0.01) (results not shown). Experiments were repeated two to three additional times using anti-gB, anti-gD, or anti-gH/gL IgG at 10 μg/ml, which showed no differences between the two viruses (results not shown). Therefore, with the exception of gD at 30 μg/ml, the antibodies to the individual glycoproteins failed to demonstrate differences in neutralization between the two viruses.
FIG. 5.
Neutralization by IgG antibodies to HSV-1 glycoproteins. (A to E) The WT and gC/gE mutant viruses were incubated with 1, 10, 30, or 100 μg/ml of anti-gB, gC, gD, gH/gL, or gI IgG. Each experiment was performed once.
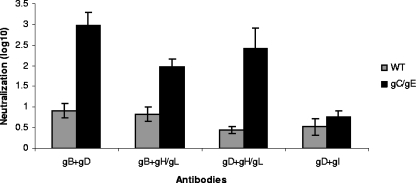
Since the human sera used in Fig. 1 and 2 contained antibodies to multiple glycoproteins, we performed neutralization experiments using antibodies in combinations. We combined 5 μg/ml of anti-gB IgG with 5 μg/ml anti-gD IgG, 5 μg/ml anti-gB with 5 μg/ml anti-gH/gL, or 5 μg/ml anti-gD with 5 μg/ml anti-gH/gL and detected much greater neutralization activity of the gC/gE mutant virus than of the WT virus (Fig. 6). This result was striking since, when antibodies were used alone at twofold higher concentrations (10 μg/ml), none of the antibodies showed greater neutralization of the gC/gE mutant virus (Fig. 5A, C, and D). As a control, antibodies to a glycoprotein required for entry were combined with one that is dispensable. Five micrograms of anti-gD IgG per milliliter and 5 μg/ml anti-gI IgG were combined, which showed no enhanced neutralization of the gC/gE mutant virus (Fig. 6). Therefore, the results suggest that the gC and gE mutations expose domains on glycoproteins that interact with one another during the entry process.
FIG. 6.
Neutralization by combinations of antibodies to HSV-1 glycoproteins involved in entry. The WT and gC/gE mutant viruses were incubated with a total of 10 μg/ml of IgG consisting of 5 μg/ml each of anti-gB and anti-gD IgG, anti-gB and anti-gH/gL IgG, anti-gD and anti-gH/gL IgG, or anti-gD and anti-gI IgG. Results represent the means ± standard errors of three determinations for each combination (P < 0.005, for a comparison of the WT and gC/gE mutant viruses for each combination, except anti-gD and anti-gI, for which P = 0.2).
DISCUSSION
An interesting finding in the current study was that gC and gE protected the WT virus from antibody neutralization. Our prior studies failed to detect significant differences between antibody neutralization of the WT virus and that of the gC/gE mutant viruses. A possible explanation for the differing results is that in the prior studies, we intentionally used low antibody concentrations so that the antibody neutralized the WT virus by only ∼50% (0.3 log10) (20, 24). This approach enabled us to optimize the assay to detect the added neutralizing effects of complement (20, 24). At low antibody concentrations, neutralization of the gC/gE mutant virus was only slightly greater than that of the WT virus (20). In contrast, the current study used antibody from individual donors at a 1:100 dilution of serum. The range of neutralizing antibody titers in the sera varied, but overall, the neutralizing titers were much higher than in our prior studies, since antibody alone neutralized, on average, ∼2 log10 WT virus compared with 0.3 log10. At these higher antibody concentrations, differences between the neutralization of the WT virus and that of the gC/gE mutant virus were readily apparent.
Studies to explain the greater antibody neutralization of the gC/gE mutant than the WT virus demonstrated that fewer glycoprotein targets for neutralizing antibodies on the gC/gE mutant virus or the lack of IgG Fc binding to the mutant virus does not explain the enhanced neutralization. Antibodies that targeted only gB, gD, or gH/gL demonstrated little difference in neutralizing activity against the WT virus or the gC/gE mutant virus; however, when antibodies to these essential entry glycoproteins were used in combinations, we detected enhanced neutralization of the gC/gE mutant virus.
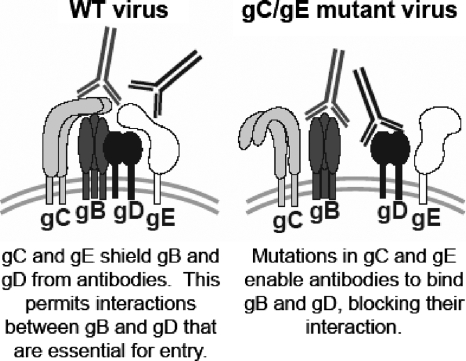
We postulate that the combinations of antibodies prevented the interactions between gB, gD, and gH/gL that are required for virus fusion with cell membranes and subsequent entry of virus into cells. Since neutralization was enhanced by each combination of antibodies to the entry molecules (gB-gD, gB-gH/gL, and gD-gH/gL), our results suggest that gB interacts with gD, gB interacts with gH/gL, and gD interacts with gH/gL. Recently, bimolecular complementation was used to evaluate cell-cell fusion mediated by gB, gD, and gH/gL. The results demonstrated that gB interacts with gD and gD interacts with gH/gL, and when gD binds to its receptor, gB interacts with gH/gL (1). Therefore, our results are entirely consistent with these observations. In addition, our results suggest that to effectively block the interactions between gB, gD, and gH/gL, antibodies must bind to more than one of the entry molecules and that gC and gE on the WT virus provide a shield that prevents the antibodies from interfering with gB-gD, gB-gH/gL, or gD-gH/gL interaction (Fig. 7).
FIG. 7.
Model of gC and gE on the virion envelope, blocking antibody access and enabling the interaction of glycoproteins that are required for fusion and entry. At the left, gC and gE on the WT virus block access of the antibodies, which enables the interaction between gB and gD that is required for fusion and entry. On the right, gC and gE on the gC/gE mutant virus fail to block antibody access. Our results suggest that antibodies must bind to at least two of the glycoproteins gB, gD, or gH/gL, to prevent glycoprotein interactions and inhibit entry.
Anti-gD IgG, when used at 30 μg/ml, was an exception to the requirement for combinations of antibodies that revealed differences between the WT and gC/gE mutant viruses. We postulate that at this high concentration of gD antibody, a critical domain of interaction between gD and either gB or gH/gL was interrupted. Experiments that use monoclonal antibodies targeted to particular domains on gB, gD, or gH/gL will be required to further define the domains of interaction unmasked in the gC/gE mutant virus.
Studies using chemical cross-linkers previously demonstrated that gC forms heterooligomers with gD, gB, and gH/gL, which suggests that gC epitopes are in close proximity to these other glycoproteins (17). Epitope masking of neutralizing domains has been described as an evasion strategy for HIV-1 gp120, in which the gp120 variable domains V1 to V3 shield conserved neutralizing epitopes elsewhere on gp120 (4, 43). Our results extend these findings to include the herpesvirus family and demonstrate masking of neutralizing domains by neighboring glycoproteins.
We previously reported that the HSV-1 FcγR protects the virus by antibody bridging, which blocks functions mediated by the IgG Fc domain, while gC binds C3b to inhibit complement activation (8, 10, 11, 15, 23-26). The current study reveals an additional immune evasion mechanism in which gC and gE prevent antibodies from disrupting the interactions between gB, gD, and gH/gL. The relative importance of these diverse immunity evasion strategies cannot be assessed by the current study; however, based on our prior results, we postulate that the virus may use different mechanisms depending on antibody concentrations (20, 24). Our findings also reveal a novel mechanism for HSV-1 neutralization by suggesting that antibodies prevent interactions between viral glycoproteins required for fusion and entry.
Acknowledgments
This work was supported by Public Health Service grants AI33063 and T32-AI07324 from the National Institute of Allergy and Infectious Diseases, by DE14152 from the National Institute of Dental and Craniofacial Research, and HL28220 from the National Heart Lung and Blood Institute.
We thank Roselyn Eisenberg and Gary Cohen for providing the rabbit antibodies to gB, gD, gH/gL, and VP5, the University of Pennsylvania Center for AIDS Research clinical core for providing serum from HIV subjects, and Robert Gross for advice on the sample sizes for the various HIV-HSV-1 coinfection cohorts.
Footnotes
Published ahead of print on 14 May 2008.
REFERENCES
- 1.Atanasiu, D., J. C. Whitbeck, T. M. Cairns, B. Reilly, G. H. Cohen, and R. J. Eisenberg. 2007. Bimolecular complementation reveals that glycoproteins gB and gH/gL of herpes simplex virus interact with each other during cell fusion. Proc. Natl. Acad. Sci. USA 10418718-18723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bender, F. C., J. C. Whitbeck, M. Ponce de Leon, H. Lou, R. J. Eisenberg, and G. H. Cohen. 2003. Specific association of glycoprotein B with lipid rafts during herpes simplex virus entry. J. Virol. 779542-9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benedetti, J. K., J. Zeh, and L. Corey. 1999. Clinical reactivation of genital herpes simplex virus infection decreases in frequency over time. Ann. Intern. Med. 13114-20. [DOI] [PubMed] [Google Scholar]
- 4.Burton, D. R., R. C. Desrosiers, R. W. Doms, W. C. Koff, P. D. Kwong, J. P. Moore, G. J. Nabel, J. Sodroski, I. A. Wilson, and R. T. Wyatt. 2004. HIV vaccine design and the neutralizing antibody problem. Nat. Immunol. 5233-236. [DOI] [PubMed] [Google Scholar]
- 5.Calistri, A., C. Parolin, and G. Palu. 2003. Herpes simplex virus type 1 can either suppress or enhance human immunodeficiency virus type 1 replication in CD4-positive T lymphocytes. J. Med. Virol. 70163-170. [DOI] [PubMed] [Google Scholar]
- 6.Calistri, A., C. Parolin, M. Pizzato, P. Calvi, I. Giaretta, and G. Palu. 1999. Herpes simplex virus chronically infected human T lymphocytes are susceptible to HIV-1 superinfection and support HIV-1 pseudotyping. J. Acquir. Immune Defic. Syndr. 2190-98. [PubMed] [Google Scholar]
- 7.Chang, Y. J., M. Jiang, J. M. Lubinski, R. D. King, and H. M. Friedman. 2005. Implications for herpes simplex virus vaccine strategies based on antibodies produced to herpes simplex virus type 1 glycoprotein gC immune evasion domains. Vaccine 234658-4665. [DOI] [PubMed] [Google Scholar]
- 8.Dubin, G., E. Socolof, I. Frank, and H. M. Friedman. 1991. Herpes simplex virus type 1 Fc receptor protects infected cells from antibody-dependent cellular cytotoxicity. J. Virol. 657046-7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisenberg, R. J., M. Ponce de Leon, H. M. Friedman, L. F. Fries, M. M. Frank, J. C. Hastings, and G. H. Cohen. 1987. Complement component C3b binds directly to purified glycoprotein C of herpes simplex virus types 1 and 2. Microb. Pathog. 3423-435. [DOI] [PubMed] [Google Scholar]
- 10.Frank, I., and H. M. Friedman. 1989. A novel function of the herpes simplex virus type 1 Fc receptor: participation in bipolar bridging of antiviral immunoglobulin G. J. Virol. 634479-4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman, H. M., G. H. Cohen, R. J. Eisenberg, C. A. Seidel, and D. B. Cines. 1984. Glycoprotein C of herpes simplex virus 1 acts as a receptor for the C3b complement component on infected cells. Nature 309633-635. [DOI] [PubMed] [Google Scholar]
- 12.Friedman, H. M., E. J. Macarak, R. R. MacGregor, J. Wolfe, and N. A. Kefalides. 1981. Virus infection of endothelial cells. J. Infect. Dis. 143266-273. [DOI] [PubMed] [Google Scholar]
- 13.Friedman, H. M., L. Wang, N. O. Fishman, J. D. Lambris, R. J. Eisenberg, G. H. Cohen, and J. Lubinski. 1996. Immune evasion properties of herpes simplex virus type 1 glycoprotein gC. J. Virol. 704253-4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman, H. M., L. Wang, M. K. Pangburn, J. D. Lambris, and J. Lubinski. 2000. Novel mechanism of antibody-independent complement neutralization of herpes simplex virus type 1. J. Immunol. 1654528-4536. [DOI] [PubMed] [Google Scholar]
- 15.Fries, L. F., H. M. Friedman, G. H. Cohen, R. J. Eisenberg, C. H. Hammer, and M. M. Frank. 1986. Glycoprotein C of herpes simplex virus 1 is an inhibitor of the complement cascade. J. Immunol. 1371636-1641. [PubMed] [Google Scholar]
- 16.Fuller, A. O., and W. C. Lee. 1992. Herpes simplex virus type 1 entry through a cascade of virus-cell interactions requires different roles of gD and gH in penetration. J. Virol. 665002-5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Handler, C. G., R. J. Eisenberg, and G. H. Cohen. 1996. Oligomeric structure of glycoproteins in herpes simplex virus type 1. J. Virol. 706067-6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herold, B. C., D. WuDunn, N. Soltys, and P. G. Spear. 1991. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J. Virol. 651090-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hung, S. L., C. Peng, I. Kostavasili, H. M. Friedman, J. D. Lambris, R. J. Eisenberg, and G. H. Cohen. 1994. The interaction of glycoprotein C of herpes simplex virus types 1 and 2 with the alternative complement pathway. Virology 203299-312. [DOI] [PubMed] [Google Scholar]
- 20.Judson, K. A., J. M. Lubinski, M. Jiang, Y. Chang, R. J. Eisenberg, G. H. Cohen, and H. M. Friedman. 2003. Blocking immune evasion as a novel approach for prevention and treatment of herpes simplex virus infection. J. Virol. 7712639-12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin, X., J. M. Lubinski, and H. M. Friedman. 2004. Immunization strategies to block the herpes simplex virus type 1 immunoglobulin G Fc receptor. J. Virol. 782562-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lubinski, J., T. Nagashunmugam, and H. M. Friedman. 1998. Viral interference with antibody and complement. Semin. Cell Dev. Biol. 9329-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lubinski, J., L. Wang, D. Mastellos, A. Sahu, J. D. Lambris, and H. M. Friedman. 1999. In vivo role of complement-interacting domains of herpes simplex virus type 1 glycoprotein gC. J. Exp. Med. 1901637-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lubinski, J. M., M. Jiang, L. Hook, Y. Chang, C. Sarver, D. Mastellos, J. D. Lambris, G. H. Cohen, R. J. Eisenberg, and H. M. Friedman. 2002. Herpes simplex virus type 1 evades the effects of antibody and complement in vivo. J. Virol. 769232-9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lubinski, J. M., L. Wang, A. M. Soulika, R. Burger, R. A. Wetsel, H. Colten, G. H. Cohen, R. J. Eisenberg, J. D. Lambris, and H. M. Friedman. 1998. Herpes simplex virus type 1 glycoprotein gC mediates immune evasion in vivo. J. Virol. 728257-8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagashunmugam, T., J. Lubinski, L. Wang, L. T. Goldstein, B. S. Weeks, P. Sundaresan, E. H. Kang, G. Dubin, and H. M. Friedman. 1998. In vivo immune evasion mediated by the herpes simplex virus type 1 immunoglobulin G Fc receptor. J. Virol. 725351-5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagot, N., A. Ouedraogo, V. Foulongne, I. Konate, H. A. Weiss, L. Vergne, M. C. Defer, D. Djagbare, A. Sanon, J. B. Andonaba, P. Becquart, M. Segondy, R. Vallo, A. Sawadogo, P. Van de Perre, and P. Mayaud. 2007. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. N. Engl. J. Med. 356790-799. [DOI] [PubMed] [Google Scholar]
- 28.Peng, T., M. Ponce de Leon, M. J. Novotny, H. Jiang, J. D. Lambris, G. Dubin, P. G. Spear, G. H. Cohen, and R. J. Eisenberg. 1998. Structural and antigenic analysis of a truncated form of the herpes simplex virus glycoprotein gH-gL complex. J. Virol. 726092-6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reynolds, S. J., and T. C. Quinn. 2005. Developments in STD/HIV interactions: the intertwining epidemics of HIV and HSV-2. Infect. Dis. Clin. North Am. 19415-425. [DOI] [PubMed] [Google Scholar]
- 30.Seidel-Dugan, C., M. Ponce de Leon, H. M. Friedman, R. J. Eisenberg, and G. H. Cohen. 1990. Identification of C3b-binding regions on herpes simplex virus type 2 glycoprotein C. J. Virol. 641897-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seidel-Dugan, C., M. Ponce de Leon, H. M. Friedman, L. F. Fries, M. M. Frank, G. H. Cohen, and R. J. Eisenberg. 1988. C3b receptor activity on transfected cells expressing glycoprotein C of herpes simplex virus types 1 and 2. J. Virol. 624027-4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sisk, W. P., J. D. Bradley, R. J. Leipold, A. M. Stoltzfus, M. Ponce de Leon, M. Hilf, C. Peng, G. H. Cohen, and R. J. Eisenberg. 1994. High-level expression and purification of secreted forms of herpes simplex virus type 1 glycoprotein gD synthesized by baculovirus-infected insect cells. J. Virol. 68766-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith, J. S., and N. J. Robinson. 2002. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J. Infect. Dis. 186S3-28. [DOI] [PubMed] [Google Scholar]
- 34.Spear, P. G., R. J. Eisenberg, and G. H. Cohen. 2000. Three classes of cell surface receptors for alphaherpesvirus entry. Virology 2751-8. [DOI] [PubMed] [Google Scholar]
- 35.Spear, P. G., and R. Longnecker. 2003. Herpesvirus entry: an update. J. Virol. 7710179-10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stanberry, L. R., S. L. Spruance, A. L. Cunningham, D. I. Bernstein, A. Mindel, S. Sacks, S. Tyring, F. Y. Aoki, M. Slaoui, M. Denis, P. Vandepapeliere, G. Dubin, and G. GlaxoSmithKline Herpes Vaccine Efficacy Study. 2002. Glycoprotein-d-adjuvant vaccine to prevent genital herpes. N. Engl. J. Med. 3471652-1661. [DOI] [PubMed] [Google Scholar]
- 37.Tal-Singer, R., C. Peng, M. Ponce De Leon, W. R. Abrams, B. W. Banfield, F. Tufaro, G. H. Cohen, and R. J. Eisenberg. 1995. Interaction of herpes simplex virus glycoprotein gC with mammalian cell surface molecules. J. Virol. 694471-4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turner, A., B. Bruun, T. Minson, and H. Browne. 1998. Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J. Virol. 72873-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wald, A., and K. Link. 2002. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J. Infect. Dis. 18545-52. [DOI] [PubMed] [Google Scholar]
- 40.Wald, A., J. Zeh, S. Selke, R. L. Ashley, and L. Corey. 1995. Virologic characteristics of subclinical and symptomatic genital herpes infections. N. Engl. J. Med. 333770-775. [DOI] [PubMed] [Google Scholar]
- 41.Wang, F., W. Tang, H. M. McGraw, J. Bennett, L. W. Enquist, and H. M. Friedman. 2005. Herpes simplex virus type 1 glycoprotein E is required for axonal localization of capsid, tegument, and membrane glycoproteins. J. Virol. 7913362-13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wasserheit, J. N. 1992. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex Transm. Dis. 1961-77. [PubMed] [Google Scholar]
- 43.Wyatt, R., N. Sullivan, M. Thali, H. Repke, D. Ho, J. Robinson, M. Posner, and J. Sodroski. 1993. Functional and immunologic characterization of human immunodeficiency virus type 1 envelope glycoproteins containing deletions of the major variable regions. J. Virol. 674557-4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu, F., M. R. Sternberg, B. J. Kottiri, G. M. McQuillan, F. K. Lee, A. J. Nahmias, S. M. Berman, and L. E. Markowitz. 2006. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA 296964-973. [DOI] [PubMed] [Google Scholar]